ABSTRACT
The biochemical and molecular basis of the defense in a mild tolerant (ICPL-332) and susceptible (ICPL-87) cultivars of pigeon pea (Cajanus cajan) due to Helicoverpa. armigera infestation was studied. We found that feeding by the larvae generated H2O2 in a localized manner and activity was observed upto 12 h (hrs) of with a sharp decline within 24 h. Similarly, PPO activity was also detected till 12 h of treatment, which decreased after 24 h of feeding by larvae. The activity of trypsin inhibitor was detected in all the treatments when assayed at 12 and 24 h after larval feeding. The expression of defense genes like the Pre-hevein-like protein PR-4 precursor (PR-4), protease inhibitor/seed storage/LTP family protein (Ltp) were significantly up-regulated in ICPL-332 upon infestation after 12 h as compared to ICPL-87, whereas the endo 1, 4 -β-glucanase (Kor-1) gene was expressed in both the cultivars after 24 h of infestation. Both the cultivars varied with respect to the induction of defense-related genes during larval feeding, both the PR-4 and Ltp genes appeared to be important for defense against H. armigera in pigeon pea. Thus, the present study revealed an insight of herbivore-induced biochemical and molecular changes in pigeon pea.
Introduction
Plants trigger the induction of defense mechanisms in response to the damage caused by insect feeding which are categorized as direct and indirect defense (Karban and Baldwin Citation1997; Thomma et al. Citation1998; Kessler and Baldwin Citation2002). Direct defenses include expression of various defensive enzymes, generation of reactive oxygen species (ROS), biosynthesis of several secondary metabolites while indirect defense is conferred by the release of various volatile compounds which act as an attractant to draw the predators to the herbivore feeding on the plant (Kessler and Baldwin Citation2001, Citation2002; War et al. Citation2012). Phytohormones are also actively involved in imparting induced resistance against herbivore attack. The major signaling molecules that chiefly regulate the defense mechanisms are jasmonic acid (JA), salicylic acid (SA) and ethylene (ET) (Koornneef and Pieterse Citation2008). These hormones either act individually or are involved in complex cross talks networks, which finely tune the defensive response (Verhage et al. Citation2010; War et al. Citation2012). While JA is involved commonly in defense against chewing insects (MacConn et al. Citation1997), SA is believed to induce a stronger response against piercing and sucking insects (War et al. Citation2012) and ET is known to act along with JA either co-actively or combatively (Kunkel and Brooks Citation2002; War et al. Citation2012). However, induced mechanisms vary according to the plant species and the kind of wounding. Plants respond differently to insect injury and mechanical wounding (Korth and Dixon Citation1997; Reymond et al. Citation2000). This difference may be because of the presence of several insect-specific elicitors in oral secretions of a herbivore (Mattiacci et al. Citation1995). The elicitor molecules in the oral secretion include various fatty acid conjugate, enzymes, and other complex molecules, which are known to generate a defensive response in plants (Mattiacci et al. Citation1995; Alborn et al. Citation1997; Funk Citation2001; Halitschke et al. Citation2001)
Insect-induced resistance is accompanied by a dynamic change in transcript profile (Kessler and Baldwin Citation2002). In many model plants such as Arabidopsis thaliana (Reymond et al. Citation2004), Nicotiana attenuata (Hermsmeier et al. Citation2001) and other plant species like in Citrus sinensis (Mozuruk et al. Citation2006), Solanum tuberosum (Lawrence et al. Citation2008), Solanum lycopersicum (Fidantsef et al. Citation1999) and poplar (Major and Constabel Citation2006) changes in transcript dynamics in response to herbivore attack have been studied. However, defense response may vary for different crop plants. Therefore, studying such plant-pest interaction in cultivated crops is important to understand the molecular mechanisms underlying the plant defense. This information would be useful to develop new strategies for crop improvement.
Pigeonpea (Cajanus cajan) is cultivated widely in developing countries in an area of about 4.57 million ha producing 3.29 million tonnes of grains, globally (Majumder and Singh Citation2005). However, the crop suffers due to considerable damage in the field by the pod borers, Helicoverpa armigera, Grapholita critica, Maruca testulalis, Lampides boeticus, Exelastis atomosa and Melanagromyza obtusa. Most significant pests are H. armigera and M. testulalis inflicting severe loss of the produce (Sharma et al. Citation2010). A few reports on the interaction of H. armigera with other legumes (Giri et al. Citation1998; Peng et al. Citation2005; Singh et al. Citation2008) but no study has been carried out in pigeon pea till date. Although, some mild resistant cultivars of pigeon pea have been identified, however, the molecular basis of their defense mechanism is not yet elucidated.
Here, we report on defense related events in two different cultivars of pigeon pea viz. ICPL-332 and ICPL-87 which is considered to be mildly tolerant and susceptible respectively. Kumari et al. Citation2006 reported that ICPL-332 suffered a lower (22.9%) pod damage when compared with a susceptible genotype, ICPL 87 (83.2%). However, the resistance mechanism underlying this is not yet known. Therefore, we studied the changes in biochemical and molecular responses in pigeon pea during H. armigera larvae infestation and using various signaling molecules like JA, SA and ET. We investigated the generation of ROS, the activity of various defense-related enzymes such as polyphenol oxidase (PPO), trypsin inhibitor and the amylase inhibitor due to feeding by pod borer larvae. Furthermore, expression of various defense-related genes was studied in both the cultivars after 12 and 24 h of feeding by H. armigera larvae. Our results suggest that the cultivar ICPL-332 induced the expression of defensive genes and various biochemical compounds to defend it against H. armigera feeding.
