 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fruit pomaces are agro-industrial by-products obtained in large quantities by the juice production industry, hence their reutilization is a suitable way to minimize environmental impact by transforming them into different value-added products. Considering the wide abundance and bioactive potential of berries pomaces, raspberry, strawberry, blackcurrant, and chokeberry pomace were chosen to examine and compare polyphenol content, antioxidant activity, and skin prebiotic capacity of their extracts. Extracts of 5.28 to 13.39 mg GAE ⋅ g−1 DM total polyphenol content were obtained, with significant differences in polyphenol abundance between classes. Relative antioxidant capacity index and global antioxidant score revealed that the blackcurrant pomace extract exhibited the highest antioxidant capacity. Staphylococcus epidermidis growth was promoted by lower extract concentrations, while higher concentrations showed inhibitory effects. On the other hand, Staphylococcus aureus was inhibited more strongly and no stimulation was detected in the tested concentration range (.025–.1 mg GAE ⋅ mL−1). In co-cultured experiments, a S. aureus/S. epidermidis rebalancing effect was proven for all tested extracts, whereas the best prebiotic capacity of 2.84 was achieved at .05 mg GAE⋅ mL−1 of raspberry pomace extract. In conclusion, berry pomace extracts could be valorized by extraction of their phytochemicals which are potent antioxidants and emerging prebiotics for topical application.
Introduction
Environmental sustainability is one of the most pressing issues confronting modern society, therefore valorizing by-products from various industries has arisen as a promising strategy to minimize waste and develop products with additional value (Iqbal et al., Citation2021). Fruit processing contributes significantly to the global food industry, but generates large amounts of pomace – the mushy residue consisting of peels, pulp, flesh, skin, stems, and seeds that remains after juice extraction (Struck et al., Citation2016). Although generally regarded as waste, in recent years fruit pomace has received increasing attention due to its unrealized potential. So far, pomace is known to be used as animal feed, an organic fertilizer, biofuel as well as food ingredient due to its diverse bioactive compounds that can promote health (Iqbal et al., Citation2021; Nirmal et al., Citation2023; Struck et al., Citation2016). The complex composition of fruit pomaces includes high concentrations of polysaccharides and phytochemical, especially polyphenols (Iqbal et al., Citation2021), which can improve the appearance and health of the skin as cosmetic compounds (Barbulova et al., Citation2015; Osorio et al., Citation2021). Polysaccharides act as natural moisturizers helping in water retention, enhancing hydration and skin barrier function (Yao and Xu, Citation2022). On the other hand, polyphenols are potent antioxidants that protect skin from oxidative stress and premature aging (Osorio et al., Citation2021). However, before applying these bioactive compounds of pomace in skin products, it is essential to isolate them through a careful extraction process (Caponio et al., Citation2023; Osorio et al., Citation2021; Plainfossé et al., Citation2020).
In addition to bioactive components, the skin microbiota, a complex ecosystem of microorganisms that reside on the surface of the skin, has increasingly attracted the interest of the cosmetic industry. Maintaining skin integrity depends on the balance between skin microbiota members. If this balance is disrupted, it may result in less microbial diversity and, consequently, to the skin disorders like psoriasis, atopic dermatitis, and acne (Byrd et al., Citation2018; Zhou et al., Citation2020).
Staphylococcus aureus and Staphylococcus epidermidis are typical species found in the skin microbiota, which is primarily composed of bacteria (Yang et al., Citation2022). S. aureus, an opportunistic pathogen, usually colonizes skin surface without hindering host health, but under certain circumstances, it can cause skin infections or exacerbate existing disorders (Edslev et al., Citation2020; Yang et al., Citation2022). On the other hand, S. epidermidis is regarded as commensal, and can prevent growth of pathogens by secreting antimicrobial compounds, activating the host’s immune response, or competing with pathogens for nutrients and space (Edslev et al., Citation2020; Severn and Horswill, Citation2022).
Therefore, in order to keep healthy skin, it is important to maintain a normal microbiota with the presence of beneficial bacteria like S. epidermidis. If S. aureus becomes overabundant, as in severe atopic dermatitis, antibiotics can be used as treatment. However, antibiotics would reduce the number of all bacteria present. Prebiotics, compounds that stimulate the growth and activity of beneficial bacteria, while inhibiting the overgrowth of harmful bacteria, are a more favorable option (Krutmann, Citation2009; Petrov et al., Citation2022). Considering that skin prebiotics are still under extensive research, besides oligosaccharides (Di Lodovico et al., Citation2020; Petrov et al., Citation2022), there are promising indications that plant extracts rich in polyphenols could be used for this purpose (Martel et al., Citation2020).
The goal of this study was to evaluate extracts of four different berry pomaces, blackcurrant (Ribes nigrum L.), raspberry (Rubus idaeus), strawberry (Fragaria × ananassa), and chokeberry (Aronia melanocarpa), as potential antioxidant cosmetic ingredients with skin microbiota rebalancing properties. Utilization of these berries as cosmetic ingredients is widespread due to their ability to protect skin from premature aging or UV damage (Gasparrini et al., Citation2017; Plainfossé et al., Citation2020). However, to the best of our knowledge, the applicability of their pomace extracts as skin prebiotics in bioactive cosmetics has not been investigated. Comparative analysis of their antioxidant capacities and bioactive contents has also not been previously performed. In this study, the extraction was performed in an organic solvent-free medium and the extracts obtained were characterized in terms of total polyphenol, flavonoid, flavonol, anthocyanin, phenolic acid, hydrolysable tannin, condensed tannin and reducing sugar content. In vitro antioxidant activity was determined by DPPH, ABTS, CUPRAC, and FRAP methods, which were integrated by using relative antioxidant capacity index (RACI) and global antioxidant score (GAS) in order to get a ranking of the antioxidant capacities of tested extracts. Afterward, their effects on the two most relevant skin Staphylococci members – beneficial S. epidermidis and pathogenic S. aureus were investigated at different concentrations. Finally, the two microorganisms were co-inoculated and the prebiotic effect of tested extracts was evaluated after 24 h of growth.
Materials and Methods
Plant Material
Pomaces of blackcurrant (Ben Nevis cultivar), raspberry (Fertodi Zamatos cultivar), strawberry (Senga Sengana cultivar) and chokeberry (Nero cultivar) were kind donation from Floriva (Ivanjica, Serbia). The propagated plantlets of all berries were from mother plants located in the experimental field of the Floriva (Ivanjica, Serbia), which are under the constant professional supervision of the authorized institutions of the State of Serbia and the Netherlands Inspection Service of Horticulture (Naktuinbouw) which guarantee high quality. Blackcurrant was planted in 2019, while raspberry, strawberry and chokeberry were planted in 2021 in Ivanjica (Serbia, 43° 34’N, 20° 13’ E) which has moderate-continental climate. All berries were harvested at full maturity in 2022, with harvesting taking place every 2 days during second half of April and first half of May for strawberries, first half of July for raspberries, and second half of August for blackcurrants and chokeberries. For each berry fruit, samples were obtained by mixing berries from three individual harvests and stored at −20°C. In October 2022, all berry samples were used for juice production, and pomaces were separated and stored at −20°C. Obtained pomaces were lyophilized in a vacuum freeze dryer (Beta 1–8 Freeze Dryer, Martin Christ, GmbH, Osteroide am Harz, Germany) and used in experiments that were conducted in March and April 2023.
