ABSTRACT
Ambient temperatures influence plant growth and development, however very little is known about changes in root growth in response to ambient temperature change. Here, we performed transcriptome profiling and compared the differences in gene expression at lower and higher temperatures compared with normal plant growth temperatures. Our analysis of the biological processes and molecular functions regulated by differentially expressed genes revealed that low temperature upregulated carbohydrate metabolism and transmembrane transport, and downregulated signal transduction and defense. High temperature upregulated metabolic processes, transport, and auxin biosynthesis, and downregulated catabolic processes. We found that increased temperature specifically affected the levels of Arabidopsis response regulators, ARR1 and ARR12, to decrease cytokinin signaling, altered the level of the brassinosteroid receptor BRI1 to downregulate brassinosteroid signaling, and changed the level of the gibberellin receptor DELLA to upregulate gibberellin signaling and mediate root elongation. These data contribute to our knowledge of how root growth adapts to elevated ambient temperature under climate warming.
1. Introduction
Global warming can have significant impacts on plant growth, development, and distribution (Hedhly et al. Citation2009; Körner and Basler Citation2010; Campitelli and Simonsen Citation2012; Liu et al. Citation2012; Huang et al. Citation2017; Nolan et al. Citation2018). Many experiments on plant adaptation to global warming are based on the prediction from the Intergovernmental Panel on Climate Change (IPCC) that the soil temperature will increase by 2°C by the end of the century (Stocker et al. Citation2013; Fang et al. Citation2017). Soil temperature has a significant effect on nutrient status, leaf length and number, stem length, and root length (Walker Citation1969). However, little is known about how plant roots adapt to ambient temperature.
Our recent study found that higher temperatures (27°C) could promote CKRC1-dependent auxin biosynthesis by enhancement of the ethylene signaling mediated by ETR1 (ETHYLENE RESPONSE1), thus maintaining normal downward root growth at higher temperatures (Fei et al. Citation2017). Comparison of the regulatory mechanisms of ckrc1-1 at three temperatures (17°C, 22°C, and 27°C) revealed temperature-dependent differences in the response patterns of in vivo signals (auxin and ethylene), the interactions between signals (auxin and ethylene), and the effects of related signals (genes) (Fei et al. Citation2019). Related results suggest complex root adaptation mechanisms at different temperatures. In addition to auxin and ethylene, recent studies have found that another plant hormone, brassinosteroid, is also involved in Arabidopsis root elongation at high temperature (Martins et al. Citation2017). However, the potential contribution of other hormones to root adaptation to higher temperature remains to be determined.
In order to better understand changes in roots in response to ambient temperature changes, we compared the transcriptome of Arabidopsis roots grown at three different ambient temperatures (17°C, 22°C, and 27°C). In experiments of Arabidopsis growth, 27°C is usually selected for high temperature experiments and 17°C is selected for low temperature experiments (Edwards et al. Citation2006; Gould et al. Citation2006; Wigge Citation2013; Ibañez et al. Citation2017; Kim et al. Citation2017a; Gyula et al. Citation2018). The differentially expressed genes were identified and then analyzed based on gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of annotated genes, revealing differential biological responses and transcription profiles. Additionally, root growth was analyzed in mutant strains with changes in hormone-related genes. These data contribute to our understanding of how root growth can adapt to warmer soil temperature conditions that are possible with climate warming.
2. Materials and methods
2.1. Plant material and growth conditions
All Arabidopsis mutants were tested in the Col-0 background. Mutants arr1-4, arr10-5, arr12-1, and the arr1,12 double mutant were provided by Guo Guangqin (Lanzhou University); rga28 was provided by Hou Suiwen (Lanzhou University); bri1-301 was provided by Li jia (Lanzhou University).
Seeds were treated at 4°C for three days in water and then sterilized with 0.1% (w/v) HgCl2 before placing on MS medium (Murashige and Skoog Basal Medium with Vitamins). Plants were cultivated at 16 h light/8 h dark cycle with a light intensity of 18 μmol m−2 s−1. Seedlings were grown vertically in a growth cabinet at different temperatures (27°C, 22°C, or 17°C, with temperature fluctuation ± 0.5°C). Plants were maintained at the same temperature for the duration of the experiment. For all comparisons, the mean was calculated for three separate experiments (from three ager plates, with approximately 28 seeds on each plate).
2.2. RNA library construction and sequencing
Total RNA was extracted from seedling roots using Trizol reagent (Sangon) according to the manufacturer’s instructions. RNA degradation and contamination were monitored by separating samples on 1% agarose gels. RNA purity was checked using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). RNA concentration was measured using the Qubit® RNA Assay Kit in Qubit®2.0 Flurometer (Life Technologies, CA USA). RNA integrity was assessed using the RNA Nano 6000Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA USA).
A total amount of 3 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA) following the manufacturer’s recommendations. The final cDNA library was enriched by PCR. Finally, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina HiSeq platform and 125 bp/150 bp paired-end reads were generated.
2.3. Differentially expressed gene analysis
Differential expression analysis of temperature conditions was performed using the edgeR package in R. The P values were adjusted using the Benjamini Hochberg method (Klipper-Aurbach et al. Citation1995). A corrected P-value of 0.05 and absolute fold change of 2 were set as the thresholds for significant differential expression. Next, GO enrichment analysis of differentially expressed genes was implemented using the cluster Profiler R package, in which gene length bias was corrected. We then used the cluster Profiler R package to test the statistical enrichment of differentially expressed genes in KEGG pathways.