Materials and methods
Plant material and insect rearing
Two different cultivars of pigeon pea, ICPL-332 (mildly tolerant) and ICPL- 87 (susceptible), were collected from the International Crops Research Institute for Semi-Arid Tropics (ICRISAT), Hyderabad and grown in pots containing mixture of autoclaved soil and coco-peat (1:1 v/v) with 16/8 h (hrs) light/dark cycle and temperature of 22–25°C. Regular watering of plants was performed during this period. The plants 4–5 weeks old were used for the experiments.
The eggs of H. armigera were also collected from the ICRISAT, Hyderabad. The larvae hatched from the eggs were reared on a artificial diet (Armes et al. Citation1992). The artificial diet mix was prepared and cut into small pieces to feed the larvae. The larvae were reared for three generations in vitro and then released for infestation.
Plant treatments
For the present study, fifteen plants were selected from each cultivar, which was subjected to four different treatments such as infestation by H.armigera larvae and treatments by methyl jasmonate (JA), salicylic acid (SA) and Ethephon (ET). The treatments were performed in three replicates. Three plants without any treatments were maintained as control plants. The exogenous treatments involved painting of the leaves of both the cultivars using methyl jasmonate (100 µM, Sigma-Aldrich), salicyclic acid (5 mM, Sigma-Aldrich) and Ethephon (50 µM, Sigma-Aldrich) as recommended by Singh et al. (Citation2008). For plant infestation, the freshly molted third (3rd) instar larvae were collected and starved overnight before they were released on the plants. We released three starved larvae on the leaves of both the cultivars of pigeon pea plants and allowed them to feed for 12 h. The plants along with the larvae were covered with a muslin cloth, which was tied at the base to prevent the escape of the larvae. The control plants were also covered with muslin cloth with no larvae in it. We also employed mechanical injury in some of the plants using a punching machine, covering around 15–20% of leaf area. A similar experiment was repeated for 24 h of treatment. The plant leaves were harvested after 12 and 24 h and assayed for various defense-related activity. All the experiments were repeated twice.
Detection of hydrogen peroxide (H2O2)
Generation of hydrogen peroxide in response to various treatments was detected by using 3, 3- diaminobenzidine (DAB) (Liu et al. Citation2012). In brief, 1 mg/ml solution of DAB (Sigma) was prepared and the pH was adjusted to 3.6 using 0.1 N HCl. Dissolving of DAB was performed at 37°C for 1 h with vigorous shaking. At least two leaves harvested from the treated plants were incubated in 10 ml of DAB solution for overnight at 37°C. The excess of DAB solution was then rinsed using distilled water. The stained leaf samples were then boiled for 3–4 h in a fixative solution containing ethanol and acetic acid in 3:1 ratio and were further incubated for an hour in Lacto-glycerol solution (lactic acid/ glycerol/water, 1:1:1). The leaf samples were then observed under white light.
Polyphenol oxidase enzyme (PPO) assay
Pigeon pea leaves were collected after 12 and 24 h of treatment and was macerated using 0.1 M phosphate buffer (pH 7) containing polyvinylpyrrolidone (5 g). Around five grams of samples were used in each case. The homogenized mixture was centrifuged at 10,000 rpm for 10 min and the supernatant was used for the determination of PPO activity following described by Arnnok and his co-workers (Citation2010). To the extract (50 µL) 1 mL of 0.1 M catechol was added, mixed thoroughly and the absorbance was recorded at 410 nm.
Trypsin (TI) and amylase inhibitor (AI) assay
The total protein extracted was also used for the detection of trypsin and amylase inhibitor activity. The trypsin inhibitor activity was carried out by incubating 10 µg of protein in a solution containing 0.1 mg/ml of trypsin, 0.5% casein and phosphate buffer (pH 7.6). Incubation was carried out at 37°C for 20 min followed by the addition of 10% trichloro acetic (TCA) acid was added. Centrifugation was carried out at 10,000 rpm for 10 min after 1 h of incubation and the absorbance was recorded at 280 nm.
Expression of defense related genes
The total RNA was extracted using RNeasy plus mini kit (Qiagen) with DNA removal column, which was then used for quantitative reverse transcriptase PCR (qPCR) using various gene-specific primers. In 2008, Singh and his co-workers reported up-regulation of several defense related genes in chickpea upon H. armigera infestation. Based on their report, we selected eight genes for studying their expression in pigeon pea upon H. armigera infestation. The primers for these genes were designed using Invitrogen’s OligoPerfectTM Designer (www.invitrogen.org) and were tested for amplification efficiency by using cDNA as a template by RT-PCR. However, only three defense-related genes such as the Pre-hevein-like protein PR-4 precursor (PR-4), Protease inhibitor/seed storage/LTP family protein (Ltp) and Endo-1, 4-β-D-glucanase KORRIGAN (kor-1 gene) showed expected amplicon size after PCR assays and were selected for the study. The remaining primers showed un-specific binding, therefore, omitted from the experiment. For an expression profile of these selected genes, 10 ng of total RNA was amplified by a two-step qPCR mix having SYBR green dye and ROX as a passive dye (SYBR® greener™ Two-Step Q-PCR Kit for ABI PRISM®, Thermo Fisher Scientific). The reactions were performed following the manufacturer’s protocol. A 5S rRNA gene was selected as an internal control and the expression analysis was performed using the standard 2−ΔΔCT method.