Chemicals
Sodium molybdate dihydrate, 3,5-dinitrosalicylic acid and, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), neocuproine, sodium molybdate dihydrate, and caffeic acid were purchased from Sigma-Aldrich (Schnelldorf, Germany). Gallic acid, quercetin, and glucose were obtained from Merck (Darmstadt, Germany). Folin – Ciocalteu phenol reagent was from Carlo Erba (Arese, Italy). Glacial acetic acid and hydrochloric acid were purchased from Zorka Pharma (Šabac, Serbia). Copper chloride, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid diammonium salt (ABTS) were purchased from Thermo Fisher Scientific (Geel, Belgium). All other chemicals and solvent methanol and n-butanol, were obtained from Centrohem (Stara Pazova, Serbia). All used chemicals and solvents were analytical grade. Trypticase soy broth, agar, yeast extract, and mannitol salt agar were purchased from the Institute Torlak (Belgrade, Serbia). Bacterial strains Staphylococcus epidermidis DSM 20,044 and Staphylococcus aureus ATCC 25,923 were purchased from Leibniz Institute DSMZ (German Collection of Microorganisms and Cell Culture GmbH, Braunschweig, Germany) and American Type Culture Collection (Rockville, Maryland, USA), respectively.
Methods
The Extraction of Bioactive Compounds from Pomaces
Before the extraction, lyophilized blackcurrant, chokeberry, red raspberry, and strawberry pomaces were ground in a mill (Retsch MM 400, Retsch GmbH, Haan, Germany) to a powder. A 10 g of sample of each powdered pomace was macerated with 100 mL of .1 M acetate buffer (pH 4.5) at 50°C with constant stirring at 150 rpm during 2 h. Then the precipitates were separated by centrifugation at 6000 rpm for 15 min and the supernatants were collected. This process was repeated three times for each pomace and all prepared extracts were stored at −20°C until analysis.
Extracts Composition Analysis
The total polyphenol content (TPC) in the extracts was assessed using a modified Folin-Ciocalteu method (Milutinović et al., Citation2015). In summary, diluted extracts (50 µL) were mixed with Folin-Ciocalteu reagent (250 µl), 15% sodium carbonate solution (1 mL), and distilled water (3.7 mL). After an incubation in the dark for 2 h, the absorbance was measured at 750 nm using buffer instead of extract as a blank sample. The results were expressed as milligrams of gallic acid equivalents (GAE) per gram of dry matter (DM) of the pomace.
The total flavonoid content (TFC) in the extracts was evaluated using the aluminum chloride colorimetric method (Marjanovic et al., Citation2021). A diluted extract (1 mL) was mixed with methanol (1.5 mL) and vortexed. After that, 10% aluminum chloride solution (.1 mL), 1 M sodium acetate solution (.1 mL) and water (2.8 mL) was added, and the mixture was then incubated in the dark for 30 min. The absorbance of the samples was measured at 415 nm against a blank sample containing buffer instead of the extract. The results were presented as milligrams of quercetin equivalents (QE) per gram of dry matter (DM) of the pomace.
The total anthocyanin content (TAC) in extracts was obtained by the pH differential method (Marjanovic et al., Citation2021). A diluted extract (.5 mL) was mixed with .025 M potassium chloride buffer pH 1.0 (1.5 mL), and simultaneously, another portion (.5 mL) of the same extract was combined with .4 M sodium acetate buffer pH 4.5 (1.5 mL). Both mixtures were vortexed for 30 s and incubated for 15 min in dark. The absorbance of samples was read at 520 and 700 nm, against buffer as a blank. The total anthocyanins concentration (C), expressed as mg of cyanidin-3-O-glucoside equivalents per L, was calculated as follows:
where A is absorbance, determined as A = (A520 – A700) pH 1.0 – (A520 – A700) pH 4.5; MW is a molecular weight of cyanidin-3-O-glucoside (449.2 g ⋅ mol−1); R is a dilution factor; ε is molar extinction coefficient for cyanidin-3-O-glucoside (26900 L ⋅ mol−1⋅ cm−1) and l is path length (1 cm). The final results were expressed as milligrams of cyanidin-3-O-glucoside equivalents (Cy3GE) per gram dry matter (DM) of the pomace.
The total flavonol content (TFLC) in the extracts was determined using the method reported previously with slight modifications (Formagio et al., Citation2014). The mixture of diluted extract (.5 mL), 2% aluminum chloride solution in methanol (.5 mL) and 5% sodium acetate solution (1.5 mL) were incubated in the dark for 2.5 h, after which the absorbance was measured at 440 nm against blank. The results were expressed as milligrams of quercetin equivalents (QE) per gram of dry matter (DM) of the pomace.
The total phenolic acid content (TPAC) in the extract was quantified using the method with Arnow’s reagent (Boroja et al., Citation2018). This reagent was prepared by dissolving 10 g of sodium nitrite and 10 g of sodium molybdate dihydrate up to 100 mL of distillate water. The mixture of diluted extract (.1 mL), distilled water (.6 mL), .5 M hydrochloric acid (.1 mL), Arnow’s reagent (.1 mL) and 1 M natrium hydroxide (.1 mL) was vortexed for 30 s and the absorbance was immediately read at 490 nm. The results were expressed as milligrams of caffeic acid (CAE) per gram of dry matter (DM) of pomace.
Total hydrolysable tannins content (THTC) in the extract was determined by the potassium iodate solution method (Willis and Allen, Citation1998). The first step consisted of heating 2.5% potassium iodate solution (2.5 mL) for 7 min at 30°C, before adding extract (.5 mL). Then, the mixture was heated for additional 2 min at 30°C and the absorbance was measured at 550 nm. The total amount of tannins in the sample was expressed in milligrams of gallic acid equivalents (GAE) per gram of dry matter (DM) of pomace.
Total condensed tannins content (TCTC) in the extract was determined by the n-butanol- hydrochloric acid assay (Vermerris and Nicholson, Citation2009). The extract (.5 mL) was combined with (5 mL) of an acidic ferrous sulfate solution, prepared by dissolving 77 mg of ferrous sulfate heptahydrate dissolved in 500 mL of 2:3 hydrochloric acid/n-butanol. The mixture was heated in a water bath at 95°C for 15 minutes and absorbance was read at 530 nm. The concentration of condensed tannins was calculated as milligrams of cyanidin equivalents (CyE) per gram of dry matter (DM) of pomace using molar extinction coefficient (34700 L ⋅ mol−1⋅ cm−1) and molar mass (287.28 g ⋅ mol−1) of cyanidin.
The total reducing sugar content in extracts was assessed by the 3,5-dinitrosalicylic acid (DNS) method (Khatri and Chhetri, Citation2020). After adding DNS reagent (.25 mL) to a diluted sample (.25 mL), the test tube with a mixture was treated in boiling water for 5 min. Then, water was added (2 mL) and the absorbances were measured at 540 nm against the blank prepared using buffer instead of a sample. The results were expressed as millimole glucose equivalents (mmol GE) per gram of dry matter (DM) of pomace.
