ABSTRACT
The presence of significant amount of heavy metals in rivers and canals due to mixing of untreated industrial effluents is a common phenomenon, especially in developing countries. The agricultural crops are influenced by the presence of various pollutants in the sewage, being applied for irrigation purpose. The effluents containing copper affect the growth and development of crop species, thereby, ought to be mitigated by foliar spray of osmoprotectants, e.g. proline. A pot culture experiment was conducted at the University of Agriculture, Faisalabad-Pakistan during the crop season 2015–2016. The treatments consisted of (a) three wheat varieties (Punjab-96, MH-97, FSD-83), (b) two levels of copper (0, 400 µM) applied through rooting medium, and (c) two levels of proline (0, 80 mM) applied through foliar application. The treatments were arranged in a completely randomized design with four replications. The results showed that application of 400 µM copper caused a reduction in biomass accumulation, chlorophyll (‘a’ and ‘b’) contents, and eventually yield (100-grain weight). There were also significant decreases in gas exchange parameters (stomatal conductance, internal CO2 concentration), photosynthetic rate, water-use-efficiency, and transpiration rate in response to copper stress. Metal toxicity caused the maximum reduction in productivity of PSII, electron transport rate, and photochemical quenching, while higher values of non-photochemical quenching were recorded in the wheat varieties. The activities of antioxidant enzymes (superoxide dismutase, catalase, peroxidase), as well as quantities of proline, protein and calcium contents were accelerated in response to copper stress. The uptake of calcium, magnesium, and potassium constituents by plants was reduced, while assimilation of calcium was increased in plants under copper stress. However, the occurrence of negative effects on these parameters due to copper stress was mitigated by foliar spray of proline at the rate of 80 mM solution. The exogenous application of proline at the rate of 80 mM resulted in the reduction of generation of reactive oxygen species and enhanced accumulation of proline and protein contents in wheat varieties under copper stress environment.
Introduction
The production of agricultural crops is significantly influenced by the presence of various pollutants in the sewage, being applied for irrigation purposes (Yadav Citation2010). The accumulation of heavy metals in the surface soil and their uptake by the plants has caused serious concern related to food safety for humans and livestock (Rehman et al. Citation2011). The agricultural production areas contaminated with heavy metals ( i.e. copper, cadmium, and nickel) have posed serious implications to sustain crop production (Singh et al. Citation2007). The farming communities in the vicinity of the industrial zones have resorted to use municipal and industrial waste as a source of irrigation water, considering it as a highly nutritive in nature for raising their crops (Jamal et al. Citation2002; Ahmad et al. Citation2013). On the other hand, these effluents are highly toxic for the growth of plants, because of containing excessive amounts of copper, lead, zinc, and nickel (Younas et al. Citation1998; Jamal et al. Citation2002). Among these heavy metals, copper is the most obnoxious metal which imposes health hazards to both humans and livestock through food and fodder chain (Houshm and Moraghebi Citation2011).
Copper (Cu) as a plant nutrient is involved in the maintenance of a number of enzymes (Hall and Williams Citation2003) and as a co-factor in protein and enzymes (Yurekli and Porgali Citation2006; Shar et al. Citation2011). However, its excessive concentration is rhizosphere causes oxidative stress and reduces the activities, e.g. physiological, photosynthesis and growth of plants (Adrees et al. Citation2015). The higher content of Cu in the root zone causes reduction in seed germination (Ouzounidou et al. Citation1992); imbalance mineral concentration in plant system (Ke et al. Citation2007); photosynthesis (Nussbaum et al. Citation1988); Chlorophyll contents (Zengin and Kirbag Citation2007); non-proliferationof roots (Sheldon and Menzies Citation2005); inhibition of plant growth (Rehman and Iqbal Citation2006); reduction in uptake of essential nutrients by roots (Michaud et al. Citation2008); and in certain cases, the death of plants. The higher concentration of Cu caused oxidative stress through generation of reactive oxygen species (ROS), such as hydroxyl radical, superoxide radicals and hydrogen peroxide, which disintegrated the cell membranes, and biological molecules through lipid peroxidation (Hall Citation2002; Mittler et al. Citation2004). The cumulative effects resulted in leaf chlorosis, reduced plant growth and yield (Chen et al. Citation2007). The negative effects could be augmented by improvements in production of antioxidants enzymes and proteins (Imlay Citation2003; Brahim and Mohamed Citation2011). The antioxidant defense systems, such as superoxide (SOD) and catalase (CAT), act as scavengers of toxic radicals and adapt to production of ROS. The SOD induces the exchange of superoxide anions to water and hydrogen peroxide, while CAT decomposes hydrogen peroxide (Xu et al. Citation2006; Frary et al. Citation2010).
The plants resort to defense mechanism through enhancing the process of osmoregulation (Szabados and Savoure Citation2010). The osmolytes maintain structure of proline and photosynthetic apparatus and detoxify ROS through cellular osmoregular in response to abiotic stresses (Ashraf and Foolad Citation2007). The adverse effects caused by copper stress could be alleviated by foliar spray of osmoprotectants, particularly proline (El-Sherbeny and Silva Citation2013). The accumulation of greater quantum of proline in the plant system resulted in enhancing the photosynthetic system and soluble protein (Shahid et al. Citation2014). Therefore, the research studies were undertaken to quantify the effects of Cu stress on various physiological, biochemical, and chemical attributes and to determine the efficacy of proline to mitigate adverse effects on wheat crop.
