ABSTRACT
The effects of arbuscular mycorrhizal fungi Glomus mosseae (+AMF) on the growth, nutrient uptake and leaf photosynthetic characteristics of alfalfa (Medicago sativa) under salt stress were studied. In alfalfa, 100 mM salt stress had no obvious effect on plant growth, nutrient content and photosynthetic capacity, however, 200 mM salt stress significantly inhibited biomass accumulation and NPK uptake. In addition, 200 mM salt stress led to decreased photosynthetic carbon assimilation capacity and lower PSII and PSI activity levels. Cultivation of alfafa with +AMF promoted biomass accumulation and uptake of nutrients, increased its underground growth, and promoted accumulation of P over N and K. +AMF increased PSII and PSI activities in alfalfa leaves under salt stress, and increased the electron transfer ability of PSII donor and acceptor sides. +AMF not only made alfalfa have relatively high stomatal conductance under salt stress, but also significantly enhanced its ability to utilize CO2.
1. Introduction
Soil salinization is one of the most important factors that affect agricultural production and the environment. Around 20% of arable land in the world is affected by soil salinization to varying degrees, especially in arid and semi-arid areas (Zhu Citation2001). Excess salt in the soil not only affects plant growth directly through ion poisoning or disturbing ion balance (Yan et al. Citation2006; Ali et al. Citation2017), but it also indirectly causes osmotic stress, which affects the water absorption of plants (Munns et al. Citation2006). Excess Na+ entering plants under salt stress can break the ion balance and disturb normal physiological activities of plants (Munns and Tester Citation2008). Photosynthesis is one of the processes that are sensitive to salt stress, and the synthesis of photosynthetic pigments is inhibited or the degradation of these pigments is accelerated under salt stress (Gong et al. Citation2013; Dąbrowski et al. Citation2016). In addition, absorption and utilization of light energy in PSII and PSI reaction centers become reduced (Zhang, Xu, Wu, et al. Citation2018), electron transport is hindered (Zhang, Xu, et al. Citation2017; Zhang, Li, et al. Citation2018), and carbon assimilation ability is limited under salt stress (Takahashi et al. Citation2017; Xu et al. Citation2018).
Arbuscular mycorrhizal fungi (AMF) acquire energy by infecting the roots of host plants (Campos-Soriano and Segundo Citation2011 ), and rely on the host plants to complete their life cycle (Borde et al. Citation2017). However, AMF also promotes the growth of host plants by promoting the uptake of nutrients (Karagiannidis et al. Citation2002; Evelin et al. Citation2009) and water (Hodge et al. Citation2009; Polcyn et al. Citation2019) by the host plants, which are mutually beneficial to the plant and fungi. Many experiments have reported that AMF can enhance plant stress resistance (Porcel et al. Citation2012; Zhang, Xu, Li, et al. Citation2018). For example, AMF can increase the accumulation of osmotic regulators such as soluble sugar (Feng et al. Citation2002), proline (Medina et al. Citation2010; Miransari Citation2010), betaine (Al-Garni Citation2006) and polyamines (Sannazzaro et al. Citation2007; Evelin et al. Citation2009) in plants under stress. AMF can reduce the level of reactive oxygen species (ROS) by increasing the activities of antioxidant enzymes (SOD, POD and CAT) under stressful conditions (Zhu et al. Citation2010; Yang et al. Citation2015). AMF can also stimulate the changes of the contents of nitric oxide (NO) (Calcagno et al. Citation2012), abscisic acid (ABA) (Ren et al. Citation2018), jasmonic acid (JA) (Hause et al. Citation2002) salicylic acid (SA) (Gutjahr and Paszkowski Citation2009), gibberellin (GA) (Shaul-Keinan et al. Citation2002) and strigolactone (SL) (Liu et al. Citation2015) in plants infected by pathogens, thus inducing defense response in plants. AMF inoculation can maintain the K+/Na+ balance under salt stress (Evelin et al. Citation2012; Garg and Bhandari Citation2016a). AMF infection effectively improves plant photosynthetic capacity (Birhane et al. Citation2012; Chandrasekaran et al. Citation2019; Li et al. Citation2019), and stomatal conductance under salt stress (Lin et al. Citation2017). AMF also improves salt tolerance by reducing non-photochemical quenching of rice leaves under salt stress (Porcel et al. Citation2015).
Alfalfa (Medicago sativa) is a perennial legume forage, and is known as ‘the king of forage’ because of its high yield and quality. Alfalfa is tolerant to barren land, saline-alkali conditions and drought (Zhang, Li, et al. Citation2017; Huang et al. Citation2018; Zhang, Xu, Sui, et al. Citation2018). However, saline-alkali stress reduces the yield and quality of alfalfa (Ruicai et al. Citation2016). AMF inoculation significantly improves the resistance of alfalfa to salt (Wei et al. Citation2018), arsenic (Li et al. Citation2018), cadmium (Liu et al. Citation2017) and atrazine (Fan and Song Citation2018; Sui et al. Citation2018). At present, many studies have shown that AMF inoculation improves salt tolerance of plants, but there are few studies on photosynthetic function of alfalfa under salt stress, especially photosynthetic activity of PSII and PSI. Therefore, the effects of AMF (Glomus mosseae) inoculation on plant growth, nutrient uptake and transport of alfalfa under different concentrations of salt stress (0, 100 and 200 mmol L−1) were studied by artificial inoculation in pots. The response of photosynthetic function of alfalfa leaves was studied to reveal the mechanism behind AMF's function in improving salt resistance of alfalfa.