Antioxidant Activity Analysis
The ferric reducing antioxidant power (FRAP) method was utilized to assess the antioxidant activity of the extracts (Milutinović et al., Citation2015). FRAP reagent was prepared by mixing 300 mM sodium acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM hydrochloric acid, and 20 mM ferric chloride hexahydrate solution in a 10:1:1 (v/v/v) ratio, respectively. Then, fresh FRAP reagent (1.5 mL) was added to diluted extract (50 µL), incubated in the dark for 5 min and the absorbance of the samples was measured at 593 nm against the FRAP reagent. The final results were expressed as micromoles of Trolox equivalent (TE) per gram of dry matter (DM) of pomace.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay (Milutinović et al., Citation2015; Petrovic et al., Citation2016) was performed to evaluate antioxidant activity of extracts. Briefly, diluted extract (50 µL) was mixed with methanol (3.95 mL) and .2 mM DPPH solution (1 mL), incubated in the dark for 30 min, and the absorbance was determined at 517 nm against methanol as a blank sample. A Trolox calibration curve was plotted as a function of the percentage of inhibition of DPPH radical using the following equation:
where A control is an absorbance of control prepared with water instead of extract and A sample is an absorbance of tested sample. The results were expressed as the micromoles Trolox equivalent (TE) per gram of dry matter (DM) of pomace.
The antioxidant activity of the extracts was evaluated using the 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging assay with minor modifications (Petrovic et al., Citation2016). The ABTS •+ stock solution, made by mixing equal volumes of 14 mM ABTS and 4.9 mM potassium persulfate in 5 mM phosphate-buffered saline (PBS) pH 7.4, then allowed to equilibrate in the dark for 16 h. The working ABTS •+ solution was prepared by diluting the stock solution in PBS with absorbance .7 ± .02 at 734 nm. The reaction mixture containing diluted extract (10 µL) and working ABTS •+ solution (1 mL) was vortexed for 30 s and incubated in the dark for 5 min. The absorbance was measured at 734 nm using PBS buffer as a blank sample. A Trolox calibration curve was plotted as a function of the percentage of inhibition of ABTS radical using EquationEquation 2(2)
(2) . where A control is the absorbance of control prepared with PBS buffer instead of extract and A sample is the absorbance of the tested sample. The results were expressed as the micromoles Trolox equivalent (TE) per gram of dry matter (DM) of pomace.
The antioxidant activity of the extracts was also evaluated using the cupric ion reducing antioxidant capacity (CUPRAC) assay (Darwish et al., Citation2021). In brief, the diluted extract (100 μL) was mixed with 10 mM copper chloride solution (1 mL), 7.5 mM neocuproine alcoholic solution (1 mL), 1 M ammonium acetate buffer solution pH 7.0 (1 mL), and distilled water (1 mL). After incubation for 30 minutes, the absorbance was measured at 450 nm against the blank. The results were expressed as the micromoles Trolox equivalent (TE) per gram of dry matter (DM) of pomace.
Relative antioxidant capacity index (RACI) was calculated according to Sun and Tanumihardjo (Sun and Tanumihardjo, Citation2007) by averaging the standard scores (SS) transformed from the raw data generated by different methods for the determination of antioxidant activity. The SS is dimensionless value calculated by EquationEquation 3(3)
(3) :
where x is the µ raw data, µ is the mean and σ is the standard deviation. The SS enables the comparison of results with different units by indicating how many units a case is smaller or larger than the mean.
For each sample, the sum of T-scores (TS) was used to determine the global antioxidant score (GAS) (Tabart et al., Citation2014). TS was calculated by transformation of raw data according to EquationEquation 4(4)
(4) :
where Xmin and Xmax represent the smallest and largest values of variable X, respectively, among tested samples. GAS allows a ranking of antioxidant activity of samples, since the highest GAS corresponds to the highest antioxidant activity.
Skin Prebiotic Activity Assays
The effect of berry pomace extracts was evaluated on two gram-positive bacterial strains: Staphylococcus epidermidis DSM 20,044 and Staphylococcus aureus ATCC 25,923. The bacterial inoculums of both strains were prepared by transferring several colonies to tryptic soy broth containing .6% (w/v) yeast extract (TSBY) and allowed to grow at 37°C with agitation overnight. The effect of extracts on bacterial growth was determined during 24 h of cultivation by optical density (OD) measurement. Briefly, each extract was added to the fresh TSBY to obtain final polyphenols concentration of .025 mg GAE ⋅ mL−1, .05 mg GAE ⋅ mL−1, .0625 mg GAE ⋅ mL−1, .08 mg GAE ⋅ mL−1and .1 mg GAE ⋅ mL−1. Then, an overnight inoculum of S. epidermidis or S. aureus was added to the growth medium in order to achieve a final concentration of 107 CFU ⋅ mL−1 (using a standard curve that establishes the relationship between colony-forming units and optical density at 600 nm) and incubated at 37°C with constant shaking at 120 rpm. The growth curves were determined by measuring optical density of aliquots on 600 nm (OD600) at specific time points. The TSBY without extracts served as the control sample.
The prebiotic capacity (PC) of tested extracts was evaluated in co-culture experiment where S. epidermidis was co-inoculated with S. aureus in TSBY with addition of extracts as previously described. The concentration of each bacterium was 107 CFU ⋅ mL−1. After 24 h of cultivation, samples were taken, diluted with saline solution, and placed on mannitol salt agar (MSA). After 48 h of incubation at 37°C, the number of grown colonies was determined (bright yellow colonies of S. aureus with yellow zones and light pink colonies of S. epidermidis without changing the medium color) and compared to the control (sample without extract) (Di Lodovico et al., Citation2020). The PC of investigated extracts was assessed by comparing the growth of beneficial bacteria (S. epidermidis) measured with and without prebiotics to the growth of pathogenic bacteria (S. aureus) under the same conditions. It was calculated for each pomace extract, using EquationEquation 5(5)
(5) :
where SEE24 and SEE0 are CFU ⋅ mL−1 of S. epidermidis with extract after 24 h and 0 h of growth, respectively. SEC24 and SEC0 are the CFU ⋅ mL−1 of S. epidermidis without extracts (control sample), after 24 h and 0 h of growth, respectively. SAE24, SAE0 and SAC24, SAC0 are the S. aureus CFU ⋅ mL−1 with and without extracts after 24 h and 0 h, respectively.
Statistical Analysis
All experiments were performed with three independent samples of each pomace, except for the experiments related to the measurement of the optical density of the bacterial medium, which were performed in duplicate. All results were presented as mean ± standard deviation from the replicates. To establish the statistical significance of mean value differences for multiple comparisons of the results (a one-way analysis of variance (ANOVA) followed by a post hoc Tuckey’s test) OriginPro 8.5 (OriginLab Corporation, Northampton, MA, USA) was used. Differences at p ≤ .05 were regarded as significant. Pearson’s correlation coefficients (r) and linear regression equations were employed to investigate the relationship between various variables by using Microsoft Excel. A negative value for r implies a negative linear correlation, while positive value suggests a positive linear correlation. If the r value is |r| < .20 correlation is very weak; .20 < |r| < .39 correlation is weak; .40 < |r| < .59 correlation is moderate; .60 < |r| < .79 correlation is strong; |r| > .8 correlation is very strong (Fogarasi et al., Citation2021).
Results and Discussion
Berries Pomace Extracts Composition
Fruit extracts contain a large number of different polyphenolic compounds that could have health-promoting effect on human skin. Polyphenols found many applications in dermo-cosmetics due to their antioxidant, anti-inflammatory, anti-microbial, anticancer and anti-allergic activities (Rajha et al., Citation2022; Sun et al., Citation2022). Beyond maintaining skin health, they can prevent premature skin aging, control skin pigmentation through tyrosinase inhibition, improve skin elasticity via anti-collagenase, anti-elastase and anti-hyaluronidase activities, and contribute to effective wound healing, among other positive effects (De Lima Cherubim et al., Citation2019; Rajha et al., Citation2022; Sun et al., Citation2022).