2.4. Quantitative real-time PCR analysis
Roots of 9-day-old Arabidopsis seedlings were removed with a scalpel, immediately frozen in liquid nitrogen, and stored at −80°C. RNA was isolated using Trizol reagent (Sangon) and reverse-transcribed into cDNA using a reverse transcription kit (DRR047A; Takara).
Quantitative RT-PCR was performed using a Bio-Rad CFX96 Real-time System (Bio-Rad) and Power SYBR green chemistry (DRR081A; Takara). There were 3 biological repetitions of the experiment, and three technical replicates of each biological sample. The primer sequences are listed in Table S1.
3. Results
3.1. Transcriptome profiling
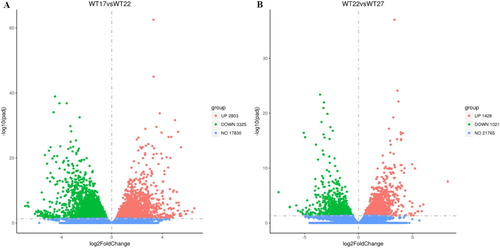
Our previous research suggests complex mechanisms by which roots adapt to different ambient temperatures (Fei et al. Citation2017, Citation2018). To comprehensively study the transcriptome and assess differential gene expression in roots in response to ambient temperature changes, wild-type (wt) seedling roots were grown in different ambient temperatures (17°C, 22°C, and 27°C) and were sequenced. The overall distribution of genes expressing significant differences was analyzed via volcano plot, revealing 2803 up-regulated genes and 3325 down-regulated genes at 17°C vs 22°C ((A)), and 1428 up-regulated genes and 1021 down-regulated genes at 22°C vs 27°C (|log2(FoldChange)| > 1 & q value < 0.05) ((B)). Significant changes in the expression levels of so many genes suggest that variations in ambient temperature can confer an extensive regulatory effect on plant root growth.
3.2. Low temperature-regulated transcripts
3.2.1. Up-regulated transcripts at low temperature compared to normal temperature
Differentially expressed genes were sorted according to biological process domain and the results showed that low temperature up-regulated expression of genes involved in carbohydrate metabolic processes (e.g. polysaccharide, glucan, and xyloglucan metabolic processes, glycolytic processes, and cofactor metabolic processes), transmembrane transport processes (e.g. organic acid, carboxylic acid, and anion transmembrane transport) and cell wall metabolic processes (e.g. cell wall organization or biogenesis and cell wall polysaccharide metabolic process). When classified according to molecular function, significantly up-regulated genes were involved in enzymatic activity (e.g. transferase, hydrolase, oxidoreductase, and lyase activity) and transporter activity (e.g. anion, carboxylic acid, and organic acid transmembrane transporter activity). Genes with increased expression played important roles in carbon metabolism, glycolysis/gluconeogenesis pathways, and amino acids biosynthesis pathways, according to KEGG enrichments (). Adaptation of plants to low temperature relies on many biological processes, such as utilization of energy resources and effective substrate transport systems (Mykytczuk et al. Citation2013). Carbohydrate metabolism, including polysaccharide, glucan, and glycolysis processes, is required to maintain cellular energy metabolism at low temperature to compensate for a low growth rate (Bakermans and Nealson Citation2004). The transcriptome results also showed enhanced carbohydrate metabolic processes, such as increased amounts of AT5G20280, encoding sucrose-phosphate synthase (SPS), a gene that was previously shown to be induced by cold temperature (Sun et al. Citation2015), AT3G44990, and AT1G65310. Transcript levels in carbohydrate metabolism increased to compensate for potential nutrient limitations at lower temperatures (Ghobakhlou et al. Citation2015). Transcriptional levels corresponding to transmembrane transport processes increased, such as AT2G37460 (encoding a nodulin MtN21-like transporter family protein), AT5G12860 (encoding a dicarboxylate transporter), AT2G41190, and AT1G25530 (encoding a transmembrane amino acid transporter family protein). Low temperature inhibited plant root growth (Zhu et al. Citation2015; Nagelmüller et al. Citation2017), as changes in the organization or biogenesis of cell wall compounds possibly restricted cell elongation. Xyloglucan endohydrolase 31 (XTH31), encoded by AT3G44990, showed significantly increased levels to modulate cell wall xyloglucan content (Table S2).
Table 1. GO and KEGG classification of low temperature-regulated transcripts.
