ABSTRACT
Herbivory damage leads to induction of rapid signals and responses in plants such as oxidative burst, accumulation of secondary metabolites and defensive proteins. Response of various defensive enzymes and secondary metabolites in flag leaf samples of six bread wheat varieties against aphid feeding was investigated. Six bread wheat varieties, namely PBW 621 and HD 2967 (timely sown irrigated), PBW 590 and PBW 658 (late sown irrigated), and PBW 644 and PBW 660 (timely sown rainfed) were grown under the aphid infested and uninfested conditions and were sampled at a regular interval to analyze the biochemical changes caused by aphid feeding. A tremendous increase in the overall activity of various enzymes namely superoxide dismutase, glutathione reductase, phenylalanine ammonia lyase and polyphenol oxidase was observed, all of which play an important role in plants defense towards aphid feeding. Each wheat genotype showed an overall difference in their defensive activity towards aphid feeding. However, certain genotypes under different conditions showed significantly less susceptibility towards aphid damage.
Abbreviations: GR: glutathione reductase; HPR: host plant resistance; PAL: phenylalanine ammonia lyase; PPO: poly phenol oxidase; POD: peroxidase; SOD: superoxide dismutase
Introduction
The aggregate area under wheat production in the world is about 225.62 million ha with an overall production of 685.6 million tons (National… Citation2015). India’s partake in world wheat area is about 12.40%, whereas it occupies 11.77% partake in the total world wheat production (Ajai et al. Citation2016). The world’s population is continuously increasing and is projected to be more than 9 billion by 2050. This population growth is co-happening with other elements such as a dietary shift in developing countries, environmental change, increasing pest population, which is compromising wheat yields due to abiotic factors, and the constant pressures of biotic hassles (Hawkesford et al. Citation2013). Among the biotic factors that limit wheat production, aphid complex is considered as a major threat, which significantly reduces grain yields if not controlled. Aphids are serious pests worldwide, able to bring about severe damage in cereal crops, especially wheat, Triticum aestivum, by direct feeding and by transmitting plant pathogenic viruses (Thackray et al. Citation2009). In India, wheat (T. aestivum) is attacked by more than 11 aphid species, out of which four species namely Sitobion avenae (Fabricius), Sitobion miscanthi, Rhopalosiphum padi and Rhopalosiphum maidis are reported to be most predominant (Jarosik et al. Citation2003) and a combined population of these four is designated as wheat aphid complex (Aslam et al. Citation2004).
Plants are sessile organisms and, with their inability to move, they have created a wide range of barriers against assault by pathogens and insects alike. A quick and transient generation of reactive oxygen species (ROS) is a typical phenomenon in plants on account of oxidative stress because of biotic and abiotic factors (Maffei et al. Citation2007; Torres Citation2010). Superoxide dismutase (SOD) has been proposed to be essential in plant stress resistance and provides the first line of defense against the harmful effects of elevated levels of ROS. Glutathione reductase (GR) and glutathione (GSH) play a crucial part in determining the resistance of a plant under different stresses. The enzyme phenylalanine ammonia lyase (PAL) catalyzes deaminating reaction of the amino acid phenylalanine from the primary metabolism into the important secondary phenylpropanoid metabolism in plants (Hahlbrock and Scheel Citation1989). Phenylpropanoid compounds not only satisfy various key functions during plant development but also act as critical protectants against various biotic and abiotic environmental stresses. Among the secondary metabolites, plant phenols constitute a standout among the most common and widespread group of defensive compounds, which play an important role in host plant resistance (HPR) against herbivores, including insects (Sharma and Garg Citation2009; Usha Rani et al. Citation2010). Qualitative and quantitative modifications in phenols and elevation in activities of oxidative enzyme in response to insect attack is a general phenomenon (War et al. Citation2012). Oxidation of phenols catalyzed by polyphenol oxidase (PPO) and peroxidase (POD) is a potential defense mechanism in plants against herbivorous insects.