The concentration of extracted polyphenols from the four berry pomaces ranged from 5.28 mg GAE ⋅ g−1 DM to 13.39 mg GAE ⋅ g−1 DM (). Total polyphenol content was highest in the chokeberry pomace extract (chokeberry), followed by blackcurrant pomace extract (blackcurrant), while raspberry pomace extract (raspberry) and strawberry pomace extract (strawberry) had approximately two times lower TPC than blackcurrant and chokeberry. Regarding flavonoids, anthocyanins, flavonols, phenolic acids and hydrolysable tannins, chokeberry emerged as the richest source. On the other hand, raspberry had lowest concentration of flavonoids and anthocyanins, with levels of flavonols and phenolic acids showing no significant difference (p > .05) compared to those found in strawberry. Interestingly, blackcurrant showed the lowest hydrolysable tannin levels but the highest content of condensed tannins among the extracts ().
Table 1. Berries pomace extracts composition.
Polyphenol content and profile in berries extracts is strongly dependent on the extraction method and fruit variety used. Some authors reported both significantly lower and higher polyphenol concentrations in berries pomaces extracts (2.71–25.40 mg GAE ⋅ g−1 DM) compared to the results obtained in the present study (Kavela et al., Citation2023; Pukalskienė et al., Citation2021; Yao et al., Citation2021). Wide range of the polyphenol concentration was reported in studies using fresh berries (4.89–19.09 mg GAE⋅ g−1 DM), as well (Georgescu et al., Citation2022; Kim, Citation2018; Su et al., Citation2023).
Regarding reducing sugars content, strawberry had highest concentration, then raspberry, followed by blackcurrant and chokeberry. Based on the comparison of obtained results with literature data (Kavela et al., Citation2023; Pukalskienė et al., Citation2021; Yao et al., Citation2021) in which 2.71–25.40 mg GAE ⋅ g−1 DM of polyphenols were extracted, we concluded that an organic-solvent-free method is providing satisfying polyphenol yields, especially when it comes to chokeberry (13.39 mg GAE ⋅ g−1 DM) and blackcurrant (11.26 mg GAE ⋅ g−1 DM) pomaces. Therefore, this approach was adopted as an environmentally sound and easy way to extract polyphenols from berries pomaces that would be used for examining their antioxidant and skin prebiotic potential.
Antioxidant Capacity of Berries Pomace Extracts
Antioxidants have the ability to prevent oxidative stress, which can harm cells and have a negative influence on health (Kotha et al., Citation2022). Oxidative stress occurs when there are more highly reactive compounds, known as free radicals, than antioxidants (Kotha et al., Citation2022; Neha et al., Citation2019). Polyphenols can neutralize free radicals, and their antioxidant activity is enhanced when with an increase in the number of phenolic hydroxyl groups they contain. Topical application of polyphenols suggests that they are effective in protecting the skin from UV radiation, resulting in reduced UV-induced skin photodamage and a lower risk of skin cancer (De Lima Cherubim et al., Citation2019; Rajha et al., Citation2022; Sun et al., Citation2022).
The highest antioxidant activity of 53.56 µmol TE ⋅ g−1 DM was achieved by blackcurrant according to the FRAP assay that assesses the ability of antioxidants to reduce ferric iron (Fe3+) to ferrous iron (Fe2+) (). This result differs significantly (p ≤ .05) from the antioxidant activity of other extracts, which was 20.84–33.87 µmol TE ⋅ g−1 DM. In the ABTS assay, blackcurrant and chokeberry showed the highest ABTS radical cation scavenging capacities, followed by strawberry and raspberry, with scores ranging from 27.22 to 72.81 µmol TE ⋅ g−1 DM. The CUPRAC assay confirmed that raspberry has the lowest antioxidant activity compared to the other tested samples, only 64.77 µmol TE ⋅ g−1 DM. Its ability to reduce cupric (Cu2+) ions to cuprous (Cu1+) ions was 63–65% lower than blackcurrant and chokeberry, the pomace extracts with the highest antioxidant activity among the tested samples. According to the DPPH test, the antioxidant activities of blackcurrant and chokeberry, as well as those of strawberry and raspberry, were comparable and not statistically different from each other (p > .05) ().
Table 2. Antioxidant capacity of berries pomace extracts.
The results from this study are consistent with findings from a previous study, which demonstrated that the antioxidant activity determined by the DPPH method, was similar between blackcurrant and chokeberry (67.0–68.2% inhibition of DPPH radicals) and nearly 1.8 times higher than strawberry (39.4% inhibition of DPPH radicals) (Pieszka et al., Citation2015). On the other hand, in a study that also examined the characteristics of several dried fruit pomaces by DPPH method, the antioxidant activity of the pomace infusion showed a decreasing trend as follows: chokeberry < strawberry < raspberry < blackcurrant (Pachołek et al., Citation2014). Discrepancies in the results can be attributed to the different composition of the pomaces, possibly influenced by cultivar, variations in degree of ripeness or in the fruit processing method, growing environment (year, location, cultivation method), age of pomace, etc.
The correlation coefficients, which range from .831 (between DPPH and FRAP method) to .965 (between DPPH and CUPRAC method), reveal a strong high and statistically significant positive correlation between the antioxidant tests (). This suggests that the antioxidant capacity determined by various procedures is very comparable, and compounds demonstrating strong antioxidant activity in one test are likely to demonstrate so in the others as well. When it comes to TPC, the correlations observed with the DPPH and CUPRAC methods (r = .963 and r = .920, respectively, p ≤ .01) were very strong, while the correlations with the ABTS and FRAP methods (r = .767, p ≤ .01 and r = .692, p ≤ .05, respectively) were strong.
Table 3. Correlation matrix among antioxidant capacities and polyphenols.
On the other hand, the FRAP assay showed a very strong positive correlation (r = .972, p ≤ .01) with TCTC (), indicating that this method is suitable for evaluating the antioxidant potential of a specific polyphenol class – condensed tannins. This could potentially explain the high antioxidant activity of blackcurrant, which was 36.76% higher compared to chokeberry and 2.6 and 2.2 times higher than raspberry and strawberry, respectively. Although its total polyphenol content and concentrations of other polyphenol classes are significantly lower compared to chokeberry, blackcurrant contains higher levels of condensed tannins (), and its antioxidant activity is significantly (p ≤ .05) greater as measured by the FRAP method (). In other words, condensed tannins may be responsible for the increased antioxidant activity of blackcurrant, as reported in previous studies (Pap et al., Citation2021; Paunović et al., Citation2022). Moreover, it is well documented that oligomerized forms of various flavonoids, type of polyphenol compounds which include condensed tannins, could exhibit significantly increased antioxidant capacity compared to its monomeric units, which is in line with results of the statistical analysis performed within the current study (De Lima Cherubim et al., Citation2019; Jadhaw and Singhal, Citation2014; Desentis-Mendoza et al., Citation2006). Additionally, TPC had a very strong positive correlation with all classes of polyphenols (r = .833–.989, p ≤ .01), except with THTC (r = .565, p > .05), which suggests that this type of tannin may not contribute significantly to the antioxidant activity of the extracts.
