ABSTRACT
Aim
This study aims to explore the expression and role of CD72 in B lymphocytes in immune thrombocytopenic purpura (ITP).
Methods
The expression level of CD72 in B lymphocytes was detected by flow cytometry in 18 ITP patients and 19 controls of healthy donor or iron-deficiency anemia patients. B cell proliferation was determined by 5-bromo-2′-deoxyuridine incorporation (BrdU) in the culture of 17 ITP patients’ and 11 controls’ peripheral mononuclear cells (PMNCs). The secretion levels of antibodies against human platelet antigens (HPA), as well as B cell proliferation-related cytokine interleukin 1(IL-1) and macrophage migration inhibitory factor (MIF) in culture supernatants were measured by ELISA.
Results
CD72 was significantly increased in B cells of newly diagnosed or persistent ITP compared with ITP in remission. B cell proliferation in culture with CD72 antibody addition was significantly decreased both in ITP patients and in controls compared with isotype antibody addition. CD72 antibody did not significantly alter HPA antibody level in ITP patients. CD72 antibody increased IL-1 and MIF levels in ITP patients’ cell culture supernatant but not in controls.
Conclusion
CD72 expression elevation accompanies the active status of ITP. In vitro addition of CD72 antibody has a negative impact on B cell proliferation. The function of CD72 in B cell proliferation in ITP may be related to IL-1 and MIF secretion.
Introduction
Immune thrombocytopenic purpura (ITP), an immune-mediated acquired disease is characterized by the accelerated platelet destruction in the spleen caused by B cell-derived anti-platelet antibodies, resulting in thrombocytopenia and a varying propensity for bleeding [Citation1,Citation2]. The prevalence of ITP in adults is about 10/105 [Citation3] with an incidence rate ranging from 1.6 to 3.9/105 every year [Citation4]. ITP may be classified as acute or chronic ITP (thrombocytopenia of less or more than 6 months in duration) [Citation5]. The goal of management for ITP is to increase the platelet count and prevent serious hemorrhage while minimizing treatment-related toxicity from therapy [Citation6].
B cells, T cells, macrophages, and bone marrow megakaryocytes may all play roles in ITP [Citation6]. Abnormal T cell response, notably supported by splenic T follicular helper (TFH) cells, stimulates the proliferation and differentiation of autoreactive B cells which produce anti-platelet antibodies [Citation1]. Numerous researches reported that B cell proportion is significantly elevated in ITP patients’ peripheral blood and even spleen [Citation7–9]. The production of B cells in ITP patients is examined by Sarina Levy-Mendelovich et al, and they reveal that the overproduction of B cells is a remarkable culprit in the pathogenesis of ITP [Citation10]. The research of in vivo experiment, research has found that B cell depletion can rescue mice from developing T cell-mediated ITP by reducing the ability of CD8+ T cell activation [Citation11]. The maintenance of TFH cells requires B cells, and B cell depletion decreases the number of TFH cells in patients with ITP [Citation12]. All these results suggest the critical role of B cells in the development of ITP.
CD72 is a B cell transmembrane protein containing a C-type lectin-like domain in the extracellular region and an immunoreceptor tyrosine-based inhibition motif in the cytoplasmic region [Citation13]. CD72 is commonly considered as a negative regulator for B cell receptor (BCR) signaling. However, it has been demonstrated to exert dual effects on B lymphocyte development and function. In mature B cells, CD72 acts a positive effect by transient recruitment of CD19 [Citation14]. In immature B cells, CD72 negatively regulates BCR signaling by its simultaneous phosphorylation and recruitment of SHP-1 [Citation15]. The interaction between CD72 and its ligand CD100 is required for proper B cell homeostasis [Citation16] and propagated not only B cell initial activation but also B cell differentiation into plasma cells [Citation17]. This interaction even promotes the secretion of interferon and tumor necrosis [Citation18]. Thus, CD72 plays a critical role in B cell survival and function in vivo.
Previous research suggests that CD72 polymorphism is related to child onset of ITP [Citation19]. Upregulation of CD72 in CD27+ memory B cells of ITP occurs in active ITP and is related to platelet counts and anti-platelet auto-antibody levels [Citation20]. But the direct impacts of CD72 on ITP patients’ B cell growth or function have not been fully reported. In this study, we used the in vitro culture of patients’ peripheral mononuclear cells (PMNCs) to explore whether interference of CD72 interaction with its ligand influences B cell proliferation and platelet antibody or cytokines secretion in ITP.