Keeping in view the importance of the disease, the present study was carried out with the objective to evaluate the various biochemical changes caused by the infestation of aphids in bread wheat varieties during the crop development.
Materials and methods
Experimental design and plant material
This field experiments were conducted under irrigated conditions at Plant Breeding Research Farm, Punjab Agricultural University, Ludhiana, India (30° 55′ N 75° 54′ E and 250 m above sea level) during 2014–2015. Six bread wheat varieties PBW 621 and HD 2967 (timely sown irrigated), PBW 590 and PBW 658 (late sown irrigated), and PBW 644 and PBW 660 (timely sown rainfed) were sown in factorial randomized complete block design under insecticide-protected and unprotected conditions. Insecticide protection and genotypes were the two factors and each treatment was replicated thrice in experimentation. Imidacloprid (Confidor 17.8 SL) was sprayed to control aphids in insecticide-protected conditions and kept as control, whereas another plot was not sprayed to observe the biochemical changes caused by the attack of aphid complex under the natural conditions. The crop was raised by the following recommended standard package of practices except for the usage of pesticides (Package… Citation2014). The sample of growing flag leaves was collected randomly from uninfested and infested plants from 90 days after sowing with an interval of 7 days for the biochemical assays.
Extraction and assay of SOD, GR, PAL and PPO
For the extraction of antioxidative enzyme, 100 mg of sample tissue was homogenized in 2 ml of ice-cold potassium phosphate buffer (0.1 M, pH 7.5) containing 10 mM mercaptoethanol, 1% PVP and 1 mM EDTA. The homogenate was centrifuged at 10,000× g for 20 min at 4°C. The supernatant obtained was used for the assay of antioxidative enzymes, as described below.
SOD activity was determined by following the procedure of Marklund and Marklund (Citation1974). SOD activity was expressed as units min−1 g−1 fresh weight. GR activity was determined as per the procedure given by Esterbauer and Grill (Citation1978). GR activity was expressed as nmoles of NADP+ formed min−1 g−1 fresh weight. PAL activity was measured following the method of Burrell and Rees (Citation1974) and was expressed as mg of t-cinnamic acid formed h−1 g−1.
For the extraction of oxidative enzyme, 100 mg of the sample tissue was homogenized in 2 ml of ice-cold sodium phosphate buffer (0.1 M, pH 7) at 4°C. The homogenate was centrifuged at 10,000 × g for 20 min at 4°C. The supernatant obtained was used for the assay of oxidative enzymes. Polyphenoloxidase activity was determined as per the procedure of Archana et al. (Citation2011). The enzyme activity expressed as change in absorbance at 495 nm min−1 g−1 fresh weight of tissue.
Total phenol content
The dried tissue sample (400 mg) was refluxed with 5 ml of 80% aqueous methanol for 1 h. The refluxed material was then filtered and diluted with hot 80% methanol to a volume of 10 ml. The extract obtained was used for the estimation of total phenols by the method of Swain and Hillis (Citation1959).
Proline content
Approximately 100 mg of the sample was homogenized with 4 ml of 3% aqueous sulfosalicylic acid and the homogenate was filtered through Whatman filter paper and the filtrate was used for proline estimation by the procedure of Bates et al. (Citation1973).
Tannin content
The tissue (50 mg) was refluxed with 7.5 ml of H2O at 100°C for 30 min. The content was then cooled and centrifuged at 10,000 × g for 20 min. The supernatant thus obtained was used for the estimation of tannins by the method of Sadasivam and Manickam (Citation1992) after dilution with water to obtain a final volume of 10 ml.
Statistical analysis
The results are expressed as mean ± SD. Data were analyzed by two-way ANOVA to determine the difference between the treatments as well as genotypes (SPSS 16.0).
Mean values of timely sown irrigated, late sown irrigated and timely sown rainfed genotypes for different parameters were calculated and fold increase/decrease of infested plants was determined from their respective uninfested plants.