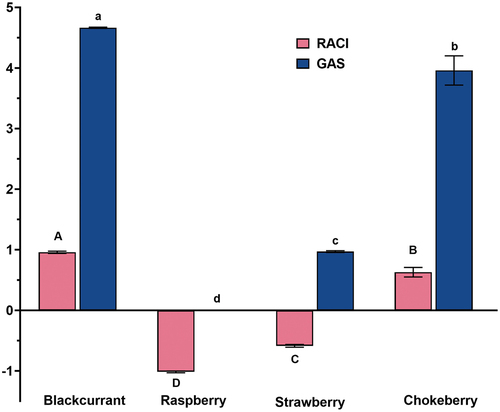
The highest positive RACI value was attributed to blackcurrant (.96), followed by chokeberry (.63) (). Conversely, negative RACI values were ascribed to strawberry (−.59) and raspberry (−1.01). Similarly, the GAS values exhibited the same order, with chokeberry and blackcurrant having values 3.98 and 4.68, respectively, which was significantly higher than raspberry (.00) and strawberry (.97) (). RACI and GAS represent statistical approaches that integrate antioxidant results obtained from different in vitro tests, offering a reasonably accurate ranking of the antioxidant capacity of tested samples (Faraone et al., Citation2018; Petrovic et al., Citation2016; Sun and Tanumihardjo, Citation2007; Tabart et al., Citation2014). Recent research suggests that the Folin-Ciocalteu reagent method, originally used for polyphenol determination, can also be employed as an alternative way to evaluate the total antioxidant activity of samples, so it was included in the calculation of dimensionless RACI and GAS (Faraone et al., Citation2018; Russo et al., Citation2015). The trend of matching order between GAS and RACI was observed in several studies (Giovagnoli-Vicuña et al., Citation2021; Todorovic et al., Citation2017). Based on the results of the current study, both RACI and GAS indicate the higher antioxidant capacity of blackcurrant and chokeberry compared to raspberry and strawberry (). As a result, it should be highlighted that the antioxidant activities of the pomace extracts, which show statistically significant differences, follow the order: blackcurrant > chokeberry > strawberry > raspberry.
Figure 1. Relative antioxidant capacity index (RACI) and global antioxidant score (GAS) of blackcurrant, raspberry, strawberry and chokeberry. Different lowercase and capital characters indicate statistically significant difference (p ≤ .05) considering RACI and GAS, respectively. Bars represent the mean values of three independent experiments, and error bars indicate the standard deviations.
Skin Prebiotic Potential of Berries Pomace Extracts
In order to examine influence of four extracts on skin microbiota composition, skin commensal (S. epidermidis) and opportunistic pathogen (S. aureus) were grown in medium with and without supplemented extracts in single culture and co-cultured experiments and their stimulatory/inhibitory effect on two microorganisms was monitored. These two bacterial strains were chosen since their imbalance is associated with one of the most common skin inflammatory diseases – atopic dermatitis (Edslev et al., Citation2020; Kim et al., Citation2019; Yang et al., Citation2022).
Influence on S. epidermidis Growth
All berry pomace extracts showed concentration-dependent effect on S. epidermidis growth (). Blackcurrant demonstrated the increase of microbial growth rate at concentrations of .025 and .05 mg GAE ⋅ mL−1, no effect at .0625 mg GAE ⋅ mL−1, and a decrease at concentrations of .08 and .1 mg GAE ⋅ mL−1. The other three extracts did not show significant effects on microbial growth rate for concentration ranging from .025 to .0625 mg GAE ⋅ mL−1. At a concentration of .08 mg GAE ⋅ mL−1 growth rate was moderately decreased for raspberry and strawberry, while further increases of extract concentration led to more pronounced reducing of the growth rate (81.8% for raspberry and 41.2% for strawberry). On the other hand, chokeberry demonstrated a slight growth rate decrease (12.3%) only at the highest extract concentration ().
Figure 2. Influence of: (a) blackcurrant, (b) raspberry, (c) strawberry and (d) chokeberry pomace extract on S. epidermidis growth. The legend symbols meaning: ![]()
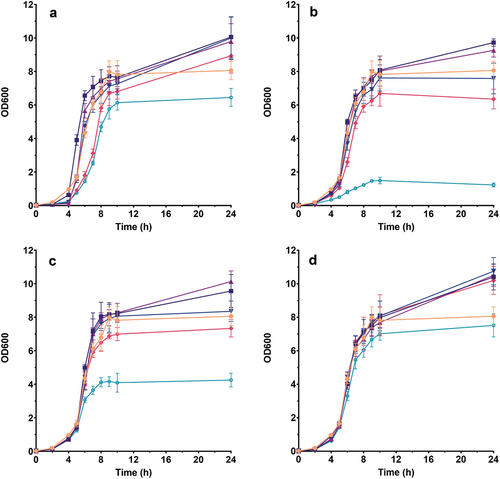
After 24 h of growth, blackcurrant and chokeberry led to S. epidermidis stimulation at all tested concentrations, except .1 mg GAE ⋅ mL−1 (). For blackcurrant, maximum stimulation was achieved at .025 mg GAE ⋅ mL−1GAE (24.7%), while there was no notable difference between concentrations in the range .025–.0625 mg GAE ⋅ mL−1 for chokeberry (~30% on average). Raspberry showed moderate stimulatory effect (20.1 and 14.9%) at the two lowest concentrations, and an increase in extract amounts resulted in an inhibition increase up to 84.8% at .1 mg GAE ⋅ mL−1. Similarly, strawberry did not influence OD600 value after 24 h at .0625 mg GAE ⋅ mL−1, lower concentrations led to ~ 20% stimulation, while higher amounts caused concentration-dependent inhibition (maximum of 47.3% at .1 mg GAE ⋅ mL−1) (). Based on the influence of four extracts on S. epidermidis growth at varying concentrations it is clear that a delicate balance between stimulatory and inhibitory effects is present which may be caused by their complexity. Namely, these extracts are mixtures of different polyphenols that can exert both positive and negative impacts on the human microbiome. A positive effect of certain polyphenols (coumaric acid, caffeic acid, vanillic acid, delphinidin, and malvidin glycosides) and polyphenol-rich extracts was previously observed on gut microbiota representatives via different metabolic pathways of degradation depending on their structure (Makarewicz et al., Citation2021). On the other hand, many studies proved the antimicrobial properties of polyphenol-rich extracts against both skin commensals and pathogens (Sun et al., Citation2022).
Influence on S. aureus Growth
All tested extracts demonstrated antimicrobial properties for S. aureus at all tested amounts above .025 mg GAE ⋅ mL−1 except for chokeberry which did not show significant influence at any of the examined concentrations (). Dose-dependent decrease of microbial growth rate was detected, with a maximum of 89.1% at .1 mg GAE ⋅ mL−1 for raspberry. The same trend was observed after 24 h of growth where raspberry demonstrated the highest inhibition degree (up to 91.9%), followed by strawberry (up to 61.6%), blackcurrant (up to 36.1%), and chokeberry (up to 33.5%). A similar effect of higher extract concentrations on the two microorganisms was observed – a stronger inhibitory effect by strawberry and raspberry and milder inhibition by blackcurrant and chokeberry ().