Materials and methods
Patients
The patients diagnosed as ITP from June 2021 to September 2021 in our hospital were enrolled for this study. First, to make clear the expression characteristics of CD72 on B cells of ITP, the peripheral blood samples of 11 newly diagnosed or persistent ITP (npITP), 7 ITP with remission by steroid drugs (ITPr), and 19 healthy donor or iron-deficiency anemia patients serving as controls were consecutively collected. The persistent ITP patients had not been treated with glucorticosteroids or other immune suppressant for at least 1 month and never treated by rituximab at the time of sampling. All of these patients had their whole blood cell counts tested by auto-analyzer simultaneously. Then to detect the CD72 blockage effects on lymphocytes, 17 ITP patients were included in this study among whom 12 patients were np ITP, 5 patients were ITPr, and 11 donors were included as controls. The diagnosis of ITP was made according to the bleeding signs and duration, at least two times platelet counts less than 100,000 per μl, the bone marrow test to exclude other obvious reasons for thrombocytopenia, and the recorded platelet recovery over 100,000 per μl with steroid drug or other immune suppressant treatments in two weeks. The ITPr were those with solid platelet counts of no less than 30,000 per μl and at least a 2-fold increase of the baseline count without bleeding. The patients’ diagnosis and response definition were referred to the criteria previously reported [Citation21]. The control group included seven iron-deficiency anemia patients, two pseudo thrombocytopenic patients, and two healthy donors. The detailed status of ITP patients was listed in . The study had the approval of the Ethics Review Board of Zhujiang Hospital of Southern Medical University, China. And each patient and healthy volunteer signed a consent form of enrollment.
Table 1. The status of ITP patients at recruitment and tests underwent.
Cell culture
The peripheral blood samples were drawn from each patient or donor and anticoagulated with heparin. Mononuclear cells were enriched by Ficoll-Hypaque density gradient centrifugation. After being washed with phosphate buffer saline three times, the PMNCs were suspended in RPMI 1640 medium (Solarbio, Beijing, China) with a supplement of cytokines referring to a previous report [Citation20]. The density of cells was adjusted to 1 × 106 cells/ml. After being allocated to 96 well plates by 200 μl/well, the cell suspensions were added with rabbit polyclonal antibody directing human CD72 extramembrane region (AA range:170–220) (Abbkine Scientific Co., Wuhan, China) or isotypic rabbit immunoglobulin with a concentration of 5 μg/μl referring to the article reported [Citation22] and placed in 5% CO2 incubator at 37°C for 72 h.
Flow cytometry analysis
For expression of CD72 determination, peripheral whole blood cells were incubated with APC-conjugated CD45 antibody, PE-conjugated CD19 monoclonal antibody(BD Biosciences, San Jose, USA), and FITC-conjugated CD72 antibody (BD Biosciences) in dark for 30 min at room temperature. Then the red cells were treated with lysis buffer and disposed of to let the white cells be left. After being washed with flow cytometry staining buffer three times, the left cells were loaded onto FACSCalibur cytometry (BD Biosciences) for analysis.
B cell proliferation detection
For proliferation assays, the in vitro culture cells were incubated with 10 μmol BrdU (ABCONE, Shanghai, China) for 18 h before they were harvested. Then they were fixed and permeabilized in dark for 15 min by a BrdU staining buffer kit (ebioscience) and treated with DNase at 37°C for 1 h. Afterward, the cells were incubated with 5 μl APC-conjugated BrdU antibody (ebioscience) at room temperature for 20 min. Cells were analyzed on a FACSCalibur cytometry (BD Biosciences). The cultured viable B cells were gated as CD19-positive, CD3-negative and fixable viability dye (ebioscience) negatively labeled group.
Evaluation of the anti-platelet antibodies and interleukin
The human anti-platelet antibody levels in the supernatant of the cultured cells were detected by an ELISA kit (Yutong, Jiangsu province, China) according to the manufacturer’s instructions. Briefly, the supernatant collected from the cultures with CD72 rabbit antibody or with isotypic rabbit immunoglobulin was added into microwell strips coated with platelet-specific antigens and then incubated with horseradish peroxidase-labeled platelet antigens. The substrates were added, and OD value was measured at 450 nm wave by Multiskan GO (Thermo Fisher Scientific, Cleveland, USA). The platelet antibody concentration was computed referring to standard samples. As to the detections of IL-1, HGF, migration inhibitory factor (MIF), and midkine, the corresponding kits were used following their respective instructions (Dogesce Biotech Co. Beijing, China for IL-1Solarbio Life Sciences Beijing, China for HGF, MULTISCIENCES BIOTECH CO Shanghai, China for midkine, and Joyee Biotechnics Co. Anhui, China for MIF).
