ABSTRACT
Plant protein–protein interaction (PPI) networks provide valuable information for the understanding of plant biological processes. In this study, we summarized and constructed the nuclear-protein interactome of Arabidopsis based on PPIs, and preliminarily analyzed the relationships among the main pathways involving these protein interactions. These proteins are involved mainly in the plant hormone signal transduction and plant-pathogen interaction, and these pathways are linked closely by co-pathway and nodal proteins. The key nodal proteins play important roles in communication among hormonal pathways. Further analysis revealed that all of these key nodal proteins were localized in multiple organelles. High-frequency protein multi-localization analysis suggested that the multi-location proteins may play key roles in organelle communication and plant growth. Thus, the screening and study of multi-location proteins may be an important research direction. Our findings can inform new research based on protein interactions and provide novel directions for plant proteome research.
Introduction
Protein is a base material of life, the basic organic substance that constitutes cells, and the main undertaker of life activities (Ruan et al. Citation2006; Wu et al. Citation2014; Subba et al. Citation2019). As the main performers of biological functions, proteins seldom work alone; they often interact with other proteins to carry out their functions (Sun et al. Citation2020). Protein–protein interactions (PPIs) can be found in all biological processes, including DNA replication and transcription, enzyme-controlled metabolic reactions, signal transduction, protein transport, protein degradation, and cell-cycle regulation (Klingstrom and Plewczynski Citation2011). The study of PPI networks can be useful for the identification of multimeric protein complexes (King et al. Citation2004; Habibi et al. Citation2010) and elucidation of protein functions at the molecular level (Hawkins and Kihara Citation2007; Khan and Kihara Citation2016), revealing the vital roles that proteins play in life activities such as growth, development, differentiation, and apoptosis (Zhu et al. Citation2011). Thus, the identification and analysis of PPI networks provide valuable information for the understanding of plant biological processes.
Currently known PPIs are classified as verified and predicted. Experimental methods applied for PPI identification include yeast two-hybrid system analysis (Rodriguez-Negrete et al. Citation2014), co-immunoprecipitation (Phee et al. Citation2006), fluorescence resonance energy transfer (Kenworthy Citation2001), and affinity chromatography combined with mass spectrometry (Joseph et al. Citation2006). Several complementary computational methods, including those employing machine-learning frameworks that consider various protein features as inputs, have been developed. The protein features used for PPI prediction include the occurrence of functional domains (Chen and Liu Citation2005), short sequence patterns (e.g. n-grams, auto-covariation) (Shen et al. Citation2007), interlogs (interactions inferred from homology) (Walhout et al. Citation2000; Gu et al. Citation2011), codon usage (Najafabadi and Salavati Citation2008), function (Zhang and Tang Citation2016), similarity in phylogenetic trees (Juan et al. Citation2008), phylogenetic profiles (Szklarczyk et al. Citation2017; Xiong et al. Citation2020), gene expression (Soong et al. Citation2008), and tertiary structures (Zhang et al. Citation2012). Because this topic has been explored using many approaches, extensive PPI data have been obtained. Thus, the systematic analysis of PPI networks is needed to mine information that can help to elucidate the mechanisms underlying biological processes.
With the development of large-scale protein-interaction detection technology, protein-interaction maps have been drawn for many model species, including Drosophila melanogaster (Giot et al. Citation2003), Caenorhabditis elegans (Li et al. Citation2004), Homo sapiens (Rual et al. Citation2005), Saccharomyces cerevisiae (Assenov et al. Citation2008b), and Arabidopsis thaliana (Geisler-Lee et al. Citation2007). Arabidopsis, the best-characterized dicotyledonous plant, is a prime model system in plant biology and beyond (Zobel Citation2016; Volkening et al. Citation2019; Zhou et al. Citation2020). As a model plant species, Arabidopsis has substantial advantages over other plants. It is also the first model organism used for the study of plant interactomes. Recently, a massive plant proteomics project, in which co-fractionation mass spectrometry was used to measure the amounts and associations of more than two million proteins from 13 diverse plant species, revealed that stable protein complexes are shared across plant cells, providing a framework for the interpretation of plant genetics and mutant phenotypes (McWhite et al. Citation2020). Although recent reports have described the predicted protein-interaction network in Arabidopsis (Geisler-Lee et al. Citation2007), no in-depth analysis of this network, especially at the organelle and/or sub-organelle level, has been conducted. Although predictions can inform future research, the prediction method used can limit the utility of the results. An analysis and summary of current research findings on known interactions should provide more accurate information to guide future research. In addition, the construction of a network diagram with known interactions for the model plant Arabidopsis will help to inform further research on crop plants.
