 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effects of salicylic acid (SA) on growth parameters and enzyme activities were investigated in salt-stressed safflower (Carthamus tinctorius L.). Twenty-five days after sowing, seedlings were treated with NaCl (0, 100, and 200 mM) and SA (1 mM), and were harvested at 21 days after treatments. Results showed that some growth parameters decreased under salinity, while malondialdehyde (MDA) and hydrogen peroxide (H2O2) content, phenolic compounds, and some enzyme activities increased. SA application increased some growth parameters, MDA and H2O2 content, and enzyme activities except catalase (CAT), which was different from the other enzymes and SA significantly reduced CAT activity in plants. These results suggest that SA-induced tolerance to salinity may be related to regulation of antioxidative responses and H2O2 level. Our study suggested that the resistant safflower can direct reactive oxygen species from a threat to an opportunity by using SA. Therefore, exogenous application of SA played this role through regulation of the antioxidant system.
Introduction
Salinity is an environmental factor that limits crop production and soil fertility in many areas in the world (Silveira et al. Citation2001; Khan and Panda Citation2007; Aftab et al. Citation2011). It causes deficiency of some nutrients and an increase in Na+ levels in plants (Grattan and Grieve Citation1998; Ramezani et al. Citation2012; Zahedi et al. Citation2012). Salinity is a significant problem in safflower (Carthamus tinctorius L.) production in many areas in the world. Safflower is a herbaceous plant that belongs to Asteraceae family (Sadeghi et al. Citation2013). It has been widely cultivated for its flowers and oil (Işigigür et al. Citation1995). Safflower is known as a moderately salt-tolerant plant (Bassil and Kaffka Citation2002). Its salt tolerance is more than that exhibited by some other oilseed plants, but like the majority of the cultivated plants, its growth and yield decrease depending on the salinity level.
Plants generally protect themselves against salinity by many strategies such as the production of reactive oxygen species (ROS), synthesis of defense proteins, and accumulation of some secondary metabolites (Sorahinobar et al. Citation2016). Several factors associated with salinity can lead to an increase in ROS formation, which can damage membrane lipids, nucleic acids, and proteins (Liang et al. Citation2003; Patade et al. Citation2011). On the other hand, specific levels of ROS are essential and lead to an increase in antioxidative protection (Stevens et al. Citation2006; Vital et al. Citation2008).
To prevent from damaging effects of ROS, plants possess antioxidative mechanisms to scavenge excess ROS in plant cells. Several antioxidative enzymes, including superoxide dismutase (SOD), peroxidase (POX), and polyphenol oxidase (PPO), are involved in detoxification of ROS (Zhang and Kirkham Citation1996; Lee and Lee Citation2000). Additionally, in order to protect against overproduction of ROS, plants synthesize some low-molecular compounds, such as ascorbate and phenolic compounds (Kim et al. Citation2007; Kováčik and Bačkor Citation2007). Phenolic compounds serve as the potent non-enzymatic antioxidant and therefore extinguish oxidative free radicals in plant cells (Rice-Evans et al. Citation1996; Grassmann et al. Citation2002). The phenylpropanoid pathway is one of the important pathway in plant cells. Phenylalanine ammonia-lyase (PAL) is another important enzyme in plant cells, which acts in this pathway and is involved in the synthesis of compounds such as phenolics and lignin (Hemm et al. Citation2004).
Manipulation of crop production with some chemical compounds or growth regulators has a main role in development of plants. The plant yield under stress condition can be increased by exogenous application of some growth regulators such as salicylic acid (SA). SA, a well-known signaling messenger, is able to reduce symptoms of several environmental stresses in plant tissues (Horváth et al. Citation2007; Hayat et al. Citation2010). It acts as a protector against various stresses and has a key role in defense mechanism in plants (Klessig and Malamy Citation1994; Gunes et al. Citation2007). It has been shown that SA is amongst the most important compounds involved in plant resistance against salinity.
Safflower (C. tinctorius L.) is an important oilseed plant whose growth and development can be affected by salt stress. Here, we performed a study to compare the effects of exogenous application of SA on safflower plants to distinguish the effects of some physiological and biochemical parameters in response to salinity and to assess the possibility of improving salt tolerance in safflower plants.
Materials and methods
Plant cultivation and chemical treatments
Seeds of safflower (C. tinctorius L.) CV. Goldasht were obtained from the Seed and Plant Improvement Institute of Karaj, Iran. Seeds were surface sterilized for 5 min in 10% sodium hypochlorite solution and then in 96% ethanol for 1 min and thoroughly washed with distilled water. Seeds were sown in Tref peat in a greenhouse with a 15 h light/9 h dark photoperiod at 27 ± 2°C temperature. Plastic pots were filled with perlite and seedlings were thinned to 5 per pot 25 days after sowing. Each pot was considered as one replicate and there were four replicates for each treatment.
Sodium chloride (0, 100, and 200 mM) and SA (1 mM) (Aftab et al. Citation2011) were applied for 21 days during vegetative growth of plants. Each pot was treated with different salt concentration with 100 ml of half-strength Hoagland’s nutrient solution (pH = 6.8–7.0) at alternative days (Hoagland and Arnon Citation1950). The nutrient solution was replaced every alternate day with fresh one. A foliar spray of SA (Aftab et al. Citation2011) was applied uniformly to the plants three times (at 1-week intervals), using an atomizer. The final harvest was performed after 21 days of treatment and leaves were collected. Five plants per treatment were used for analyses in all the experiments. Then they were oven-dried at 60°C for 3 days for the determination of dry weight (five replicates per treatment). Besides, fresh leaf samples from plants were stored at −70°C until the biochemical analysis.