3.2.2. Down-regulated transcripts at low temperature compared to normal temperature
Low temperature negatively regulated expression of genes involved in signal transduction (e.g. response to salicylic acid and jasmonic acid, and the abscisic acid-activated signaling pathway), defense processes (e.g. response to fungi and bacteria,) and rhythm processes (e.g. photoperiodism) when sorted according to biological process domain. When classified according to molecular function, enzymatic activity (e.g. phospholipase activity and phosphatase activity), and signal factor activity (e.g. signal transducer activity and receptor activity) exhibited negative correlation. KEGG enrichment analysis showed that differentially expressed genes were involved in the endocytosis pathway and the plant-pathogen interaction pathway (). Previous work showed changes in salicylic acid immunity pathway genes after exposure to low temperature and pathogen infection and jasmonic acid-dependent signaling is typically activated in response to pathogens (Pieterse et al. Citation2009; Martínez-Medina et al. Citation2017; Kim et al. Citation2017b). Consistent with those results, we observed significantly down-related expression of salicylic acid and jasmonic acid in response to low temperature treatment, leading to weakened defenses. For example, HR4 (AT3G50480 (Sáenzmata and Jiménezbremont Citation2012)) is involved in salicylic acid and jasmonic acid signaling and was down-regulated. Exogenous abscisic acid may enhance freeze tolerance (Liu et al. Citation2013; Vishwakarma et al. Citation2017). In this study, genes in the abscisic acid-activated signaling pathway (e.g. AT2G38310 and AT4G37790) were down-regulated at low temperature. Additionally, cold temperatures can disrupt circadian rhythms in many organisms (Murayama et al. Citation2017). Circadian 1 (CIR1) is involved in circadian regulation in Arabidopsis (Zhang et al. Citation2010), and our results indicate significant down-regulation of CIR1(AT5G37260) upon exposure to low temperature, so low temperature could regulate roots growth via rhythm processes (Table S2).
Overall, our results indicated that carbohydrate metabolic processes were up-regulated by low temperature to maintain cellular energy metabolism, and that salicylic acid and jasmonic acid signal transduction and defense processes were down-regulated by low temperature, resulting in weakened defenses to fungal or bacterial attack.
3.3. High temperature-regulated transcripts
3.3.1. Up-regulated transcripts by high temperature compared to normal temperature
GO analysis based on of biological process domain classification revealed that the genes that were up-regulated at high temperature were principally involved in metabolic processes (e.g. glycosinolate, glucosinolate, and glycosyl compound biosynthetic processes and secondary metabolic processes), transport processes (e.g. anion, organic acid, carboxylic acid, and malate transport), and auxin biosynthesis and auxin-activated signaling pathways. When sorted on the basis of the molecular function domain, most genes were involved in transporter activity (e.g. anion and organic acid transporter activity) and protein domain specific binding. Differentially expressed genes were significantly enriched in the photosynthesis, glucosinolate biosynthesis, and circadian rhythm pathways according to KEGG enrichments (). Glucosinolate plays roles in plant defense against bacterial and fungal pathogens (Ludwig-Müller et al. Citation2000). AT1G62540 and AT2G43100 were both up-regulated and are involved in the glycosinolate metabolic process. Previous studies reported that enhancement of glycosyl compound biosynthetic processes and secondary metabolic process may indicate accelerated biological processes and structure stabilization in response to high temperature (Dixon and Paiva Citation1995; Oh et al. Citation2009; Dixon et al. Citation2010). Cellular phospholipids may affect vesicular trafficking (Mcmahon and Gallop Citation2005), autophagy (Holland et al. Citation2016), and membrane secretion (Monteiro et al. Citation2005). These conclusions agreed with our results showing accelerated metabolic processes at higher temperature. In particular, transport processes regulated by genes (e.g. AT1G08430, AT1G51340, AT1G17840 and AT1G25530) were enhanced. High temperature can also promote flowering and modulate the circadian clock (Liu et al. Citation2015). Our results also showed that the transcription abundance of AT1G22770 was upregulated to promote flowering under long days in a circadian clock-controlled flowering pathway. The observed increase in auxin biosynthesis and the auxin-activated signaling pathway under high temperature can promote fertility to maintain good crop yields under global warming (Atsushi Citation2013). In roots adapting to high temperature response, these changes of auxin biosynthesis and the auxin-activated signaling pathway also included several significantly up-regulated genes (e.g. AT1G16400, AT5G12330, AT4G36260, AT5G16530, and AT1G19790) (Table S2).
Table 2. GO and KEGG classification of high temperature-regulated transcripts.
