 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the effects of calcium (Ca2+) application on acquired systemic tolerance mechanism to cadmium (Cd) stress in sesame (Sesamum indicum L.) were studied. The Cd stress reduced the root and shoot growth of sesame, and plant contents of photosynthetic pigments; however, the application of Ca2+ improved these parameters under Cd stress condition. The hydrogen peroxide, malondialdehyde and soluble sugar contents were higher under Cd stress, and were reduced by Ca2+ treatment. The antioxidant enzyme activities in the leaves of sesame, superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) were higher under Cd stress, whereas reduced concentration was observed in Ca2+-treated plants. Cd stress increased the contents of diacylglycerol and sterol ester; however Ca2+ treatment resulted in a significant increase in phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol and phosphatidylserine. Our results indicated that application of calcium enables sesame plants to withstand the deleterious impact of cadmium through upregulating acquired systemic tolerance system as lipid fractions (galactolipids, phospholipids, neutral lipids), antioxidant enzymes (SOD, POD, CAT, APX, GR) hence protect membrane functions.
Introduction
Cadmium stress is among the important abiotic factors that restrict plant growth. Its uptake by plants is rather quick because of its high mobility, which is, in turn, responsible for its rapid transportation to above-ground plant parts (Asgher et al. Citation2014). The uptake and subsequent accumulation of Cd in plant tissues result in the production of excess reactive oxygen species (ROS), which can obstruct the absorption of essential elements such as zinc, calcium and iron from proteins and inhibit the chloroplast and mitochondrial electron transport chain (Gallego et al. Citation2012). Leaf necrosis, impeded nutrient uptake and reduced enzyme activity are widely accepted effects of cadmium stress (Abd_Allah et al. Citation2015; Hashem et al. Citation2016). Cadmium induces cell death by exerting oxidative damage on membranes, proteins and nucleic acids, thereby restricting the growth and yield of plants (Nazar et al. Citation2012). It also has negative effects on membranes by inducing alterations in the lipid composition. Heavy metals undergo direct competition with calcium at the plasma membrane sites, which mediates its easy replacement and thereby alters plant metabolism. It has been observed that stress-induced alterations in membrane compositions might have both adaptive and deleterious effects on the overall physiological performance of a plant (Mansour & Salama Citation2004).
There are several strategies to overcome the stress-induced harmful effects, including the use of nutrients, which have a broad effect on the metabolism of plants under Cd stress (Chen et al. Citation2007). Among mineral nutrients, Ca2+ has a clear role in the growth and developmental regulation of plants, in heavy metal detoxification and in tolerance to Cd stress (Suzuki Citation2005). The amelioration of the negative effect of stress by Ca2+ is, in part, attributed to maintaining antioxidant enzyme activities and reducing the lipid peroxidation of cell membranes (Khan et al. Citation2010; Ahmad et al. Citation2015).
Several studies have reported that the application of Ca2+ resulted in an increased activity of antioxidant enzymes and improved physiological and biochemical processes, leading to the tolerance of the faba bean to Cd stress (Siddiqui et al. Citation2012). Moreover, some research reported positive effects of Ca2+ application on maize growth under Cd stress by improving the potassium uptake of plants and decreasing the Cd concentration in the plant root (Kurtyka et al. Citation2008).
However, the effect of Ca2+ application on acquired systemic tolerance mechanism under Cd stress is still unclear in sesame plant. Thus, the objectives of our study were to determine the interactive efficiency of Ca2+ application both in the metabolism of lipids in sesame (Sesamum indicum L.) and in plant tolerance to Cd stress. Sesame (S. indicum L.), an herbaceous annual plant, was chosen for this study because of its high content of oil and polyunsaturated fatty acids (up to 90%), which include linoleic, oleic, stearic and palmitic acids (Uzun et al. Citation2008).