SA quantification by HPLC
According to the method of Wen et al. (Citation2005), sample preparation for extraction and quantification of SA was performed. Treated and control leaf tissues (1 g) were extracted with methanol/water/trifluoroacetic acid (TFA) (50:50:0.1) mixed solvent, and the volume of the turbid fluid was adjusted to 10 ml. The mixture was centrifuged at 3000 rpm for 5 min, and filtered through a nylon filter. Three replications (per each treatment) were used for the estimation of total SA content. Chromatographic separations were performed on an Agilent 1200 series high-performance liquid chromatography (HPLC), including a quaternary pump and a degasser equipped with a G1321A fluorescence detector and a G1315D diode array detector. Separation process was carried out on a C18 column (250 9 4.6 mm, with 5.0 m particle size) from Waters Company (Massachusetts, USA). The flow rate of the mobile phase was kept at 0.5 ml/min. Phase A was water containing 0.02% TFA, and phase B was methanol containing 0.02% TFA. The column temperature was controlled at 25°C. Injection volume was 10 µl and samples were detected at 305 nm (Wen et al. Citation2005).
Determination of malondialdehyde content
Malondialdehyde (MDA) content in this experiment was measured in relation to thiobarbituric acid (TBA) reactive substances (Heath and Packer Citation1968). Leaf samples (0.2 g) of plants were homogenized in 2 ml of 0.1% (w/v) trichloroacetic acid (TCA) and the homogenate was centrifuged for 20 min at 10,000×g. To 1 ml of the aliquot of the supernatant, 4 ml of 20% (TCA) containing 0.5% TBA was added. The mixture was heated at 95°C for 30 min and was quickly chilled in an ice bath. After that the mixture centrifuged at 10,000×g for 15 min. The absorbance of the mixture was evaluated at 532 and 600 nm. The value for non-specific absorption at 600 nm was then subtracted from that of 532 nm. The concentration of MDA content was calculated by using an absorption coefficient of 155 mM−1 cm−1.
Determination of hydrogen peroxide content
For determination of H2O2 (hydrogen peroxide) levels, plant materials (0.5 g) were homogenized in 1 ml of 0.1% (w/v) TCA and centrifuged for 15 min at 12,000×g (Velikova et al. Citation2000). An estimated 0.5 ml of the extract was added to 1 ml of 1 M potassium iodide (KI) and 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0). Eventually, the absorbance was recorded at 390 nm and then H2O2 content was calculated using a standard curve.
Determination of antioxidant enzymes’ activity
Plant leaf tissues were homogenized at 4°C in 1 M Tris-HCl (pH 6.8) to estimate different enzyme activities. The Tris-HCl buffer contained 5 mM 1.4 dithiotheritol (DTT), 0.5 mM NaCl, and 5 mM ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifuged at 13,000×g (J2-21 M, Beckman, Palo Alto, USA) for 30 min at 4°C. The obtained supernatant was kept at −70°C and used for enzyme assays and protein determination. A UV–Vis spectrophotometer (UV-160, Shimadzu, and Tokyo, Japan) was used for detecting enzyme activity. Protein was determined according to the Bradford assay (Bradford Citation1976), using bovine serum albumin (BSA) as standard.
Estimation of SOD (EC 1.15.1.1) activity was performed by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) in a reaction mixture (Giannopolitis and Ries Citation1977). The reaction mixture contained 50 mM sodium phosphate buffer (pH 7.5), 75 µM NBT, 75 µM riboflavin, 0.1 mM EDTA, 13 mM l-methionine, and 0.1 ml of enzyme extract. For 18 min, the reaction mixture was irradiated and absorbance was recorded at 560 nm against the nonirradiated blank. One unit of SOD was defined as the amount of enzyme, which caused 50% inhibition of NBT reduction under the assay condition, and the results were reported in the [Unit mg−1 (protein)].
The reaction mixture for POX (EC 1.11.1.7) activity measurement comprised 4 ml of 0.2 M acetate buffer (pH 4.8), 0.2 ml of 20 mM benzidine, 0.4 ml of H2O2 (3%), and 50 µl of enzyme extract (Abeles and Biles Citation1991). The increase in absorbance was recorded at 530 nm. The POX activity was defined as 1 µM of benzidine oxidized per min per mg protein [Unit mg−1 (protein)].
The reaction mixture for PPO (E.C. 1.14.18.1) activity measurement contained 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.8), 0.2 ml of 20 mM pyrogallol and 50 µl of enzymes extract at 40°C (Raymond et al. Citation1993). The increase in absorbance was recorded at 430 nm. The PPO activity was defined as 1 µM of pyrogallol oxidized per minutes per mg protein [unit mg−1 (protein)].