Results
SOD activity
Developing flag leaves of all the test genotypes showed an increase in SOD activity following aphid infestation (). A 1.1-fold increase in SOD activity was observed in the infested flag leaf samples when compared to uninfested flag leaf samples of all the test genotypes. Maximum SOD activity was observed in infested leaves of PBW 658 at 28 days after emergence (DAE). This might be due to less susceptibility of this particular genotype against feeding effect of aphids. Overall, maximum activity of SOD in all the genotypes was observed at 28 DAE and this could be the higher incidence of aphids at this particular stage. Timely sown rainfed genotypes were found to show better results followed by timely sown irrigated and late sown irrigated genotypes. PBW 644, a timely sown rainfed genotype, exhibited the highest increase in SOD activity as compared to all other genotypes under infested condition irrespective of time intervals.
Table 1. SOD activity in developing flag leaves of six test genotypes under infested and uninfested conditions at different growth stages (units min−1 g−1 FW).
GR activity
Aphid infestation increases the activity of GR in developing flag leaves of all the test genotypes (). A 1.3-fold increase in GR activity was observed in the infested flag leaf samples than the uninfested flag leaf samples of all the test genotypes. Timely sown rainfed genotypes were again recorded to show significantly increasing results than timely sown irrigated and late sown irrigated genotypes. Irrespective of genotypes, maximum overall GR activity was observed at 28 DAE which may indicate higher aphid incidence at this particular stage. Average maximum GR activity throughout the growth period was observed in infested flag leaves of PBW 644 and was found minimum in infested flag leaves of PBW 590. Thus, PBW 644 could be considered less susceptible than the other genotypes.
Table 2. GR activity in developing flag leaves of six test genotypes under infested and uninfested conditions at different growth stages (nmoles of NADP+ formed/min/g FW).
PAL activity
Infested wheat genotypes showed a significant increase in PAL activity as compared to the uninfested genotypes (). A 1.4-fold increase in PAL activity was observed in the infested developing flag leaf samples as compared to the uninfested flag leaf samples of all the genotypes. Maximum SOD activity was observed in infested leaves of PBW 658 at 28 DAE. Increase in PAL activity was observed to be maximum in late sown irrigated genotypes followed by timely sown rainfed and timely sown irrigated genotypes. PBW 658, a late sown irrigated genotype, showed overall higher PAL activity throughout the growth period as compared to other genotypes.
Table 3. PAL activity in developing flag leaves of six test genotypes under infested and uninfested conditions at different growth stages (µg g−1 FW).
PPO activity
Infested wheat plants showed a significant increase in PPO activity (1.4-fold) as compared to the respective uninfested plants of all the genotypes (). Average increase in PPO activity was seen to be maximum at 28 DAE and was observed to be maximum in HD 2967 and minimum in PBW 658. Considering three conditions, mean PPO activity was observed significantly higher in timely sown irrigated and timely sown rainfed genotypes. Overall an average increase in PPO activity was seen maximum in infested flag leaves of HD 2967 throughout the growth period.
Table 4. PPO activity in developing flag leaves of six test genotypes under infested and uninfested conditions at different growth stages (units min−1 mg−1 proteins).
Total phenols
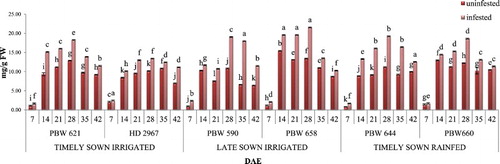
Aphid infestation resulted in a significant increase in the content of total phenols in the developing flag leaves of all the test genotypes (). A 1.4-fold increase in total phenols was recorded in infested flag leaves as compared to uninfested flag leaves of all the wheat genotypes. Maximum increase in total phenols was observed at 28 DAE and was observed to be maximum in PBW 658. This increase in phenol content at 28 DAE may be due to higher aphid feeding at this particular stage. Average total phenols were observed to be maximum in late sown irrigated wheat genotypes followed by timely sown rainfed and timely sown irrigated genotypes. Comprehensively, an increase in total phenols was observed to be maximum in PBW 658 as compared to other genotypes throughout the growth period.