The nucleus is the largest and most important eukaryotic cell structure (Janota et al. Citation2020). It is the regulatory center of cell genetics and metabolism and consists of a nuclear envelope (the outer nuclear membrane, inner nuclear membrane, nuclear pore membrane, and embedded nuclear pore complexes), a nuclear lamina, chromosomes, a nucleolus, and other nuclear bodies. Each of these substructures performs specific functions, yet all of them are connected, and the proteins of different substructures interact with one another (Petrovska et al. Citation2015; Meier et al. Citation2017). Many of these aspects are less understood for the nuclei of plants than for those of animals and fungi. Thus, the systematic analysis of the nuclear-protein interactome can aid the understanding of nucleus-based plant biological processes. Like all proteins, nuclear proteins are synthesized in the cytoplasm and then transported to the nucleus with the help of the nuclear pore complex, which acts as a barrier between the cytoplasm and nuclear membrane. The import and export of proteins through the nuclear pore complex play fundamental roles in gene regulation and other biological functions (Freitas and Cunha Citation2009; Juhlen and Fahrenkrog Citation2018). Different organelles have a unique significance in cells, so as proteins in different organelles. In the preliminary work, we statistically analyzed the PPI network of all organelles in Arabidopsis. It was found that the reviewed proteins in nucleus accounts for 37.8% of the total reviewed proteins in Arabidopsis, and the protein interaction items in nucleus accounts for 69.7% of the total. However, the reviewed proteins and related interactions in other organelles were lower than 22% and 37.8%, respectively. From the current research progress and data volume, the nucleus is more suitable for statistical analysis for the study of PPI network for a single organelle. The Universal Protein Resource (UniProt; https://www.uniprot.org/) is a well-known protein database. The protein knowledge database UniProtKB is a major UniProt component that contains information compiled from manual reading and computer extraction from the academic literature and other databases, including data on Gene Ontology annotation, species names and classifications, and protein subcellular localizations, interactions, processing, modification, and expression. Currently, approximately 35,000 genes encoding 172,139 proteins in Arabidopsis are listed in UniProtKB. Among Arabidopsis organelles, the nucleus has the largest number of proteins listed in UniProt. For this study, we retrieved all nuclear proteins (n = 14,529, including 9,777 unreviewed and 3,580 reviewed proteins) of Arabidopsis from UniProt (Table S1). To obtain more accurate and reliable results from molecular mechanism analysis, we screened the reviewed nuclear proteins of Arabidopsis and their interaction partners. Based on the results, we performed related bioinformatics analyses, focusing on the interactions and pathways in which these proteins are involved.
Materials and methods
Data acquisition
We searched for Arabidopsis nuclear proteins on UniProt on 2 February 2021, and downloaded data on the reviewed proteins. We identified 3580 reviewed proteins, and retrieved information on their interactions from the ‘Subunit structure’ subsection of the ‘Interaction’ section in UniProt. This subsection contains information on protein quaternary structure and interaction with other proteins and protein complexes (PPIs, host–pathogen PPIs, and protein–complex interactions, with the exception of physiological receptor–ligand interactions, which are annotated in the ‘Function’ section). Interacting proteins from other species, such as those participating in host–pathogen PPIs, were excluded from the analysis. Interactions involving proteins from closely related species (due to the ‘By similarity’ parameter) were also excluded; only interactions associated with ‘Publications’ were retained for further analysis.
Nuclear-protein interactome network visualization
Reviewed Arabidopsis nuclear proteins that were classified as participating in protein interactions were used for protein interactome network construction with the network visualization program Cytoscape 3.8.1 (Shannon et al. Citation2003). Duplicate interactions, which would cause self-looping, were removed before network construction. The network topology was analyzed using the Network Analyzer plug-in (Assenov et al. Citation2008a).