Ascorbate peroxidase (APX; EC 1.11.1.1) activity was measured using the reaction mixture comprised 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM H2O2, 0.5 mM ascorbate, and 10 µl protein extract in a total volume of 1 ml. (Jebara et al. Citation2005). The reaction was initiated by the addition of H2O2, and the concentration of oxidized ascorbate was measured by a decrease in absorbance at 290 nm for 1 min. The concentration of oxidized ascorbate was calculated by using the molar extinction coefficient (2.8 mM−1 cm−1). The results were expressed as 1 µM of ascorbate oxidized per minutes per mg protein [Unit mg−1 (protein)].
Total catalase (CAT; EC 1.11.1.6) activity was assayed from the H2O2 decomposition rate as measured by a decrease in absorbance at 240 nm (Aebi Citation1984). The reaction mixture comprised 0.625 ml of 50 mM potassium phosphate buffer (pH 7.0), 75 µl H2O2 (3%), and 10 µl of protein extract. CAT activity was expressed as units (1 mol of H2O2 decomposed per minutes per mg protein [Unit mg−1 (protein)].
Determination of PAL activity
PAL activity was measured based on the rate of cinnamic acid production in reaction (Ochoa-Alejo and Gómez-Peralta Citation1993). One milliliter of the extraction buffer, 0.4 ml of double distilled water, 0.5 ml of 10 mM l-phenylalanine, and 20 µl of enzyme extract was incubated at 37°C for 1 h. Then, 0.5 ml of HCl (6 M) was added to the solution and the product was extracted using 5 ml of ethyl acetate. Thus, the extracting solvent removed by evaporation. The residue was suspended in 3 ml of NaOH (0.05 M) and the cinnamic acid concentration in it was calculated with absorbance measured at 290 nm with a spectrophotometer. The results were expressed according to each unit of PAL activity that is equal to 1 µmol of cinnamic acid produced per minutes.
Scavenging ability on DPPH radical
In order to free radical scavenging activity measurement, leaf tissue (0.1 g) was extracted in 1 ml methanol 80% (Shimada et al. Citation1992). Then 0.1 ml of plant extract was added to 3.9 ml of 80 ppm of 1,1-diphenyl-2-picrylhydrazyl (DPPH) solution. The mixture was vigorously shaken and then was allowed to sit at room temperature for 30 min in the dark. The absorbance was recorded at 517 nm and corresponds to the extract ability to reduce the radical DPPH to the yellow-colored diphenylpicrylhydrazine. The free radical scavenging activity was calculated using the following equation:
Total phenolic concentration
Total phenolics were extracted with 80% (v/v) methanol at 70°C water bath for 3 h (Niknam and Ebrahimzadeh Citation2002). The suspensions of methanolic extraction were filtered, the methanol was removed by vacuum distillation, and the aqueous solutions were used for quantitative determination. Total phenolics were assayed using the Folin–Ciocalteu reagent (Singleton and Rossi Citation1965), which was slightly modified (Ranganna Citation1986). An aliquot of 250 µL of extract was added to 2 ml distilled water, 250 µL folin reagent, and 0.5 ml Na2CO3 (7%). The solution was adjusted with distilled water to a final volume of 3 ml and thoroughly mixed. After 30 min the absorbance was recorded at 760 nm. Aqueous solutions of gallic acid (0–200 µg ml−1) were used as standards for plotting working curve and leaf total phenolic concentration was expressed as GAE in µg g−1 FW.
Statistical analysis
Statistical analysis was performed with a randomized complete block design. Experiments were repeated three times, with three replications in each group. Tests for significant differences among treatments were conducted using analysis of variance (ANOVA) using SPSS (version 18) with Duncan’s multiple range tests and P values ≤ .05 are considered to be significant.
Results and discussion
In the present study, some physiological parameters were investigated to better understand the effects of exogenous application of SA in safflower under salinity. Plant adaptations to salinity are affected by several environmental factors. In our experiment, growth parameters which were followed by measuring FW and DW were remarkably inhibited under different NaCl concentrations in C. tinctorius L. and were severe at the concentration of 200 mM. (). Reduction of growth under stress have been previously observed in different studies (Jaleel et al. Citation2007; Shaheen et al. Citation2013; Merati et al. Citation2014).
Figure 1. Effects of NaCl (0, 100, and 200 mM) and SA on growth parameters at 21 days after treatments in C. tinctorius L. The groups are −SA (plants with no SA treatment), +SA (plants sprayed with 1 mM sodium salicylate three times a week for every other day). Data are the means ± SE. Means with different letters indicate a significant difference at P ≤ .05 using Duncan’s multiple range test.
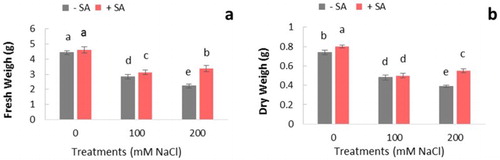
Application of SA in plants improved the negative effect of salinity by increasing leaf growth, especially in 200 mM NaCl-treated plants. It was found that SA treatment had more effect on stress tolerance at severe stress conditions. Promoting effect of SA on growth aspects has been reported in many other crop species (Fariduddin et al. Citation2003; Shakirova et al. Citation2003; Khodary Citation2004; El-Tayeb Citation2005; Dicko et al. Citation2006). The increase of growth induced by SA may be due to the induction of antioxidant function and metabolic activity that increase plant tolerance (Wang and Li Citation2006). It was also reported that SA application in plants is concomitant with the accumulation of active oxygen species (Gunes et al. Citation2007).