Materials and methods
A pot culture experiment was conducted to quantify the effects of proline under copper stress conditions on wheat crop during crop season 2015–2016 at University of Agriculture, Faisalabad-Pakistan. The treatments consisted of three wheat varieties (‘Punjab-96g,’ ‘MH-97,’ ‘FSD-83’); two levels of copper (0, 400 µM, CuSO4.5H2O) applied through rooting medium and two levels of proline (0, 80 mM) applied exogenously and arranged in a completely randomized design with factorial arrangement with four replications.
The seed of wheat varieties was surface sterilized with 5.0 g L−1 sodium hypochlorite solution for five minutes and air-dried at room temperature before dibbling in the experimental pots. Ten kilograms of washed river sand was filled in plastic pots measuring 24.5 × 28.0 cm2 and having a drainage hole at the bottom. The impurities from the river sand were removed by a 10-hour leaching with 10% HCl followed by thorough washing with deionized water. Approximately, 15 seeds of wheat varieties were dibbled at 5 mm depth in each pot during November 2015. The pots were irrigated with modified half-strength Hoagland’s nutrient solution at every two days interval (Hoagland and Arnon Citation1950; Epstein Citation1972). The evaporated water was replenished with distilled water at other times. After complete germination, five healthy and uniform in size seedlings were retained in each pot. The seedlings were treated with various levels of copper nutrient solution through rooting medium, while foliar spray of proline was carried out at 12th day after complete germination. Proline was applied in combination with 0.1% (v/v) Tween-80 (polyoxythylene sorbiton monoleate) surfactant to maximize penetration into leaf tissues. Moreover, the untreated plants were also sprayed with distilled water. A constant volume of 20 ml proline and/or distilled water was sprayed per pot to ensure full foliage coverage.
Morphological attributes
Biological yield and plant height
The plants were harvested at day 35 after sowing. The material was washed with deionized water and blotted. The plants were divided into leaves and roots. The quantum of fresh weight of shoot and root was recorded. The material was oven dried at 70°C for 24 h till the constant weight was obtained.
Gas exchange characteristics
The measurements on various gas exchange characteristics were recorded by employing LCA-4 ADC portable gas analyzer (IRGA) by selecting flag leaf as the diagnostic leaf. The instrument was calibrated at 403.3 mmol m−2 s−1 for molar flow of air, 99.9 kPa atmospheric pressure, 6.0–8.9 mbar water vapor pressure, 1711 µmolm−2 s−1 PAR, 28.4–27.9°C leaf temperature and 352 µmol mol−1 ambient CO2 concentration.
Chlorophyll contents
Data for chlorophyll content were collected by acetone method. The 0.1 g of flag leaves were ground using pestle and mortar in 5 ml of 80% acetone. The centrifuged extract was then used for chlorophyll estimation. The readings were recorded by employing U-2001 Spectrophotometer and calculated by an equation:
Estimation of total soluble proteins
Total soluble proteins of fresh leaf samples were recorded by method (Bradford Citation1976). For this assay, 0.5 g fresh leaves were ground using a tissue grinder in 5 ml of 50 mM cooled phosphate buffer (pH 7.8) placed in an ice bath. The homogenate was centrifuged at 15,000 rpm for 15 min at 4°C. The supernatant was used for protein determination. Each sample (0.1 µl) was taken in a test tube and mixed with 5 ml of Bradford reagent and incubated at 37°C for 10–15 min along with blank. Reading for absorbance was recorded at 595 nm employing spectrophotometer (IRMECO U2020).
Proline determination
Proline contents were determined by Bates et al. (Citation1973). 0.5 g fresh healthy leaf tissue was homogenized in 10 ml of 30% sulpho-salicylic acid and filtered with filter paper. Two milliliters of acid ninhydrin (prepared by mixing 1.25 g ninhydrin in 30 ml glacial acetic acid) and 2 ml of glacial acetic acid were added to the filtrate. The filtrate was mixed and heated in water bath for 60 min at 100°C. The mixture was then cooled and 4 ml of toluene was added and mixed. The chromophore containing toluene was separated from the aqueous phase and its absorbance was recorded at 520 nm with a spectrophotometer (IRMECO U2020). Proline concentration was determinate by using following equation:
Determination of inorganic ions
Sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) were used in the process of digestion of plant material. 0.1 g dried plant material (leaf and root) from oven-dried samples and 2 ml of conc. H2SO4 were put into the digestion flasks and incubated it overnight at 25°C temperature. 1 ml of H2O2 was poured down through the sides of digestion flasks, mixed and flasks were placed onto the hot plate and warmed at 250°C until fume formation occurred. Heating was continuously done for 30 min and then digestion flasks were removed from the plate. Above process was repeated, with the addition of 1 ml H2O2 when needed, until the material became colorless. Then the extract was put into the volumetric flasks and made the volume up to 50 ml, filtered and used for inorganic ion determination.