Biological pathway analysis of proteins in the nuclear-protein interactome
Proteins are the basic components of biological pathways, and PPIs play fundamental roles in these pathways. The KEGG is a database resource for the elucidation of high-level functions and utilities of biological systems, such as the cell, organism, and ecosystem, from molecular-level information, especially large-scale molecular datasets generated via genome sequencing and other high-throughput experimental technologies (Kanehisa Citation2002). At present, the KEGG contains data on 70 biological pathways related to Arabidopsis that involve 2005 proteins in the Arabidopsis proteome. Arabidopsis nuclear proteins and their interaction partners were reorganized and subjected to KEGG pathway analysis.
Results and discussion
Overview of the nuclear-protein interactome network
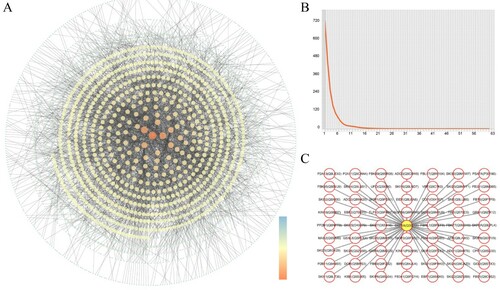
Among 3580 reviewed nuclear proteins of Arabidopsis (Table S1), 2005 nuclear proteins (Table S2) were found to have 3169 interactions (Table S3). These binary interactions were used to construct a nuclear-protein interactome network via Cytoscape. More than 80% of the proteins form an internal core network, and some proteins also participate in several sub-networks ((A)). The proteins in this core network have high connectivity, whereas independent distributions of proteins account for a small part of the whole network. The results indicate that Arabidopsis nuclear-protein interactions require in-depth study. A protein’s degree of connectivity in a network is an important topological property that indicates the number of partners with which it interacts (Friedel and Zimmer Citation2007). Protein interactomes typically contain a few highly connected hubs (proteins with >10 partners), numerous smaller hubs (proteins with 3–10 partners), pipes (connecting 2 partners), and free ends (proteins without partners). This distribution reflects an inverse power relationship between nodal frequency and connectivity ((B)), and is similar to those of other small-world network structures, such as social networks and electrical power grids (Watts and Strogatz Citation1998; de Silva et al. Citation2006; Gu et al. Citation2011). In the network, highly connected central nodal proteins in a scale-free PPI network were defined as hub proteins, with numerous interaction partners and high correlation in candidate modules (Ota et al. Citation2016). Hub proteins are considered to play a role in the plant stress response, including transcription factor hubs, protein kinase and phosphatase hubs, ubiquitin system associated hubs, chaperone and co-chaperone hubs, redox signaling hubs. However, there are still some functionally unclear hubs with uncharacterized or unraveled function in the plant stress response, which could be potential research targets for the next step (Vandereyken et al. Citation2018). These nodal proteins are conserved across different organisms (Batada et al. Citation2006; Zotenko et al. Citation2008; Ning et al. Citation2010). In this network, the average degree (AD) is 3.56, which is close to those of the Arabidopsis (AD = 4) (Consortium, 2011) and C. elegans (AD = 4) interactome maps (Li et al. Citation2004), but lower than those of D. melanogaster (AD = 7) (Giot et al. Citation2003), S. cerevisiae (AD = 61) (Assenov et al. Citation2008b), and H. sapiens (AD = 23). The current network’s AD is also lower than those of predicted rice (AD = 29) (Zhu et al. Citation2011) and maize (AD = 21.8) interactome networks (Musungu et al. Citation2015).