2. Materials and methods
2.1. Experimental materials
The experiment was conducted in the Plant Physiology Laboratory of Northeast Forestry University (Harbin, Heilongjiang) from March to June 2017. The culture matrix was peat soil sterilized at 121 °C for 2 h. The tested mycorrhizal fungus was Glomus mosseae, which was purchased from the Arbuscular Mycorrhizal Fungi Germplasm Resource Bank of China, numbered ‘BGCAH01.’ Glomus mosseae was propagated for 4 months using shamrock (Oxalis acetosella L.) as the host plant, and the soil containing fungal spores, mycelia and infected roots of host plant was obtained.
The alfalfa variety Zhaodong (Medicago sativa CV. Zhaodong) was used in this study, and its seeds were obtained from the Crop Research Institute of Heilongjiang Academy of Land Reclamation Sciences. Mature and plump seeds with similar size were selected, sterilized with 10% H2O2 for 10 min and cleaned with sterile water. Seeds were then sown in the culture matrices inoculated with AMF (+AMF) and without AMF (-AMF), respectively. In the +AMF treatment, 50 g of soil containing Glomus mosseae was added to the culture matrix per kg, while in -AMF treatment, the same amount of sterilized soil was added. Every 10 seeds were sown in a pot with the diameter of 12 cm and the height of 15 cm. Pots were incubated in an artificial climate chamber with 25°C, 400μmol m−2 s−1 light intensity, 12/12 h photoperiod (light/darkness) and 75% relative humidity. Water and seedling management were carried out regularly.
Fifteen pots each were prepared for +AMF and -AMF treatments, and the +AMF and -AMF treatments were divided into three groups, where each group contained 5 pots. One liter of NaCl solution with the concentration of 100 and 200 mmol L−1 was irrigated per pot in +AMF and -AMF treatments, and the same amount of distilled water was irrigated per pot as control (CK). A tray was placed under each culture pot to retrieve the water that ran off the pots, and these solutions were poured back into the pots later. The data of seedling culture were measured at 45 dpi.
2.2. Measurement parameters and methods
Determination of chlorophyl fluorescence parameters: Fully unfolded leaves of alfalfa in each treatment were selected and treated with dark adaptation clip for 30 min. OJIP curves and modulated reflection at 820 nm (MR820 nm) of leaves in each treatment were measured by M-PEA (Multi-Function Plant Efficiency Analyser) (Hansatech) after dark adaptation. Five repetitions were carried out for each treatment. The maximum photochemical efficiency of PSII (Fv/Fm) and the relative drop (△I/Io) of MR820 nm curve signal were obtained by JIP-test of the curves. Fv/Fm and △I/Io were used to characterize the photochemical activity of PSII and PSI, respectively, of which Fv/Fm = (Fm-Fo)/Fm, where Fm and Fo are the relative fluorescence intensities of P point (1000 ms) and O point (0.01 ms) on the OJIP curve, Io represents the maximum value of the MR820 nm signal, and △I represents the difference between the maximum and the minimum of the MR820 nm signal (Zhang, Xu, Wu, et al. Citation2018). Relative fluorescence intensity of the O point on the OJIP curve was defined as 0, and that of P point and J point (2 ms) was defined as 1. The OJIP curve was standardized according to the formulas VO−P = (Ft-Fo)/(Fm-Fo) and VO−J = (Ft-Fo)/(FJ-Fo) to obtain the relative variable fluorescence VJ at J point (2 ms) on VO−P curve and relative variable fluorescence VK at K point (0.3 ms) on VO−J curve. The standardized VO−P and VO−J curves of alfalfa leaves at different salt concentrations were compared with those of CK and the differences were denoted as △VO−P and △VO−J, respectively.
Determination of photosynthetic gas exchange parameters: Fully unfolded leaves of alfalfa in were collected, using the Li-6400 photosynthesis measurement system, the light intensity PFD was set to 1000 μmol m−2·s−1 and the gas path of the instrument was connected to the atmosphere by an air buffer bottle to measure the parameters of alfalfa leaves, such as net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr) and intercellular CO2 concentration (Ci). The leaf area of alfalfa was too small to fill the leaf chamber of photosynthesis measurement system, therefore the leaf area was measured after the aboveground parameters were measured. The actual parameters of photosynthetic gas exchange in leaves were obtained by multiplying the parameters of photosynthetic gas exchange that were directly obtained by the instrument by the ratio of leaf chamber area to leaf area. Each treatment was replicated three times.
Determination of plant biomass and nutrient content: After the photosynthesis and chlorophyl fluorescence parameters were measured, the plants were taken out from the matrix, and the root surface was carefully washed to remove substrates. The aboveground and underground parts of plants were kept separately in different envelopes, and the biomass was weighed after drying the samples at 80 °C to a constant weight. The aboveground and underground parts of alfalfa in different treatments were ground down and sieved using 40 meshes. After digesting the aboveground and underground samples using H2SO4-H2O2, N content was determined by the Kjeldahl method, P content was measured by Mo-Sb Anti-colorimetry, and K content was determined by the flame photometric method (Bao Citation2005). Each treatment was measured three times.
2.3. Data analysis
Excel (2003) and SPSS (22.0) software were used for statistical analysis. Two-way ANOVA and least significant difference (LSD) were used for the comparison of the differences between different data sets.