Determination of mineral nutrients
The amount of dissolved mineral nutrients like potassium (K+), calcium (Ca2+) and magnesium (Mg2+) was recorded by using flame photometer (Model PFPI-7, Jenway, UK), while copper (Cu2+) determination was done by Atomic Absorption Spectrum.
Antioxidant enzyme extraction
For the determination of antioxidant enzymes, 0.5 g fresh leaf samples were ground in tissue grinder in 5 ml of 50 mM cooled phosphate buffer (pH 7.8) placed in an ice bath. The homogenate was centrifuged at 15,000 rpm for 15 min at 4°C. The supernatant was used for the determination of enzyme activities.
Superoxide dismutase
The activity of SOD was determined by measuring its ability to inhibit production of nitro blue tetrazolium (NBT) (Giannopolitis and Ries Citation1977). The 3 ml reaction solution contained 50 µM NBT, 1.3 µM riboflavin, 13 mM methionine, 75 nM EDTA, 50 mM phosphate buffer (pH 7.8) and 20–50 µl enzyme extract. Test tubes containing the reaction solution were irradiated under light (15 fluorescent lamps) at 78 µmol m−2 s−1 for 15 min. The absorbance of irradiant solution was taken at 560 nm by spectrophotometer (IRMECO U2020). One unit of SOD activity was defined as the amount of enzyme that inhibited 50% NBT photo-reduction.
Catalase and peroxidase
The CAT reaction solution (3 ml) contained 50 mM phosphate buffer (pH 7.0), 5.9 mM H2O2 and 0.1 mM enzyme extract. Change in the reaction solution at 240 nm was recorded after every 20 s. One unit CAT activity was defined as an absorbance range of 0.01 units per min. Peroxidase (POD) reaction solution (3 ml) contained 50 mM phosphate buffer (pH 5.0), 20 mM guaiacol, 40 mM H2O2 and 0.1 ml enzyme extract. Change in the reaction solution at 470 nm was recorded after every 20 s. One unit POD activity was defined as an absorbance range of 0.01 units per min (Chance and Maehly Citation1955).
Yield
At maturity, plants were harvested and data for 100-grain weight from each pot were quantified.
Statistical analysis
The three-way analysis of variance of data for all the parameters was computed by using a COSTAT computer program (Cohort software Berkeley, California). The least significance differences between mean values were calculated according to (Steel et al. Citation1997).
Results
Morphological attributes
Plant height
Data for plant height differed significantly (p < 0.05) in response to wheat varieties, proline and copper levels, and their interactive effects. The foliar spray of proline at the rate 80 mM caused an increase in plant height by 19.41% in ‘Punjab-96’ over the unsprayed crop. The addition of copper at the rate of 400 µM caused a reduction in plant height by 29.38% in variety ‘FSD-83’ over untreated plants ().
(b) Shoot and root fresh and dry weight
Table 1. Effect of exogenously applied proline on fresh and dry matter yield under copper stress condition.
Data for fresh and dry weight of shoots differed significantly (p < 0.05) due to different wheat varieties, levels of proline and copper and their interaction as well. The foliar spray of proline at the rate of 80 mM resulted in increased in shoot fresh and dry weight by 19.7% and 18.0% in variety ‘FSD-83,’ respectively over untreated plants. The addition of copper at the rate of 400 µM caused a reduction in shoot fresh and dry weight by 42.1% and 42.3% in variety MH-97, respectively over control plants. Similarly, the foliar spray of proline at the rate of 80 mM resulted in increased in root fresh and dry weight by 10.7% and 13.3% in variety ‘FSD-83,’ respectively over untreated plants. The addition of copper at the rate of 400 µM caused a reduction in root fresh and dry weight by 25.9% and 34.0% in variety ‘MH-97,’ respectively over control plants. Results showed that variety ‘FSD-83’ showed better accumulation of biomass when compared to other two wheat varieties ().
Chlorophyll constituents
Data for chlorophyll contents ‘a’ and ‘b’ differed significantly (p < 0.05) due to wheat varieties, proline and copper levels and their interactive effects. Averaged across proline and copper level, wheat variety ‘FSD-83’ maintained higher chlorophyll contents ‘a’ and ‘b’ by 17.8% and 10.1%, respectively, when compared to other varieties. While the application of copper decreased chlorophyll contents by 35.0% in variety Punjab-96 and 27.0% in variety ‘MH-97’ over untreated plants ().
Table 2. Effect of exogenously applied proline on chlorophyll constituents.
Total soluble protein and proline contents
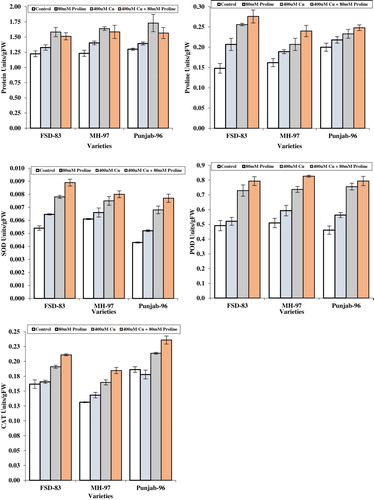
Data for proline content differed significantly (p < 0.05) in response to wheat varieties, proline and copper levels and their interaction too. The values of proline content in leaf tissues was increased significantly (72.9%) in variety ‘FSD-83’ due to copper presence in growing media. Similarly, total soluble protein contents were also increased significantly in all varieties especially in variety ‘MH-97’ (33.0%) due to copper presence in growing media ().