Statistics
Statistical analysis was performed with Graphpad Prism 5 (Graphpad Software, San Diego, CA). All values were expressed as median and range. One-way ANOVA with Tukey’s test was used in comparing the median CD72 expression on subgroups of ITP patients and control B cells. The differences in B cell proliferation rates, anti-platelet antibodies, and cytokines were compared using Student’s t-test. P < 0.05 was considered statistically significant.
Results
ITP patients have a higher percent and absolute count of B cells, and the CD72 expression on B cells in npITP is higher than ITPr
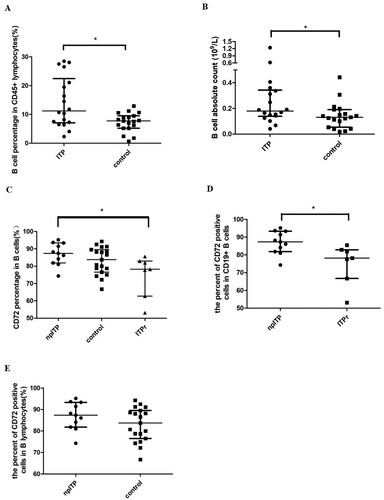
The percentage of B cells in CD45+ lymphocytes was significantly higher in ITP patients than in controls (median percent of 11.84% vs 7.6%, respectively, P = 0.021, (a)). The absolute counts of CD19+ B cell subsets were calculated by multiplying the percentage (results of flow cytometer) by absolute lymphocyte counts (results of blood cell auto-analyzer). B cell subset count was also significantly higher in ITP patients than in controls (median count of 0.179 and 0.131 109/l respectively, P = 0.032, (b)). The CD72-positive B cell percentage in npITP patients was significantly higher than that in ITPr patients (P = 0.049, (c)). The median percentage of CD72+ cells in CD19+ B cells was obviously increased in npITP compared with that in ITPr (87.35% vs 78.19%, respectively, P = 0.018, (d)). But there was no significant difference of CD72+ B cells between npITP and controls (P = 0.18, (e)).
Figure 1. Flow cytometry analysis of ITP and control peripheral lymphocytes. (a,b) The percentage and absolute count of B cells in ITP patients were significantly increased compared with that in control (P = 0.021 and 0.032, respectively, bars at median with interquartile range). (c) CD72 expression in B cells was significantly different between npITP, ITPr, and control with median of 87.35%, 78.19%, and 83.72%, respectively (P = 0.049, bars at median with interquartile range). (d) The percentage of CD72-positive B cells of npITP was significantly higher than that of ITPr (P = 0.018, bars at median with interquartile range). (e) There is no significant difference in CD72-positive B cell percentages between npITP and control (P = 0.18, bars at median with interquartile range).
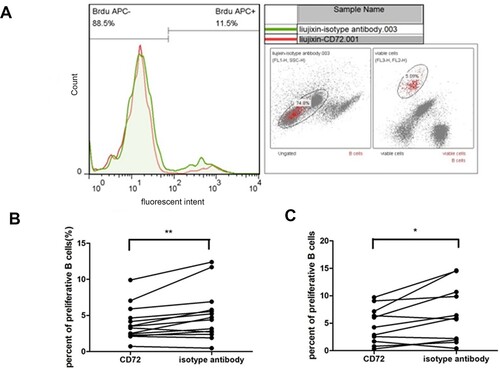
CD72 antibody negatively regulated B cell proliferation
CD72 antibody was added to the in vitro culture of mononuclear cells to explore the specific impact of CD72 blockage on cell proliferation, and isotype antibody added to an in vitro parallel culture of the same person’s cells was regarded as controls. A typical result of flow cytometry was depicted in (a). CD72 antibody significantly decreased the ITP patients’ B cell proliferation compared with the isotype antibody (mean of difference −1.142%, P = 0.009, (b)). B cell proliferation in control group was also obviously decreased by CD72 antibody (the mean of differences −2.1%, P = 0.026, (c)).