Figure 3. Influence of: (a) blackcurrant, (b) raspberry, (c) strawberry and (d) chokeberry pomace extract on S. aureus growth. The legend symbols meaning: ![]()
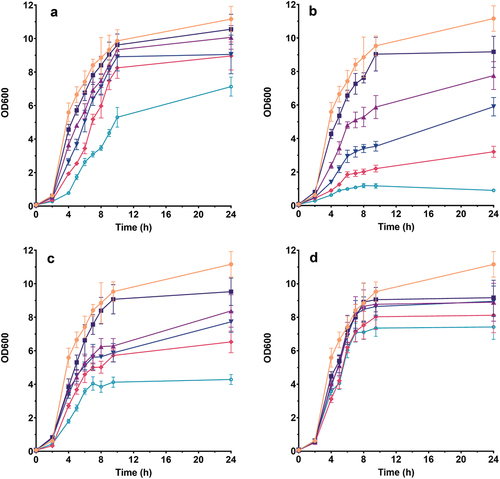
A higher inhibition degree of S. aureus was caused by tested extracts compared to S. epidermidis (). It was previously reported that some Gram-positive strains, including S. epidermidis and S. aureus, were sensitive to extracts rich in certain tannin types such as gallo-tannins. Pentagalloyl-O-β-D-glucose was associated with antimicrobial properties against two microorganisms, with bactericidal effect against S. aureus shown after only 3 h of incubation, while S. epidermidis was more resistant and 1.5 log reduction in the CFU number after 24 h was observed (Maisetta et al., Citation2019). Strawberries and raspberries are known as natural sources of galloyl glucose (https://foodb.ca/compounds/FDB000233); therefore, the pronounced inhibitory effect of their extracts against S. aureus could be associated with this particular compound.
All obtained results imply that finding specific conditions at which S. epidermidis growth will be promoted while S. aureus will be inhibited leading to their rebalancing is very complex, but crucial for future application. Since these two microorganisms live together on the human skin and it is known that they interact with each other, the final assessment of the prebiotic capacity of four pomace extracts was done in a co-cultured experiment.
Prebiotic Potential of Extracts of Co-Cultured Medium
All tested extracts showed different PC values after 24 hours of growth in co-cultured medium and statistical analysis revealed different influence of extracts concentrations on their prebiotic potential (). Positive PC values imply that tested the compound possesses prebiotic potential against the examined strains, while a value below zero suggests a negative effect on the balance of tested microorganisms.
Figure 4. Prebiotic capacity of different berries pomace extracts concentrations in S. epidermidis and S. aureus co-culture. Different lowercase characters indicate statistically significant difference (p ≤ .05) between tested concentrations of each pomace extracts. Data represent the mean values of three independent experiments, and error bars indicate the standard deviations.
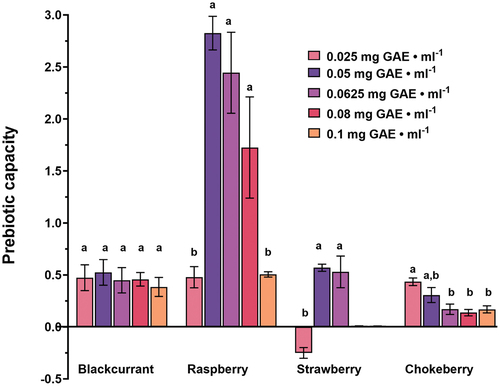
Strawberry was the only extract demonstrating a negative PC value indicating that the corresponding concentration (.025 mg GAE ⋅ mL−1) will alter the S. aureus/S. epidermis ratio in favor of the harmful microorganism. At high concentrations (.08 and .1 mg GAE ⋅ mL−1) this extract had no statistically significant influence on PC since both bacteria were strongly inhibited, while concentrations of .05 and .0625 mg GAE ⋅ mL−1 showed prebiotic potential with PC values around .5. Chokeberry showed the highest PC of around .5 at the lowest tested extract concentration, while higher concentrations demonstrated a less pronounced effect on the two microorganisms’ ratio. For blackcurrant extract, concentration did not show a statistically significant influence in the tested range and a PC plateau was observed at a value of ~ .5. The best results were obtained with raspberry extract which demonstrated a dose-dependent effect on PC values with sharp maximum at polyphenol concentration of .05 mg GAE ⋅ mL−1. The highest and lowest concentrations showed a moderately positive effect – PC of .5, while concentrations of .08, .0625 and .05 mg GAE ⋅ mL−1 gave 1.73, 2.44 and 2.85 values of PC, respectively (). This results accentuated raspberry as the most promising skin prebiotic source among tested extracts.
This result showed that previously documented antagonistic effect of S. epidermidis against S. aureus is even more pronounced in a medium supplemented with raspberry extract, and indicates a potential synbiotic effect of S. epidermidis and raspberry pomace extract (Woo and Sibley, Citation2020). In addition to polyphenols and gallo-tannins, raspberry extract is known as a source of xylitol, a proven skin prebiotic. Xylitol demonstrated antimicrobial and antibiofilm effects on S. aureus, and had no statistically significant impact on S. epidermidis when combined with various oligosaccharides, suggesting a possible explanation for the high PC value observed in the current study (Di Lodovico et al., Citation2020; Woo and Sibley, Citation2020). Since the amount of raspberry used was two times higher than that of blackcurrant and chokeberry (due to its lower polyphenol content), it probably provided a suitable ratio of all biomolecules which influenced microbial growth and was thus singled out as the best potential skin prebiotic.
Conclusions
Almost half of all the fruit and vegetables produced are wasted, therefore it is clear that the valorization of their agro-industrial by-products is of global importance. This study proved that blackcurrant, raspberry, strawberry, and chokeberry pomace from the juice production industry can be reutilized. Organic solvent-free extraction of these berry pomaces yielded polyphenol-rich extracts with demonstrated antioxidant activity. RACI and GAS statistical analysis revealed that blackcurrant is the most powerful antioxidant among tested extracts. All extracts demonstrated a dose-dependent effect on skin beneficial (S. epidermidis) and harmful (S. aureus) bacteria growth, whereas raspberry pomace extract showed the highest potential based on prebiotic capacity achieved in co-culture of the two microorganisms. Overall, the results provide strong evidence for berries pomaces as valuable sources of bioactive compounds and suggest potential application in functional foods and prebiotic cosmetics. Future investigations would be directed toward additional extraction process optimization in order to maximize its efficiency. Furthermore, a detailed prebiotic activity screening of berry pomace extracts on in vitro skin models and other cutaneous microbiota representatives should be performed to obtain more comprehensive evidence of these extracts on the entire skin microbiome.
Abbreviation
| TPC | = | Total polyphenol content |
| TFC | = | Total flavonoid content |
| TPC | = | Total anthocyanin content |
| TFLC | = | Total flavonol content |
| TPAC | = | Total phenolic acid content |
| THTC | = | Total hydrolysable tannin content |
| TCTC | = | Total condensed tannin content |
| Cy3GE | = | Cyanidin-3-O-glucoside equivalent |
| CyE | = | Cyanidin equivalent |
| TE | = | Trolox equivalent |
| Blackcurrant | = | Blackcurrant pomace extract |
| Chokeberry | = | Chokeberry pomace extract |
| Raspberry | = | Raspberry pomace extract |
| Strawberry | = | Strawberry pomace extract |
| RACI | = | Relative antioxidant capacity index |
| GAS | = | Global antioxidant score |
| PC | = | Prebiotic capacity |
| TSBY | = | Tripton soy broth with yeast |
| MSA | = | Mannitol salt agar |
Acknowledgments
The authors acknowledge and thank to Floriva (Ivanjica, Serbia) for their kind donation of blackcurrant, chokeberry, strawberry and raspberry pomaces. Icons in the graphical abstract were made by Marcovector from http://www.freepik.com and Dinosoftlab from http://www.flaticon.com.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barbulova, A., G. Colucci, and F. Apone. 2015. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2(2):82–92. doi: 10.3390/cosmetics2020082.