3. Results and analysis
3.1. Biomass
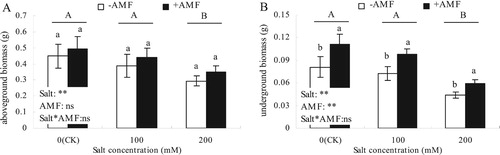
As shown in , salt concentration had a significant effect on the aboveground biomass of alfalfa (P < 0.05), +AMF had an insignificant effect on the aboveground biomass (P > 0.05), while salt concentration and +AMF had a very significant effect on the underground biomass (P < 0.01). In addition, salt × AMF did not show a significant interaction on the aboveground and underground biomass of alfalfa. There was no significant difference between aboveground and underground biomass of 100 mM salt treatment and CK, but there was a significant decrease in aboveground and underground biomass in the 200 mM salt treated samples. Although +AMF promoted the accumulation of aboveground biomass of alfalfa, there was no significant difference between +AMF and -AMF. However, the underground biomass of the +AMF treatment was significantly higher than the -AMF treatment under different salt concentration conditions.
3.2. N-P-K nutrient content
Different concentrations of salt stress significantly affected the N and P contents in aboveground and underground parts and K content in underground parts of alfalfa (; P < 0.01), and had significant effects on K content of the aboveground parts (P < 0.05). +AMF did not affect N and K contents in aboveground and underground parts of alfalfa, but had significant effects on P content. There was no significant interaction between salt × AMF and the NPK content of the aboveground and underground parts of alfalfa.
Figure 2. Effects of AMF inoculation on N (A, B), P (C, D), and K (E, F) contents in the aboveground and underground parts of alfalfa under salt stress.
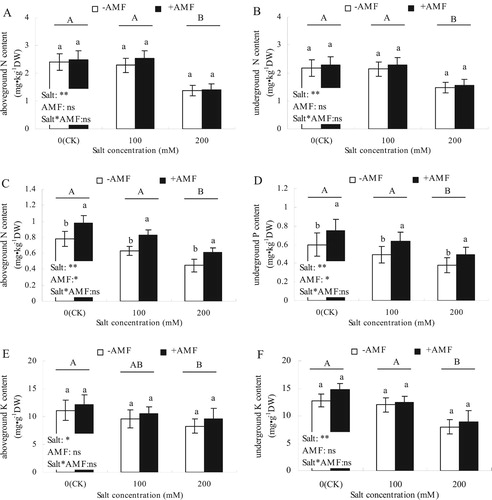
With the increase in salt concentration, the content of NPK in the aboveground and underground parts of alfalfa decreased to varying degrees. The N and K contents in the aboveground and underground parts of alfalfa increased to different degrees after +AMF treatment under different salt concentrations, but the difference was not statistically significant when compared with -AMF. However, the P content in aboveground and underground parts increased significantly under different salt concentrations in the +AMF treatment compared with the -AMF treatment.
3.3. OJIP curve, MR820 curve and photochemical activities of PSII and PSI
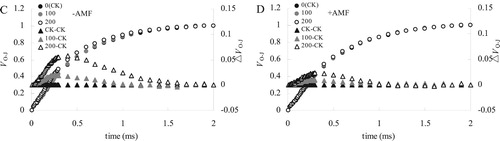
At the 100 mM salt concentration, the change of OJIP curve of alfalfa leaves was smaller than that CK ((A)). At higher salt concentrations of 200 mM, the relative fluorescence intensity of the P point of the OJIP curve decreased significantly ((B)). The relative fluorescence intensity at the J point showed an increasing trend in the -AMF treatment, but +AMF treatment did not result in that increase.
Figure 3. Effects of-AMF (A) and +AMF (B) on OJIP curves and MR820 curves of alfalfa leaves under salt stress.
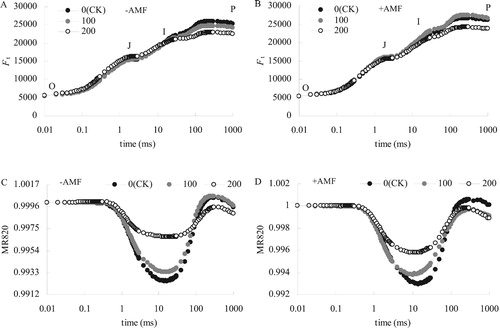
At the 100 mM salt concentration, the MR820 curve of alfalfa treated with +AMF and -AMF decreased slightly ((C and D)). Howver, treated with 200 mM salt, the MR820 curve decreased greatly, and +AMF had significantly lower MR820 curve than -AMF.
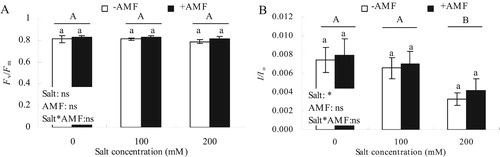
Under different salt concentrations, Fv/Fm of alfalfa leaves in the +AMF treatment was slightly higher than the -AMF treatment, however the difference was not statistically different ((A)). Different salt concentrations also did not affect Fv/Fm, and there was no obvious interaction between salt × AMF and Fv/Fm. There was no significant difference between △I/Io at 100 mM salt treatment and CK ((B)). In contrast, plants treated with 200 mM salt concentration showed significantly lower △I/Io compared with CK ((B); P < 0.05). Under different salt concentrations, △I/Io of alfalfa leaves increased to varying degrees when treated with +AMF compared with the -AMF treatment, however, the difference was not statistically significant.