Results
Generation of hydrogen peroxide
The induction of defense response upon H. armigera infestation and exposure to the signaling molecules in pigeon pea was assayed by detection of H2O2, polyphenol oxidase activity (PPO), the amylase inhibitor (AI) and trypsin inhibitor (TI) expression after 12 and 24 h of treatments.
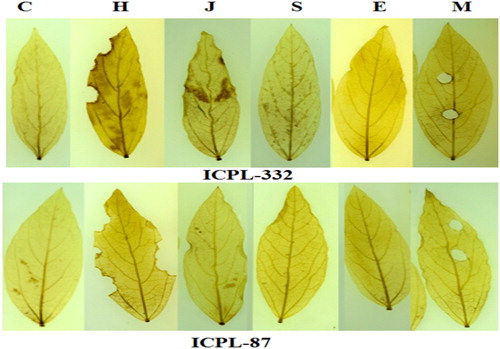
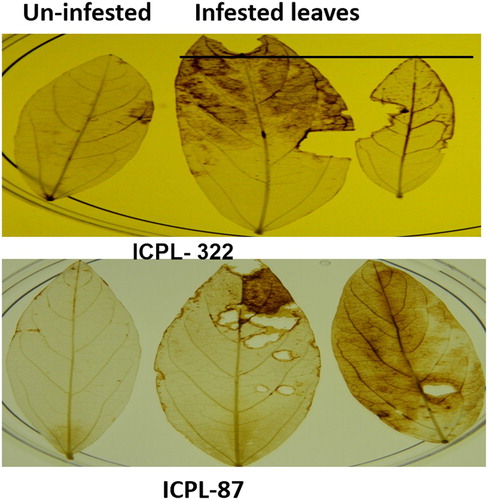
To determine the potential role of H2O2 in plant samples, treated leaf samples of both the cultivars were stained with DAB solution. The H2O2 accumulation was detected as dark brown spots (). The H2O2 accumulation was detected after 12 h of treatment in both the cultivars due to infestation by H. armigera and JA and ET treated leaves. Also, accumulation of H2O2 was observed to be significantly increased upon mechanical wounding. After 24 h of infestation, the accumulation of H2O2 decreased considerably (data not included). When H2O2 accumulation was compared among the samples, the accumulation was found to be maximum in H. armigera infested leaves of ICPL-332. We also compared the infested and uninfested leaves from the same plants and we found that the H2O2 accumulation in the infested leaves only () but not in the uninfested leaves.
Polyphenol oxidase and trypsin inhibitor expression upon infestation
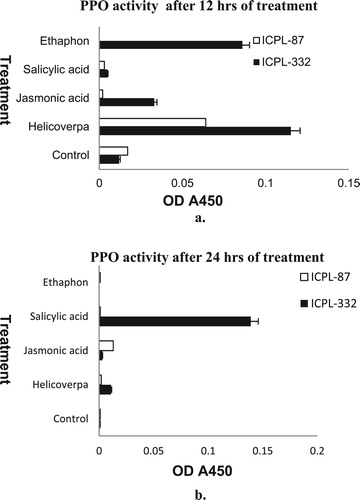
Assay for PPO enzyme activity was performed after 12 and 24 h of treatments using 20 µg of leaf protein. The PPO activity in the two cultivars was compared after 12 h of treatment and found that the PPO activity was highest in the H. armigera infested leaves of ICPL-332 than ICPL-87 (a). However, after 24 h of treatment, PPO activity declined in the treated samples except in leaves of ICPL-332 treated with salicylic acid (b).
Figure 3. Polyphenol oxidase activity in pigeon pea in response to 12 (a) and 24 h (b) treatments. The means ± SE were calculated from data pooled from three experimental repeats.
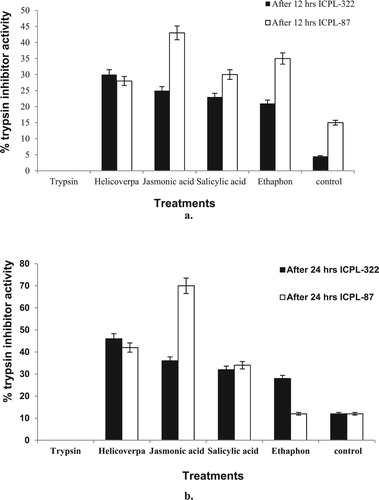
Presence of trypsin inhibitor (TI) was also detected by performing the enzyme assay. TI activity was detected in both the cultivars and in all the leaf samples subjected to different treatments. The activity was found to be maximum in JA treated samples of ICPL–87 (a). The trypsin inhibitor activity in all the samples increased after 24 h in all the treatments except in ET treated samples (b).
Expression of the various defense related genes
Three genes, namely PR-4, Kor-1, and Ltp family protein were selected after analyzing the PCR amplification efficiency of the primers. To check the expression of these genes upon various treatments, qPCR was performed after 12 h (a) and 24 h (b) of treatment. After 12 h of treatment, the expression of PR-4 gene was observed in most of the treated samples of both the cultivars. In ICPL-332, expression of the PR-4 gene was found to be higher (2 fold) due to H. armigera infestation and appeared to be regulated by both JA and SA when compared with ICPL-87 and controls.
Figure 5. Levels of expression of defense related transcript in pigeon pea due to feeding on leaves by Helicoverpa larvae and SA, JA, ET treatments. The means ± SE were calculated from data pooled from three experimental repeats. a. Expression of PR-4 (a), Kor-1 (b) and Ltp (c) gene after 12 h of infestation by Helicoverpa larvae and SA, JA, ET treatments in cultivars ICPL-332 and 87; b. Expression of PR-4 (a), Kor-1 (b) and Ltp (c) gene after 12 h of infestation by Helicoverpa larvae and SA, JA, ET treatments in cultivars ICPL-332 and 87.