Materials and methods
Plant, soil, treatments and growth conditions
Seeds of sesame (S. indicum L.) variety Giza 32 were provided by Agriculture Research Center, Giza, Egypt. The soil used in the present study was collected from the Agricultural Research Station of Derab, Riyadh, Saudi Arabia. With a sandy texture, the native composition (%) of the experimental soil was sand, 93.1; clay, 4.2; silt, 2.7; organic carbon, 0.07; total nitrogen, 0.003 and pH 7.4. The seeds were surface-sterilized with 85% ethanol, followed by 10% v/v NaOCl and were rinsed several times with sterile, distilled water. Sterile seeds were germinated in a blotter at 25°C for seven days; then, similar healthy germinated seeds were selected and sown in pots (30 cm in diameter, 15 kg capacity) filled with soil. Plants at the third-leaf stage were divided into two main groups; one group was irrigated with full strength Hoagland solution (control plants), and the second group was irrigated with water supplemented with modified Hoagland solution containing 100 µM CdCl2. Both groups were further subdivided into two groups, and either 10 ml of 50 mM CaCl2 per pot (30 cm in diameter) or distilled water was applied. Plants were grown for eight weeks in growth chambers maintained at 30 ± 2°C with an 18-h light (2000 μmol m−2 S−1) and 6-h dark photo-cycle and an relative humidity (RH) of 70–75%. Plants were carefully removed, and different morphological characteristics were determined. The plant parts were oven dried at 70°C for 48 h to determine the dry weight.
Leaf area
Leaf area per plant was determined following the formula of Carleton and Foote (Citation1965):
Cadmium (Cd) content in plants
The concentration of Cd in plant tissue was determined following the methods of Burd et al. (Citation2000) and Jackson (Citation1962). The Cd concentration in plant tissue was evaluated using a flame atomic absorption spectrophotometer (T80 UV/VIS Spectrophotometer, PG Instruments Ltd, UK).
Concentration of photosynthetic pigments
For the extraction and determination of photosynthetic pigments, a known weight of fresh leaf sample was extracted in acetone (80%, v/v), and the extract was centrifuged at 10,000 × g for 10 min. The clear supernatant was adjusted to a known volume with acetone, and the optical density was recorded at 480, 645 and 663 nm (Arnon Citation1949).
Concentration of hydrogen peroxide, malondialdehyde, free proline and soluble sugars
Hydrogen peroxide (H2O2) was determined according to Sergiev et al. (Citation1997), and the concentration of H2O2 was calculated from a standard curve; the results were expressed as nmol g−1 fresh weight. Lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA) produced, as described by Heath and Packer (Citation1968), in the thiobarbituric acid reaction. The absorbance was recorded at 600 and 532 nm after boiling the contents at the boiling temperature. The MDA concentration was calculated using an extinction coefficient of 155 mM cm−l. The estimation of free proline was conducted according to the methods of Bates et al. (Citation1973). Total soluble sugars (TSS) were estimated following the method of Stewart (Citation1989). A standard curve (10–100 µg ml−l) was used as a reference.
Concentration of lipid fractions
The lipid fraction was extracted from the leaves of cassia using chloroform following the methods of Fölch et al. (Citation1957) and Cachorro et al. (Citation1993). The total lipid content was estimated according to Marsh and Weinstein (Citation1966) using stearic acid [C18] (Sigma) as a standard. The total galactolipid, natural lipid and phospholipid contents were estimated according to Maxwell and Williams (Citation1968), Amenta (Citation1964) and Rouser et al. (Citation1970), respectively. After separation using thin layer chromatography (TLC) plates (G60 Silica Gel, Merck, Germany), the qualitative and quantitative estimation of TLC plates was carried out according to Amenta (Citation1964). Two-dimensional chromatography was used to separate phospholipid classes (Rouser et al. Citation1970), and identification was made by comparing the Rf-values to those of pure standards and by a specific staining reaction (molybdenum reagent) (Dittmer and Lester Citation1964). Spots were outlined with a pencil and scraped off the plates, and total phospholipids were determined by Dittmer and Wells (Citation1969).
Extraction and measurement of antioxidant enzymes
Fresh leaf sample (0.5 g) was homogenized in a mixture of 50 mM tris-HCl buffer (pH 7.0) containing 3.0 mM MgCl2, 1.0 mM ethylenediaminetetraacetic acid (EDTA), 1.0% (w/v) polyvinylpyrrolidone (PVP) and homogenate and was centrifuged at 10,000 rpm for 15 min at 4°C (Kar & Mishra Citation1976). Supernatant was used as the enzyme source to assess the antioxidant enzymes (peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR)). Estimation of protein was carried out using the method of Lowry et al. (Citation1951).
Peroxidase (POD) activity was measured according to the method described by Kar and Mishra (Citation1976). CAT activity was assayed as described by Aebi (Citation1984). SOD activity was assayed as described by Marklund and Marklund (Citation1974).