3.4. Standardized O-P and O-J curves and VJ and VK
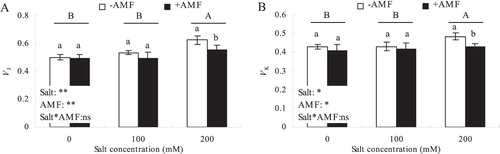
With increasing salt concentrations, VJ on VO−P curve of alfalfa leaves increased significantly, but +AMF treatment alleviated the increase of VJ under salt stress ((A and B)). Both salt and +AMF treatments significantly affected VJ (P < 0.01), but there was no significant interaction between salt and +AMF treatments ((A)).
VK on the VO−J curve of alfalfa leaves increased with increasing salt concentrations, and the +AMF treatment significantly reduced VK at different salt concentrations ((A and B)). VK of alfalfa leaves treated with 100 mM salt was similar to CK, but VK increased significantly when plants were treated with 200 mM salt ((B)). +AMF significantly affected VK of alfalfa leaves, and VK under +AMF treatment at 200 mM was 11.34% lower than the -AMF treatment (P < 0.05).
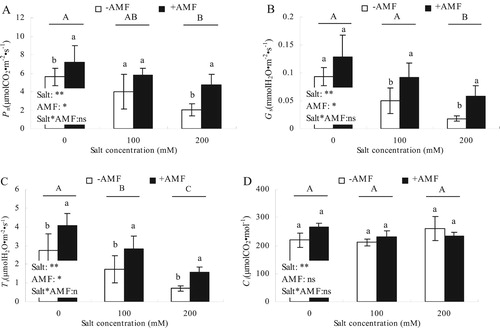
3.5. Gas exchange parameters of photosynthesis
Pn, Gs and Tr of alfalfa leaves were significantly affected by salt concentration (P < 0.01), while Ci was not affected ((A–D); P > 0.05). +AMF also significantly affected Pn, Gs and Tr, but did not affect Ci. With increasing salt concentrations, Pn, Gs and Tr decreased, but each parameter was affected at different salt concentrations. Pn, Gs and Tr of the +AMF treatment were significantly higher than the -AMF treatment. Only at 100 mM salt treatment, there was no significant difference between Pn in the +AMF and -AMF treatments. Different salt concentrations did not affect Ci, but Ci in the -AMF treatment was significantly higher than the +AMF treatment when plants were also treated with 200 mM salt.
4. Discussion
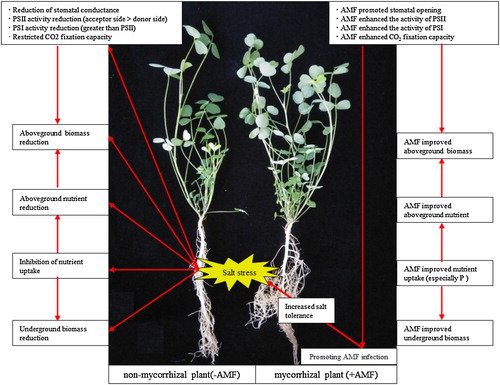
Growth inhibition is the most common phenotypic response to salt stress. Higher salt concentration directly stimulates plant roots and inhibits the growth of shoots (Galvan-Ampudia and Testerink Citation2011). As a cash crop for stem and leaf, alfalfa biomass directly affects the yield of alfalfa. In this study, salt concentration had a significant effect on biomass accumulation of aboveground and underground parts of alfalfa. Especially at the 200 mM salt concentration, the biomass of aboveground and underground parts decreased significantly. Interestingly, treatment with 100 mM salt solution did not affect alfalfa biomass, indicating that alfalfa has strong salt tolerance. Inoculation of plants with fungi such as Funneliformis mosseae and Rhizophagus irregularis can promote the accumulation of biomass under salt stress (Sheng et al. Citation2011; Aroca et al. Citation2013; Porcel et al. Citation2016). In this experiment, +AMF promoted the accumulation of aboveground biomass of alfalfa at different salt concentrations, but there was no significant difference compared with the -AMF treatment. Underground biomass was significantly greater in the +AMF treatment than the -AMF treatment at different salt concentrations, suggesting that +AMF promoted the growth of underground parts of alfalfa more than the aboveground parts.
Soil salinization inhibits the plant's ability to uptake nutrients (Uygur and Yeti˙Si˙R Citation2009; Yadav et al. Citation2011; Zhang, Li, et al. Citation2018), while AMF symbiosis promotes nutrient uptake by the roots of the host plant because the extraradical mycelium (ERM) enlarges the root contact area (Evelin et al. Citation2009). In addition, +AMF promotes the growth of alfalfa roots under salt stress, as it is conducive to the absorption of nutrients and water by the roots. Except at excessive salt concentrations, NP is one of the limiting factors for alfalfa growth and yield in saline-alkali soil (He et al. Citation2017). AMF can increase the contents of major elements and trace elements of plants by selective ion absorption, especially for P content, which has low mobility (Evelin et al. Citation2009). AMF increased N uptake of the host plant by 20%−50% (Toussaint et al. Citation2004; Jin et al. Citation2005). Under salt stress, +AMF significantly promotes N content in plants such as Sesbania aegyptiaca (Giri and Mukerji Citation2004), Trigonella foenum-graecum (Evelin et al. Citation2012) and Triticum aestivum (Talaat and Shawky Citation2014) and P content in plants such as Oryza sativa (Porcel et al. Citation2016), Acacia auriculiformis (Giri et al. Citation2003) and Acacia nilotica (Giri et al. Citation2007). +AMF increases K uptake under salt stress, thus increasing the K/Na ratio of plants (Wu et al. Citation2010), and this is caused by AMF's regulation of the expression of K/Na balance-related membrane transporters, such as SOS1, HKT1 and SKOR (Porcel et al. Citation2016). In this study, the NPK content of the aboveground and underground parts of alfalfa decreased to varying degrees under salt stress, but +AMF treatment increased the NPK content. Under different salt concentrations, only P content in the aboveground and underground parts of alfalfa treated with +AMF was significantly different from -AMF, therefore the effect of +AMF on P uptake and transport under salt stress was significantly greater than N and K.