Figure 1. Change in phenol content in uninfested and infested developing flag leaves of wheat genotypes at different growth stages. Error bars denote ±SD of three replicates, bars with same letter(s) at particular day are not significantly different at P ≤ .05 (CD at 5% between ABC = 0.33; A: timely sown irrigated; B: late sown irrigated; C: timely sown rainfed).
Proline
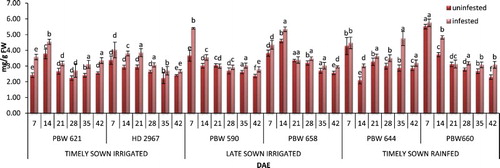
Infested flag leaves of all the test genotypes showed a moderate increase in proline content as compared to the uninfested flag leaves (). A 1.2-fold increase in proline content was observed in all the flag leaves infested by aphids as compared to the uninfested flag leaves. Maximum proline content was observed at 28 DAE of the flag leaves, with maximum content recorded in PBW 660 and minimum in HD 296. Overall, higher proline content was observed in infested leaves of PBW 660 as compared to all other genotypes irrespective of growth stages. Timely sown rainfed genotypes showed significantly better results followed by late sown irrigated and timely sown irrigated genotypes.
Figure 2. Change in proline content in uninfested and infested developing flag leaves of wheat genotypes at different growth stages. Error bars denote ±SD of three replicates, bars with same letter(s) at particular day are not significantly different at P ≤ .05 (CD at 5% between ABC = 0.61; A: timely sown irrigated; B: late sown irrigated; C: timely sown rainfed).
Tannins
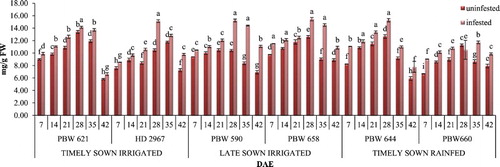
The content of tannins increased after the aphid infestation. A 1.2-fold increase in tannin content was recorded in the flag leaves infested by aphids as compared to the uninfested flag leaves of all the genotypes (). This increase in tannin content after aphid infestation indicates their protective role in plants. Maximum average increase in tannins was observed in PBW 658 throughout the growth period. Late sown irrigated genotypes showed significantly higher results than timely sown irrigated and timely sown rainfed genotypes.
Figure 3. Change in tannin content in uninfested and infested developing flag leaves of wheat genotypes at different growth stages. Error bars denote ±SD of three replicates, bars with same letter(s) at particular day are not significantly different at P ≤ .05 (CD at 5% between ABC = 0.58; A: timely sown irrigated; B: late sown irrigated; C: timely sown rainfed).
Discussion
Herbivory damage leads to induction of rapid signals and responses in plants such as oxidative burst, accumulation of secondary metabolites and defensive proteins (Gill et al. Citation2010). Rapid and brief generation of ROS is a typical phenomenon in plants on account of oxidative stress because of biotic and abiotic factors (Maffei et al. Citation2007; Torres Citation2010). NADPH oxidase generates superoxide anion at the plasma membrane or in the apoplast extracellularly, which is then converted to H2O2 by SOD (Maffei et al. Citation2007; Torres Citation2010). Other important antioxidative enzymes include PAL, GR, etc. In the present study, an increase in SOD, GR and PAL activity was observed after the aphid infestation which indicates their defensive role during herbivory attack; however, the strength in induction of enzymatic activities varied among genotypes. This might be due to differences in sensitive upregulation response of genotypes against insect pest (War et al. Citation2012).