The method described by Foyer and Halliwell (Citation1976) was used to estimate the GR. The estimation of APX was carried out following the method of Nakano and Asada (Citation1981) by monitoring the decrease in absorbance at 290 nm. The activities of all enzymes were expressed as EU mg−1 protein.
Statistical analysis
The data presented represent the mean of three replicates and were statistically evaluated using one-way analysis of variance (ANOVA) using the SPSS-21 software. Least significant differences (LSD) (p = .05) were calculated.
Results
The response of sesame to cadmium stress was studied in pot experiments. The results showed that cadmium stress reduced the length of the root and shoot of sesame by 36.9% and 25.2%, respectively (). Calcium supplementation caused an increase of 5.9% and 16.3% in the root and shoot length of sesame, respectively (). The length of lateral roots was reduced by 55.1% upon cadmium stress, but it was increased by 43.7% following calcium treatment ().
Table 1. Effect of Cd (0.1 mM CdCl2) stress on length, dry weight, number of branches and leaf area of S. indicum L. grown with and without calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5), LSD at 0.05 (one-way ANOVA by SPSS-21).
Cd stress also reduced the dry weight of the root and shoot by 51.9% and 24.3%, respectively. Calcium treatment increased the weight of the root and shoot by 12.7% and 69.6%, respectively (). The leaf area and number of branches of sesame were affected by Cd stress and were increased by 15% after application of Ca2+ ().
The accumulation of Cd in plant roots and shoots is shown in . The content of Cd was higher in roots (372.3 µg g−1) than in leaves (153.2 µg g−1). The application of Ca2+ reduced the Cd accumulation in leaves (up to 80%) but increased it in root systems ().
Table 2. Cd (µg g−1 dry wt) content in leaf and root of S. indicum grown with and without calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5), LSD at 0.05 (one-way ANOVA by SPSS-21).
Photosynthetic pigments were also enhanced by Ca2+ application. Cd stress reduced chlorophylls a, b, total chlorophyll and carotenoid content by 39.1%, 48.7%, 43.7% and 53.1%, respectively (), whereas their content increased with Ca2+ application by 19.3%, 9.4%, 17.1% and 22.2%, respectively ().
Table 3. Effect of cadmium (0.1 mM CdCl2) stress on chlorophyll a (Chl. a), chlorophyll b (Chl. b), carotenoid (Carot.) and total chlorophyll (Total Chls.) contents in leaves of S. indicum L. grown with and without calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5), LSD at 0.05 (one-way ANOVA by SPSS-21).
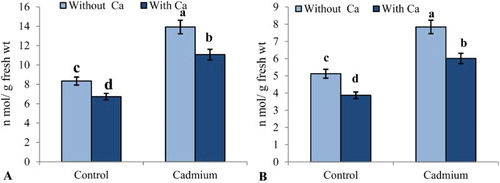
Cd addition significantly increased H2O2 and MDA content ((A,B)). By contrast, the application of Ca2+ decreased those parameters by 20.4% and 23.3%, respectively, compared to Cd-stressed plants.
Figure 1. Effect of Cd stress (0.1 mM CdCl2) on (A) H2O2 (nmol g−1 fresh wt) and (B) MDA (nmol g−1 fresh wt) in leaves of S. indicum with and without added calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5).
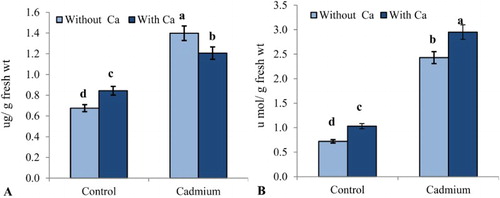
The soluble sugar and proline concentrations of leaves are shown in , and their concentration increased in Cd-stressed plants by 51.71% and 70.37%, respectively. However, supplementation of Ca2+ to Cd-treated plants decrease increment of both soluble sugar and proline in comparison with the plants treated with Cd alone. In absence of Cd stress soluble sugar and proline concentrations of Ca2+-treated plants, showed increased slightly in comparison to control ().
Figure 2. Effect of cadmium stress (0.1 mM CdCl2) on (A) TSS (µg g−1 fresh wt) and (B) proline (µmol g−1 fresh wt) in leaves of S. indicum with and without added calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5).