Figure 2. The effect of anti-CD72 on B cell proliferation during in vitro culture of ITP patients’ mononuclear cells. (a) Histograms represent CD72 antibody cultured cells (red line) or isotype antibody cultured cells (green-filled histogram). (b) Anti-CD72 significantly decreased B cell proliferation of ITP patients in vitro (**P = 0.009). (c) Anti-CD72 decreased B cell proliferation from control in culture (*P = 0.021).
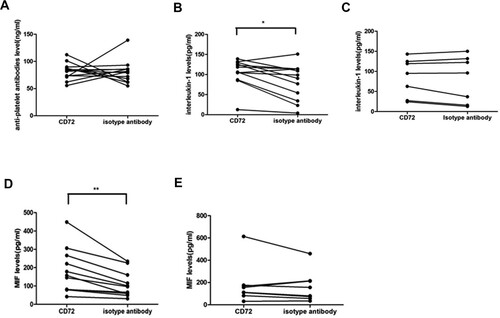
Blockage of CD72 does not affect anti-platelet antibodies’ secretion but increased IL-1 and MIF levels
The cultured cells supplemented with CD72 antibody or isotype antibody were centrifuged, and the supernatant was collected for tests. The platelet antibody levels in the supernatant of CD72 antibody and isotype antibody-treated ITP patients’ mononuclear cells showed no significant difference (P = 0.91, (a)). But IL-1 level was significantly elevated in the CD72 antibody-added culture supernatant of npITP compared with isotype antibody (mean of difference 23.77 ng/ml, P = 0.014, (b)). Meanwhile, no significant difference in IL-1 level was found in the supernatant of CD72 antibody and isotype antibody-treated mononuclear cells in control group (mean of difference 4.418 ng/ml, P = 0.379, (c)). Furthermore, MIF level in the CD72 antibody-treated culture supernatant of npITP patients was significantly elevated compared with that in the isotype antibody-treated culture supernatant (mean of difference 74.04 ng/ml, P = 0.002, (d)). In control group, CD72 antibody did not significantly alter MIF level compared with isotype antibody (mean of difference 19.93 ng/ml, P = 0.416, (e)). We also tested other B cell proliferation-related cytokines like midkine and HGF in the culture supernatant, but we did not find any obvious difference in these cytokines between CD72 antibody and isotype antibody groups (data not shown).
Figure 3. The effect of anti-CD72 on the levels of platelet antibody, interleukin 1(IL-1), and macrophage migration inhibitory factor (MIF) during in vitro culture. (a) Platelet antibody levels were not significantly different between CD72 and isotype antibody-added cultures in ITP. B. IL-1 was significantly elevated by the addition of CD72 antibody in the group of ITP (*P = 0.014). (c) IL-1 levels were not significantly different between CD72 and isotype antibody-added cultures in control group. (d) MIF was significantly increased in CD72 added culture compared with isotype antibody in ITP group (**P = 0.002). (e) MIF was not obviously different between CD72 and isotype antibody-added cultures in control group.
Discussion
CD72 plays dual roles in B lymphocyte development and function by interacting with different modulators of BCR signaling [Citation23]. ITP is a B cell-mediated autoimmune disease, and the role of CD72 in ITP is little known. Our present data supported the increment of B cells in ITP patients from the view of percentage as well as absolute count. We further made a study on CD72 expression characteristics in ITP B cells.
Abnormal expression and function of CD72 on B cells have been reported in some autoimmune diseases, such as primary Sjogren’s syndrome [Citation24] and systemic lupus erythematosus [Citation25]. The serum level of soluble CD72 in systemic lupus erythematosus patients is increased compared with rheumatoid arthritis patients or healthy people [Citation26]. The percentage of CD72+ B cells and serum soluble CD72 level are also reported to be increased in primary Sjogren’s syndrome patients [Citation24]. The mRNA expression of CD72 in active ITP patients is reported to be lower than that in patients in remission and controls [Citation27]. But our present data demonstrated a higher expression of CD72 in active ITP patients’ B lymphocytes than in ITP in remission. The result was consistent with a previous report demonstrating that active ITP is more highly expressed CD72 compared with ITP in remission and healthy controls [Citation20]. Comparing with the former mRNA expression which was detected basing on peripheral mononuclear cells, our data and others’ directly reflected the expression characteristics of CD72 in ITP B cells. Meanwhile, our present data did not show that CD72 expression on B cells of active ITP was significantly increased compared with controls. The discrepancy might come from the limited sample number of our patients. The finding may also suggest that the CD72 expression increment is more closely related to developing phase of ITP than pathogenic features of ITP.