Salt stress significantly inhibits the photosynthetic capacity of plants, resulting in the reduction of photochemical activity of PSII and photoinhibition (Zhang, Xu, et al. Citation2017; Xu et al. Citation2018; Zhang, Li, et al. Citation2018). In addition to PSII, PSI is sensitive to stress, and PSI is more likely to experience photoinhibition than PSII (Zhang et al. Citation2010). In this study, the shape of the OJIP curve of alfalfa leaves changed with increasing salt concentration, and Fv/Fm decreased, but the difference was not statistically significant (). Fv/Fm and △I/Io are important indicators of PSII and PSI photoinhibition in plants under stress. Although salt stress inhibited PSII activity of alfalfa leaves, it did not cause significant photoinhibition. In contrast, the photoinhibition of PSI was significantly greater than PSII. Studies have shown that PSII photoinhibition of plant leaves under stress is often related to strong light induction. The photosynthetic parameters in this study were measured in an artificial climate chamber. Although salt stress inhibited PSII function of alfalfa leaves, low light intensity (400 μmol·m−2·s−1) did not cause PSII photoinhibition. +AMF can improve photosynthesis of plants under salt stress by promoting stomatal conductance, water use efficiency and photochemical activity of PSII in plant leaves (Hajiboland et al. Citation2010; Talaat and Shawky Citation2014; Porcel et al. Citation2015). Some studies have reported that +AMF increases chlorophyl synthesis by promoting the absorption of Mg2+, thereby improving the light capture ability of plant leaves (Hashem et al. Citation2015). +AMF can increase the electron transfer rate and photochemical efficiency of PSII in plant leaves (Tsimilli-Michael et al. Citation2000; Zhu et al. Citation2014), as well as the photosynthetic capacity of plants under salt stress to alleviate photoinhibition (Zhu et al. Citation2016). These results are consistent in findings in this study, where +AMF alleviated the decrease of Fv/Fm and △I/Io under salt stress, suggesting that +AMF increases the activity of PSII and PSI under salt stress.
The decrease of PSII activity under stress is related to the blockage of electron transport. Electron transport is blocked when electron transfer from QA to QB becomes inhibited, causing a decrease in oxygen evolution complex (OEC) activity on the PSII donor side and the degradation of D1 protein on PSII acceptor side (Cheng et al. Citation2016; Liu et al. Citation2019). VJ and VK of alfalfa leaves increased significantly under salt stress, and the increase of VJ was greater than that of VK. The increase of VJ reflects the inhibition of electron transfer from QA to QB on the PSII acceptor side, and the increase of VK is considered to be a marker for damaged OEC activity on the PSII donor side (Kalaji et al. Citation2014; Zhang, Feng, et al. Citation2018). Therefore, salt stress can inhibit the electron transfer ability on the PSII donor and acceptor sides of alfalfa leaves, especially the sensitivity of PSII acceptor side is greater than that of donor side. The increase of VJ and VK in alfalfa leaves treated with +AMF was significantly lower than -AMF, which indicated that +AMF improved the electron transfer ability on the PSII donor side and acceptor side under salt stress. This is similar to a previous study, which showed that AMF promoted electron transfer on the PSII donor and acceptor side of pistachio rootstocks under salt stress (Shamshiri and Fattahi Citation2016). The promotion in electron transfer may be related to the fact that AMF can increase the expression of psbA and psbD, which encode the core protein D1 and D2 of the PSII reaction center of the host plant under salt stress (Chen et al. Citation2017).
The decrease of the activity of photochemical reaction centers in plant leaves under salt stress restricts the supply of assimilation (ATP and MDPH), and the closure of stomata under stress leads to decreased carbon assimilation capacity. In this study, increasing salt concentrations led to significant decreases in the Gs of alfalfa leaves, which led to the decrease of Pn and Tr. Pn, Gs and Tr of alfalfa leaves treated with +AMF were higher than -AMF to varying degrees, which indicated that +AMF improved photosynthetic gas exchange ability of alfalfa under salt stress by increasing stomatal conductance of leaves. The decrease of Gs is often related to the water status of plants. Under water deficit conditions, plants reduce transpiration rate by closing stomata to maintain the normal swelling pressure of cells. Therefore, the relative high Gs in the +AMF treatment under salt stress may be related to the increased water absorption under salt stress by +AMF (Hodge et al. Citation2009; Polcyn et al. Citation2019). In addition to the limitation of stomatal factors, the photosynthetic capacity of plants is often reduced by non-stomatal factors under severe stress, such as decreased activity of the dark reaction-related enzymes. Farquhar and Sharkey (Citation1982) found that when there were non-stomatal constraints, Ci is often increased. In this study, Ci of alfalfa leaves increased significantly in plants treated with salt 200 mM salt and -AMF, but it increased slightly in the +AMF treatment. Hence, +AMF-treated alfalfa had relatively high carbon assimilation ability under high salt stress (200 mM), which was caused by changes in stomatal factors, and +AMF alleviated CO2 fixation ability under salt stress. +AMF can alleviate the decrease of carbon assimilation-related enzymes activities, such as RuBisCO (Garg and Bhandari Citation2016b) and phospho-enol-pyruvate carboxylase (PEPC) (Hashem et al. Citation2015), induced by salt stress. Therefore, increasing the activities of enzymes involved in the dark reaction by +AMF under salt stress may underlie the enhancement of photosynthetic capacity of alfalfa leaves, however, further research is needed to examine this.
summarizes how +AMF promotes plant growth and photosynthetic capacity of alfalfa under salt stress.