3.3.2. Down-regulated transcripts by high temperature compared to normal temperature
Classification on the basis of biological process domain revealed that genes that were downregulated in response to exposure to high temperature were involved principally in catabolic processes (e.g. protein ubiquitination, proteasomal protein) and rhythmic processes. When sorted on the basis of molecular function domain, most genes were related to enzyme activity (e.g. ubiquin/ubiquitin-like protein ligase activity) and phospholipid binding. Differentially expressed genes were significantly enriched in valine, leucine, and isoleucine degradation and protein processing in endoplasmic reticulum pathways according to KEGG enrichments (). The plant ubiquitination pathway is involved in the regulation of morphogenesis, the circadian clock, and response to hormone or pathogen signal molecules (Nelson et al. Citation2000; Osterlund et al. Citation2000; Stone et al. Citation2006; Rosebrock et al. Citation2007; Takahashi et al. Citation2009). Ubiquitylation and proteasome-mediated ubiquitin-dependent protein catabolic processes are required for protein ubiquitination and degradation (Callis and Vierstra Citation2000). Previous work found that U-box E3 ubiquitin ligases AtCHIP and PLANT U-BOXs (PUBs) are involved in temperature stress tolerance and the AtCHIP transcript was upregulated by high temperature (Yan et al. Citation2003; Cho et al. Citation2006; Min et al. Citation2016; Byun et al. Citation2017). We found that elevated ambient temperature had the same effect on AtCHIP (AT3G07370) expression, and that genes encoding RING/U-box superfamily proteins involved in protein ubiquitination (e.g. AT3G18710 (PUB29) AT5G37490 (PUB21), AT3G11840 (PUB24), AT1G60190 (PUB19), AT2G35930 (PUB23), AT5G55970, AT1G14200, AT1G05880, and AT1G55530) were dominantly downregulated in response to high temperature treatment. Previous studies showed increased valine, leucine, and isoleucine degradation in extended darkness to compensate for limits in respiration (Däschner et al. Citation2001; Binder Citation2010; Schertl et al. Citation2017). The downregulated genes involved in the valine, leucine, and isoleucine degradation pathway (e.g. AT3G06850, AT1G03090, AT1G21400, and AT1G10070) may indicate that the availability of carbohydrates for respiration was less limited at higher temperature. Expression of BT2 (AT3G48360), a regulator of circadian rhythm, was also downregulated and may influence multiple biological processes (Table S2). BT2 also participates in hormone signaling transduction, nutrient status, and stress responses (Mandadi et al. Citation2009). Therefore, high temperature also could regulate roots growth via rhythm processes.
Analysis of differentially expressed genes under high temperature suggested that glycosyl compound biosynthetic processes and secondary metabolic processes were enhanced by high temperature and accelerated biological processes and promoted structure stabilization to strengthen defense. Additionally, the protein ubiquitination pathway in roots was downregulated by high temperature.
3.4. Identification of hormone-related genes regulated at elevated ambient temperature
Interactions of plant hormones constitute a complex regulation network to respond to ambient temperature. Previous studies have shown that auxin, ethylene, cytokinin, and brassinosteroid hormones are involved in ambient temperature-mediated root growth (Zhu et al. Citation2015; Fei et al. Citation2017; Martins et al. Citation2017). And phenotypes of ga3ox1/2 and ga20ox1/2 mutants producing reduced gibberellin levels and signaling have been shown to contribute to plant tolerance exposure to abiotic stresses, while ga2ox mutant producing high gibberellin levels can’t survive under same condition (Colebrook et al. Citation2014). To confirm that GA signaling also engages in ambient temperature regulation, transcript abundance of gibberellin biosynthetic and metabolic genes was compared. The KEGG analysis and functional annotation results indicated higher levels of plant hormone signal transduction at 27°C compared to 22°C (). To analyze hormone-signaling response to elevated ambient temperature in roots, we compared the relative expression levels of hormone-related genes (). When transcript abundance of seedling roots was analyzed at 27°C and compared to that at 22°C, gibberellin biosynthesis genes (GA3OX2, GA2OX1, and GA2OX4) were upregulated and genes encoding DELLA proteins (RGL3 and RGA) were downregulated. Cytokinin biosynthesis genes (LOG1, LOG7, and CYP735A2) were downregulated, degradation genes (CKX4 and CKX7) were upregulated, and signaling genes (AHK2, ARR1, ARR2, ARR4, ARR7, and ARR12) were downregulated. Brassinosteroid signaling genes (BRI1, BSK3) were also downregulated. Overall, the results suggest that DELLA-mediated gibberellin signaling may enhance response to elevated ambient temperature, and that ARR1 and ARR12-mediated cytokinin and BRI1-mdiated brassinosteroid signaling may reduce this response.
Table 3. Identification of hormone-related genes elevated ambient temperature.
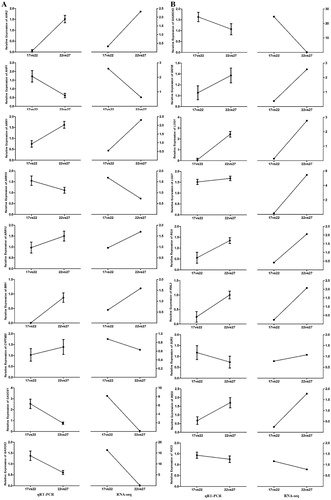
To verify the accuracy and repeatability of the RNA-seq results, 18 genes related to hormones were selected for qRT-PCR analysis. The results indicated that the relative variation of genes treated at different temperatures was highly consistent with the variation observed by sequencing ((A,B)). Therefore, the transcriptome profiling data can be used to investigate gene expression patterns and compare differentially expressed genes.
3.5. Phenotypes of mutant response to elevated ambient temperatures
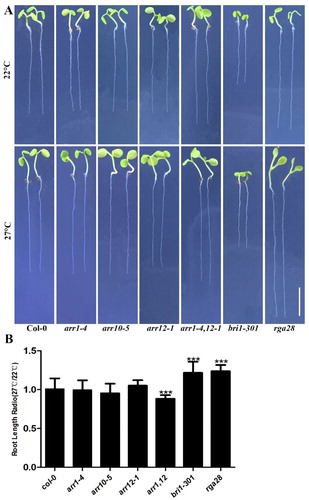
To further characterize hormone response to elevated ambient temperature, we dissected the phenotypes of cytokinin, gibberellin, and brassinosteroid-related mutants at 22°C and 27°C. A previous report showed that BRI1-dependent brassinosteroid signaling is involved in root response to prolonged elevated ambient temperature (Martins et al. Citation2017). The root growth of arr1-4, arr10-5, arr12-1, arr1,12, bri1-301 and rga28 mutants were analyzed at both 22°C and 27°C ((A,B)). Root growth in bri1-301 and rga28 was more sensitive to elevated ambient temperatures than that of wild-type plants, and arr1,12 was less sensitive to elevated ambient temperatures than that of wild-type plants, as revealed by the ratio of the root lengths of plants grown at 27°C and 22°C ((B)). This is consistent with our transcriptome data that cytokinin, gibberellin, and brassinosteroid hormones have important effects on root response to elevated ambient temperature.