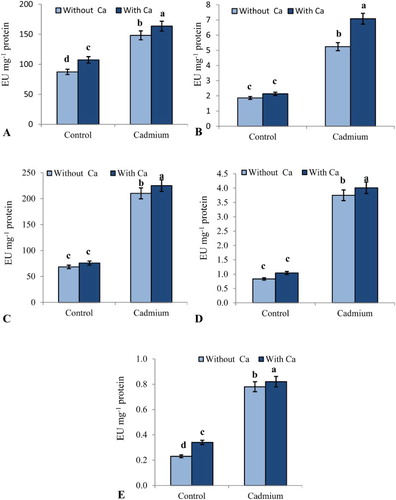
The antioxidant enzyme activities in the leaves of sesame are shown in . Cd stress affected those enzymes by increasing SOD, POD, CAT, APX and GR by 41.04%, 65.0%, 67.4%, 77.8% and 70.5%, respectively. However, the activities of antioxidant enzymes were reduced by the application of Ca2+.
Figure 3. Effect of Cd stress (0.1 mM CdCl2) on (A) SOD (B) POD (C) CAT (D) APX and (E) GR in leaves of S. indicum with and without added calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5).
The lipid fractions of leaves of sesame were sensitive to Cd stress (). Several groups of lipids, such as galactolipids, phospholipids and neutral lipids, were reduced by Cd stress and drastically increased in Ca2+-treated plants, being higher (up to 30%) compared to the Cd control plants.
Table 4. Effect of Cd (0.1 mM CdCl2) stress on lipid fractions (mg g−1 fresh weight) in leaves of S. indicum L. grown with and without calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5), LSD at 0.05 (one-way ANOVA by SPSS-21).
We also observed changes in the neutral lipid composition, as shown in . Diacylglycerol, sterol ester and non-esterified fatty acids decreased with calcium treatment by 1.7%, 6.5% and 3.2%, respectively, whereas triacylglycerol and sterol increased by 58.1% and 55.07%, respectively ().
Table 5. Effect of cadmium (0.1 mM CdCl2) stress on neutral lipids (mg g−1 fresh wt) diacylglycerol (DG), triacylglycerol (TG), sterol (S), sterol ester (SE) and non-esterified fatty acids (FAA) in leaves of S. indicum L. grown with and without calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5), LSD at 0.05 (one-way ANOVA by SPSS-21).
Relative to cadmium-stressed plants, calcium-treated cadmium-stressed plants had 28.03% and 19.1% higher diacylglycerol and sterol ester contents (). Total phospholipids were reduced by 29.8% due to cadmium stress, but calcium alone increased phospholipids by 11.8% (). Calcium supplementation to cadmium-stressed plants caused a 14.4% increase compared to cadmium-treated plants (). Furthermore, calcium supplementation caused an increase of up to 42% in the phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol and phosphatidylserine concentrations ().
Table 6. Effect of cadmium (0.1 mM CdCl2) stress on phospholipids (mg g−1 fresh wt), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS) and phosphatidic acid (PA) in leaves of S. indicum L. grown with and without calcium (50 mM CaCl2). Data presented are the means ± SE (n = 5), LSD at 0.05 (one-way ANOVA by SPSS-21).
Discussion
Reduced height and biomass due to cadmium stress corroborates with the findings in Triticum aestivum (Ci et al. Citation2009), Brassica juncea (Irfan et al. Citation2014) and Helianthus annuus (Abd_Allah et al. Citation2015). The reduced height, leaf area and biomass in S. indicum due to cadmium stress were reversed following calcium application. Cadmium stress causes perturbations in key metabolic pathways and triggers necrosis, thereby inducing growth retardation (Amaya-Carpio et al. Citation2009). In Cassia italica, Hashem et al. (Citation2016) demonstrated drastic reductions in growth due to cadmium application. During stress, the cell cycle progression and cell elongation rates are hindered because stresses exert irreversible effects on membrane potential and the functioning of the proton pumps responsible for cell growth (Karcz & Kurtyka Citation2007). Calcium protects plants from deleterious impacts of stress by acting in signaling pathways and regulating calmodulin-like proteins to promote several growth mechanisms in plants (Sarwat et al. Citation2013). Ahmad et al. (Citation2015) also demonstrated reductions in biomass accumulation in mustard due to cadmium application and its subsequent amelioration by Ca2+ application. Exposure of plants to stress brings down the hydraulic conductivity and hampers cell wall extensibility, which decreases the growth rate (Ehlert et al. Citation2009). Cadmium transport toward shoots was reduced by calcium application, and similar to our earlier results, Ci et al. (Citation2009), Elloumi et al. (Citation2014) and Abd_Allah et al. (Citation2015) reported reduced cadmium accumulation in the upper parts of wheat, almond and sunflower plants. Plants are very sensitive to cadmium, and even low concentrations of cadmium can prove toxic to major metabolic pathways. The calcium-induced growth enhancement in plants could be attributed to restricted cadmium uptake in calcium-treated plants (Ahmad et al. Citation2015). In the present study, calcium helped sesame plants displace cadmium quickly to maintain cellular metabolism.