5. Conclusion
Salt stress inhibits plant growth and nutrient uptake of alfalfa, and leads to decreased PSII and PSI photochemical activity and photosynthetic capacity. +AMF promoted the accumulation of alfalfa biomass under salt stress, especially of the underground parts. The facilitation of +AMF on P uptake of alfalfa under salt stress was significantly greater than N and K uptake. Although +AMF had no significant effect on PSII and PSI photochemistry activity under different salt concentrations, it significantly alleviated the inhibition of electron transfer on the PSII donor side and acceptor side of alfalfa leaves under salt stress. +AMF also increased the stomatal constraints of alfalfa leaves under salt stress, as well as the CO2 fixation capacity.
Acknowledgments
This research was supported by ‘Young Talents’ Project of Northeast Agricultural University (18QC12) and The National Natural Science Fund (31901088)
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Liang Shi-chu
Liang Shi-chu, 1965.3, Doctor degree, Plant Physiology and Ecology.
Jiang Yong
Jiang Yong, 1981.4, Doctor degree, Plant Physiology and Ecology.
Li Ma-bo
Li Ma-bo, 1998.10, Bachelor degree, Plant Physiology.
Zhu Wen-xu
Zhu Wen-xu, 1986.4, Doctor degree, Plant Physiology and Ecology.
Xu Nan
Xu Nan, 1982.8, Doctor degree, Plant Physiology and Ecology.
Zhang Hui-hui
Zhang Hui-hui, 1986.10, Doctor degree, Plant Physiology and Ecology.
References
- Al-Garni SMS. 2006. Increasing NaCl-salt tolerance of a halophytic plant Phragmites australis by mycorrhizal symbiosis. Am-Eurasian J Agric Environ Sci. 1(2):119–126.
- Ali S, Rizwan M, Qayyum MF, Ok YS, Ibrahim M, Riaz M, Arif MS, Al-Wabel M, Shahzad AN. 2017. Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ Sci Pollut Res Int. 24(14):12700–12712.
- Aroca R, Ruiz-Lozano JM, Zamarreño ÁM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA. 2013. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol. 170(1):47–55.
- Bao SD. 2005. Soil analysis. Beijing: China agriculture press.
- Birhane E, Sterck FJ, Fetene M, Bongers F, Kuyper TW. 2012. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 169(4):895–904.
- Borde M, Dudhane M, Kulkarni M. 2017. Role of arbuscular mycorrhizal fungi (AMF) in salinity tolerance and growth response in plants under salt stress conditions. Mycorrhiza-Eco-Physiology, Secondary Metabolites. Nanomaterials. 71–86.
- Calcagno C, Novero M, Genre A, Bonfante P, Lanfranco L. 2012. The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatularoots. Mycorrhiza. 22(4):259–269.
- Campos-Soriano L, Segundo BS. 2011. New insights into the signaling pathways controlling defense gene expression in rice roots during the arbuscular mycorrhizal symbiosis. Plant Signal Behav. 6(4):553–557.
- Chandrasekaran M, Chanratana M, Kim K, Seshadri S, Sa T. 2019. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress–a meta-analysis. Front Plant Sci. 9:457.
- Chen J, Zhang H, Zhang X, Tang M. 2017. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front Plant Sci. 8:1739.
- Cheng DD, Zhang ZS, Sun XB, Zhao M, Sun GY. 2016. Photoinhibition and photoinhibition-like damage to the photosynthetic apparatus in tobacco leaves induced bypseudomonassyringaepv.tabaciunder light and dark conditions. BMC Plant Biol. 16(1):1–11.
- Dąbrowski P, Baczewska AH, Pawluśkiewicz B, Paunov M, Alexantrov V, Goltsev V, Kalaji MH. 2016. Prompt chlorophyll a, fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in perennial ryegrass. J Photochem Photobiol B. 157(7):22–31.
- Evelin H, Giri B, Kapoor R. 2012. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza. 22(3):203–217.
- Evelin H, Kapoor R, Giri B. 2009. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot. 104(7):1263–1280.
- Fan XX, Song FQ. 2018. Responses of nonenzymatic antioxidants to atrazine in arbuscular mycorrhizal roots of Medicago sativa L. Mycorrhiza. 28(3):1–5.
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 33(1):317–345.
- Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z. 2002. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza. 12(4):185–190.
- Galvan-Ampudia CS, Testerink C. 2011. Salt stress signals shape the plant root. Curr Opin Plant Biol. 14(3):296–302.
- Garg N, Bhandari P. 2016a. Interactive effects of silicon and arbuscular mycorrhiza in modulating ascorbate-glutathione cycle and antioxidant scavenging capacity in differentially salt-tolerant Cicer arietinum L. genotypes subjected to long-term salinity. Protoplasma. 253(5):1325–1345.