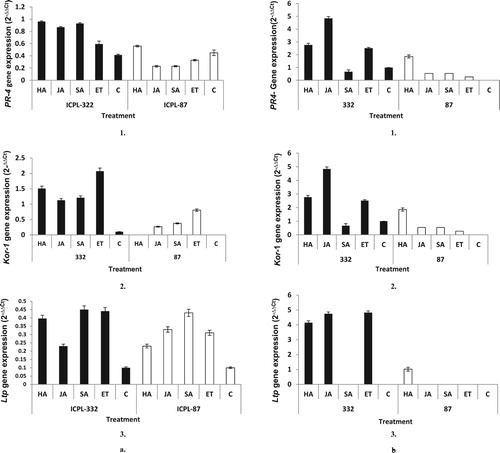
After 12 h of treatment, Kor-1 gene appeared to be up-regulated by all the treatment in ICPL-332, but its level of expression was higher in the leaf samples treated with ET and leaves infested by H. armigera. However, on the contrary, the expression of Kor-1 was significantly less in ICPL-87 and was not observed in leaves infested by H. armigera. For Ltp gene, its expression was observed in both the cultivars, but the expression was higher in ICPL-332 which was mediated by SA and ET. After 24 h of treatment, the expression of PR-4, Kor-1, and Ltp were observed in ICPL-332, whereas the level declined, significantly, in ICPL-87.
Discussion
To gain insights of the responses of pigeon pea against H. armigera feeding, we studied the changes in the generation of H2O2, polyphenol oxidase (PPO) activity, trypsin and amylase inhibitor activity, and expression of three key defense related genes. Moreover, the role of various signaling molecules was also studied which are the key regulators of plant defense (Arimura et al. Citation2000; Winz and Baldwin Citation2001; De Vos et al. Citation2005).
Generation of H2O2 in pigeon pea-Helicoverpa interaction
The generation of various reactive oxygen species (ROS) including H2O2 is one of the earliest known defense responses, which is associated with plant pathogen/pest relationship (Lamb and Dixon Citation1997; Li et al. Citation2011). The stimulation of ROS production can also be due to drought (Loggini et al. Citation1999), salinity (Valderrama et al. Citation2006) or by various phytohormones (Pei et al. Citation2000; Nafie et al. Citation2011). The H2O2 is a component of complex ROS network that triggers programed cell death (Jabs Citation1999), which is mediated by certain systematic cellular and molecular events comprising of complex networks of ROS (Jacobson et al. Citation1997; Dangl et al. Citation2000).
In the present study, the H2O2 accumulation was detected using DAB staining and was visible as brown coloration at the site of wounding (chewed and scraped leaf surface). After 12 h of treatment, the pattern of H2O2 accumulation varied for all the treatments in both the cultivars, but the generation of H2O2 was comparatively less in the susceptible cultivar. The generation of H2O2 was found to be mostly mediated by H. armigera infestation and application of JA and ET. SA is also known to promote H2O2 production at an early event of signaling (Garreton et al. Citation2002; Lee et al. Citation2010; Khokon et al. Citation2011). Similarly, exogenous application of JA enhances the hydrogen peroxide activity (Nafie et al. Citation2011). The generation of H2O2 after 24 h of treatment was very less in all the samples except in the samples infested by H. armigera. The generation of H2O2 may be because of continuous feeding by the larvae. The decline in the H2O2 accumulation after 24 h of treatment may be because of the fact that the generation of H2O2 is maximum within 4–6 h of infection/infestation and then decline steadily (Bergey et al. Citation1999; Orozco-Cardenas and Ryan Citation1999). The generation of high H2O2 in our study in response to mechanical wounding is consistent with the H2O2 generation in tomato in response to wounding (Orozco-Cardenas et al. Citation2001). It is reported that H2O2 generated in response to a wound can be detected both locally and systematically in plant tissues (Bergey et al. Citation1999; Orozco-Cardenas et al. Citation2001; Choi et al. Citation2007), however, when tested for systemic spread of H2O2 due to H. armigera infestation we found that the generation of H2O2 was localized around the wounded tissues.
Defense enzyme activities upon H. armigera infestation
Polyphenol oxidase (PPO) is a recognized oxidative enzyme which plays a very important role in plant pest defense (Felton et al. Citation1989; Duffey and Felton Citation1991; Haruta et al. Citation2001). In plants, polyphenol oxidase (PPO) and peroxidase (POD) are potential defense enzymes that cause oxidation of phenols during infestation by herbivorous insects. PPO is involved in the oxidation of phenols, which produces quinones that are responsible for toxicity against insects (War et al. Citation2012). In addition, nutritional value of plant proteins for insects reduces due to alkylation of amino acids (Bhonwong et al. Citation2009). Phenols also mediate reduction of ROS (Maffei et al. Citation2007). Several reports suggest that PPO activity increases when plants are subjected to various biotic and abiotic stresses (Coley Citation1980; Mayer Citation1986). In the present study, spectral analysis of PPO activity after 12 h of treatment revealed that the enzyme activity increased in both the cultivars in response to H.armigera infestation and the activity declined after 24 h of treatment. Similar results were reported during interaction between tomato and Leptinotarsa decemlineata (Felton and Duffey, Citation1992), poplar (hybrid) and Malacosoma disstria (Constabel et al. Citation2000), trembling aspen and Malacosoma disstria together with tea and Helopeltis theivora (Chakraborty and Chakraborty, Citation2005). Although PPO activity is induced by various signaling molecules (Thipyapong and Steffens Citation1997; Constabel and Ryan Citation1998) but we have found that the PPO activity in pigeon pea is mediated by ethylene and jasmonic acid. There was a significant decrease in the PPO activity with time (after 24 h). After 24 h of treatment, the PPO activity was found to be elevated only in the tolerant variety on SA treatment. This might be because of application of SA in higher amounts. SA is known to induce PPO activity when used in higher concentration (War et al. Citation2011).