A significant difference was observed in SOD activity of infested and uninfested flag leaf samples of all the genotypes. Flag leaf samples obtained from infested wheat plants showed an increase in SOD activity. Feeding activity by aphids might have resulted in upregulation of SOD activity. Simova-Stoilova et al. (Citation2009) studied the antioxidative activities of wheat subjected to field drought and reported well-expressed SOD and CAT activity in the stresses plants, showing that the antioxidative defense is an indispensable part of the basic metabolism, empowering the plant to cope instantaneously with rapid environmental stresses. SOD activity has been reported to increase in plants exposed to various environmental stresses (Sharma and Dubey Citation2005; Mishra et al. Citation2011). Overproduction of SOD was reported to result in enhanced oxidative stress tolerance in plants (Gupta et al. Citation1993). Kaur et al. (Citation2014) reported an increase in SOD activity was observed in the test genotypes of pigeon pea in response to Helicoverpa armigera feeding.
Another important component of the oxygen scavenging system is GR that catalyzes the reduction of glutathione disulfide to the sulfhydryl form GSH, which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell (Deponte Citation2013). After the infestation of aphids, an increase in GR activity was observed in the infested flag leaf samples of all the test genotypes. The increase in GR activity under stress condition was also supported by Desingh and Kanagaraj (Citation2007), who showed a significantly increased activity of SOD and GR in cotton plants under salt stress when compared to control plants.
Studies have shown that PAL activity responds to various stresses, such as wounding, drought, salinity, heavy metals and infection by viruses, bacteria or fungi; therefore, PAL is involved in the defense response of plant cells (MacDonald and D’Cunha Citation2007; Gao et al. Citation2008). A significant increase in PAL activity was observed in the infested flag leaves of all the test genotypes during the current study. A higher level of PAL activity was also observed in pea seedling leaves infested by pea aphids when compared with the control plants, and the alteration in PAL activity was proportional to the population sizes of pea aphid and the duration of infestation (Mai et al. Citation2014). The biosynthesis of PAL and aggregation of phenylpropanoid structures have been reported up on pathogenic attack including viruses, tissue wounding, UV irradiation, low temperatures, low levels of nitrogen, phosphate and iron (Dixon and Paiva Citation1995; Gholizadeh et al. Citation2004; Ritter and Schulz Citation2004). Thus, incline in PAL activity after the infestation of aphids could be linked to defense response of the plant against the pathogen feeding activity. Since PAL enzyme is involved in phenylpropanoid biosynthesis, a positive correlation could be observed in the activity of PAL and total phenols, suggesting increased content of total phenols due to upregulation of PAL.
PPO is known to catalyze the oxidation of phenols to quinones, which are toxic to pathogens. An increase in PPO activity was observed after the infestation of aphids. A significant increase in PPO activity was observed in the infested flag leaves as compared to the uninfested flag leaves of all the test genotypes. A similar increasing trend was observed by Sarwar et al. (Citation2006) during their studies on chickpea. Studies describing correlations of high PPO levels in cultivars or lines with high pathogen resistance also provide support for a pathogen defense role of PPO (Raj et al. Citation2006). PPOs can function in the following ways: (a) PPO-generated quinines may alkylate essential amino acids, decreasing plant nutritional quality, (b) quinones may produce oxidative stress in the gut lumen through redox cycling and (c) quinones and ROS produced by phenolic oxidation may be absorbed and have toxic effects on herbivores (Helmi and Mohamed Citation2016).
Phenolic acids are known to contribute significantly to the total antioxidant activity of wheat (Baublis et al. Citation2000; Yu et al. Citation2003). Current studies revealed an increase in phenol content after the infestation of aphids in different wheat genotypes. The elevation of phenols could be explained as a mechanism of defense that acts as a barrier to insect feeding. These phenolic compounds are known to inhibit the larval development and growth by acting as feeding deterrents. Phenolic compounds induced in plants are either directly toxic to insects or mediate the signaling of various transduction pathways, which in turn produce toxic secondary metabolites and activate defensive enzymes (Helmi and Mohamed Citation2016). Quinones formed by oxidation of phenols bind covalently to leaf proteins and inhibit protein digestion in herbivores (Bhonwong et al. Citation2009).