In our study, Cd stress decreased the chlorophyll contents, and similar observations have been reported in H. annuus (Abd_Allah et al. Citation2015). In another study, the photosynthetic pigments in Abelmoschus esculentus L. and Cyamopsis tetragonoloba L. were reduced by Cd stress (Mangal et al. Citation2013). Supplementation of sufficient amounts of mineral elements such as K, Ca, Mn and Mg maintains an optimal composition of photosynthetic pigments for the sufficient synthesis of photoassimilates (Elloumi et al. Citation2014; Ahmad et al. Citation2015).
In the present study, treatment with cadmium (100 µM CdCl2) considerably increased the accumulation of H2O2, resulting in the increased peroxidation of lipids. The results of the present study support the findings of Elloumi et al. (Citation2014), Abd_Allah et al. (Citation2015) and Ahmad et al. (Citation2015). Plants that are exposed to extreme environments exhibit an increased accumulation of free radicals, which can alter the metabolism and membrane functioning (Khan et al. Citation2015). Biological membranes are very sensitive to free radicals and are easily denatured when attacked (Sofo et al. Citation2016). In the present investigation, calcium supplementation reduced the production of H2O2, thereby providing protection to cells from oxidative damage. Our results of reduced H2O2 and lipid peroxidation in calcium-supplemented plants are in agreement with those obtained for Arabidopsis thaliana (Suzuki Citation2005), Vicia faba (Siddiqui et al. Citation2012) and B. juncea (Ahmad et al. Citation2015). Adequate mineral additions of Ca2+ protect membranes and the photosynthetic apparatus from stress-induced peroxidation (Ahmad et al. Citation2015). Cadmium toxicity caused destabilization of membranes, as reflected in the reduced chloroplast pigments (Ahmad et al. Citation2015). Such deleterious changes resulting from the peroxidation of lipids are widely accepted as important parameters for measuring the intensity and magnitude of stress factors. The reduced production of H2O2 and lipid peroxidation in calcium-treated plants suggest the potential of calcium in reversing ROS-induced oxidative damage. The increased peroxidation of membrane lipids in cadmium-stressed plants is due to the increased lipoxygenase activity, which results in the rapid generation of peroxides and hydroxyl radicals and thus imposes deteriorating effects on cellular organelles (Djebali et al. Citation2005). Recently, it was demonstrated that the application of calcium to cadmium-stressed mustard (Ahmad et al. Citation2015) and faba bean (Siddiqui et al. Citation2012) plants caused significant reductions in lipid peroxidation by downregulating the production rates of ROS.
The calcium-induced accumulation of proline and soluble sugars indicates the positive role of calcium in mitigating the negative impact of cadmium stress. The accumulation of osmotic constituents such as proline and soluble sugars helps plants maintain their cellular function by maintaining the water content in the tissue. It has been established that stress results in upregulated proline synthesis with a concomitant reduction in catabolism (Iqbal et al. Citation2015). Our observation of proline and sugar accumulation in cadmium-stressed plants supports similar previous findings (Irfan et al. Citation2014; Hashem et al. Citation2016). The actual mechanism of cadmium toxicity is not well understood, and improving cadmium tolerance in plants through conventional methods may be the best alternative. Proline and other important osmolytes, such as sugars, protect membranes and other cellular structures by neutralizing ROS (Abd_Allah et al. Citation2015; Ahmad et al. Citation2015). Proline accumulation regulates protein turnover, regulates stress protective proteins and is involved in ROS scavenging (Ahmad et al. Citation2015). In the present study, calcium application caused further increases in proline and sugars, thus helping nullify the effects of cadmium. Changes in the sugar fraction due to stress involve alterations in carbon assimilation and the partitioning between source and sink tissues (Gupta and Kaur Citation2005; Rosa et al. Citation2009).