Highly connected proteins in the nuclear-protein interactome network
Highly connected hubs are reported to represent the most evolutionarily conserved proteins and to form the backbones of core processes (Evlampiev and Isambert Citation2008). In plants, proteins interact with one another to perform functions; thus, the plant protein network should contain key proteins that perform important functions in many life processes. In this study, each of 114 proteins had more than 10 interaction partners, giving rise to 3169 pairs of interacting proteins that are involved in functions such as ubiquitination, energy homeostasis, hormone activity, and cell division (Table S2). SKP1-like protein 1A (SKP1A; Q39255), which belongs to the ubiquitin protein family, has the highest connectivity in our network, with 63 different protein partners ((C)). Other SKP1 proteins (e.g. Q9FHW7, 50 partners; O49484, 21 partners; Q9M1X5, 14 partners) also form highly connected hubs. The SKP1 protein is involved in ubiquitination and subsequent proteasomal degradation of target proteins. Together with CUL1, RBX1, and an F-box protein, SKP1 forms SCF E3 ubiquitin ligase complexes, such as SCFUFO(Wang et al. Citation2003), SCFTIR1 (Salehin et al. Citation2015; Dezfulian et al. Citation2016), SCFCOI1 (Van der Does et al. Citation2013; Yan et al. Citation2013), SCFEID1 (Marrocco et al. Citation2006), SCFAFR (Harmon and Kay Citation2003), SCFORE9 (Woo et al. Citation2001), and SCFEBF1/EBF2 (Dong et al. Citation2017; Pham et al. Citation2018). These E3 ligases interact with multiple key pathway proteins to participate in 26S proteasome-mediated ubiquitination, thereby regulating the cell cycle, proliferation, apoptosis, differentiation, transfer, gene expression, transcription regulation, signal transmission, damage repair, and many other life processes. These key roles could explain the frequent presence of ubiquitination-related proteins among highly interacting proteins. Similarly, in the predicted interactome networks of rice and maize, the putative ubiquitin family proteins Q6H7T6 and K7U7X5 interact with the largest numbers of proteins (Zhu et al. Citation2011; Musungu et al. Citation2015). These results indicate that the ubiquitin pathway is highly conserved in eukaryotes and plays key roles in plant growth and development.
Other highly connected proteins in the network are SNF1-related protein kinase catalytic subunit alpha (KIN10; Q38997, 49 partners), IAA16 (O24407, 36 partners), CDKA1 (P24100, 34 partners), TPL (Q94A17, 33 partners), and SPL (O81836, 31 partners; , Table S2). KIN10 is a catalytic subunit of the probable trimeric SNF1-related protein kinase (SnRK) complex, which is a central regulator of cellular energy homeostasis involved in the response to darkness, sugar levels, and stress conditions (which are seemingly unrelated); activation of energy-producing pathways; and inhibition of energy-consuming processes (Farras et al. Citation2001; Tsai and Gazzarrini Citation2012; Zhai et al. Citation2017). The overexpression of AtKIN10 leads to delayed leaf senescence; enhanced tolerance to nutrient starvation, drought, and submergence; and the inhibition of sucrose-induced hypocotyl elongation in Arabidopsis (Chen et al. Citation2017; Soto-Burgos and Bassham Citation2017; Noriane et al. Citation2018). Aux/IAA proteins are short-lived transcriptional factors that function as repressors of early auxin response genes at low auxin concentrations. Repression is thought to result from the interaction with auxin response factors, proteins that bind to the auxin-responsive promoter element (Liscum and Reed Citation2002). CDKA1 is involved in the control of the cell cycle, cell morphogenesis, and cell proliferation (Hemerly et al. Citation2000), and is essential for G1/S and G2/M phase transitions in mitosis (Zhao et al. Citation2012). The CDKD-dependent activation of CDKA1 controls microtubule dynamics and cytokinesis during meiosis (Takatsuka et al. Citation2015). In addition, the CDK inhibitor SIAMESE targets CDKA1 and CDKB1 complexes to induce endoreplication in trichomes(Wang et al. Citation2020). During plant meiosis, the Arabidopsis Cdk1/Cdk2 homolog CDKA controls chromosome axis assembly (Wijnker et al. Citation2019; Yang et al. Citation2020). Due to redundancy with the other TPR proteins, TPL regulates apical embryonic fate in Arabidopsis (Long et al. Citation2006), mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis (Szemenyei et al. Citation2008), and interacts with GIR1 and GIR2 to promote histone deacetylation of target chromatin (Wu and Citovsky Citation2017). SPL acts as an adapter-like transcriptional repressor, recruiting TPL/TPR corepressors to inhibit TCP transcription factors required for nucellus and embryo sac development. It is also involved in the establishment of the prospective chalaza of the ovule and control of funiculus cell number and length (Balasubramanian and Schneitz Citation2000, Citation2002). Additionally, during ovule development, SPL plays a central role in generating patterns related to the proximo–distal and adaxial-abaxial axes (Bencivenga et al. Citation2012; Wei et al. Citation2015).