Antioxidant enzymes
Data for antioxidant enzymes was significantly (p < 0.05) enhanced due to the imposition of copper in growing media in all wheat varieties. The foliar spray of proline produced a little effect on increasing the levels of SOD. However, the addition of copper at the rate of 400 µM caused an increase in SOD by 58.14% in Punjab-96 over the untreated crop. Amount of peroxidase (POD) differed significantly in response to various treatments. The wheat variety ‘Punjab-96’ maintained higher POD contents by 64.1% under 400 µM copper stress followed by ‘FSD-83’ and ‘MH-97’ varieties. Data for catalase (CAT) differed significantly due to the imposition of copper treatments by 25.4% in variety ‘MH-97’ when compared to untreated crop. Averaged across wheat varieties and spray of proline caused an increase in SOD, POD and CAT levels by 20.9%, 22.2%, and 9.1%, respectively ().
Gas exchange characteristics
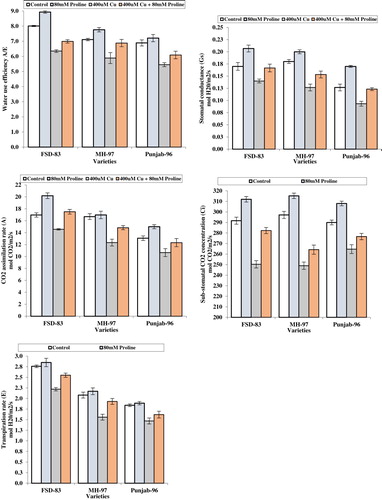
Data for photosynthetic rate differed significantly (p < 0.05) in response to wheat varieties, proline and copper levels, and their interactive effects. The foliar spray of proline at the rate of 80 mM caused an increase in photosynthetic rate by 19.0%, transpiration rate by 6.8%, sub-stomatal CO2 concentration by 6.9%, stomatal conductance by 21.2%, and water-use-efficiency by 11.4% over untreated crop. The addition of copper at the rate of 400 µM caused a reduction in photosynthetic rate by 26.06%, transpiration rate by 25.0%, sub-stomatal CO2 concentration by 16.2%, stomatal conductance by 25.4%, and water-use-efficiency by 20.9% over untreated crop. Based on gas exchange characteristics variety, ‘FSD-83’ showed better results as compared to varieties ‘MH-97’ and ‘Punjab-96’ ().
Chlorophyll flourescence
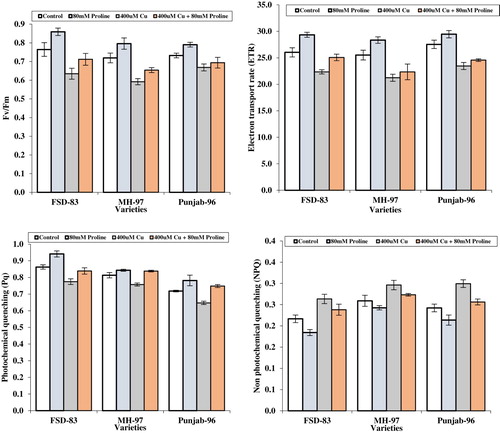
Data for non-photochemical quenching co-efficient differed significantly (p < 0.05) in response to wheat varieties, proline and copper levels as well as their interaction. The values of non-photochemical quenching co-efficient were decreased by 14.9% in FSD-83 due to spray of proline chemical as compared to non-sprayed plants. However, the addition of copper at the rate of 400 mM caused an increase in non-photochemical quenching co-efficient by 23.6% in Punjab-96 over control. Data for efficiency of photosystem-II differed significantly in response to treatments among varieties, the wheat variety‘FSD-83’ maintained higher level of efficiency of photosystem-II by 12.3% followed by varieties ‘MH-97’ and ‘Punjab-96’ varieties, whereas, the imposition of copper decreased PII efficiency by 17.6% in variety ‘MH-97’ over unsprayed crop. Similarly, the values for electron transfer rate (ETR) were enhanced by foliar application of proline by 12.7% in variety ‘FSD-83’ while the addition of copper caused a reduction in ETR by 16.7% over untreated crop ().