- Garg N, Bhandari P. 2016b. Silicon nutrition and mycorrhizal inoculations improve growth, nutrient status, K+/Na+ ratio and yield of Cicer arietinum L. genotypes under salinity stress. Plant Growth Regul. 78(3):371–387.
- Giri B, Kapoor R, Mukerji KG. 2003. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol Fertil Soils. 38(3):170–175.
- Giri B, Kapoor R, Mukerji KG. 2007. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol. 54(4):753–760.
- Giri B, Mukerji KG. 2004. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandifloraunder field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza. 14(5):307–312.
- Gong B, Wen D, Vandenlangenberg K, Wei M, Yang FJ, Shi QH, Wang XF. 2013. Comparative effects of NaCl and NaHCO3, stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci Hortic. 157(3):1–12.
- Gutjahr C, Paszkowski U. 2009. Weights in the balance: jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol Plant-Microbe Interact. 22(7):763–772.
- Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C. 2010. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 331(1–2):313–327.
- Hashem A, Abd-Allah EF, Alqarawi AA, Aldubise A, Egamberdieva D. 2015. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J Plant Interact. 10(1):230–242.
- Hause B, Maier W, Miersch O, Kramell R, Strack D. 2002. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol. 130(3):1213–1220.
- He H, Peng Q, Wang X, Fan C, Pang J, Lambers H, Zhang X. 2017. Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil. 416(1–2):565–584.
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. 2009. Plant root growth, architecture and function. Plant Soil. 321(1–2):153–187.
- Huang Z, Liu Y, Cui Z, Fang Y, He HH, Liu BR, Wu GL. 2018. Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). Field Crops Res. 221(15):1–6.
- Jin H, Pfeffer PE, Douds DD, Piotrowski E, Lammers PJ, Shachar-Hill Y. 2005. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 168(3):687–696.
- Kalaji HM, Schansker G, Ladle RJ, Video G, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dabrowski P, et al. 2014. Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res. 122(2):121–158.
- Karagiannidis N, Bletsos F, Stavropoulos N. 2002. Effect of Verticillium wilt (Verticillium dahliae Kleb.) and mycorrhiza (Glomus mosseae) on root colonization, growth and nutrient uptake in tomato and eggplant seedlings. Sci Hortic. 94(1–2):145–156.
- Li JQ, Meng B, Chai H, Yang XC, Song WZ, Li SX, Lu A, Tao Zhang T, Sun W. 2019. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front Plant Sci. 9:499.
- Li J, Sun Y, Jiang X, Chen B, Zhang X. 2018. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol Environ Saf. 157:235–243.
- Lin J, Wang Y, Sun S, Mu C, Yan X. 2017. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci Total Environ. 576:234–241.
- Liu Z, Li YJ, Wang J, He XY, Tian CJ. 2015. Different respiration metabolism between mycorrhizal and non-mycorrhizal rice under low-temperature stress: a cry for help from the host. J Agric Sci. 153(04):602–614.
- Liu M, Sun J, Li Y, Xiao Y. 2017. Nitrogen fertilizer enhances growth and nutrient uptake of Medicago sativa inoculated with Glomus tortuosum grown in Cd-contaminated acidic soil. Chemosphere. 167:204–211.
- Liu XJ, Zhang HH, Wang JR, Wu XY, Ma SL, Xu ZS, Zhou T, Xu N, Tang XD, Baiyi A. 2019. Improvement of drought resistance by increasing water use efficiency and PSII function of mulberry seedling leaves under drought stress with increased CO2 concentrations. J Plant Interact. 14(1):219–228.
- Medina A, Roldán A, Azcón R. 2010. The effectiveness of arbuscular-mycorrhizal fungi and Aspergillus niger or Phanerochaete chrysosporium treated organic amendments from olive residues upon plant growth in a semi-arid degraded soil. J Environ Manag. 91(12):2547–2553.
- Miransari M. 2010. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 12(4):563–569.
- Munns R, James RA, Läuchli A. 2006. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 57(5):1025–1043.
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 59(1):651–681.
- Polcyn W, Paluch-Lubawa E, Lehmann T, Mikuła R. 2019. Arbuscular mycorrhiza in highly fertilized maize cultures alleviates short-term drought effects but does not improve fodder yield and quality. Front Plant Sci. 10:496.
- Porcel R, Aroca R, Azcon R, Ruiz-Lozano JM. 2016. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza. 26(7):673–684.
- Porcel R, Aroca R, Ruiz-Lozano JM. 2012. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustainable Dev. 32(1):181–200.
- Porcel R, Redondo-Gómez S, Mateos-Naranjo E, Aroca R, Garcia R, Ruiz-Lozano JM. 2015. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J Plant Physiol. 185:75–83.
- Ren CG, Kong CC, Xie ZH. 2018. Role of abscisic acid in strigolactoneinduced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 18:74.
- Ruicai L, Mingna L, Tiejun Z, Kang J, Sun Y, Cong L, Gao Y, Liu F, Yang Q. 2016. Comparative proteomic analysis reveals differential root Proteins in Medicago sativa and Medicago truncatula in response to salt stress. Front Plant Sci. 7:424.
- Sannazzaro AI, Echeverría M, Albertó EO, Ruiz OA, Menéndez AB. 2007. Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol Biochem. 45(1):39–46.