Protease and amylase inhibitors are one of the important components of the plant defense system (Hermosa et al. Citation2006; Kansal et al. Citation2008; Hägg et al. Citation2013) and hence they are believed to be one of potential candidates for protection of plants against insects (Ryan Citation1990; Franco et al. Citation2002). Most proteases inhibit larval midgut digestive enzymes (Tamhane et al. Citation2005; Zhua et al. Citation2007). The expression of protease inhibitors also differs in response to various signaling molecules (Rakwala et al. Citation2001; Casaretto et al. Citation2004). In our experiment, TI activity was found to increase in all the treated samples with respect to the control. A similar induced response was reported in rice (Casaretto et al. Citation2004; Wang et al. Citation2011). In addition amylase inhibitors, which act as an inhibitor of larval midgut amylase (Kluh et al. Citation2005) accumulates due to foliar treatment with exogenous jasmonic acid was observed, previously (Délano-Frier et al. Citation2004).
Differential expression of various defense- related genes
Singh and his co-workers in 2008 prepared a forward Suppression Subtractive Hybridization library of chickpea upon Helicoverpa infestation and found defense-related genes along with genes involved in detoxification, protein synthesis, and photosynthesis. Based on the experiments it appeared that pathogenesis related proteins (PR-10 and PR-5), pre-hevein-like protein (PR-4), and LTP/protease inhibitor (Ltp) were directly involved in Helicoverpa defense in chickpea.
The expression of defense-related genes, PR-4, kor -1 and Ltp was studied by quantitative PCR. The PR genes are an important element of plant defense and are found to be up-regulated by a pathogen or insect attack (Bonasera et al. Citation2006), various signaling molecules like SA (Zhang et al. Citation2010). During insect infestation, a Pre-hevein-like protein was found to be up-regulated, however, mechanical damage did not induce such proteins (Reymond et al. Citation2000). In pigeon pea, the expression of the PR-4 gene was induced by H. armigera feeding in both ICPL 332 and ICPL-87. However, the level of the PR-4 gene was highly up-regulated in the cultivar, ICPL 332, and appeared to be mediated by both JA and SA.
The Ltp gene plays a significant role in plant responses to various kinds of stress (Hughes et al. Citation1992; Trevino and O’Connell Citation1998). The Ltp gene was reported to have membrane permeabilization effect on pathogens (Cammue et al. Citation1995; Regente et al. Citation2005). The Ltp genes are reported to be induced in response to pathogen attack and signaling molecules like JA and ET (Jung et al. Citation2003). Similarly, the Kor1 gene was reported to be regulated by pathogen attack and signaling molecules like JA and SA (López-Cruza et al. Citation2014). Kor1 gene is known to be involved in cellulose biosynthesis process and cell wall editing properties (López-Cruza et al. Citation2014).Moreover, both Ltp and kor-1 genes were also found to be up-regulated in chickpea due to insect, Helicoverpa feeding on leaves (Singh et al. Citation2008).
Thus, it was found that the defense response due to H.armigera infestation varied in susceptible (ICPL-87) and the mildly tolerant (ICPL-332) cultivar of pigeon pea. The higher levels of expression of PPO, amylase inhibitor and PR-4 and Kor-1 gene in ICPL -332 appeared to be contributing to the resistance characteristics of this cultivar. Although this is a preliminary study on pigeon pea-Helicoverpa armigera interaction, but could be useful to understand defense mechanism of pigeon pea.
Acknowledgments
We are grateful to the International Crop Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, for providing the seeds of a susceptible and a mild tolerant genotype of pigeon pea. ALM would like to thank the Department of Biotechnology (DBT), Govt of India for support in the form of the fellowship to pursue his postgraduate studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 276:945–949. doi: 10.1126/science.276.5314.945
- Arimura G, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J. 2000. Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochem Biophys Resh Comm. 277:305–310. doi: 10.1006/bbrc.2000.3672
- Armes NJ, Bond GS, Cooters RJ. 1992. The laboratory culture and development of Helicoverpa armigera. Natural Resources Institute Bulletin No. 57. Chatham (UK): Natural Resources Institute.
- Arnnok P, Ruangviriyachai C, Mahachai R, Techawongstien S, Chanthai S. 2010. Optimization and determination of polyphenol oxidase and peroxidase activities in hot pepper (Capsicum annuum L.) pericarb. Intl Food Res J. 17:385–392.