Figure 3. Phenotypes of mutant response to different ambient temperatures. (A) Seedlings were grown on vertical MS plates at 22°C, and 27°C for 7 days. Bar = 5 mm. (B) Relative ratios of root lengths in mutants grown under the same conditions. (n = 25–50; P < 0.001, based on t-test).
Besides, previous reports showed that arr1-3 arr12-1 was less sensitive to low temperature than wild-type seedlings (Zhu et al. Citation2015). To analyze how bri1-301 and rga28 respond to low temperatures, we also observed the phenotype of these mutants at low temperatures (17°C). The result revealed that the root length of bri1-301 was less sensitive to low temperatures than that of wild-type plants, and that rga28 was more sensitive to low temperatures than that of wild-type plants, as revealed by the ratio of the root lengths of corresponding lines grown at 17°C and 22°C (Figure S1(A,B)). Therefore, we speculate that different hormones respond differently to different ambient temperatures.
4. Discussion
Overall, roots show differential response to low and high temperature. Low temperature enhanced carbohydrate metabolism to maintain cellular energy metabolism and compensate for nutrient limitations (Bakermans and Nealson Citation2004; Ghobakhlou et al. Citation2015) and inhibited root growth (Zhu and Geisler Citation2015; Nagelmüller et al. Citation2017). In contrast, high temperature increased glycosyl compound biosynthetic processes and secondary metabolic processes to facilitate stabilization (Dixon and Paiva Citation1995; Oh et al. Citation2009; Dixon et al. Citation2010). Therefore, enhanced defense against bacterial and fungal pathogens at high temperature was required for plant roots, and low temperature decreased defenses with reduction of salicylic acid and jasmonic acid signals. The root response patterns to low and high temperature also revealed similar regulation of biological processes, with changes in expression of genes involved in transport processes (anion, organic acid, and carboxylic acid transport) and circadian rhythm. Lower and higher temperature conditions both alter gene expression to influence circadian rhythm in roots. It was shown previously that auxin and ethylene are involved in root growth regulated by temperature (Fei et al. Citation2017, Citation2018), and that increased temperature downregulated brassinosteroid signaling mediated by BRI1 to regulate root growth (Martins et al. Citation2017). Our data are consistent with brassinosteroid signaling reducing the response to elevated ambient temperature. ARR1 and ARR12-mediated cytokinin signaling was reduced under continuous high temperature treatment, the root growth of arr1,12 was inhibited at 27°C compared to 22°C, gibberellin signaling was enhanced by elevated ambient temperature, and the root growth of rga28 was promoted at 27°C compared to 22°C. Overall, our results show extensive regulation of hormones at elevated ambient temperature during root growth.
5. Conclusion
Plant roots efficiently uptake water and mineral nutrients from soil (Augstein and Carlsbecker Citation2018). Ambient temperature regulates physiological and biochemical processes in plant roots by influencing the soil microenvironment. Through transcriptome sequencing analysis, we found changes in regulatory mechanisms at different ambient temperatures.
Understanding the molecular mechanisms of plant root function and adaption provide insight into how plants adapt to environmental temperature change. Global warming between the Last Glacial Maximum (LGM) and the early Holocene (10,000 years before the present) was on the order of 4–7°C, and warming is predicted to continue (Annan and Hargreaves Citation2013; Massondelmotte and Schulz Citation2013; Stocker et al. Citation2013; Nolan et al. Citation2018). Thus, it is important to learn how plant roots respond to changes in ambient temperatures. Our results indicate that temperature variations of only a few degrees have significant effects on gene expression in plant roots, indicating complex responses through multiple mechanisms. Comparison of the changes in biological processes and hormonal functions at different temperatures should provide insight into how plants adapt to global temperature changes. It is becoming increasingly important to see what the future of agriculture, phenology, and ecology might look like by understanding the effects of slightly higher ambient temperatures on plant physiology.
Author contributions
X.F.L. designed the experiments; Q.H.F. performed most of the experiments, interpreted data, generated figures, and wrote the manuscript; others performed some of the experiments.
Acknowledgments
This work was supported by grants from the Chinese National Science Foundation (31201062), Fund for Fostering Talents in Basic Science of the National Natural Science Foundation of China (J1210077, J1210033, and J110350), the Fundamental Research Funds for the Central Universities (lzujbky-2018-109 and lzujbky-2019-it14), and the foundation of the Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations (lzujbky-2018-kb05). The funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Thanks to the Core Facility of the School of Life Sciences, Lanzhou University. Prof Guangqin Guo, Li jia, and Suiwen Hou provided necessary materials.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Xiaofeng Li http://orcid.org/0000-0003-3017-0855
Additional information
Funding
Notes on contributors
Qionghui Fei
Qionghui Fei is graduate student of Lanzhou University. Fei is a member of Xiaofeng Li’s team.