B cells from CD72-deficient mice are hyper-responsive to BCR stimulation [Citation28], suggesting that CD72 may negatively modulate BCR signaling to improve proliferation. Additionally, CD72 apparently positively regulates BCR-mediated signaling pathways in murine mature B cells [Citation29]. This pathway induces growth and apoptosis in mature B cells and immature B cells, respectively [Citation23]. Although CD72 expression on CD19+/CD27+ memory B cells or even in gross B cells was reported to be significantly increased in ITP patients compared with controls [Citation20], the role of high CD72 expression in ITP was not reported before. Our present results suggested that the blockade of CD72 binding with its ligand restricted the proliferation of B cells of ITP in vitro. Combining the results of CD72 expression in ITP patients, we speculated that the high expression of CD72 in B cells may support the high proliferation of B cells in ITP patients. These findings were inconsistent with the previous research indicating that CD72 expression reduces the cellular proliferation of mature B cells, and ligation of CD72 using anti-CD72 monoclonal antibodies contributes to B cell proliferation upon Ag stimulation [Citation30]. Our findings revealed a novel role of anti-CD72 in B cell proliferation in ITP but the mechanism was unclear. Besides BCR signaling, CD72 is also reported to signal B cells by inducing BCR-independent positive signaling pathways [Citation31]. Therefore, the function of CD72 in ITP may be mediated by BCR signaling as well as BCR-independent signaling.
The production of antibodies against platelets is a feature of ITP. In our present study, the blockage of CD72 was not found to influence the secretion levels of platelet antibodies. It may be correlated with the finding that expression of CD72 on B cells is linked to the immunoglobulin class switching [Citation32], and the reduction of CD72 expression is associated with the increased expression of cell surface IgG on B cells from systemic lupus erythematosus [Citation33]. So the isotypes of immunoglobulin may be influenced by CD72 expression rather than the gross immunoglobulin levels. It may also be related to the lack of simultaneous stimulation to B cells from ITP with in vitro cultured mature dendritic cells or Staphylococcal aureus Cowan I which promotes the platelet antibody secretion more efficiently [Citation34,Citation35]. Anyway, the spontaneous secretion of platelet autoantibodies from ITP patients’ lymphocytes was not found to be impacted by the blockade of CD72 in our culture conditions.
The inflammatory cytokines IL-1 and MIF are crucial in B cell proliferation. In the current research, we observed the obvious elevation of B cell proliferation-related cytokines MIF and IL-1 caused by anti-CD72 addition into the cell culture of ITP patients. There are some reports indicating the negative role of MIF on B cell proliferation. For example, negative modulation of MIF by an anti-sense transfectant in a B cell line WEH1-231 results in more cells entering S-phase [Citation36]. Human amniotic cells culture supernatant containing bio-active MIF significantly reduces B cell proliferation after mitogenic stimulation [Citation37]. Recently, MIF deficiency is reported to favor a relative increase of B cells in the atheroprone brachiocephalic artery region and the appearance of periadventitial B cell-rich clusters in atherosclerosis [Citation38]. These reports suggest that MIF may be related to the proliferation suppression of B cells with autoimmune pathogenic features. Moreover, MIF and CD74 are downregulated in blood B cells from early-onset multiple sclerosis patients [Citation39], and iTRAQ-based quantitative proteomics analysis of ITP patients reveals that MIF is decreased significantly compared with normal control but is elevated obviously in ITP remission by effective treatment [Citation40]. IL-1 is also reported to be associated with the suppression of B cell generation. For example, it is reported that myeloid-derived suppressor cells (MDSCs) inhibit B cell development in vitro via soluble factors, and the addition of anti-IL-1 Abs restored B lymphopoiesis in BM cultures containing MDSCs, showing that MDSCs inhibition of B lymphopoiesis is mediated by IL-1 [Citation41]. So our present results and the former reports supported that MIF and IL-1 elevation by CD72 blockade may play a role in the mechanism of suppressing B cell proliferation in ITP patients. We did not find a similar elevation of MIF or IL-1 by CD72 blockade in control cell cultures although both groups showed B cell proliferation decreased by CD72 antibody. This supported that ITP has different immune stable status from control, and there are other mechanisms involved in the suppression of B cell proliferation by CD72 blockade in control.