S. indicum exposed to cadmium stress exhibited enhancements in the antioxidant enzyme activities analyzed, and calcium application caused a further increase in their activities, which suggested a protective role of calcium in mitigating oxidative stress. Such an enhancement in the activity of antioxidant enzymes in response to cadmium stress has been reported in T. aestivum (Milone et al. Citation2003); B. juncea (Asgher et al. Citation2014) and C. italica (Hashem et al. Citation2016). Maximal increases in activity were observed in plants treated with cadmium and calcium ((A–E)). SOD, POD, CAT, APX and GR detoxify free radicals and keep their levels within a non-toxic range (Abd_Allah et al. Citation2015; Hashem et al. Citation2016). Ahmad et al. (Citation2015) demonstrated a higher activity of antioxidant enzymes due to calcium treatment, which contributed to the increased protection of membranes. SOD eliminates superoxide radicals, whereas CAT and APX contribute to radical scavenging by further terminating the superoxide radical generated chain reactions (Siddiqui et al. Citation2012; Sarwat et al. Citation2013; Sofo et al. Citation2016). In our experiments, calcium addition helped plants resist stress and stimulated plant growth and development. Such positive effects of calcium can be due to its impact on mineral uptake, which results in improved antioxidant defense systems (Siddiqui et al. Citation2012; Asgher et al. Citation2014; Ahmad et al. Citation2015; Iqbal et al. Citation2015). This stress-ameliorating role of calcium can be ascribed to the enhancement of antioxidant activities, which was shown to reduce lipid peroxidation in calcium-treated plants. In addition, increased GR activity in calcium-treated plants could contribute to maintaining the redox buffer and coordinating electron transport during photosynthetic processes (Asgher et al. Citation2014). This increased antioxidant metabolism supports the role of calcium in mitigation of cadmium stress in S. indicum, which can contribute significantly to the protection of membrane lipids.
Cadmium stress drastically reduced the lipid content in S. indicum. The total phospholipid and neutral lipids were observed to be higher in calcium-treated plants, and calcium application ameliorated the cadmium-induced decline to some extent. Our present observations agree with previous reports (Elloumi et al. Citation2014; Abd_Allah et al. Citation2015). Among the phospholipids estimated, phosphatidic acid showed a slight increase with cadmium stress; a similar increment was reported by Ammar et al. (Citation2008) in tomato. Modifications in the lipid environment due to stress can directly affect the activity of intrinsic membrane proteins (Quartacci et al. Citation2000). Calcium induces the adhesion and collapse of phosphatidylserine bilayers into multilamellar structures. Previously, it has also been demonstrated that Cd stress increases saturated fat components and reduces unsaturated components (Kuznetsova et al. Citation2008; Abd_Allah et al. Citation2015). Improved lipid synthesis can mediate and rectify ROS or hormone-regulated signaling (Wi et al. Citation2014). Reduced phospholipid and unsaturated fatty acid biosynthesis due to Cd stress has also been reported in different plants (Ben Ammar et al. Citation2007; Elloumi et al. Citation2014; Sofo et al. Citation2016). Calcium application mitigated the cadmium stress on phospholipids and neutral lipids, all of which play an important role in membrane functioning (Kuznetsova et al. Citation2008; Abd_Allah et al. Citation2015).
Conclusion
Cd stress resulted in oxidative stress, which caused increased lipid peroxidation and membrane dysfunction, as observed in the changes in membrane compositions. Cd considerably affected the lipid concentrations in leaves of sesame, whereas calcium ameliorated this effect and protected membrane function. Antioxidant enzymes were upregulated due to cadmium stress and were further enhanced due to calcium treatment. In the present study, we observed a strong positive effect of calcium on S. indicum growth under cadmium stress.
Additional information
Funding
References
- Abd_Allah EF, Hashem A, Alqarawi AA, Alwathnani HA. 2015. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak J Bot. 47(2):785–795.
- Aebi H. 1984. Catalase in vitro. Meth Enzymol. 105:121–126. doi: 10.1016/S0076-6879(84)05016-3
- Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LS, Zhang J-S. 2015. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. &Coss.) by calcium application involves various physiological and biochemical strategies. PLoS One. 10(1):e0114571. doi: 10.1371/journal.pone.0114571.