- Bergey DR, Orozco-Cardenas M, De Moura DS, Ryan CA. 1999. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proc Natl Acad Sci USA. 96(4):1756–1760. doi: 10.1073/pnas.96.4.1756
- Bhonwong A, Stout MJ, Attajarusit J, Tantasawat P. 2009. Defensive role of tomato polyphenol oxidases against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J Chem Ecol. 35:28–38. doi: 10.1007/s10886-008-9571-7
- Bonasera JM, Kim JF, Beer SV. 2006. PR genes of apple: identification and expression in response to elicitors and inoculation with Erwinia amylovora. BMC Plant Biol. 6:23–34. doi: 10.1186/1471-2229-6-23
- Cammue BP, Thevissen K, Hendriks M, Eggermont K, Goderis IJ, Proost P, Van Damme J, Osborn RW, Guerbette F, Kader JC. 1995. A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiol. 109:445–455. doi: 10.1104/pp.109.2.445
- Casaretto JA, Zúñiga GE, Corcuera LJ. 2004. Abscisic acid and jasmonic acid affect proteinase inhibitor activities in barley leaves. J Plant Physiol. 161:389–396. doi: 10.1078/0176-1617-01236
- Chakraborty U, Chakraborty N. 2005. Impact of environmental factors on infestation of tea leaves by Helopeltis theivora, and associated changes in flavonoid flavor components and enzyme activities. Phytoparasitica. 33:88–96. doi: 10.1007/BF02980930
- Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK. 2007. Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol. 145:890–904. doi: 10.1104/pp.107.103325
- Coley PD. 1980. Effects of leaf age and plant life history patterns on herbivory. Nature. 284:545–546. doi: 10.1038/284545a0
- Constabel CP, Ryan CA. 1998. A survey of wound- and methyl jasmonate-induced leaf polyphenol oxidase in crop plants. Phytochem. 47:507–511. doi: 10.1016/S0031-9422(97)00539-6
- Constabel CP, Yip L, Patton JJ, Christopher ME. 2000. Polyphenol oxidase from hybrid poplar. Cloning and expression in response to wounding and herbivory. Plant Physiol. 124:285–296. doi: 10.1104/pp.124.1.285
- Dangl JL, Dietrich RA, Thomas H. 2000. Senescence and programmed cell death. In: Buchanan B, Gruissem W, Jones R, editor. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists Press; p. 1044–1100.
- Délano-Frier JP, Martínez-Gallardo NA, Martínez-de la VO, Salas-Araiza MD, Barbosa-Jaramillo ER, Torres A, Vargas P, Borodanenko A. 2004. The effect of exogenous jasmonic acid on induced resistance and productivity in amaranth (Amaranthus hypochondriacus) is influenced by environmental conditions. J Chem Ecol. 30(5):1001–1034. doi: 10.1023/B:JOEC.0000028464.36353.bb
- De Vos M, Van Oosten VR, Van Poecke RMP. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant–Microbe Interact. 18:923–937. doi: 10.1094/MPMI-18-0923
- Duffey SS, Felton GW. 1991. Enzymatic antinutritive defenses of the tomato plant against insects. In: Hedin PA, editor. Naturally occurring pest bioregulators. Washington, DC: ACS Press; p. 167–197.
- Felton GW, Donato KK, Vecchio RJD, Duffey SS. 1989. Activation of foliar oxidases by insect feeding reduces nutritive quality of dietary protein for foliage for noctuid herbivores. J Chem Ecol. 15:2667–2694. doi: 10.1007/BF01014725
- Felton GW, Duffey SS. 1992. Avoidance of anti-nutritive plant defense: role of midgut pH in Colorado potato beetle. J Chem Ecol. 18:571–583. doi: 10.1007/BF00987820
- Fidantsef AL, Stout MJ, Thaler JS, Duffey SS, Bostock RM. 1999. Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesis-related protein P4 in the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol. 54:97–114. doi: 10.1006/pmpp.1998.0192
- Franco OL, Rigden DJ, Melo FR, Grossi-De-Sá MF. 2002. Plant alpha-amylase inhibitors and their interaction with insect alpha-amylases. Eur J Biochem. 269(2):397–412. doi: 10.1046/j.0014-2956.2001.02656.x
- Funk CJ. 2001. Alkaline phosphatase activity in white-fly salivary glands and saliva. Archives of Insect Biochem Physiol. 46:165–174. doi: 10.1002/arch.1026
- Garreton V, Carpinelli J, Jordana X, Holuigue L. 2002. The as-1 promoter element is an oxidative stress-responsive element and salicylic acid activates it via oxidative species. Plant Physiol. 130:1516–1526. doi: 10.1104/pp.009886
- Giri AP, Harsulkar AM, Deshpande VV, Sainani MN, Gupta VS, Ranjekar PK. 1998. Chickpea defensive proteinase inhibitors can be inactivated by pod borer gut proteinases. Plant Physiol. 116:393–401. doi: 10.1104/pp.116.1.393
- Hägg JF, Zagrobelny M, Bak S. 2013. Plant defense against insect herbivores. Int J Mol Sci. 14(5):10242–10297. doi: 10.3390/ijms140510242
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 125:711–717. doi: 10.1104/pp.125.2.711
- Haruta M, Pedersenb JA, Constabela CP. 2001. Polyphenol oxidase and herbivore defense in trembling aspen (Populus tremuloides): cDNA cloning, expression, and potential substrates. Physiol Plantarum. 112:552–558. doi: 10.1034/j.1399-3054.2001.1120413.x
- Hermosa MR, Turrà D, Fogliano V, Monte E, Lorito M. 2006. Identification and characterization of potato protease inhibitors able to inhibit pathogenicity and growth of Botrytis cinerea. Physiol Mol Plant Pathol. 68:138–148. doi: 10.1016/j.pmpp.2006.09.004
- Hermsmeier D, Schittko U, Baldwin IT. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 125:683–700. doi: 10.1104/pp.125.2.683
- Hughes MA, Dunn MA, Pearce RS, White AJ, Zhang L. 1992. An abscisic-acid-responsive, low temperature barley gene has homology with a maize phospholipid transfer protein. Plant Cell Environ. 15:861–865. doi: 10.1111/j.1365-3040.1992.tb02155.x
- Jabs T. 1999. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 57:231–245. doi: 10.1016/S0006-2952(98)00227-5
- Jacobson MD, Weil M, Raff MC. 1997. Programmed cell death in animal development. Cell. 88:347–354. doi: 10.1016/S0092-8674(00)81873-5
- Jung HW, Kim W, Hwang BK. 2003. Three pathogen-inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant, Cell and Environ. 26:915–928. doi: 10.1046/j.1365-3040.2003.01024.x
- Kansal R, Kumar M, Kuhar K, Gupta RN, Subrahmanyam B, Koundal KR, Gupta VK. 2008. Purification and characterization of trypsin inhibitor from Cicer arietinum L. and its efficacy against Helicoverpa armigera. Brazilian J Plant Physiol. 20(4):313–322. doi: 10.1590/S1677-04202008000400007
- Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago: University of Chicago Press.