Liyuan Liang
Liyuan Liang is graduate student of Lanzhou University. Liang is a member of Xiaofeng Li’s team.
Fan Li
Fan Li is graduate student of Lanzhou University. Li is a member of Xiaofeng Li’s team.
Li Zhang
Li Zhang is graduate student of Lanzhou University. Zhang is a member of Xiaofeng Li’s team.
Youxia Li
Youxia Li is graduate student of Lanzhou University. Li is a member of Xiaofeng Li’s team.
Yuman Guo
Yuman Guo is graduate student of Lanzhou University. Guo is a member of Xiaofeng Li’s team.
Lei Wu
Lei Wu is graduate student of Lanzhou University. Wu is a member of Xiaofeng Li’s team.
Xueqin Meng
Xueqin Meng is graduate student of Lanzhou University. Meng is a member of Xiaofeng Li’s team.
Huanhuan Gao
Huanhuan Gao is graduate student of Lanzhou University. Gao is a member of Xiaofeng Li’s team.
Xiaofeng Li
Xiaofeng Li is Assistant Professor at Lanzhou University in the Department of cell biology. His research examines the interaction between plant endogenous hormones and environmental ambient temperature. His research aims to reveal how ambient temperature changes regulate plant growth and development.
References
- Annan JD, Hargreaves JC. 2013. A new global reconstruction of temperature changes at the Last Glacial Maximum. Clim Past. 9(1):367–376. doi: 10.5194/cp-9-367-2013
- Atsushi H. 2013. High temperature injury and auxin biosynthesis in microsporogenesis. Front Plant Sci. 4:47.
- Augstein F, Carlsbecker A. 2018. Getting to the roots: a developmental genetic view of root anatomy and function from Arabidopsis to lycophytes. Front Plant Sci. 9:1410. doi:10.3389/fpls.2018.01410.
- Bakermans C, Nealson KH. 2004. Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobacter cryopegella. J Bacteriol. 186:2340–2345. doi:10.1128/jb.186.8.2340-2345.2004.
- Binder S. 2010. Branched-chain amino acid metabolism in Arabidopsis thaliana. Arabidopsis Book. 8:e0137. doi: 10.1199/tab.0137
- Byun MY, Cui LH, Oh TK, Jung YJ, Lee A, Park KY, Kang BG, Kim WT. 2017. Homologous U-box E3 ubiquitin ligases OsPUB2 and OsPUB3 are involved in the positive regulation of low temperature stress response in rice (Oryza sativa L.). Front Plant Sci. 8:16. doi: 10.3389/fpls.2017.00016
- Callis J, Vierstra RD. 2000. Protein degradation in signaling. Curr Opin Plant Biol. 3:381–386. doi: 10.1016/S1369-5266(00)00100-X
- Campitelli BE, Simonsen AK. 2012. Plant evolutionary ecology: molecular genetics, global warming and invasions, and the novel approaches we are using to study adaptations. New Phytol. 196:975–977. doi: 10.1111/nph.12028
- Cho SK, Chung HS, Ryu MY, Park MJ, Lee MM, Bahk Y-Y, Kim J, Pai HS, Kim WT. 2006. Heterologous expression and molecular and cellular characterization of CaPUB1 encoding a hot pepper U-Box E3 ubiquitin ligase homolog. Plant Physiol. 142:1664–1682. doi:10.1104/pp.106.087965.
- Colebrook EH, Thomas SG, Phillips AL, Hedden P. 2014. The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol. 217:67–75. doi:10.1242/jeb.089938.
- Däschner K, Couée I, Binder S. 2001. The mitochondrial isovaleryl-coenzyme a dehydrogenase of Arabidopsis oxidizes intermediates of leucine and valine catabolism. Plant Physiol. 126:601–612. doi:10.1104/pp.126.2.601.
- Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, Wang L. 2010. The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol. 3:371–390. doi: 10.1046/j.1364-3703.2002.00131.x
- Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7:1085. doi: 10.2307/3870059
- Edwards KD, Anderson PE, Hall A, Salathia NS, Locke JCW, Lynn JR, Straume M, Smith JQ, Millar AJ. 2006. FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell. 18:639–650. doi: 10.1105/tpc.105.038315
- Fang C, Li F, Pei J, Ren J, Gong Y, Yuan Z, Ke W, Zheng Y, Bai X, Ye JS. 2017. Impacts of warming and nitrogen addition on soil autotrophic and heterotrophic respiration in a semi-arid environment. Agric For Meteorol. 248:449–457. doi: 10.1016/j.agrformet.2017.10.032
- Fei Q, Li J, Luo Y, Ma K, Niu B, Mu C, Gao H, Li X. 2018. Plant molecular responses to the elevated ambient temperatures expected under global climate change. Plant Signal Behav. 13:e1414123. doi:10.1080/15592324.2017.1414123.