Ionic concentration in leaf tissues
Data for metal elements in leaf differed significantly (p < 0.05) in response to proline, copper levels and wheat varieties, as well as their interaction. Data for calcium content differed significantly in leaf organs due to differed treatments. The wheat variety‘FSD-83’ maintained higher leaf Ca2+ content followed by ‘MH-97’ and ‘Punjab-96’ varieties. The foliar spray of proline caused an increase in Ca2+ content by 40.3% over untreated crop. The addition of copper caused a reduction in Ca2+ content by 42.8% in variety ‘Punjab-96’ over untreated crop. Data for magnesium content in leaf organ differed greatly in response to the imposition of different treatments. The variety ‘FSD-83’ maintained higher Mg+ content 11.4% compared to other varieties. Moreover, the addition of copper caused a reduction in Mg+ content by 20.5% in Punjab-96 over the untreated crop. Data for potassium content differed significantly in response to treatments. The wheat variety ‘FSD-83’ maintained maximum K+ content by 16.7% due to foliar application of proline as compared to other two varieties. However, the addition of Cu caused a reduction in K+ content by 36.3% in Punjab-96 over the untreated crop. Data for copper content in leaf organ also enhanced significantly due to copper treatments. The addition of Cu2+ at the rate of 400 µM caused an increase in Cu2+ contents by 87.5% in leaf tissues of variety Punjab-96 over control plants ().
Table 3. Effect of exogenously applied proline on nutrient concentration in shoot organ.
Ionic concentration in root tissues
Data for metal elements in root differed significantly (p < 0.05) in response to proline, copper levels, and wheat varieties, as well as their interaction. Data for Ca2+ content differed significantly in root organs due to differed treatments. The wheat variety ‘FSD-83’ maintained higher root Ca2+ content followed by ‘MH-97’ and ‘Punjab-96’ varieties. The foliar spray of proline caused increase Ca2+ content by 17.0% over untreated crop. The addition of copper caused a reduction in Ca2+ content by 26.6% in variety ‘MH-97’ over untreated crop. Data for magnesium content in root organ differed greatly in response to imposition of different treatments. The variety ‘FSD-83’ maintained higher Mg+ content 10.4% compared to other varieties. Moreover, the addition of copper caused a reduction in Mg+ content by 24.1% in variety MH-97 over untreated crop. Data for potassium content differed significantly in response to treatments. The wheat variety ‘FSD-83’ maintained maximum K+ content by 13.9% due to foliar application of proline when compared to other two varieties. However, the addition of Cu caused a reduction in K+ content by 19.4% in variety ‘MH-97’ over untreated crop. Data for copper content in root organ also enhanced significantly due to copper treatments. The addition of Cu2+ at the rate of 400 µM caused increase in 177.7% Cu2+ contents in root tissues of variety ‘MH-97’ when compared to untreated control plants ().
Table 4. Effect of exogenously applied proline on nutrient concentration in root organ.
Yield component
Data for 100-grain weight differed significantly in response to different treatments. The variety ‘FSD-83’ maintained maximum 100-grain weight by 4.15% when compared to other two varieties, while, the values of 100-grain weight were decreased by 18.75% in Punjab-96 due to the addition of copper compared to untreated check ().
Table 5. Effect of exogenously applied proline on 100-grain weight under copper stress.
Discussion
The productivity of crop species is affected to a greater proportion due to biotic (diseases, insect pests) and abiotic (eco-edaphic factors, salinity, drought, temperature, and heavy metal pollutants) in the agricultural production system (Lawlor and Cornic Citation2002). In the recent years, the rapid industrialization, the outflow of effluents from different industries has caused havoc in polluting the irrigation water and surface soils of agricultural areas. These pollutants contain heavy toxic metals, which not only affect the crop yield but also add poison to the food chain for causing abnormalities on the healthiness of humans and livestock. Among these heavy metals, the copper metal plays a vital role in growth and development of crops as it is the main constituent of many proteins and enzymes processes. On the other hand, high concentration results in toxicity in plants, thereby growth is inhibited (Hall Citation2002). Various researchers (Hall and Williams Citation2003; Shar et al. Citation2011) reported that excessive concentration of copper caused disruption in protein and nitrogen metabolism, by disrupting the photosynthesis and respiration processes. The occurrence of chlorosis of leaves caused inhibition of plant growth (Fariduddin et al. Citation2009). Janas et al. (Citation2010) reported that metabolic processes such as leaf and root growth, photosynthetic rate, production of biomass and pigment contents were hindered. The growth and development of crop species is an outcome of various morphological parameters i.e. plant height, number of leaves per plant and flag leaf area in wheat. The results of the study indicated reduced plant height in response to a higher content of copper in the rhizosphere. The findings corroborate with those of Houshm and Moraghebi (Citation2011) that plant height was reduced due to the presence of higher content of copper in the root zone. The spray of proline caused an increase in apical meristem and cell division, which improved the plant height. These results agree with those of Ali et al. (Citation2013).
The copper stress caused significant reduction in fresh and dry weights of shoots and root organs. Various researchers (El-Tayeb et al. Citation2006; Sonmez et al. Citation2006; Muslu and Ergun Citation2013) also reported that biological yield was depressed in response to copper stress. Furthermore, the foliar spray of proline caused improvement in shoot and root weights. The stress due to higher quantity of copper caused nutrients imbalance in the plant system, which caused reduction in seedlings growth and germination rate. Various researchers (Imlay Citation2003; Ouzounidou Citation1995; Yadav Citation2010; Sethy and Ghosh Citation2013; Kalai et al. Citation2014) reported that osmotic stress impacted negatively on the growth of seedlings in response to specific ion toxicity.