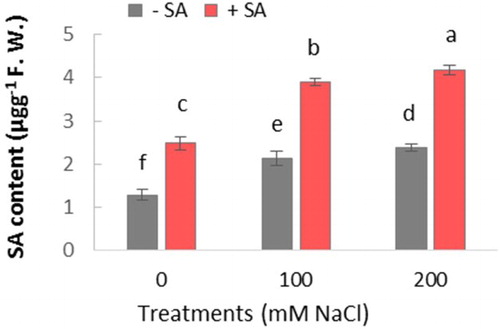
HPLC analysis showed that SA content in leaves of salt-stressed plants (both 100 and 200 mM NaCl) was significantly higher in comparison with controls (). Following SA treatment of plants, their SA content was significantly induced, in both salt-stressed and unstressed plants, as compared to the controls. Its content increased about 2 fold in all SA-treated plants.
Figure 2. Effect of salinity (0, 100, and 200 mM NaCl) and exogenous application of on SA content in leaves of safflower plants at 21 days after treatments. The groups are −SA (plants with no SA treatment) and SA (plants sprayed with 1 mM sodium salicylate three times a week for every other day). Columns indicate mean ± SE. Means with different letters indicate a significant difference at P ≤ .05 using Duncan multiple range test.
It was found that salinity causes a significant increase of MDA production in safflower ((a)). Exogenous application of SA showed an increase in MDA content in salt-stressed plants but there was no significant difference between 100 and 200 mM NaCl treatments after SA application.
Figure 3. Effect of salinity (0, 100, and 200 mM NaCl) and exogenous application of SA on content of (a) MDA and (b) H2O2 in leaves of safflower plants at 21 days after treatments. The groups are −SA (plants with no SA treatment) and +SA (plants sprayed with 1 mM sodium salicylate three times a week for every other day). Columns indicate mean ± SE based on three replicates. Means with different letters indicate a significant difference at P ≤ .05 using Duncan multiple range test.
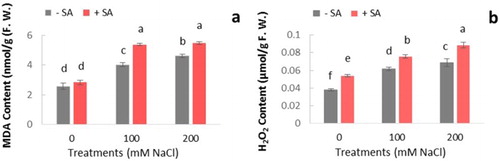
For determination of ROS scavenging capacity, H2O2 levels of plants were estimated under stress conditions. Basal H2O2 content in salt-stressed plants was higher compared to unstressed plants ((b)). Both salt treatments showed a significant increase in H2O2 at 21 days. Plants treated with SA enhanced H2O2 production significantly in comparison with controls. Peroxidation of lipid in cell membranes under oxidative stress conditions reflects oxidative damage induced by free radicals (Demiral and Türkan Citation2004). H2O2 triggers many defense responses and is involved in SA accumulation in plants (Leon et al. Citation1995). As reported in other studies (Harfouche et al. Citation2008; Chao et al. Citation2010; Wang and Liu Citation2012), similarly an increase in H2O2 level was observed in the present study following SA treatment. Moreover, it was suggested that the promotion of H2O2 accumulation by SA is related to the inhibition of enzymes responsible for H2O2 scavenging or an increase in SOD activity (Kang et al. Citation2003; Krantev et al. Citation2008; Chao et al. Citation2010; Hayat et al. Citation2010). This might be a reason for the general increase of H2O2 content in plants ((b)).
The differences in antioxidant enzyme activities are depicted in . During the experimental period, SA treatment caused more induction of these activities except CAT in plants. In salt-stressed plants, all enzyme activities were significantly increased. The highest induction of enzyme activity was observed in PPO in 200 mM NaCl-treated plants with SA application. In our study, results for CAT activity were different from the other enzymes. SA treatment significantly reduced CAT activity in plants especially in 200 mM NaCl treatment.
Table 1. Specific activity of five antioxidative enzymes in safflower treated with NaCl (0, 100, and 200 mM) and SA treatment at 21 days after treatments.
Plants respond to stress by increasing the antioxidant activity to restore the cellular equilibrium between production and scavenging of ROS (Salah et al. Citation2011; Bano et al. Citation2014). Decreased antioxidant activity can lead to overproduction of ROS and lipid peroxidation of cell membranes, which would result in harmful ion leakage.
SOD protects cells from oxidative stress by converting the destructive superoxide radical into molecular oxygen and H2O2, which are less dangerous (Scandalios Citation1993). Hence, it would decrease the risk of hydroxyl radical formation from superoxide. Subsequently, H2O2 molecules are degraded by CAT and POX (Xu et al. Citation2013). According to our results, activity of SOD in safflower was enhanced after exposure to salinity (). Similarly, increased activity of SOD was reported in M. pulegium under drought stress (Hassanpour et al. Citation2012), and in Zea mays (Kaya et al. Citation2013) and M. pulegium L. (Merati et al. Citation2014) under salt stress. Hence, it is plausible that the increase in SOD activity can be an attempt to overcome the oxidative stress. A similar result was presented in wheat plants treated with SA (Sorahinobar et al. Citation2016).