- Boroja, T., V. Mihailović, J. Katanić, S.P. Pan, S. Nikles, P. Imbimbo, D.M. Monti, N. Stanković, M.S. Stanković, and R. Bauer. 2018. The biological activities of roots and aerial parts of Alchemilla vulgaris L. S. Afr. J. Bot. 116:175–184. doi: 10.1016/j.sajb.2018.03.007.
- Byrd, A.L., Y. Belkaid, and J.A. Segre. 2018. The human skin microbiome. Nat. Rev. Microbiol. 16(3):143–155. doi: 10.1038/nrmicro.2017.157.
- Caponio, G.R., F. Minervini, G. Tamma, G. Gambacorta, and M. De Angelis. 2023. Promising application of grape pomace and its agri-food valorization: Source of bioactive molecules with beneficial effects. Sustain.(switzerland) 15(11):9075. doi: 10.3390/su15119075.
- Darwish, A.G., P.R. Das, A. Ismail, P. Gajjar, S.P. Balasubramani, M.B. Sheikh, V. Tsolova, S.M. Sherif, and I. El-Sharkawy. 2021. Untargeted metabolomics and antioxidant capacities of muscadine grape genotypes during berry development. Antioxidants 10(6):914. doi: 10.3390/antiox10060914.
- De Lima Cherubim, D.J., C.V. Buzanello Martins, L. Oliveira Fariña, and R.A. da Silva de Lucca. 2019. Polyphenols as natural antioxidants in cosmetics applications. J Cosmet Dermatol 19(1):33–37. doi: 10.1111/jocd.13093.
- Desentis-Mendoza, R.M., H. Hernandez-Sanchez, A. Moreno, C. Rojas, L. Chel-Guerrero, J. Tamariz, and M.E. Jaramillo-Flores. 2006. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from ustilago maydis. Biomacromolecules 7(6):1845–1854. doi: 10.1021/bm060159p.
- Di Lodovico, S., F. Gasparri, E. Di Campli, P. Di Fermo, S. D’Ercole, L. Cellini, and M. Di Giulio. 2020. Prebiotic combinations effects on the colonization of staphylococcal skin strains. Microorganisms 9(1):37. doi: 10.3390/microorganisms9010037.
- Edslev, S., T. Agner, and P. Andersen. 2020. Skin microbiome in atopic dermatitis. Acta Derm. Venereol. 100(12):adv00164. doi: 10.2340/00015555-3514.
- Faraone, I., D. Rai, L. Chiummiento, E. Fernandez, A. Choudhary, F. Prinzo, and L. Milella. 2018. Antioxidant activity and phytochemical characterization of Senecio clivicolus wedd. Molecules 23(10):2497. doi: 10.3390/molecules23102497.
- Fogarasi, M., M.-I. Socaciu, C.-D. Sălăgean, F. Ranga, A.C. Fărcaș, S.A. Socaci, C. Socaciu, D. Țibulcă, S. Fogarasi, and C.A. Semeniuc. 2021. Comparison of different extraction solvents for characterization of antioxidant potential and polyphenolic composition in boletus edulis and cantharellus cibarius mushrooms from Romania. Molecules 26(24):7508. doi: 10.3390/molecules26247508.
- Formagio, A., C. Volobuff, M. Santiago, C. Cardoso, M. Vieira, and Z. Valdevina Pereira. 2014. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in Psychotria leaf extracts. Antioxidants 3(4):745–757. doi: 10.3390/antiox3040745.
- Gasparrini, M., T. Forbes-Hernandez, S. Afrin, P. Reboredo-Rodriguez, D. Cianciosi, B. Mezzetti, J. Quiles, S. Bompadre, M. Battino, and F. Giampieri. 2017. Strawberry-based cosmetic formulations protect human dermal fibroblasts against UVA-induced damage. Nutrients 9(6):605. doi: 10.3390/nu9060605.
- Georgescu, C., A. Frum, L.I. Virchea, A. Sumacheva, M. Shamtsyan, F.G. Gligor, N.K. Olah, E. Mathe, and M. Mironescu. 2022. Geographic variability of berry phytochemicals with antioxidant and antimicrobial properties. 2022. Molecules 27(15):4986. doi: 10.3390/molecules27154986.
- Giovagnoli-Vicuña, C., P. Velásquez, G. Montenegro, J. Espejo, M. Gómez, G. Cabrera-Barjas, and A. Giordano. 2021. Nutritional and antioxidant potential of Chilean native fruits: Lleuque (prumnopitys andina) and copihue (lapageria rosea). J. Food Nutr. Res 60:352–362.
- Iqbal, A., P. Schulz, and S.S.H. Rizvi. 2021. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present insights and future challenges. Food Biosci. 44:101384. doi: 10.1016/j.fbio.2021.101384.
- Jadhaw, S.B., and R.S. Singhal. 2014. Laccase-gum Arabic conjugate for preparation of water-soluble oligomer of catehin with enhances antioxidant activity. Food Chem. 150:9–16. doi: 10.1016/j.foodchem.2013.10.127.
- Kavela, E.T.A., L. Szalóki-Dorkó, and M. Máté. 2023. The efficiency of selected green solvents and parameters for polyphenol extraction from chokeberry (aronia melanocarpa (michx)) pomace. Foods 12(19):3639. doi: 10.3390/foods12193639.
- Khatri, D., and S.B.B. Chhetri. 2020. Reducing sugar, total phenolic content, and antioxidant potential of Nepalese plants. Biomed. Res. Int. 2020:1–7. doi: 10.1155/2020/7296859.
- Kim, J.S. 2018. Antioxidant activities of selected berries and their free, esterified, and insoluble-bound phenolic acid contents. Prev. Nutr. Food Sci 23(1):35–45. doi: 10.3746/pnf.2018.23.1.35.
- Kim, J., B.E. Kim, K. Ahn, and D.Y.M. Leung. 2019. Interactions between atopic dermatitis and staphylococcus aureus infection: Clinical implications. Allergy Asthma Immunol. Res. 11(5):593–603. doi: 10.4168/aair.2019.11.5.593.
- Kotha, R.R., F.S. Tareq, E. Yildiz, and D.L. Luthria. 2022. Oxidative stress and antioxidants—a critical review on in vitro antioxidant assays. Antioxidants 11(12):2388. doi: 10.3390/antiox11122388.
- Krutmann, J. 2009. Pre- and probiotics for human skin. J. Dermatol. Sci. 54(1):1–5. doi: 10.1016/j.jdermsci.2009.01.002.
- Maisetta, G., G. Batoni, P.E. Caboni, S. Rinaldi, and A.C. Zucca. 2019. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant cytinus. BMC Complement Altern Med 19(1):82. doi: 10.1186/s12906-019-2487-7.
- Makarewicz, M., I. Drożdż, T. Tarko, and A. Duda-Chodak. 2021. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 10(2):188. doi: 10.3390/antiox10020188.
- Marjanovic, A., J. Djedjibegovic, A. Lugusic, M. Sober, and L. Saso. 2021. Multivariate analysis of polyphenolic content and in vitro antioxidant capacity of wild and cultivated berries from Bosnia and Herzegovina. Sci. Rep. 11(1):19259. doi: 10.1038/s41598-021-98896-8.
- Martel, J., D.M. Ojcius, Y.-F. Ko, and J.D. Young. 2020. Phytochemicals as prebiotics and biological stress inducers. Trends Biochem. Sci. 45(6):462–471. doi: 10.1016/j.tibs.2020.02.008.