The chlorophyll contents ‘a’ and ‘b’ were decreased in response to copper stress. The reduction in photosynthetic pigments might be due to the destruction of thylakoid membranes and destruction of chlorophyll apparatus in response to copper stress. The similar results were reported by Michaud et al. (Citation2008) and Ahmed et al. (Citation2010). Furthermore, foliar spray of proline at the rate of 80 mM alleviated the copper stress and maintained higher contents of chlorophyll. These results agreed with those of Shahid et al. Citation2014 that spray of proline maintained sub-cellular structures under saline environments. The accumulation of copper in the rhizosphere resulted in enhanced production of anti-oxidants, protein and proline contents in three wheat varieties. Various researchers (Ben Ahmad et al. Citation2010; Brahim and Mohamed Citation2011; Muslu and Ergun Citation2013; Sharma and Singh Citation2013) reported that increase in antioxidant enzymes (SOD, POD, CAT) and protein contents provided protection to avoid possible destruction in important enzymes. Furthermore, accumulation of proline protected the plants against damage of ROS caused by copper stress (Matysik et al. Citation2002; Verma et al. Citation2011; Azooz et al. Citation2012).
Some of the gas exchange characteristics (i.e. stomatal conductance (gs), photosynthetic rate (A), internal CO2 concentration (Ci), transpiration rate (E) and water-use-efficiency) were greatly reduced due to copper stress. However, the negative effects were ameliorated in response to spray of proline. The similar results have been reported by Ali et al. (Citation2007) and Ben Ahmad et al. (Citation2010). Kaymakanova et al. (Citation2008) reported that stomatal conductance was reduced by abiotic stress while CO2 concentration and biochemical capability was reduced (Khan and Panda Citation2008). Janas et al. (Citation2010) reported that photosynthetic activity was reduced due to inhibition of Rubisco activity. Chlorophyll fluorescence parameters were a little affected due to copper stress. Jamil et al. (Citation2007) reported that values of electron transfer (ETR), efficiency of photosystem-II (Fv/Fm), photochemical quenching co-efficient (qP) and non-photochemical quenching co-efficient (qN) were slightly reduced, while, the activity of non-photochemical quenching (NPQ) were slightly enhanced in response to copper stress. The reduction in values of Fv/Fm ratio was mainly due to the reduction in Fm value. The reduction resulted due to increase in energy dissipation, disintegration of accessory pigments for capturing light from photosystem-II (Maxwell and Johnson Citation2000).
The uptake of nutrients was greatly impacted in response to copper stress. The uptake of calcium, magnesium, and potassium ions by root, shoot, and leaf organs was reduced due to copper stress. On the other hand, copper content substantially increased in root organs compared to shoot and leaf organs under copper stress environment. The maintenance of higher content of copper by roots inhibited the uptake of other nutrients. The results agree with those of Michaud et al. (Citation2008) and Karimi et al. (Citation2012) that presence of copper in excessive amount in roots inhibited the other nutrients for translocation from roots to above ground parts. Contrary to it, the foliar spray of proline caused an increase in uptake of Ca2+, Mg2+, Na+, and K+ by root, shoot and leaf organs, while the uptake of copper was also reduced by all parts. Ashraf and Foolad (Citation2007) reported that uptake of ions by plants was regulated by proline spray under stress environment. The copper stress caused a reduction in 100-grain weight. Different researchers (Athar and Ahmad Citation2002; Ahmad et al. Citation2013; Gang et al. Citation2013) also reported that yield and yield components of wheat were reduced under copper stress.
The results of the study indicated that presence of higher copper ion in the rhizosphere resulted in reduction of plant growth, physiological and biochemical parameters. However, the stress could be mitigated by foliar spray of proline at the rate of 80 mM under copper stress conditions. In the present study wheat variety ‘FSD-83’ produced showed better biomass accumulation and biochemical attributes when compared to varieties ‘MH-97’ and ‘Punjab-96.’
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-ur-Rehman M, Irshad MK, Bharwana SA. 2015. The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res. 22:8148–8162. doi: 10.1007/s11356-015-4496-5
- Ahmad K, Khan ZI, Ashraf S, Ejaz A, Shaheen M, Raza SH, Abbas F, Tahir HM. 2013. Effect of sewage water irrigation on the uptake of some essential minerals in canola (Brassica napus L.): A potential forage crop for ruminants. Pak J Life Soc Sci. 11:42–47.
- Ahmed A, Hasnain A, Akhtar S, Hussain A, Yasin G, Wahid A, Mahmood S. 2010. Antioxidant enzymes as bio-markers for copper tolerance in safflower (Carthamus tinctorius L.). Afr J Biotechnol. 9:5441–5444. doi: 10.5897/AJB10.638
- Ali Q, Ashraf M, Athar H-U-R. 2007. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak J Bot. 39:1133–1144.
- Ali HM, Siddiqui MH, Al-Whaibi MH, Basalah MO, Sakran AM, El-Zaidy M. 2013. Effect of proline and abscisic acid on the growth and physiological performance of faba bean under water stress. Pak J Bot. 45:933–940.