Biological pathways involved by proteins in the nuclear-protein interactome
PPIs are the basic elements of biological pathways and play fundamental roles in almost all biological processes, the identification of PPI subnetworks in a given biological process is vital to achieve a better understanding of that process and how it connects to other processes (Szklarczyk et al. Citation2019; Xiong et al. Citation2020). Arabidopsis data were derived from the most comprehensive biological pathway database available, the Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.kegg.jp/kegg/tool/map_pathway1.html) (Kanehisa Citation2002). High-frequency protein analysis showed that these proteins are involved mainly in ubiquitination, hormones, and the cell cycle, and thus affect a variety of metabolic pathways. KEGG analysis of the pathways of the 3169 pairs of protein interactions was performed. In this study, all KEGG pathways refer to nuclear interaction related proteins from Arabidopsis. Taking each pair of interacting proteins as an object, 2005 proteins, including Arabidopsis nuclear proteins and their partners, were matched to 70 pathways (Table S4). Plant hormone signal transduction (PHST) pathways had the greatest coverage of target protein interactions, including the biosynthesis and metabolism of auxin (IAA), cytokinin (CYT), gibberellic acid (GA), abscisic acid (ABA), brassinosteroid (BR), jasmonic acid (JA), salicylic acid (SA), and ethylene (ETH). These signal transduction pathways are diverse and sophisticated; they control many developmental and physiological responses, including pistil development (Li et al. Citation2020), reproductive growth (Guo et al. Citation2020), early ripening (Nawaz et al. Citation2020), and homeotic transformation from stamen to petal (Jing et al. Citation2020). Although only terrestrial plants have complete nuclear auxin response systems (Mutte et al. Citation2018), individuals of charophycean algae (which lack full response systems) responded to auxin by activating and repressing hundreds of genes within 1 h (Ohtaka et al. Citation2017), suggesting that this response involved an alternative pathway incorporating other functions (e.g. PPIs). Plant hormone signaling crosstalk can occur in response to biotic and abiotic stresses (Altmann et al. Citation2020; Blazquez et al. Citation2020). A secondary pathway related to the Arabidopsis nuclear-protein interactome is plant–pathogen interaction (PPAI), which also interacts with the MAPK signaling pathway.
Among hormone signaling mechanisms, those of auxin, JA, and GA involve hormone-activated targeting of transcriptional regulators for degradation, with the primary mechanism involving an Skp1/Cullin/F-box-type ubiquitin ligase (Blazquez et al. Citation2020). In addition to the control of signal transduction pathways (Kelley and Estelle Citation2012), the ubiquitin proteolytic system plays important roles in activities such as the regulation of the cell cycle (Marrocco et al. Citation2010) and the modulation of plant immunity (Zhang et al. Citation2014). In our KEGG analysis, the ubiquitin-mediated proteolysis (UBMP) pathway was ranked fourth. As proteins are processed and modified mainly in the endoplasmic reticulum (ER) (Brandizzi Citation2018; Afrin et al. Citation2020), the protein processing in endoplasmic reticulum (PPER) pathway also comprises a major proportion of the network, given the functions of the nucleus in transcription and translation.