An increase in the amount of total tannins after aphid feeding indicated that tannins might play a role as feeding deterrents. Tannins have the capacity to interact with proteins suggesting that tannins affected insect herbivores by inactivating insect enzymes as well as dietary proteins (Robbins et al. Citation1987). It has been reported that condensed tannins reduce the growth and survival of many pests (Grayer et al. Citation1992; Bernards and Bastrup-Spohr Citation2008; Sharma and Garg Citation2009) as they precipitate proteins nonspecifically by hydrogen bonding or covalent bonding with proteins, thereby reducing nitrogen mineralization and/or digestion in the herbivore midgut (Bernards and Bastrup-Spohr Citation2008).
A large body of data suggests a positive correlation between proline accumulation and plant stress (Hayat et al. Citation2012). Numerous studies have reported proline as an antioxidant suggesting its role as ROS scavenger and singlet oxygen quencher (Smirnoff and Cumbes Citation1989; Matysik et al. Citation2002). An increased production of proline in infested flag leaves of all the test wheat genotypes is in agreement with the findings of many authors. Considering genotypes, overall mixed results were obtained in terms of resistance towards aphid feeding; however, PBW 658, PBW 644 and HD 2967 gave significantly better results under infested conditions
Thus, in conclusion, we could say that infestation by aphids alters the biochemical system of the plants. An increase in all the defense enzymes, phenols and proline was observed during the current studies. Interestingly, most of these biochemicals were observed to increase at 28 DAE in all the test genotypes (). Muhammad et al. (Citation2013) also observed maximum aphid population during the mid-weeks of March. He reported that this increase in aphid population might be due to favorable temperature for aphid reproduction during this period. Temperature ranging from 7.7°C to 25.2°C is favorable for aphid growth (Chander Citation1996), while the optimum temperature for aphid growth is 23.44°C (Miller and Smith Citation1998). The amount with which these biochemicals elevated varied in different genotypes. This may be due to the difference in the duration and effectiveness of defense responses against aphid feeding in each genotype. However, the genotypes PBW 658 (late sown irrigated), HD 2967 (timely sown irrigated) and PBW 644 (timely sown rainfed) showed significantly better results than the other genotypes, and thus could be considered to have better defense response than the other genotypes. Since so far all the released wheat varieties are susceptible against aphids (), therefore these varieties under different sowing conditions could be utilized for further research to generate wheat varieties resistant against aphids. Considering the above results aphid seems to be an important pest, which could cause considerable harm to the wheat plant. Thus, further work is required either to develop more resistant wheat genotypes or to develop a method to control the continuously increasing aphid attacks so as to protect the quality and yield of the wheat plants.
Table 5. Meteorological data for different days of flag leaf sampling.
Table 6. Response of tested genotypes against aphid complex.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ajai S, Sudheer R, Vijay R, Sandeep M. 2016. Efficacy of quinalphos 25 EC for the management of wheat aphid. Agric Sci Digest. 36:337–339.
- Archana S, Prabakar K, Raguchander T, Hubballi M, Valarmathi P, Prakasam V. 2011. Defense responses of grapevine to Plasmoparaviticola induced by azoxystrobin and Pseudomonas fluorescens. Int J Sustainable Agric. 3:30–38.
- Aslam M, Razaq M, Ahmad F, Faheem M, Akhtar W. 2004. Population of aphids (Schizaphis graminum R) on different varieties of wheat (Triticum aestivum L). Intl J Agric Bio. l6:974–977.
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39:205–207. doi: 10.1007/BF00018060
- Baublis A, Decker EA, Clydesdale FM. 2000. Antioxidant effect of aqueous extracts from wheat based ready-to-eat breakfast cereals. Food Chem. 68:1–6. doi: 10.1016/S0308-8146(99)00142-9
- Bernards M, Bastrup-Spohr L. 2008. Phenylpropanoid metabolism induced by wounding and insect herbivory. In: Schaller A, editor. Induced plant resistance to herbivory. NY: Springer-Verlag; p. 189–211.