- Shamshiri MH, Fattahi M. 2016. Effects of arbuscular mycorrhizal fungi on photosystem II activity of three pistachio rootstocks under salt stress as probed by the OJIP-test. Russ J Plant Physiol. 63(1):101–110.
- Shaul-Keinan O, Gadkar V, Ginzberg I, Grünzweig JM, Chet I, Elad Y, Wininger S, Belausov E, Eshed Y, Atzmon N, et al. 2002. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol. 154(2):501–507.
- Sheng M, Tang M, Zhang F, Huang Y. 2011. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza. 21(5):423–430.
- Sui X, Wu Q, Chang W, Fan XX, Song FQ. 2018. Proteomic analysis of the response of Funnelifor mismosseae/Medicago sativa to atrazine stress. BMC Plant Biol. 18:289.
- Takahashi M, Shigeto J, Sakamoto A, Morikawa H. 2017. Selective nitration of PsbO1, PsbO2, and PsbP1 decreases PSII oxygen evolution and photochemical efficiency in intact leaves of Arabidopsis. Plant Signal Behav. 12(10):e1376157.
- Talaat NB, Shawky BT. 2014. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot. 98(1):20–31.
- Toussaint JP, St-Arnaud M, Charest C. 2004. Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can J Microbiol. 50(4):251–260.
- Tsimilli-Michael M, Eggenberg P, Biro B, Köves-Pechy K, Vörös I, Strasser RJ. 2000. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol. 15(2):169–182.
- Uygur V, Yeti˙Si˙R H. 2009. Effects of rootstocks on some growth parameters, phosphorous and nitrogen uptake watermelon under salt stress. J Plant Nutr. 32(4):629–643.
- Wei C, Xin S, Fan XX, Song FQ. 2018. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front Microbiol. 9:652.
- Wu QS, Zou YN, He XH. 2010. Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant. 32(2):297–304.
- Xu N, Zhang HH, Zhong HX, Wu YN, Li JB, Li X, Yin ZP, Zhu WX, Qu Y, Sun GY. 2018. The response of photosynthetic functions of F1 cutting seedlings from Physocarpus amurensis Maxim (♀) × Physocarpus opulifolius “Diabolo” (♂) and the parental leaves to salt stress. Front Plant Sci. 9:714.
- Yadav S, Irfan M, Ahmad A, Hayat S. 2011. Causes of salinity and plant manifestations to salt stress: A review. J Environ Biol. 32(5):667–685.
- Yan SH, Ji J, Wang G. 2006. Effects of salt stress on plants and the mechanism of salt tolerance. World Sci Technol Res Dev. 28(4):70–76.
- Yang Y, Han X, Liang Y, Ghosh A, Chen J, Tang M. 2015. The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. Plos One. 10(12):e0145726.
- Zhang HH, Feng P, Yang W, Sui X, Li X, Zhang RT, Gu SY, Xu N. 2018. Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J For Res. 29(4):1049–1059.
- Zhang ZS, Jia YJ, Gao HY, Zhang LT, Li HD, Meng QW. 2010. Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis sativus L.) leaves. Planta. 234(5):883–889.
- Zhang HH, Li X, Xu N, Sun ML, Cai DJ, Sun GY, Gu SY. 2017. Alkalinity and salinity tolerance during seed germination and early seedling stages of three alfalfa (Medicago sativa L.) cultivars. Legume Research. 40(5):853–858.
- Zhang HH, Li X, Zhang SB, Yin ZP, Zhu WX, Li JB, Meng L, Zhong HX, Wu YN, Xu N, Sun GY. 2018. Rootstock alleviates salt stress in grafted mulberry seedlings: physiological and PSII function responses. Front Plant Sci. 9:1806.
- Zhang HH, Xu N, Li X, Jin WW, Tian Q, Sun GY, Gu SY. 2017. Overexpression of 2-Cys Prx increased salt tolerance of photosystem II in tobacco. Int J Agric Biol. 19(4):735–745.
- Zhang HH, Xu N, Li X, Long JH, Sui X, Wu YN, Li JB, Wang JF, Qu Y, Sun GY. 2018. Arbuscular mycorrhizal fungi (Glomus mosseae) improves growth, photosynthesis and protects photosystem II in leaves of Lolium perenne L. under cadmium contaminated soil. Front Plant Sci. 9:1156.
- Zhang HH, Xu N, Sui X, Zhong HX, Yin ZP, Li X, Sun GY. 2018. Photosynthesis response to drought stress in leaves of two alfalfa (Medicago sativa L.) varieties. International Journal of Agriculture and Biology. 20(5):1012–1020.
- Zhang HH, Xu N, Wu XY, Wang JR, Ma SL, Li X, Sun GY. 2018. Effects of four types of sodium salt stress on plant growth and photosynthetic apparatus in sorghum leaves. Journal of Plant Interaction. 13(1):506–513.
- Zhu JK. 2001. Plant salt tolerance. Trends Plant Sci. 6(2):66–71.
- Zhu X, Song F, Xu H. 2010. Influence of arbuscular mycorrhiza on lipid peroxidation andantioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza. 20:325–332.
- Zhu XQ, Tang M, Zhang HQ. 2016. Arbuscular mycorrhizal fungi enhanced the growth, photosynthesis, and calorific value of black locust under salt stress. Photosynthetica. 55(2):1–8.
- Zhu XQ, Wang CY, Chen H, Tang M. 2014. Effects of arbuscular mycorrhizal fungi on photosynthesis, carbon content, and calorific value of black locust seedlings. Photosynthetica. 52(2):247–252.