Metabolic pathways are complex and essential to life processes (Tatsis and O'Connor Citation2016; Kumari et al. Citation2020; Zhang et al. Citation2020). Metabolic pathways ranked third in the KEGG analysis here (Table S4), with pathway modules carotenoid biosynthesis, zeatin biosynthesis, fatty acid biosynthesis and degradation, fructose and mannose metabolism, photosynthesis, carbon fixation in photosynthetic organisms, the TCA cycle, oxidative phosphorylation, glutathione metabolism, nitrogen metabolism, amino-acid metabolism, and starch and sucrose metabolism (Figure S1). There are evidences showed that nuclear metabolism has a link with cell fate (Yanes et al. Citation2010; Boon et al. Citation2020b). Metabolism epigenetically determines cellular fate (Boon et al. Citation2020a). Methylation of DNA and histones, and acetylation of histones contribute to chromatin dynamics. Histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. Histones undergo posttranslational modifications that alter their interaction with DNA and nuclear proteins. Here, there are 120 histone related proteins in the reviewed nuclear proteins of Arabidopsis, including histone-lysine N-methyltransferase, lysine-specific demethylase, histone acetyltransferase, H3, H2A, H2B, H1, and were found to have 139 interaction items. Among them, histone-lysine N-methyltransferase family member SUVH2 (O22781) together with MORC6 and SUVH9 regulates the silencing of some transposable elements (Liu et al. Citation2016). SUVH2 interacts with DNA-directed RNA polymerase V subunit NRPE1 and with DRD1 and DMS3 (Johnson et al. Citation2014; Liu et al. Citation2014). It is required for normal methylation of ‘Lys-9’ and ‘Lys-27’ of histone H3, ‘Lys-20’ of H4, and cytosine (Naumann et al. Citation2005), but there is no significant effect on histone methylation when the gene is mutated (Johnson et al. Citation2008). SUVH2 does not bind S-adenosyl-L-methionine and lacks methyltransferase activity (Johnson et al. Citation2008). Instead, it may function downstream of DRM2 in RNA-directed DNA methylation, binding to methylated DNA and recruiting DNA-directed RNA polymerase V to chromatin (Johnson et al. Citation2014; Liu et al. Citation2016). For the metabolic pathways here, although most pathways were enriched, some were not, implying that further research is needed. The unenriched metabolism pathways may function in other organelles, as we screened only the proteins interacting in the nucleus.
Co-pathway nuclear proteins play key roles in various biological pathways
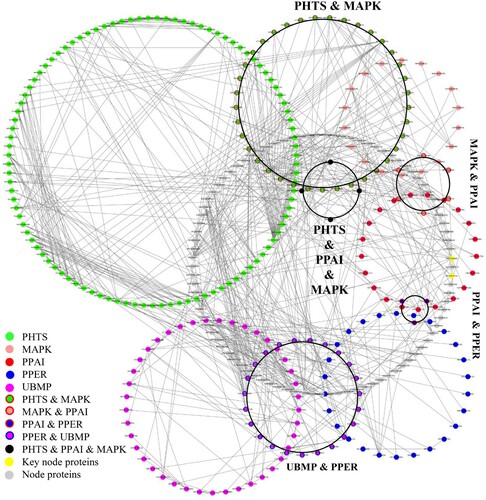
To further analyze the relationships between Arabidopsis nuclear proteins and their interaction partners, we selected the top five pathways for deep analysis, inlcuding PHST (135 proteins), PPAI (36 proteins), UBMP (65 proteins), PPER (54 proteins), and MAPK (65 proteins). In addition to the proteins involved in these pathways, there are nodal proteins among these five pathways and acting as bridge connecting them directly or indirectly. The shortest protein-interaction paths were selected to reveal the connections among these pathways and communities (). Among them, 90 proteins were involved only in the PHST pathways, 23 proteins were involved only in the PPAI pathway, 42 proteins were involved only in the UBMP pathway, 25 proteins were involved only in the PPER pathway, and 14 were involved only in the MAPK pathway. In addition, four proteins are shared by the PHST, PPAI, and MAPK pathways, 37 proteins are shared by the PHST and MAPK pathways, six proteins are shared by the MAPK and PPAI pathways, three proteins are shared by the PPAI and PPER, and 23 proteins are shared by the UBMP and PPER pathways (). The data revealed direct correlations between the PHST and MAPK, MAPK and PPAI, PPAI and PPER, and UBMP and PPER pathways. Interestingly, the UBMP pathway has no direct relationship with PHST pathways through co-pathway proteins (). This finding raised the question of whether a pathway starts with a signal and ends with ubiquitin, or whether the result is an artifact of the analytical procedure. Hence, we further analyzed the functions of co-pathway proteins.