- Milutinović, M., N. Radovanović, M. Ćorović, S. Šiler-Marinković, M. Rajilić-Stojanović, and S. Dimitrijević-Branković. 2015. Optimisation of microwave-assisted extraction parameters for antioxidants from waste Achillea millefolium dust. Ind. Crops Prod. 77:333–341. doi: 10.1016/j.indcrop.2015.09.007.
- Neha, K., M.R. Haider, A. Pathak, and M.S. Yar. 2019. Medicinal prospects of antioxidants: A review. Eur J Med Chem 178:687–704. doi: 10.1016/j.ejmech.2019.06.010.
- Nirmal, N.P., A.C. Khanashyam, A.S. Mundanat, K. Shah, K.S. Babu, P. Thorakkattu, F. Al-Asmari, and R. Pandiselvam. 2023. Valorization of fruit waste for bioactive compounds and their applications in the food industry. Foods 12(3):556. doi: 10.3390/foods12030556.
- Osorio, L.L.D.R., E. Flórez-López, and C.D. Grande-Tovar. 2021. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 26(2):515. doi: 10.3390/molecules26020515.
- Pachołek, B., K. Krawczyk, and E. Żak. 2014. Potential use of dried fruit pomaces to create sensory properties and antioxidant activity of fruit teas. Towaroznawcze Problemy Jakości. Polish J. commod. sci 3:77–84.
- Pap, N., D. Reshamwala, R. Korpinen, P. Kilpeläinen, M. Fidelis, M.M. Furtado, A.S. Sant’ana, M. Wen, L. Zhang, J. Hellström, et al. 2021. Toxicological and bioactivity evaluation of blackcurrant press cake, sea buckthorn leaves and bark from scots pine and Norway spruce extracts under a green integrated approach. Food Chem. Toxicol. 153:112284. doi: 10.1016/j.fct.2021.112284.
- Paunović, S., P. Mašković, and M. Milinković. 2022. Phytochemical and antimicrobial profile of black currant berries and leaves. Acta. Agric. Serb. 27(53):25–29. doi: 10.5937/AASer2253025P.
- Petrov, A., M. Ćorović, A. Milivojević, M. Simović, K. Banjanac, R. Pjanović, and D. Bezbradica. 2022. Prebiotic effect of galacto‐oligosaccharides on the skin microbiota and determination of their diffusion properties. Int J Cosmet Sci 44(3):309–319. doi: 10.1111/ics.12778.
- Petrovic, M., D. Suznjevic, F. Pastor, M. Veljovic, L. Pezo, M. Antic, and S. Gorjanovic. 2016. Antioxidant capacity determination of complex samples and individual phenolics - multilateral approach. Comb. Chem. High Throughput Screen. 19(1):58–65. doi: 10.2174/1386207318666151102094227.
- Pieszka, M., P. Gogol, M. Pietras, and M. Pieszka. 2015. Valuable components of dried pomaces of chokeberry, black currant, strawberry, apple and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann. Anim. Sci. 15(2):475–491. doi: 10.2478/aoas-2014-0072.
- Plainfossé, H., M. Trinel, G. Verger-Dubois, S. Azoulay, P. Burger, and X. Fernandez. 2020. Valorisation of ribes nigrum L. Pomace, an agri-food by-product to design a New Cosmetic Active. Cosmetics 7(3):56. doi: 10.3390/cosmetics7030056.
- Pukalskienė, M., A. Pukalskas, L. Dienaitė, S. Revinytė, C.V. Pereira, A.A. Matias, and P.R. Venskutonis. 2021. Recovery of bioactive compounds from strawberry (fragaria × ananassa) pomace by conventional and pressurized liquid extraction and assessment their bioactivity in human cell cultures. Foods 10(8):1780. doi: 10.3390/foods10081780.
- Rajha, H.N., A. Paule, G. Aragonès, M. Barbosa, C. Caddeo, E. Debs, R. Dinkova, G.P. Eckert, A. Fontana, P. Gebrayel, et al. 2022. Recent advances in research on polyphenols: Effects on microbiota, metabolism, and health. Mol. Nutr. Food Res. 66(1):e2100670. doi: 10.1002/mnfr.202100670.
- Russo, D., P. Valentão, P. Andrade, E. Fernandez, and L. Milella. 2015. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 16(8):17696–17718. doi: 10.3390/ijms160817696.
- Severn, M.M., and A.R. Horswill. 2022. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 21(2):97–111. doi: 10.1038/s41579-022-00780-3.
- Struck, S., M. Plaza, C. Turner, and H. Rohm. 2016. Berry pomace – a review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol 51(6):1305–1318. doi: 10.1111/ijfs.13112.
- Su, J., L. Jin, R. Yang, Y. Liang, S.H. Nile, and G. Kai. 2023. Comparative studies on selection of high polyphenolic containing Chinese raspberry for evaluation of antioxidant and cytotoxic potentials. J. Agric. Food. Res. 12:100603. doi: 10.1016/j.jafr.2023.100603.
- Sun, M., Y. Deng, X. Cao, L. Xiao, Q. Ding, F. Luo, P. Huang, Y. Gao, M. Liu, and H. Zhao. 2022. Effects of natural polyphenols on skin and hair health: A review. Molecules 27(22):7832. doi: 10.3390/molecules27227832.
- Sun, T., and S.A. Tanumihardjo. 2007. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 72(9):R159–R165. doi: 10.1111/j.1750-3841.2007.00552.x.
- Tabart, J., C. Kevers, N. Dardenne, V. Schini-Kerth, A. Albert, J. Dommes, J.-O. Defraigne, and J. Pincemail. 2014. Deriving a global antioxidant score for commercial juices by multivariate graphical and scoring techniques: Applications to blackcurrant juice. Process. And Impact Antioxid. Beverages 301–307. doi: 10.1016/b978-0-12-404738-9.00030-1.
- Todorovic, V., M. Milenkovic, B. Vidovic, Z. Todorovic, and S. Sobajic. 2017. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 82(4):1020–1027. doi: 10.1111/1750-3841.13672.
- Vermerris, W., and R. Nicholson. 2009. Phenolic compound biochemistry. Isolation and identification of phenolic compounds, pp. 154–156. Dordrecht, Netherlands: Springer Dordrecht.
- Willis, R.B., and P.R. Allen. 1998. Improved method for measuring hydrolyzable tannins using potassium iodate. Analyst (Lond) 123(3):435–439. doi: 10.1039/a706862j.
- Woo, T.E., and C.D. Sibley. 2020. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J. Am. Acad. Dermatol. 82(1):222–228. doi: 10.1016/j.jaad.2019.08.078.
- Yang, Y., L. Qu, I. Mijakovic, and Y. Wei. 2022. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Fact. 21(1):176. doi: 10.1186/s12934-022-01901-6.
- Yao, J., J. Chen, J. Yang, Y. Hao, Y. Fan, C. Wang, and N. Li. 2021. Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT 135:109995. doi: 10.1016/j.lwt.2020.109995.
- Yao, Y., and B. Xu. 2022. Skin health promoting effects of natural polysaccharides and their potential application in the cosmetic industry. Polysaccharides 3(4):818–830. doi: 10.3390/polysaccharides3040048.
- Zhou, H., L. Shi, Y. Ren, X. Tan, W. Liu, and Z. Liu. 2020. Applications of human skin microbiota in the cutaneous disorders for ecology-based therapy. Front Cell Infect Microbiol 10:570261. doi: 10.3389/fcimb.2020.570261.