- Ashraf M, Foolad M. 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 59:206–216. doi: 10.1016/j.envexpbot.2005.12.006
- Athar R, Ahmad M. 2002. Heavy metal toxicity: effect on plant growth and metal uptake by wheat, and on free living Azotobacter. Water, Air, Soil Pollut. 138:165–180. doi: 10.1023/A:1015594815016
- Azooz MM, Abou-Elhamd MF, Al-Fredan MA. 2012. Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Aust J Crop Sci. 6:688.
- Bates L, Waldren R, Teare I. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39:205–207. doi: 10.1007/BF00018060
- Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss M, Ben Abdullah F. 2010. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J Agric Food Chem. 58:4216–4222. doi: 10.1021/jf9041479
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Brahim L, Mohamed M. 2011. Effects of copper stress on antioxidative enzymes, chlorophyll and protein content in Atriplex halimus. Afr J Biotechnol. 10:10143–10148. doi: 10.5897/AJB10.1804
- Chance B, Maehly A. 1955. Assay of catalase and peroxidase. Methods Enzymol. 2:764–775.
- Chen W, Chang AC, Wu L. 2007. Assessing long-term environmental risks of trace elements in phosphate fertilizers. Ecotoxicol Environ Saf. 67:48–58. doi: 10.1016/j.ecoenv.2006.12.013
- El-Sherbeny MR, Silva JATd. 2013. Foliar treatment with proline and tyrosine affect the growth and yield of beetroot and some pigments in beetroot leaves. J Hortic Res. 21:95–99. doi: 10.2478/johr-2013-0027
- El-Tayeb M, El-Enany A, Ahmed N. 2006. Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.). Plant Growth Regul. 50:191–199. doi: 10.1007/s10725-006-9118-2
- Epstein F. 1972. Mineral nutrition of plants: principles and prospectives. New York (NY): Wiley.
- Fariduddin Q, Yusuf M, Hayat S, Ahmad A. 2009. Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environ Exp Bot. 66:418–424. doi: 10.1016/j.envexpbot.2009.05.001
- Frary A, Göl D, Keleş D, Ökmen B, Pınar H, Şığva HÖ, Yemenicioğlu A, Doğanlar S. 2010. Salt tolerance in Solanum pennellii: antioxidant response and related QTL. BMC Plant Biol. 10:01–16. doi: 10.1186/1471-2229-10-58
- Gang A, Vyas A, Vyas H. 2013. Toxic effect of heavy metals on germination and seedling growth of wheat. J Env Res Dev. 8:206.
- Giannopolitis CN, Ries SK. 1977. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 59:309–314. doi: 10.1104/pp.59.2.309
- Hall J. 2002. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 53:1–11. doi: 10.1093/jexbot/53.366.1
- Hall J, Williams LE. 2003. Transition metal transporters in plants. J Exp Bot. 54:2601–2613. doi: 10.1093/jxb/erg303
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Circular, California Agricultural Experiment Station, 347.
- Houshm A, Moraghebi F. 2011. Effect of mixed cadmium, copper, nickel and zinc on seed germination and seedling growth of safflower. Afr J Agric Res. 6:1463–1468.
- Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol. 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938
- Jamal A, Ayub N, Usman M, Khan AG. 2002. Arbuscular mycorrhizal fungi enhance zinc and nickel uptake from contaminated soil by soybean and lentil. Int J Phytoremediation. 4:205–221. doi: 10.1080/15226510208500083
- Jamil M, Rehman S, Lee KJ, Kim HS, Rha ES. 2007. Salinity reduced growth PS2 Photochemistry and chlorophyll content in radish. Sci Agric. 64:111–118. doi: 10.1590/S0103-90162007000200002
- Janas K, Zielińska-Tomaszewska J, Rybaczek D, Maszewski J, Posmyk M, Amarowicz R, Kosińska A. 2010. The impact of copper ions on growth, lipid peroxidation, and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol. 167:270–276. doi: 10.1016/j.jplph.2009.09.016
- Kalai T, Khamassi K, Silva JATd, Gouia H, Ben-Kaab LB. 2014. Cadmium and copper stress affect seedling growth and enzymatic activities in germinating barley seeds. Arch Agron Soil Sci. 60:765–783. doi: 10.1080/03650340.2013.838001
- Karimi P, Khavari-Nejad R, Niknam V, Ghahremaninejad F, Najafi F. 2012. The effects of excess copper on antioxidative enzymes, lipid peroxidation, proline, chlorophyll, and concentration of Mn, Fe, and Cu in Astragalus neo-mobayenii. Scientific World J. 2012:01–06.
- Kaymakanova M, Stoeva N, Mincheva T. 2008. Salinity and its effects on the physiological response of bean (Phaseolus vulgaris L.). J Cent Eur Agric. 9:749–755.
- Ke W, Xiong Z, Xie M, Luo Q. 2007. Accumulation, subcellular localization and ecophysiological responses to copper stress in two Daucus carota L. populations. Plant Soil. 292:291–304. doi: 10.1007/s11104-007-9229-1
- Khan M, Panda S. 2008. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant. 30:81. doi: 10.1007/s11738-007-0093-7
- Lawlor DW, Cornic G. 2002. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x
- Matysik J, Alia, Bhalu B, Mohanty. 2002. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci. 82:525–532.