There are obvious shortcomings in this research. First, a small sample size is a problem. Although it usually leads us to accept a null hypothesis and our data revealed some obvious differences in respect of B cell level, CD72 expression in B cell, B cell proliferation change on CD72 antibody addition, etc., the results could still be related to some extreme values. Moreover, the lack of statistical significant difference such as the secretion difference of platelet antibody under the conditions of with and without CD72 antibody addition may also be related to the small sample size. So more samples are needed to verify the results. Second, although our findings revealed CD72 antibody influenced ITP patients’ B cell proliferation and elevated IL-1 and MIF secretion, their relationship was not directly elicited and the underlining mechanism of the impacts was not exploited. Further experimental design is needed to clarify the relationship and mechanism.
Anyway, our research demonstrated CD72 expression elevation in ITP patients’ B cells is associated with the immune abnormality of active patients. CD72 blockage suppresses B cell proliferation but not anti-platelet antibody production in vitro. Cytokines MIF and IL-1 are likely to function as act suppressors in B cell proliferation in ITP patients.
Acknowledgment
We are particularly grateful to Guangdong Science and Technology Department for its financial support to our study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or used during the study appeared in the submitted article.
Additional information
Funding
References
- Audia S, Mahévas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–632.
- Kuwana M, Okazaki Y, Kaburaki J, et al. Spleen is a primary site for activation of platelet-reactive T and B cells in patients with immune thrombocytopenic purpura. J Immunol. 2002;168(7):3675–3682.
- Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4(11):2377–2383.
- Deane S, Teuber SS, Gershwin ME. The geoepidemiology of immune thrombocytopenic purpura. Autoimmun Rev. 2010;9(5):A342–A349.
- Kühne T. Idiopathic thrombocytopenic purpura in childhood: controversies and solutions. Pediatr Blood Cancer. 2006;47(5 Suppl):650–652.
- Psaila B, Bussel JB. Immune thrombocytopenic purpura. Hematol Oncol Clin North Am. 2007;21(4):743–759., vii.
- Geng Y, Sun XL, Su AY, et al. Expression of CD19(+)B cells and involvement of serum breg in pathogenesis of immune thrombocytopenia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(3):911–915.
- Alavi S, Aryan Z, Ghazizadeh F, et al. The immunophenotype of bone marrow lymphocytes in children with immune thrombocytopenic purpura. Pediatr Hematol Oncol. 2014;31(6):548–554.
- Mahévas M, Patin P, Huetz F, et al. B cell depletion in immune thrombocytopenia reveals splenic long-lived plasma cells. J Clin Invest. 2013;123(1):432–442.
- Levy-Mendelovich S, Lev A, Aviner S, et al. Quantification of specific T and B cells immunological markers in children with chronic and transient ITP. Pediatr Blood Cancer. 2017;64(12):e26646.
- Guo L, Kapur R, Aslam R, et al. CD20+ B-cell depletion therapy suppresses murine CD8+ T-cell-mediated immune thrombocytopenia. Blood. 2016;127(6):735–738.
- Audia S, Rossato M, Trad M, et al. B cell depleting therapy regulates splenic and circulating T follicular helper cells in immune thrombocytopenia. J Autoimmun. 2017;77:89–95.
- Tsubata T. CD72 is a negative regulator of B cell responses to nuclear lupus self-antigens and development of systemic lupus erythematosus. Immune Netw. 2019;19(1):e1.
- Venkataraman C, Lu P-J, Buhl AM, et al. CD72-mediated b cell activation involves recruitment of CD19 and activation of phosphatidylinositol 3-kinase. Eur J Immunol. 1998;28(10):3003–3016.
- Wu Y, Nadler MJS, Brennan LA, et al. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr Biol. 1998;8(18):1009–1017.
- Kumanogoh A, Shikina T, Watanabe C, et al. Requirement for CD100-CD72 interactions in fine-tuning of B-cell antigen receptor signaling and homeostatic maintenance of the B-cell compartment. Int Immunol. 2005;17(10):1277–1282.
- Kuklina Е, Nekrasova IV, Valieva YV. Involvement of semaphorin (Sema4D) in T-dependent activation of B cells. Bull Exp Biol Med. 2017;163(4):447–450.
- Li BJ, He Y, Zhang Y, et al. Interferon-α-induced CD100 on naïve CD8(+) T cells enhances antiviral responses to hepatitis C infection through CD72 signal transduction. J Int Med Res. 2017;45(1):89–100.