- Bhonwong A, Stout MJ, Attajarusit J, Tantasawat P. 2009. Defensive role of tomato polyphenol oxidase against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J Chem Ecol. 35:28–38. doi: 10.1007/s10886-008-9571-7
- Burrell MM, Rees TA. 1974. Metabolism of phenylalanine and tyrosine by rice leaves infected by Piricularia oryzae. Physiol Plant Pathol. 4:497–508. doi: 10.1016/0048-4059(74)90035-6
- Chander S. 1996. Aphid infestation of wheat in relation to climatic factors and predators. J Annals Plant Protect Sci. 4:148–150.
- Deponte M. 2013. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochem Biophys Acta. 1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018
- Desingh R, Kanagaraj G. 2007. Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. Gen Plant Physiol. 33:221–234.
- Dixon RA, Paiva N. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7:1085–1097. doi: 10.1105/tpc.7.7.1085
- Esterbauer H, Grill D. 1978. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physio. 61:119–121. doi: 10.1104/pp.61.1.119
- Gao S, Ouyang C, Wang S, Xu Y, Tang L, Chen F. 2008. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 54:374–381.
- Gholizadeh A, Kumar M, Balasubrahmanyam A, Sharma S, Narwal S, Lodha ML, Kapoor HC. 2004. Antioxidant activity of antiviral proteins from Celosia cristata. J Plant Biochem Biotechnol. 13:13–18. doi: 10.1007/BF03263184
- Gill RS, Gupta K, Taggar GK, Taggar MS. 2010. Review article: role of oxidative enzymes in plant defenses against insect herbivory. Acta Phyto Pathol Entomol Hung. 45:277–290. doi: 10.1556/APhyt.45.2010.2.4
- Grayer RJ, Kimmins FM, Padgham DE, Harborne JB, Ranga Rao DV. 1992. Condensed tannin levels and resistance in groundnuts (Arachis hypogoea (L.) against Aphis craccivora (Koch). Phytochemistry. 31:3795–3799. doi: 10.1016/S0031-9422(00)97530-7
- Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD. 1993. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 90:1629–1633. doi: 10.1073/pnas.90.4.1629
- Hahlbrock K, Scheel D. 1989. Physiology and molecular biology of phenylpropanoid metabolism. Ann Rev Plant Physiol Plant Mol Biol. 40:347–369. doi: 10.1146/annurev.pp.40.060189.002023
- Hawkesford MJ, Araus JL, Park R, Calderini D, Miralles D, Shen T, Zhang J, Parry MA. 2013. Prospects of doubling global wheat yields. Food Energy Secur. 2:34–48. doi: 10.1002/fes3.15
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. 2012. Role of proline under changing environments. Plant Signal Behav. 7:1456–1466. doi: 10.4161/psb.21949
- Helmi A, Mohamed HI. 2016. Biochemical and ultrastructural changes of some tomato cultivars after infestation with Aphis gossypii Glover (Hemiptera: Aphididae) at Qalyubiyah, Egypt. Gesunde Pflanzen. 68:41–50. doi: 10.1007/s10343-016-0361-9
- Jarosik V, Honek A, Tichopad A. 2003. Comparison of field population growths of three cereal aphid species on winter wheat. Plant Protect Sci. 39:61–64.
- Kaur R, Gupta AK., Taggar GK. 2014. Role of catalase, H2O2 and phenolics in resistance of pigeonpea towards Helicoverpa armigera (Hubner). Acta Physiol Plant. 36:1513–1527. doi: 10.1007/s11738-014-1528-6
- MacDonald MJ, D’Cunha GB. 2007. A modern view of phenylalanine ammonia lyase. Biochem Cell Biol. 85:273–282. doi: 10.1139/O07-018
- Maffei ME, Mithöfer A, Boland W. 2007. Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release. Phyto Chem. 68:2946–2959.