POX can catalyze the oxidation of some compounds such as lignin, suberin, and phenolics in the cell wall and also has a main role in removing of H2O2 from cytosol and chloroplasts (Jbir et al. Citation2001; Dicko et al. Citation2006). These are effective in construction and lignification of the cell wall in stress conditions. Plants increase lignin synthesis in the cell wall in stress condition in order to maintain water (Garcia et al. Citation1997). Increasing the POX activity induced by salinity was reported in rice (Demiral and Türkan Citation2004), safflower (Hosseini et al. Citation2010; Karray-Bouraoui et al. Citation2011), Artemisia annua L. (Aftab et al. Citation2011), and pennyroyal (Merati et al. Citation2014). Similar findings were observed in A. annua L. treated with exogenous SA in salt-stressed plants (Aftab et al. Citation2011). In our study, the activities of POX as an ROS scavenger increased in SA-treated plants compared to controls; hence, it could provide a mechanism for enhanced resistance of safflower plants in salinity.
PPO is an enzyme responsible for oxidation of phenolic compounds. PPO activity considerably increased under salinity especially at 200 mM NaCl in safflower (). Increased PPO activity may reduce the phenolics, thereby protecting the content of Indole-3-acetic acid (IAA) (Merati et al. Citation2014), and this can enhance cell wall growth. The observation that PPO activity was affected by the application of SA in both salt-stressed and unstressed plants supported the idea that its response can be SA dependent. A similar result was also reported in chamomile plants using exogenous application of SA (Kováčik et al. Citation2009).
APX and CAT are two other enzymes responsible for removing H2O2 from cells (Dewir et al. Citation2006). In our experiment, APX activity significantly increased under different NaCl concentrations and more induced by SA treatment but as mentioned, results for CAT activity were different. SA treatment significantly reduced CAT activity in plants under 200 mM NaCl treatment (). H2O2 is an integral component of cell signaling cascades (Mittler Citation2002; Pastori and Foyer Citation2002). Similar findings were presented in other plants treated with SA (Kováčik et al. Citation2009; Sorahinobar et al. Citation2016). It seems that APX and CAT regulations serve to limit excessive H2O2 accumulation in the cells.
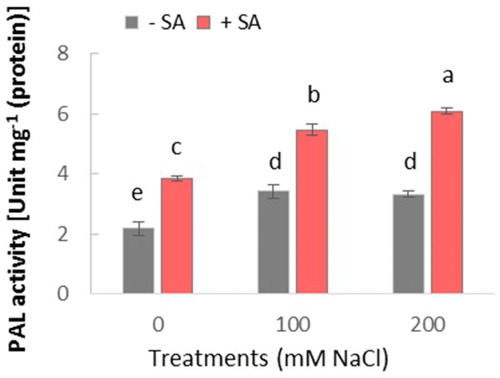
PAL is a key enzyme in the phenylpropanoid pathway. It should be noted that an increase in PAL activity may be related to the plant defense system through biosynthesis of some metabolites, such as SA, phenols, and lignin in defense pathways (Mandal et al. Citation2009). This study demonstrated that PAL activity was induced by salinity, while this increase was stronger in plants treated by SA in comparison with controls. Furthermore, SA-induced PAL activity in plants suggests a positive feedback in SA production.
To better understand the underlying mechanisms of resistance against salinity, the activity of PAL was investigated as a key enzyme in the production of SA. Salinity significantly increased PAL activity in salt-stressed and unstressed plants (). However, no significant difference was observed between 100 and 200 mM NaCl treatments. SA treatment significantly increased PAL activity in both salt-stressed and unstressed plants. This increase was more pronounced in plants treated with SA, under 200 mM NaCl salinity.
Figure 4. Effect of salinity (0, 100, and 200 mM NaCl) and exogenous application of SA on PAL activity in leaves of safflower plants at 21 days after treatments. The groups are −SA (plants with no SA treatment) and +SA (plants sprayed with 1 mM sodium salicylate three times a week for every other day). Columns indicate mean ± SE based on three replicates. Means with different letters indicate a significant difference at P ≤ .05 using Duncan’s multiple range test.
Besides the enzymatic antioxidant system, induction of non-enzymatic antioxidant compounds in plants was observed. Our result showed that significant changes in DPPH radical scavenging activity occurred in response to salinity ((a)). Besides, SA treatment increased DPPH radical scavenging activity in both salt-stressed and unstressed plants in comparison with controls. This increase in 200 mM NaCl treatment plants was about two-fold of controls.
Figure 5. Effect of salinity (0, 100, and 200 mM NaCl) and exogenous application of SA on (a) DPPH scavenging activity and (b) total phenolics content in leaves of safflower plants at 21 days after treatments. The groups are −SA (plants with no SA treatment) and +SA (plants sprayed with 1 mM sodium salicylate three times a week for every other day). Columns indicate mean ± SE based on three replicates. Means with different letters indicate a significant difference at P ≤ .05 using Duncan multiple range test.