- Xu J, Lu S, Tao J, et al. CD72 polymorphism associated with child-onset of idiopathic thrombocytopenic purpura in Chinese patients. J Clin Immunol. 2008;28(3):214–219.
- Lyu M, Hao Y, Li Y, et al. Upregulation of CD72 expression on CD19(+) CD27(+) memory B cells by CD40L in primary immune thrombocytopenia. Br J Haematol. 2017;178(2):308–318.
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393.
- Jiang X, Björkström NK, Melum E. Intact CD100-CD72 interaction necessary for TCR-induced T cell proliferation. Front Immunol. 2017;8:765.
- Wu HJ, Bondada S. CD72, a coreceptor with both positive and negative effects on B lymphocyte development and function. J Clin Immunol. 2009;29(1):12–21.
- Shen Y, Ma Y, Xie J, et al. A regulatory role for CD72 expression on B cells and increased soluble CD72 in primary Sjogren's syndrome. BMC Immunol. 2020;21(1):21.
- Asmiyou A, Bakr Ashraf M, Shahin DA, et al. CD40 and CD72 expression and prognostic values among children with systemic lupus erythematosus: a case-control study. Lupus. 2020;29(10):1270–1276.
- Vadasz Z, Goldeberg Y, Halasz K, et al. Increased soluble CD72 in systemic lupus erythematosus is in association with disease activity and lupus nephritis. Clin Immunol. 2016;164:114–118.
- Zhou H, Qi A-P, Li H-Y, et al. CD72 gene expression in immune thrombocytopenia. Platelets. 2012;23(8):638–644.
- Pan C, Baumgarth N, Parnes JR. CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity. 1999;11(4):495–506.
- Ogimoto M. Impairment of B cell receptor-mediated Ca2 + influx, activation of mitogen-activated protein kinases and growth inhibition in CD72-deficient BAL-17 cells. Int Immunol. 2004;16(7):971–982.
- Li DH, Tung JW, Tarner IH, et al. CD72 down-modulates BCR-induced signal transduction and diminishes survival in primary mature B lymphocytes. J Immunol. 2006;176(9):5321–5328.
- Wu HJ, Venkataraman C, Estus S, et al. Positive signaling through CD72 induces mitogen-activated protein kinase activation and synergizes with B cell receptor signals to induce X-linked immunodeficiency B cell proliferation. J Immunol. 2001;167(3):1263–1273.
- Wakabayashi C, Adachi T, Wienands J, et al. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science. 2002;298(5602):2392–2395.
- Nakano S, Morimoto S, Suzuki J, et al. Down-regulation of CD72 and increased surface IgG on B cells in patients with lupus nephritis. Autoimmunity. 2007;40(1):9–15.
- Yu H, Liu Y, Han J, et al. TLR7 regulates dendritic cell-dependent B-cell responses through BlyS in immune thrombocytopenic purpura. Eur J Haematol. 2011;86(1):67–74.
- Hou M, Lv B, He Q, et al. Both splenic CD5(+) B and CD5(-) B cells produce platelet glycoprotein-specific autoantibodies in chronic ITP. Thromb Res. 2003;110(1):1–5.
- Takahashi A, Iwabuchi K, Suzuki M, et al. Antisense macrophage migration inhibitory factor (MIF) prevents anti-IgM mediated growth arrest and apoptosis of a murine B cell line by regulating cell cycle progression. Microbiol Immunol. 1999;43(1):61–67.
- Li H, Niederkorn JY, Neelam S, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–907.
- Schmitz C, Noels H, Bounkari OE, et al. Mif-deficiency favors an atheroprotective autoantibody phenotype in atherosclerosis. Faseb J. 2018;32(8):4428–4443.
- Rijvers L, Melief M-J, van der Vuurst de Vries RM, et al. The macrophage migration inhibitory factor pathway in human B cells is tightly controlled and dysregulated in multiple sclerosis. Eur J Immunol. 2018;48(11):1861–1871.
- Wang Y, Wang S, Gong C, et al. iTRAQ-based quantitative proteomics analysis of immune thrombocytopenia patients before and after Qishunbaolier treatment. Rapid Commun Mass Spectrom. 2021;35(3):e8993.
- Kennedy DE, Knight KL. Inhibition of B lymphopoiesis by adipocytes and IL-1-producing myeloid-derived suppressor cells. J Immunol. 2015;195(6):2666–2674.