- Mai VC, Drzewiecka K, Jeleń H, Narożna D, Rucińska-Sobkowiak R, Kęsy J, Floryszak-Wieczorek J, Gabryś B, Morkunas I. 2014. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 221–222:1–12. doi: 10.1016/j.plantsci.2014.01.011
- Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
- Matysik J, Alia, Bhalu B, Mohanty P. 2002. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci. 82:525–532.
- Miller RM, Smith AW. 1998. The greenbug aphid and its control. Fact sheet extension horticulture and crop sciences. Ohio State Univ Ext. Fact Sheet, 43210–1086.
- Mishra S, Jha AB, Dubey RS. 2011. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma. 248:565–577. doi: 10.1007/s00709-010-0210-0
- Muhammad W, Nasir M, Abbas SK., Irshad M, Abbas MW, Nawaz A, Rehman A. 2013. Resistance pattern against aphid (Diuraphis noxia) in different wheat varieties/lines at district Layyah. Acad J Ent. 6:116–120.
- National Agricultural Statistics Service (NASS). 2015. Agricultural Statistics Board, USDA (quoted from www.nue.osstate.edu/crop-information/world-wheat-production.htm).
- Package of practices for crops of Punjab, Rabi. 2014. Ludhiana: Punjab Agricultural University.
- Raj SN, Sarosh BR, Shetty HS. 2006. Induction and accumulation of polyphenol oxidase activities as implicated in development of resistance against pearl millet Downy mildew disease. Funct Plant Biol. 33:563–571. doi: 10.1071/FP06003
- Ritter H, Schulz GE. 2004. Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell. 16:3426–3436. doi: 10.1105/tpc.104.025288
- Robbins CT, Hanley TA, Hagerman AE, Hjeljord O, Baker DL, Schwartz CC, Mautz WW. 1987. Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology. 68:98–107. doi: 10.2307/1938809
- Sadasivam S, Manickam A. 1992. Biochemical methods for agricultural sciences. New Delhi: Wiley Eastern; p. 187–188.
- Sarwar H, Sarwar M, Jamil FF. 2006. Role of polyphenoloxidase and catalase in ascochyta blight resistance in Chickpea. Pak J Bot. 35:111–115.
- Sharma P, Dubey RS. 2005. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 46:209–221. doi: 10.1007/s10725-005-0002-2
- Sharma N, Garg V. 2009. Antihyperglycemic and antioxidative potential of hydroalcoholic extract of Butea monosperma Lam flowers in alloxan-induced diabetic mice. Indian J Exp Biol. 47:571–576.
- Simova-Stoilova L, Demirevska K, Petrova T, Tsenov N, Feller U. 2009. Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (Triticum aestivum L.) varieties subjected to long-term field drought. Plant Growth Regul. 58:107–117. doi: 10.1007/s10725-008-9356-6
- Smirnoff N, Cumbes QJ. 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 28:1057–1060. doi: 10.1016/0031-9422(89)80182-7
- Swain T, Hillis WE. 1959. The phenolic constituents of Prunus domestica. I.—the quantitative analysis of phenolic constituents. J Sci Food Agric. 10:63–68. doi: 10.1002/jsfa.2740100110
- Thackray DJ, Diggle AJ, Jones RAC. 2009. BYDV PREDICTOR: a simulation model to predict aphid arrival, epidemics of Barley yellow dwarf virus and yield losses in wheat crops in a Mediterranean-type environment. Plant Path. 58:186–202. doi: 10.1111/j.1365-3059.2008.01950.x
- Torres MA. 2010. ROS in biotic interactions. Physiol Plant. 138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x
- Usha Rani P, Jyothsna Y. 2010. Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiol. 32:695–701. doi: 10.1007/s11738-009-0449-2
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 7:1306–1320. doi: 10.4161/psb.21663
- Yu L, Perret J, Harris M, Wilson J, Haley S. 2003. Antioxidant properties of bran extracts from “Akron” wheat grown at different locations. J Agri Food Chem. 51:1566–1570. doi: 10.1021/jf020950z