- Kessler A, Baldwin IT. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 291:2141–2144. doi: 10.1126/science.291.5511.2141
- Kessler A, Baldwin IT. 2002. Plant responses to insect herbivory, the emerging molecular analysis. Annual Review of Plant Biol. 53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207
- Khokon AR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y. 2011. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34:434–443. doi: 10.1111/j.1365-3040.2010.02253.x
- Kluh I, Horn M, Hýblová J, Hubert J, Dolečková-Marešová L, Voburka Z. 2005. Inhibitory specificity and insecticidal selectivity of α-amylase inhibitor from Phaseolus vulgaris. Phytochem. 66(1):31–39. doi: 10.1016/j.phytochem.2004.11.001
- Koornneef A, Pieterse CMJ. 2008. Cross talk in defense signaling. Plant Physiol. 146:839–844. doi: 10.1104/pp.107.112029
- Korth KL, Dixon RA. 1997. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol. 115:1299–1305. doi: 10.1104/pp.115.4.1299
- Kumari AD, Reddy DJ, Sharma HC. 2006. Effect of Grain yield in pigeonpea genotypes with different levels of resistance to the pod borer, Helicoverpa armigera. Indian J of Plant Protech. 34(2):184–187.
- Kunkel BN, Brooks DM. 2002. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 5:325–331. doi: 10.1016/S1369-5266(02)00275-3
- Lamb C, Dixon RA. 1997. The oxidative burst in plant disease resistance. Ann Rev Plant Physiol Plant Mol Biol. 48:251–275. doi: 10.1146/annurev.arplant.48.1.251
- Lawrence SD, Novak NG, Ju CJ, Cooke JE. 2008. Potato, Solanum tuberosum, defense against Colorado potato beetle, Leptinotarsa decemlineata (Say): microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. J Chem Ecol. 34:1013–1025. doi: 10.1007/s10886-008-9507-2
- Lee S, Kim SG, Park CM. 2010. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 188:626–637. doi: 10.1111/j.1469-8137.2010.03378.x
- Li JT, Qiu ZB, Zhang XW, Wang LS. 2011. Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol Plant. 33:835–842. doi: 10.1007/s11738-010-0608-5
- Liu Z, Zhang Z, Faris JD, Oliver RP, Syme R, McDonald MC, McDonald BA, Solomon PS, Lu S, Shelver WL, et al. 2012. The cysteine rich nechrotrophic effector SnTox1 produced by Stagnospora noduram triggers susceptibility of wheat lines harboring Snn1. PLos Pathog. 8(1):e1002467. doi: 10.1371/journal.ppat.1002467
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. 1999. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 119:1091–1100. doi: 10.1104/pp.119.3.1091
- López-Cruza J, Finitia I, Fernández-Crespob E, Crespo-Salvadora O, García-Agustínb P, González-Boscha C. 2014. Absence of endo-1,4-β-glucanase KOR1 alters the Jasmonate-dependent defense response to Pseudomonas syringae in Arabidopsis. Journal of Plant Physiol. 171(16):1524–1532. doi: 10.1016/j.jplph.2014.07.006
- Maffei ME, Mithöfer A, Boland W. 2007. Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release. Phytochemistry. 68:2946–2959. doi: 10.1016/j.phytochem.2007.07.016
- Major IT, Constabel CP. 2006. Molecular analysis of poplar defense against herbivory: comparison of wound- and insect elicitor-induced gene expression. New Phytol. 172:617–635. doi: 10.1111/j.1469-8137.2006.01877.x
- Majumder ND, Singh F. 2005. Pigeonpea improvement in India. Souvenir, 4th international food legume research conference on food legumes for nutritional security and sustainable agriculture; Oct 18–22; New Delhi (India). p. 53–65.