- Amaya-Carpio L, Davies FT Jr, Fox T, He C. 2009. Arbuscular mycorrhizal fungi and organic fertilizer influence photosynthesis, root phosphatase activity, nutrition, and growth of Ipomoea carnea ssp. fistulosa. Photosynthetica. 47(1):1–10. doi: 10.1007/s11099-009-0003-x
- Amenta JS. 1964. A rapid method for quantification of lipids separated by thin layer chromatography. J Lipid Res. 5:270–272.
- Ammar WB, Nouairi I, Zarrouk M, Jemal F. 2008. The effect of cadmium on lipid and fatty acid biosynthesis in tomato leaves. Biologia. 63(1):86–93.
- Arnon DI. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24:1–15. doi: 10.1104/pp.24.1.1
- Asgher M, Khan NA, Khan MIR, Fatma M, Masood A. 2014. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotox Environ Saf. 106:54–61. doi: 10.1016/j.ecoenv.2014.04.017
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39:205–207. doi: 10.1007/BF00018060
- Ben Ammar W, Nouairi I, Zarrouk M, Jemal F. 2007. Cadmium stress induces changes in the lipid composition and biosynthesis in tomato (Lycopersicon esculentum Mill.) leaves. Plant Growth Regul. 53(2):75–85. doi: 10.1007/s10725-007-9203-1
- Burd GI, Dixon GD, Glick BR. 2000. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol. 46:237–245. doi: 10.1139/w99-143
- Cachorro P, Ortiz A, Cerda A. 1993. Effects of saline stress and calcium on lipid composition in bean roots. Phytochem. 32:1131–1136. doi: 10.1016/S0031-9422(00)95077-5
- Carleton M, Foote K. 1965. A comparison of methods for estimating total leaf area of barley plants. Crop Sci. 5(6):602–603. doi: 10.2135/cropsci1965.0011183X000500060041x
- Chen S, Sun L, Sun T, Chao L, Guo G. 2007. Interaction between cadmium, lead and potassium fertilizer (K2SO4) in a soil-plant system. Environ Geochem Health. 29:435–446. doi: 10.1007/s10653-007-9088-y
- Ci D, Jiang D, Dai T, Jing Q, Cao W. 2009. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere. 77(11):1620–1625. doi: 10.1016/j.chemosphere.2009.08.062
- Dittmer JC, Lester RL. 1964. A simple, specific spray for the detection of phospholipids on thin layer chromatograms. J Lipid Res. 5:126–127.
- Dittmer JC, Wells MA. 1969. Quantitative and qualitative analysis of lipid and lipid components. Methods Enzymol. 14:482–530. doi: 10.1016/S0076-6879(69)14055-0
- Djebali W, Zarrouk M, Brouquisse R, El Kahoui S, Limam F, Ghorbel MH, Chaibi W. 2005. Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol. 7:358–368. doi: 10.1055/s-2005-837696
- Ehlert C, Maurel C, Tardieu F, Simonneau T. 2009. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 150:1093–1104. doi: 10.1104/pp.108.131458
- Elloumi N, Zouari M, Chaari L, Jomni C, Marzouk B, Elloumi FBA. 2014. Effects of cadmium on lipids of almond seedlings (Prunus dulcis). Bot Stud. 55(61):1–9.
- Fölch J, Lees M, Sloane-Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 226:497–509.
- Foyer CH, Halliwell B. 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 133:21–25. doi: 10.1007/BF00386001
- Gallego SM, Pena LB., Barcia RA., Azpilicueta CE., Iannone MF., Rosales EP., Zawoznik MS., Groppa MD., Benavides MP. 2012. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ Exp Bot. 83:33–46. doi: 10.1016/j.envexpbot.2012.04.006
- Gupta AK, Kaur N. 2005. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci. 30:761–76. doi: 10.1007/BF02703574
- Hashem A, Abd_Allah EF, Alqarawi AA, Egamberdieva D. 2016. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J Biol Sci. 23:39–47. doi: 10.1016/j.sjbs.2015.11.007
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophysic. 125:189–198. doi: 10.1016/0003-9861(68)90654-1
- Iqbal N, Umar S, Khan NA. 2015. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol. 178:84–91. doi: 10.1016/j.jplph.2015.02.006
- Irfan M, Ahmad A, Hayat S. 2014. Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci. 21:125–131. doi: 10.1016/j.sjbs.2013.08.001
- Jackson ML. 1962. Soil chemical analysis. New York (NY): Prentice Hall; p. 263–268.