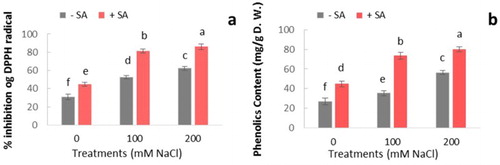
In our experiment, the phenolic contents significantly increased under salinity ((b)). The highest amount of phenolic compounds was observed in plants under 200 mM NaCl with application of SA. Phenolic compounds are potent inhibitors of oxidative stress in cells (Rice-Evans et al. Citation1996). The key step in the biosynthesis of phenolics, which is converting phenylalanine to trans-cinnamic acid, is controlled by PAL. Phenolics can also cooperate with POX in H2O2 scavenging in the cells. According to our results, accumulation of phenolic compounds in salt-stressed plants treated with SA can play an important role in resistance against salinity. With regard to DPPH-free radical scavenging activity and phenolics content in response to salinity, SA-treated plants in comparison with controls are more potent in controlling ROS production.
The rise in phenolic compounds after treatment with SA may be due to increased PAL activity, as PAL was reported to be associated with the synthesis of phenolic compounds via phenylpropanoid pathway (Hahlbrock and Scheel Citation1989). Our results are also in accordance with the study on pennyroyal plants (Hassanpour et al. Citation2012). They observed that higher levels of phenolics are associated with higher levels of antioxidant enzyme activity. Since, the phenolic compounds have an antioxidative role in plants; therefore, the simultaneous increase in phenolics level and DPPH-free radical scavenging activity suggests that the increase in free radical scavenging activity might be due to the increase in phenolics level which is induced by exogenous SA application.
Conclusion
Salinity tolerance is associated with the activity of some antioxidant enzymes and with the accumulation of non-enzymatic antioxidant compounds (Asada Citation1999). From our findings, it can be concluded that the exogenous application of SA in combination with salinity ameliorated stress effects in C. tinctorius by improving antioxidant enzymes and regulation of H2O2 level in plant cells. Our study provides an overview of the salt-stressed safflower interaction by analysis of enzymatic and non-enzymatic antioxidative pathways and some enzymes such as PAL, which is involved in the production of signaling molecules, such as SA. In addition, our results showed that there are different physiological and biochemical response patterns in stress condition in SA-treated plants. The present findings suggest that these differences are probably associated with salinity resistance. It is indicated that a central role for the SA signaling pathway in activating safflower response against salt stress is possible. The current results can provide new insights to better realizing the responsible mechanisms to regulate salt stress resistance in C. tinctorius.
Finally, our study suggested that the resistant safflower plants can direct ROS from a threat to an opportunity by using some key regulators such as SA. We propose that exogenous application of SA in plants played this role through regulation of the antioxidant enzymes. Therefore, these components can be considered to ameliorate salinity effects in safflower, due to low price and their availability. Further work on the signaling systems and gene expression of enzymes involved in salt stress is required to obtain more information about how SA treatment ameliorate salinity effects in C. tinctorius plants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abeles FB, Biles CL. 1991. Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol. 95:269–273. doi: 10.1104/pp.95.1.269
- Aebi H. 1984. Catalase in vitro. Methods Enzymol. 105:121–126. doi: 10.1016/S0076-6879(84)05016-3
- Aftab T, Khan MMA, da Silva JAT, Idrees M, Naeem M, Moinuddin M. 2011. Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J Plant Growth Regul. 30:425–435. doi: 10.1007/s00344-011-9205-0
- Asada K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 50:601–639. doi: 10.1146/annurev.arplant.50.1.601
- Bano S, Ashraf M, Akram NA. 2014. Salt stress regulates enzymatic and nonenzymatic antioxidative defense system in the edible part of carrot (Daucus carota L.). J Plant Interact. 9:324–329. doi: 10.1080/17429145.2013.832426
- Bassil ES, Kaffka SR. 2002. Response of safflower (Carthamus tinctorius L.) to saline soils and irrigation: I. consumptive water use. Agric Water Manag. 54:67–80. doi: 10.1016/S0378-3774(01)00148-2
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Chao YY, Chen CY, Huang WD, Kao CH. 2010. Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil. 329:327–337. doi: 10.1007/s11104-009-0161-4
- Demiral T, Türkan I. 2004. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J Plant Physiol. 161:1089–1100. doi: 10.1016/j.jplph.2004.03.009
- Dewir Y, Chakrabarty D, Ali M, Hahn E, Paek K. 2006. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ Exp Bot. 58:93–99. doi: 10.1016/j.envexpbot.2005.06.019
- Dicko MH, Gruppen H, Traoré AS, Voragen AG, van Berkel WJ. 2006. Phenolic compounds and related enzymes as determinants of sorghum for food use. Biotechnol Mol Biol Rev. 1:21–38.