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence – a practical guide. J Exp Bot. 51:659–668. doi: 10.1093/jexbot/51.345.659
- Michaud AM, Chappellaz C, Hinsinger P. 2008. Copper phytotoxicity affects root elongation and iron nutrition in durum wheat (Triticum turgidum durum L.). Plant Soil. 310:151–165. doi: 10.1007/s11104-008-9642-0
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends Plant Sci. 9:490–498. doi: 10.1016/j.tplants.2004.08.009
- Muslu A, Ergün N. 2013. Effects of copper and chromium and high temperature on growth, proline and protein content in wheat seedlings. Bangladesh J Bot. 42:105–112. doi: 10.3329/bjb.v42i1.15871
- Nussbaum S, Schmutz D, Brunold C. 1988. Regulation of assimilatory sulfate reduction by cadmium in Zea mays L. Plant Physiol. 88:1407–1410. doi: 10.1104/pp.88.4.1407
- Ouzounidou G. 1995. Effect of copper on germination and seedling growth of Minuartia, Silene, Alyssum and Thlaspi. Biol Plant. 37:411–416. doi: 10.1007/BF02913990
- Ouzounidou G, Eleftheriou E, Karataglis S. 1992. Ecophysical and ultrastructural effects of copper in Thlaspi ochroleucum (Cruciferae). Can J Bot. 70:947–957. doi: 10.1139/b92-119
- Rehman SA, Iqbal MZ. 2006. Seed germination and seedling growth of trees in soil extracts from Korangi and Landhi industrial areas of Karachi, Pakistan. J New Seeds. 8:33–45. doi: 10.1300/J153v08n04_03
- Rehman F, Khan F, Varshney D, Naushin F, Rastogi J. 2011. Effect of cadmium on the growth of tomato. Biol Med. 3:187–190.
- Sethy SK, Ghosh S. 2013. Effect of heavy metals on germination of seeds. J Nat Sci Biol Med. 4:272. doi: 10.4103/0976-9668.116964
- Shahid MA, Balal RM, Pervez MA, Abbas T, Aqeel MA, Javaid MM, Garcia-Sanchez F. 2014. Exogenous proline and proline-enriched Lolium perenne leaf extract protects against phytotoxic effects of nickel and salinity in Pisum sativum by altering polyamine metabolism in leaves. Turk J Bot. 38:914–926. doi: 10.3906/bot-1312-13
- Shar GQ, Kazi TG, Shah FA, Shar AH, Soomro FM. 2011. Variable uptake and accumulation of essential and heavy metals in maize (Zea mays L.) grains of six maize varieties. Aust J Basic Appl Sci. 5:117–121.
- Sharma A, Singh G. 2013. Studies on the effect of Cu (II) ions on the antioxidant enzymes in chickpea (Cicer arietinum L.) cultivars. Журнал стресс-физиологии ибиохимии. 9:5–13.
- Sheldon A, Menzies N. 2005. The effect of copper toxicity on the growth and root morphology of Rhodes grass (Chloris gayana Knuth.) in resin buffered solution culture. Plant Soil. 278:341–349. doi: 10.1007/s11104-005-8815-3
- Singh D, Nath K, Sharma YK. 2007. Response of wheat seed germination and seedling growth under copper stress. J Environ Biol. 28:409.
- Sonmez S, Kaplan M, Sonmez NK, Kaya H, Uz I. 2006. High level of copper application to soil and leaves reduce the growth and yield of tomato plants. Scientia Agricola. 63:213–218. doi: 10.1590/S0103-90162006000300001
- Steel RGD, Torrie JH, Dickey DA. 1997. Principles and procedures of statistics: a biometrical approach. 3rd ed. New York (NY): McGraw Hill.
- Szabados L, Savouré A. 2010. Proline: a multifunctional amino acid. Trends Plant Sci. 15:89–97. doi: 10.1016/j.tplants.2009.11.009
- Verma JP, Singh V, Yadav J. 2011. Effect of copper sulphate on seed germination, plant growth and peroxidase activity of Mung bean (Vigna radiata). Int J Bot. 7:200–204. doi: 10.3923/ijb.2011.200.204
- Xu J, Yang L, Wang Z, Dong G, Huang J, Wang Y. 2006. Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere. 62:602–607. doi: 10.1016/j.chemosphere.2005.05.050
- Yadav S. 2010. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot. 76:167–179. doi: 10.1016/j.sajb.2009.10.007
- Younas M, Shahzad F, Afzal S, Khan MI, Ali K. 1998. Assessment of Cd, Ni, Cu, and Pb pollution in Lahore, Pakistan. Environ Int. 24:761–766. doi: 10.1016/S0160-4120(98)00068-3
- Yurekli F, Porgali ZB. 2006. The effects of excessive exposure to copper in bean plants. Acta Biol Cracov Ser Bot. 48:7–13.
- Zengin FK, Kirbag S. 2007. Effects of copper on chlorophyll, proline, protein and abscisic acid level of sunflower (Helianthus annuus L.) seedlings. J Environ Biol. 28:561–566.