- Mattiacci L, Dicke M, Posthumus MA. 1995. Beta-glucosidase: an elicitor of herbivore-induced plant odor that attracts host searching parasitic wasps. Proc Natl Acad Sci USA. 92:2036–2040. doi: 10.1073/pnas.92.6.2036
- Mayer AM. 1986. Polyphenol oxidases in plants-recent progress. Phytochem. 26:11–20. doi: 10.1016/S0031-9422(00)81472-7
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. 1997. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 94:5473–5477. doi: 10.1073/pnas.94.10.5473
- Mozuruk J, Hunnicutt LE, Cave RD, Hunter WB, Bauscher MG. 2006. Profiling transcriptional changes in Citrus sinensis (L.) Osbeck challenged by herbivory from the xylem feeding leaf hopper Homalodisca coagulata (Say) by cDNA macroarray analysis. Plant Sci. 170:1068–1080. doi: 10.1016/j.plantsci.2006.01.014
- Nafie E, Hathout T, Mokadem ASA. 2011. Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. Cells. Braz J Plant Physiol. 23(2):161–174. doi: 10.1590/S1677-04202011000200008
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA. 2001. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 13:179–191. doi: 10.1105/tpc.13.1.179
- Orozco-Cárdenas ML, Ryan CA. 1999. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 96:6553–6557. doi: 10.1073/pnas.96.11.6553
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cell. Nature. 406:731–734. doi: 10.1038/35021067
- Peng JY, Li ZH, Xiang H, Huang JH, Jia SH, Miao XX, Haung YP. 2005. Preliminary studies on differential defense responses induced during plant communication. Cell Res. 15:187–192. doi: 10.1038/sj.cr.7290285
- Rakwala R, Agrawala GK, Jwa NS. 2001. Characterization of a rice (Oryza sativa L.) Bowman Birk proteinase inhibitor: tightly light regulated induction in response to cut, jasmonic acid, ethylene and protein phosphatase 2A inhibitors. Gene. 263:189–198. doi: 10.1016/S0378-1119(00)00573-4
- Regente MC, Giudici AM, Villalain J, De la Canal L. 2005. The cytotoxic properties of a plant lipid transfer protein involve membrane permeabilization of target cells. Lett Appl Microbiol. 40:183–189. doi: 10.1111/j.1472-765X.2004.01647.x
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. 2004. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell. 16:3132–3147. doi: 10.1105/tpc.104.026120
- Reymond P, Weber H, Damond M, Farmer EE. 2000. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 12:707–720. doi: 10.1105/tpc.12.5.707
- Ryan CA. 1990. Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Ann Rev Phytopathol. 28:425–449. doi: 10.1146/annurev.py.28.090190.002233
- Sharma OP, Gopali JB, Yelshetty S, Bambawale OM, Garg DK, Bhosle BB. 2010. Pests of pigeon pea and their management-NCIPM-LBS Building-IARI-New Delhi-India.
- Singh A, Singh IK, Verma PK. 2008. Differential transcript accumulation in Cicer arietinum L. in response to a chewing insect Helicoverpa armigera and defense regulators correlate with reduced insect performance. J Exp Bot. 59(9):2379–2392. doi: 10.1093/jxb/ern111
- Tamhane VA, Chougule NP, Giri AP, Dixit AR, Sainani MN, Gupta VS. 2005. In vivo and In vitro effect of Capsicum annum proteinase inhibitors on Helicoverpa armigera gut proteinases. Biochimica Biophysica Acta. 1722:156–167. doi: 10.1016/j.bbagen.2004.12.017
- Thipyapong P, Steffens JC. 1997. Tomato polyphenol oxidase – differential response of the polyphenol oxidase F promoter to injuries and wound signals. Plant Physiol. 115:409–418. doi: 10.1104/pp.115.2.409
- Thomma B, Eggermont K, Pennickx I, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. 1998. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 95:15107–15111. doi: 10.1073/pnas.95.25.15107
- Trevino MB, O’Connell MA. 1998. Three drought-responsive members of the nonspecific lipid-transfer protein gene family in Lycopersicon pennellii shows different developmental patterns of expression. Plant Physiol. 116:1461–1468. doi: 10.1104/pp.116.4.1461
- Valderrama R, Corpas FJ, Carreras A, Gómez-Rodríguez MV, Chaki M, Pedrajas JR, Fernández-Ocaña A, Del Río LA, Barroso JB. 2006. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 29(7):1449–1459. doi: 10.1111/j.1365-3040.2006.01530.x
- Verhage A, Van Wees SCM, Pieterse CMJ. 2010. Plant immunity: it’s the hormones talking, but what do they say? Plant Physiol. 154:536–540. doi: 10.1104/pp.110.161570
- Wang X, Hu LC, Zhou GX, Cheng JA, Lou YG. 2011. Salicylic acid and ethylene signaling pathways are involved in production of rice trypsin proteinase inhibitors induced by the leaf folder Cnaphalocrocis medinalis (Guenée). Chinese Sci Bull. 56(22):2351–2358. doi: 10.1007/s11434-011-4568-y
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signal Behavior. 7(10):1306–1320. doi: 10.4161/psb.21663
- War AR, Paulraj MG, War MY, Ignacimuthu S. 2011. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal Behavior 6(11):1787–1792. doi: 10.4161/psb.6.11.17685
- Winz RA, Baldwin IT. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. Part IV. Insect-induced ethylene reduces its jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol. 125:2189–2202. doi: 10.1104/pp.125.4.2189
- Zhang Y, Xu S, Din P, Wang D, Cheng YT, He J, Gao M, Xu F, Li Y, Zhu Z, et al. 2010. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc Natl Acad Sci USA. 107(42):18220–18225. doi: 10.1073/pnas.1005225107
- Zhua YC, Abel CA, Chen MS. 2007. Interaction of Cry1Ac toxin (Bacillus thuringiensis) and proteinase inhibitors on the growth, development, and midgut proteinase activities of the bollworm, Helicoverpa zea. Pest Biochem and Physiol. 87:39–46. doi: 10.1016/j.pestbp.2006.05.004