- Kar M, Mishra D. 1976. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 57:315–319. doi: 10.1104/pp.57.2.315
- Karcz W, Kurtyka R. 2007. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol Plant. 51:713–719. doi: 10.1007/s10535-007-0147-0
- Khan MIR, Nazir F, Asgher M, Per TS, Khan NA. 2015. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol. 173:9–18. doi: 10.1016/j.jplph.2014.09.011
- Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MMA. 2010. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant. 32:121–132. doi: 10.1007/s11738-009-0387-z
- Kurtyka R, Małkowski E, Kita A, Karcz W. 2008. Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Polish J Environ Stud. 17:51–56.
- Kuznetsova TY, Vetchinnikova LV, Titov AF, Ilinova MK. 2008. Effect of cadmium on fatty acid composition of lipids in the shoots of Karelian birch cultured in vitro. Russ J Plant Physiol. 55:657–662. doi: 10.1134/S1021443708050099
- Lowry OH, Rosebrough NS, Farrand AL, Randall RJ. 1951. Protein measurement with folin phenol reagent. J Bio Chem. 193:263–275.
- Mangal M, Agarwal M, Bhargava D. 2013. Effect of cadmium and zinc on growth and biochemical parameters of selected vegetables. J Pharma Phytochem. 2:106–114.
- Mansour MMF, Salama KHA. 2004. Cellular basis of salinity tolerance in plants. Environ Exp Bot. 52:113–122. doi: 10.1016/j.envexpbot.2004.01.009
- Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
- Marsh JB, Weinstein DB. 1966. Simple charring method for determination of lipids. J Lipid Res. 7:574–576.
- Maxwell MAB, Williams JP. 1968. Separation and estimation of the galactolipid components of broad bean leaves. J Chromatogr. 35:223–229. doi: 10.1016/S0021-9673(01)82378-5
- Milone MT, Sgherri C, Clijsters H, Navari-Izzo F. 2003. Antioxidative responses of wheat treated with realistic concentration of cadmium. Environ Exp Bot. 50:265–276. doi: 10.1016/S0098-8472(03)00037-6
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 22:867–880.
- Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA. 2012. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci. 3:1476–1489. doi: 10.4236/ajps.2012.310178
- Quartacci MF, Pinzino C, Sgherri CLM, Dalla Vecchia F, Navari-Izzo F. 2000. Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol Plant. 108:87–93. doi: 10.1034/j.1399-3054.2000.108001087.x
- Rosa M, Hilal M, Gonzalez JA, Prado FE. 2009. Low-temperature effect on enzyme activities involved in sucrose-starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiol Biochem. 47:300–307. doi: 10.1016/j.plaphy.2008.12.001
- Rouser G, Fleischer S, Yamamoto A. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5:494–496. doi: 10.1007/BF02531316
- Sarwat M, Ahmad P, Nabi G, Hu X. 2013. Ca2+ signals: The versatile decoders of environmental cues. Crit Rev Biotechnol. 33:97–109. doi: 10.3109/07388551.2012.672398
- Sergiev I, Alxieva V, Karanov E. 1997. Effect of spermone, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Comp Rend Acad Bulg Sci. 51:121–124.
- Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. 2012. Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress. Int J Mol Sci. 13:6604–6619. doi: 10.3390/ijms13066604
- Sofo A, Scopa A, Hashem A, Abd_Allah EF. 2016. Lipid metabolism and oxidation in plants subjected to abiotic stresses. In: Mohamed MA, Ahmad P, editors. Plant-environment interaction: responses and approaches to mitigate stress. 1st ed. John Wiley & Sons.
- Stewart EA. 1989. Analysis of vegetation and other organic material. New York: Acad. Press; p. 46–60.
- Suzuki N. 2005. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotech. 22:19–25. doi: 10.5511/plantbiotechnology.22.19
- Uzun B, Arslan C, Furat S. 2008. Variation in fatty acid compositions, oil content and oil yield in a germplasm collection of sesame (Sesamum indicum L.). J Am Oil Chem Soc. 85:1135–1142. doi: 10.1007/s11746-008-1304-0
- Wi SJ, Seo SY, Cho K, Nam MH, Park KY. 2014. Lysophosphatidylcholine enhances susceptibility in signaling pathway against pathogen infection through biphasic production of reactive oxygen species and ethylene in tobacco plants. Phytochemistry. 104:48–59. doi: 10.1016/j.phytochem.2014.04.009