- El-Tayeb M. 2005. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 45:215–224. doi: 10.1007/s10725-005-4928-1
- Fariduddin Q, Hayat S, Ahmad A. 2003. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica. 41:281–284. doi: 10.1023/B:PHOT.0000011962.05991.6c
- Garcia AB, Engler J, Iyer S, Gerats T, Van Montagu M, Caplan AB. 1997. Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol. 115:159–169. doi: 10.1104/pp.115.1.159
- Giannopolitis CN, Ries SK. 1977. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 59:309–314. doi: 10.1104/pp.59.2.309
- Grassmann J, Hippeli S, Elstner EF. 2002. Plant’s defence and its benefits for animals and medicine: role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiol Biochem. 40:471–478. doi: 10.1016/S0981-9428(02)01395-5
- Grattan S, Grieve C. 1998. Salinity–mineral nutrient relations in horticultural crops. Sci Hortic. 78:127–157. doi: 10.1016/S0304-4238(98)00192-7
- Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, Cicek N. 2007. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol. 164:728–736. doi: 10.1016/j.jplph.2005.12.009
- Hahlbrock K, Scheel D. 1989. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 40:347–369. doi: 10.1146/annurev.pp.40.060189.002023
- Harfouche AL, Rugini E, Mencarelli F, Botondi R, Muleo R. 2008. Salicylic acid induces H2O2 production and endochitinase gene expression but not ethylene biosynthesis in Castanea sativa in vitro model system. J Plant Physiol. 165:734–744. doi: 10.1016/j.jplph.2007.03.010
- Hassanpour H, Khavari-Nejad RA, Niknam V, Najafi F, Razavi K. 2012. Effects of penconazole and water deficit stress on physiological and antioxidative responses in pennyroyal (Mentha pulegium L.). Acta Physiol Plant. 34:1537–1549. doi: 10.1007/s11738-012-0952-8
- Hayat Q, Hayat S, Irfan M, Ahmad A. 2010. Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot. 68:14–25. doi: 10.1016/j.envexpbot.2009.08.005
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198. doi: 10.1016/0003-9861(68)90654-1
- Hemm MR, Rider SD, Ogas J, Murry DJ, Chapple C. 2004. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 38:765–778. doi: 10.1111/j.1365-313X.2004.02089.x
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station. Circular 347.
- Horváth E, Szalai G, Janda T. 2007. Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul. 26:290–300. doi: 10.1007/s00344-007-9017-4
- Hosseini T, Shekari F, Ghorbanli M. 2010. Effect of salt stress on ion content, proline and antioxidative enzymes of two safflower cultivars (Carthamus tinctorius L.). J Food Agric Environ. 8:1080–1086.
- Işigigür A, Karaosmanoglu F, Aksoy H. 1995. Characteristics of safflower seed oils of Turkish origin. J Am Oil Chem Soc. 72:1223–1225. doi: 10.1007/BF02540994
- Jaleel CA, Gopi R, Manivannan P, Panneerselvam R. 2007. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta Physiol Plant. 29:205–209. doi: 10.1007/s11738-007-0025-6
- Jbir N, Chaïbi W, Ammar S, Jemmali A, Ayadi A. 2001. Root growth and lignification of two wheat species differing in their sensitivity to NaCl, in response to salt stress. Comptes Rendus de l'Académie des Sciences-Series III-Sciences de la Vie. 324:863–868. doi: 10.1016/S0764-4469(01)01355-5
- Jebara S, Jebara M, Limam F, Aouani ME. 2005. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol. 162:929–936. doi: 10.1016/j.jplph.2004.10.005
- Kang G, Wang C, Sun G, Wang Z. 2003. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ Exp Bot. 50:9–15. doi: 10.1016/S0098-8472(02)00109-0
- Karray-Bouraoui N, Harbaoui F, Rabhi M, Jallali I, Ksouri R, Attia H, Msilini N, Lachaâl M. 2011. Different antioxidant responses to salt stress in two different provenances of Carthamus tinctorius L. Acta Physiol Plant. 33:1435–1444. doi: 10.1007/s11738-010-0679-3
- Kaya C, Sonmez O, Aydemir S, Ashraf M, Dikilitas M. 2013. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J Plant Interact. 8:234–241. doi: 10.1080/17429145.2012.725480
- Khan M, Panda S. 2007. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant. 30:81–89. doi: 10.1007/s11738-007-0093-7
- Khodary S. 2004. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Int J Agric Biol. 6:5–8.
- Kim JK, Bamba T, Harada K, Fukusaki E, Kobayashi A. 2007. Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J Exp Bot. 58:415–424. doi: 10.1093/jxb/erl216
- Klessig DF, Malamy J. 1994. The salicylic acid signal in plants. In: Palme K, editor. Signals and signal transduction pathways in plants. Springer Netherlands; p. 203–222
- Kováčik J, Bačkor M. 2007. Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant Soil. 297:255–265. doi: 10.1007/s11104-007-9346-x
- Kováčik J, Grúz J, Bačkor M, Strnad M, Repčák M. 2009. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 28:135–143. doi: 10.1007/s00299-008-0627-5
- Krantev A, Yordanova R, Janda T, Szalai G, Popova L. 2008. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol. 165:920–931. doi: 10.1016/j.jplph.2006.11.014
- Lee DH, Lee CB. 2000. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci. 159:75–85. doi: 10.1016/S0168-9452(00)00326-5
- Leon J, Lawton MA, Raskin I. 1995. Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 108:1673–1678. doi: 10.1104/pp.108.4.1673
- Liang Y, Chen Q, Liu Q, Zhang W, Ding R. 2003. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol. 160:1157–1164. doi: 10.1078/0176-1617-01065
- Mandal S, Mallick N, Mitra A. 2009. Salicylic acid-induced resistance to Fusarium oxysporum f.sp. lycopersici in tomato. Plant Physiol Biochem. 47:642–649. doi: 10.1016/j.plaphy.2009.03.001
- Merati MJ, Hassanpour H, Niknam V, Mirmasoumi M. 2014. Exogenous application of penconazole regulates plant growth and antioxidative responses in salt-stressed Mentha pulegium L. J Plant Interact. 9:791–801. doi: 10.1080/17429145.2014.948084
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410. doi: 10.1016/S1360-1385(02)02312-9
- Niknam V, Ebrahimzadeh H. 2002. Phenolics content in Astragalus species. Pak J Bot. 34:283–289.