- Fei Q, Wei S, Zhou Z, Gao H, Li X. 2017. Adaptation of root growth to increased ambient temperature requires auxin and ethylene coordination in Arabidopsis. Plant Cell Rep. 36:1507–1518. doi:10.1007/s00299-017-2171-7.
- Fei Q, Zhang J, Zhang Z, Wang Y, Liang L, Wu L, Gao H, Sun Y, Niu B, Li X. 2019. Effects of auxin and ethylene on root growth adaptation to different ambient temperatures in Arabidopsis. Plant Sci. 281:159–172. doi:10.1016/j.plantsci.2019.01.018.
- Ghobakhlou AF, Johnston A, Harris L, Antoun H, Laberge S. 2015. Microarray transcriptional profiling of Arctic Mesorhizobium strain N33 at low temperature provides insights into cold adaption strategies. BMC Genomics. 16:1–14. doi: 10.1186/s12864-015-1611-4
- Gould PD, Locke JC, Larue C, Southern MM, Davis SJ, Hanano S, Moyle R, Milich R, Putterill J, Millar AJ. 2006. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell. 18:1177–1187. doi: 10.1105/tpc.105.039990
- Gyula P, Baksa I, Tóth T, Mohorianu I, Dalmay T, Szittya G. 2018. Ambient temperature regulates the expression of a small set of sRNAs influencing plant development through NF-YA2 and YUC2. Plant Cell Environ. 41:2404–2417. doi:10.1111/pce.13355.
- Hedhly A, Hormaza JI, Herrero M. 2009. Global warming and sexual plant reproduction. Trends Plant Sci. 14:30–36. doi: 10.1016/j.tplants.2008.11.001
- Holland P, Knævelsrud H, Søreng K, Mathai BJ, Lystad AH, Pankiv S, Bjørndal GT, Schultz SW, Lobert VH, Chan RB, et al. 2016. HS1BP3 negatively regulates autophagy by modulation of phosphatidic acid levels. Nat Commun. 7:13889. doi:10.1038/ncomms13889.
- Huang Z, Footitt S, Tang A, Finch-Savage WE. 2017. Predicted global warming scenarios impact on the mother plant to alter seed dormancy and germination behavior in Arabidopsis. Plant Cell Environ. 41:187–197. doi:10.1111/pce.13082.
- Ibañez C, Poeschl Y, Peterson T, Bellstädt J, Denk K, Gogol-Döring A, Quint M, Delker C. 2017. Ambient temperature and genotype differentially affect developmental and phenotypic plasticity in Arabidopsis thaliana. BMC Plant Biol. 17:114. doi: 10.1186/s12870-017-1068-5
- Kim YS, An C, Park S, Gilmour SJ, Wang L, Renna L, Brandizzi F, Grumet R, Thomashow M. 2017b. CAMTA-mediated regulation of salicylic acid immunity pathway genes in Arabidopsis exposed to low temperature and pathogen infection. Plant Cell. 29. doi:10.1105/tpc.16.00865.
- Kim S, Hwang G, Lee S, Zhu JY, Paik I, Nguyen TT, Kim J, Oh E. 2017a. High ambient temperature represses anthocyanin biosynthesis through degradation of HY5. Front Plant Sci. 8:1787. doi: 10.3389/fpls.2017.01787
- Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, Karp M, Benjamini Y, Hochberg Y, Laron Z. 1995. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses. 45:486–490. doi:10.1016/0306-9877(95)90228-7.
- Körner C, Basler D. 2010. Phenology under global warming. Science. 327:1461–1462. doi: 10.1126/science.1186473
- Liu L, Cang J, Yu J, Wang X, Huang R, Wang J, Lu B. 2013. Effects of exogenous abscisic acid on carbohydrate metabolism and the expression levels of correlative key enzymes in winter wheat under low temperature. J Agric Chem Soc Japan. 77:516–525.
- Liu J, Feng L, Li J, He Z. 2015. Genetic and epigenetic control of plant heat responses. Front Plant Sci. 6:267.
- Liu Y, Mu J, Niklas KJ, Li G, Sun S. 2012. Global warming reduces plant reproductive output for temperate multi-inflorescence species on the Tibetan plateau. New Phytol. 195:427–436. doi: 10.1111/j.1469-8137.2012.04178.x
- Ludwig-Müller J, Krishna P, Forreiter C. 2000. A glucosinolate mutant of Arabidopsis is thermosensitive and defective in cytosolic Hsp90 expression after heat stress. Plant Physiol. 123:949–958. doi: 10.1104/pp.123.3.949
- Mandadi KK, Misra A, Ren S, McKnight TD. 2009. BT2, a BTB protein, mediates multiple responses to nutrients, stresses, and hormones in Arabidopsis. Plant Physiol. 150:1930–1939. doi:10.1104/pp.109.139220.
- Martins S, Montieljorda A, Cayrel A, Huguet S, Roux PL, Ljung K, Vert G. 2017. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat Commun. 8:309. doi: 10.1038/s41467-017-00355-4
- Martínez-Medina A, Fernandez I, Lok GB, Pozo MJ, Pieterse CMJ, Wees SCMV. 2017. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 213:1363–1377. doi: 10.1111/nph.14251
- Massondelmotte V, Schulz M. 2013. Chapter 5: Information from Paleoclimate Archives.