- Ochoa-Alejo N, Gómez-Peralta JE. 1993. Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum L.). J Plant Physiol. 141:147–152. doi: 10.1016/S0176-1617(11)80751-0
- Pastori GM, Foyer CH. 2002. Common components, networks, and pathways of cross-tolerance to stress: the central role of “redox” and abscisic acid-mediated controls. Plant Physiol. 129:460–468. doi: 10.1104/pp.011021
- Patade VY, Bhargava S, Suprasanna P. 2011. Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: growth, osmolytes accumulation, and antioxidant defense. J Plant Interact. 6:275–282. doi: 10.1080/17429145.2011.557513
- Ramezani M, Seghatoleslami M, Mousavi G, Sayyari-Zahan MH. 2012. Effect of salinity and foliar application of iron and zinc on yield and water use efficiency of Ajowan (Carum copticum). Intl J Agric Crop Sci. 4:421–426.
- Ranganna S. 1986. Handbook of analysis and quality control for fruit and vegetable products. New Delhi: Tata McGraw-Hill Education
- Raymond J, Rakariyatham N, Azanza J. 1993. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry. 34:927–931. doi: 10.1016/S0031-9422(00)90689-7
- Rice-Evans CA, Miller NJ, Paganga G. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 20:933–956. doi: 10.1016/0891-5849(95)02227-9
- Sadeghi M, Dehghan S, Fischer R, Wenzel U, Vilcinskas A, Kavousi HR, Rahnamaeian M. 2013. Isolation and characterization of isochorismate synthase and cinnamate 4-hydroxylase during salinity stress, wounding, and salicylic acid treatment in Carthamus tinctorius. Plant Signal Behav. 8:e27335-1–e27335-9 doi: 10.4161/psb.27335
- Salah I, Mahmoudi H, Gruber M, Slatni T, Boulaaba M, Gandour M, Messedi D, Hamed K, Ksouri R, Hannoufa A, et al. 2011. Phenolic content and antioxidant activity in two contrasting Medicago ciliaris lines cultivated under salt stress. Biologia. 66:813–820. doi: 10.2478/s11756-011-0102-6
- Scandalios JG. 1993. Oxygen stress and superoxide dismutases. Plant Physiol. 101:7–12. doi: 10.1104/pp.101.1.7
- Shaheen S, Naseer S, Ashraf M, Akram NA. 2013. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact. 8:85–96. doi: 10.1080/17429145.2012.718376
- Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR. 2003. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 164:317–322. doi: 10.1016/S0168-9452(02)00415-6
- Shimada K, Fujikawa K, Yahara K, Nakamura T. 1992. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agr Food Chem. 40:945–948. doi: 10.1021/jf00018a005
- Silveira J, Melo A, Viégas RA, Oliveira J. 2001. Salinity-induced effects on nitrogen assimilation related to growth in cowpea plants. Environ Exp Bot. 46:171–179. doi: 10.1016/S0098-8472(01)00095-8
- Singleton V, Rossi JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enology Viticulture. 16:144–158.
- Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H, Behmanesh M, Enferadi ST. 2016. Central role of salicylic acid in resistance of wheat against Fusarium graminearum. J Plant Growth Regul. 35:477–491. doi: 10.1007/s00344-015-9554-1
- Stevens J, Senaratna T, Sivasithamparam K. 2006. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 49:77–83.
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 151:59–66. doi: 10.1016/S0168-9452(99)00197-1
- Vital SA, Fowler RW, Virgen A, Gossett DR, Banks SW, Rodriguez J. 2008. Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environ Exp Bot. 62:60–68. doi: 10.1016/j.envexpbot.2007.07.006
- Wang LJ, Li SH. 2006. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 48:137–144. doi: 10.1007/s10725-005-6146-2
- Wang Y, Liu JH. 2012. Exogenous treatment with salicylic acid attenuates occurrence of citrus canker in susceptible navel orange (Citrus sinensis Osbeck). J Plant Physiol. 169:1143–1149. doi: 10.1016/j.jplph.2012.03.018
- Wen D, Li C, Di H, Liao Y, Liu H. 2005. A universal HPLC method for the determination of phenolic acids in compound herbal medicines. J Agr Food Chem. 53:6624–6629. doi: 10.1021/jf0511291
- Xu J, Duan X, Yang J, Beeching JR, Zhang P. 2013. Coupled expression of Cu/Zn-superoxide dismutase and catalase in cassava improves tolerance against cold and drought stresses. Plant Signal Behav. 8:e24525. doi: 10.4161/psb.24525
- Zahedi AM, Fazeli I, Zavareh M, Dorry H, Gerayeli N. 2012. Evaluation of the sensitive components in seedling growth of common bean (Phaseolus vulgaris L.) affected by salinity. Asian J Crop Sci. 4:159–164. doi: 10.3923/ajcs.2012.159.164
- Zhang J, Kirkham M. 1996. Enzymatic responses of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Sci. 113:139–147. doi: 10.1016/0168-9452(95)04295-4