- Mcmahon HT, Gallop JL. 2005. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 438:590–596. doi: 10.1038/nature04396
- Min HJ, Ye JJ, Kang BG, Kim WT. 2016. CaPUB1, a hot pepper U-box E3 ubiquitin ligase, confers enhanced cold stress tolerance and decreased drought stress tolerance in transgenic rice (Oryza sativa L.). Mol Cells. 39:250–257. doi: 10.14348/molcells.2016.2290
- Monteiro D, Liu Q, Lisboa S, Scherer GE, Quader H, Malhó R. 2005. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J Exp Bot. 56:1665–1674. doi: 10.1093/jxb/eri163
- Murayama Y, Kori H, Oshima C, Kondo T, Iwasaki H, Ito H. 2017. Low temperature nullifies the circadian clock in cyanobacteria through Hopf bifurcation. Proc Natl Acad Sci USA. 114:5641–5646. doi: 10.1073/pnas.1620378114
- Mykytczuk NCS, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG. 2013. Bacterial growth at −15°C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 7:1211–1226. doi:10.1038/ismej.2013.8.
- Nagelmüller S, Hiltbrunner E, Körner C. 2017. Low temperature limits for root growth in alpine species are set by cell differentiation. AoB Plants. 9:plx054. doi:10.1093/aobpla/plx054.
- Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B. 2000. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell. 101:331–340. doi: 10.1016/S0092-8674(00)80842-9
- Nolan C, Overpeck JT, Allen JRM, Anderson PM, Betancourt JL, Binney HA, Brewer S, Bush MB, Chase BM, Cheddadi R, et al. 2018. Past and future global transformation of terrestrial ecosystems under climate change. Science. 361:920–923. doi:10.1126/science.aan5360.
- Oh MM, Trick HN, Rajashekar CB. 2009. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J Plant Physiol. 166:180–191. doi: 10.1016/j.jplph.2008.04.015
- Osterlund MT, Hardtke CS, Wei N, Deng XW. 2000. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 405:462–466. doi: 10.1038/35013076
- Pieterse CM, Leonreyes A, Van der Ent S, Van Wees SC. 2009. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 5:308–316. doi: 10.1038/nchembio.164
- Rosebrock TR, Zeng L, Brady JJ, Abramovitch RB, Xiao F, Martin GB. 2007. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature. 448:370–374. doi: 10.1038/nature05966
- Sáenzmata J, Jiménezbremont JF. 2012. HR4 gene is induced in the Arabidopsis-Trichoderma atroviride beneficial interaction. Int J Mol Sci. 13:9110–9128. doi: 10.3390/ijms13079110
- Schertl P, Danne L, Braun HP. 2017. 3-Hydroxyisobutyrate dehydrogenase is involved in valine and isoleucine degradation in A. thaliana. Plant Physiol. 175, 51–61. doi: 10.1104/pp.17.00649
- Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V. 2013. The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Comput Geom. 2007:1–21.
- Stone S, Williams L, Farmer L, Vierstra R, Callis J. 2006. KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell. 18:3415–3428. doi: 10.1105/tpc.106.046532
- Sun J, Zhang J, Larue CT, Huber SC. 2015. Decrease in leaf sucrose synthesis leads to increased leaf starch turnover and decreased RuBP regeneration-limited photosynthesis but not Rubisco-limited photosynthesis in Arabidopsis null mutants of SPSA1. Plant Cell Environ. 34:592–604. doi: 10.1111/j.1365-3040.2010.02265.x
- Takahashi H, Nozawa A, Seki M, Shinozaki K, Endo Y, Sawasaki T. 2009. A simple and high-sensitivity method for analysis of ubiquitination and polyubiquitination based on wheat cell-free protein synthesis. BMC Plant Biol. 9:39. doi: 10.1186/1471-2229-9-39
- Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M. 2017. Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci. 8:161.
- Walker JM. 1969. One-degree increments in soil temperatures affect maize seedling behavior. Soil Scisocamerproc. 33:729–736. doi: 10.2136/sssaj1969.03615995003300050031x
- Wigge PA. 2013. Ambient temperature signalling in plants. Curr Opin Plant Biol. 16:661–666. doi:10.1016/j.pbi.2013.08.004.
- Yan J, Wang J, Li Q, Hwang JR, Patterson C, Zhang H. 2003. AtCHIP, a U-box-containing E3 ubiquitin ligase, plays a critical role in temperature stress tolerance in Arabidopsis. Plant Physiol. 132:861–869. doi:10.1104/pp.103.020800.
- Zhang X, Chen Y, Wang ZY, Chen Z, Gu H, Qu LJ. 2010. Constitutive expression of CIR1 (RVE2) affects several circadian-regulated processes and seed germination in Arabidopsis. Plant J. 51:512–525. doi: 10.1111/j.1365-313X.2007.03156.x
- Zhu J, Geisler M. 2015. Keeping it all together: auxin–actin crosstalk in plant development. J Exp Bot. 66:4983–4998. doi: 10.1093/jxb/erv308
- Zhu J, Zhang KX, Wang WS, Gong W, Liu WC, Chen HG, Xu HH, Lu YT. 2015. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 56:727–736. doi: 10.1093/pcp/pcu217