Figure 2. A subnetwork of top five pathways of Arabidopsis nuclear proteins. Co-pathway proteins were circled together.
Total 41 proteins were commonly involved in PHST and MAPK pathways, including M2K4, M2K5, MPK6, P2C03, SRK2A/C/D/E/I, etc (Table S6). ABA differentially regulates the expression of MAPK cascade components and induces the activation of MAPKs (Danquah et al. Citation2014). MAPK cascades are also involved in several ABA responses, including antioxidant defense, guard-cell signaling, and seed germination (Xing et al. Citation2008; Jammes et al. Citation2009; Zong et al. Citation2009). In plants, PPC2s are inhibited when ABA binds to PYR/PYL/RCAR receptors, thereby allowing the activation of SnRK2s. Active SnRK2 kinases phosphorylate downstream AREB/ABF transcription factors and subsequently activate MAP3 K transcription and the next MKK3 module. ABA-induced activation of the MKK3 module may also be due to preformed MAP3Ks, which are first triggered by ABA production and then activated rapidly and directly, possibly by autophosphorylation or SnRK2s, resulting in the reinforcement of plant responses (Danquah et al. Citation2014; Choi et al. Citation2017). MYCs are key transcriptional regulators of JA-mediated gene expression (Fernandez-Calvo et al. Citation2011; Wang et al. Citation2017; Ortigosa et al. Citation2020). The repression of MYC activity is required for the resetting of JA signaling and to avoid harmful runaway responses, thereby maintaining plant fitness. Wounding induces the MAPK signaling module MAP3K14-MKK3-MPK1/MPK2/MPK7, which depends on JA signaling through the transcriptional regulation of MAP3K14 and has a slower response than the MKK4/5-MPK3/6 cascade (Horak Citation2020).
Total of 10 proteins, including BAK1, M2K4, CALM1, and RBOHD, are shared between the PPAI and MAPK pathways (Table S6). When plant cells sense stress, signals are amplified by secondary messengers such as calcium, reactive oxygen species (ROS), and some protein kinases. BAK1 functions in pathogen-associated molecular pattern-triggered immunity via its interactions with EFR and FLS2 (Roux et al. Citation2011). It is also involved in BR signal transduction (Kemmerling et al. Citation2007), positive regulation of the BR-dependent plant growth pathway, and negative regulation of the BR-independent cell-death pathway (He et al. Citation2007). Recent studies have also shown that BAK1 is involved in plant immunity (Ma et al. Citation2020) via a MAPK signaling cascade downstream of the bacterial flagellin receptor FLS2. The MKK3-MPK7 module was reported to play a role during PPAIs. MPK7 is activated by ROS, which are produced when plants are challenged with pathogenic bacteria, or by drought-induced ABA (Danquah et al. Citation2015). ROS are secondary messengers that activate Ca2+-permeable channels (Kohler et al. Citation2003), and the resulting elevated cytosolic Ca2+ levels activate S- and R-type anion channels (Roelfsema et al. Citation2004). ABA can induce ROS via mechanisms that involve the NADPH oxidases AtRBOHD and AtRBOHF in guard cells (Kwak et al. Citation2003). Although the ROS produced by the NADPH oxidases RBOHD and RBOHF seem to be involved in both pathways, RBOHD responds only to pathogens and RBOHF only to ABA signals (Mersmann et al. Citation2010). These results indicate that hormones and the MAPK pathway are closely linked, and that both participate in the plant pathogen response.
The heat shock proteins (HSPs), such as HS905 (Q9SIF2), HS906 (F4JFN3) and ENPL (Q9STX5), are common in the PPAI and PPER pathways (Table S6). HSPs/chaperones play critical roles in biogenesis, maturation, and plant immunity (Li et al. Citation2009; Park and Seo Citation2015). ER-localized HSP90 is involved in the resistance to tunicamycin- and high calcium-induced ER stresses (Chong et al. Citation2015). In addition, 23 proteins in the ubiquitin ligase complex, including UbcH5, CHIP, RBX1, Cul1, and Skp1 (Table S3), are frequently involved in the UBMP and PPER pathways for ER-associated degradation in the cytoplasm. The ER is the site of protein processing and modification; there, proteins are folded with the help of lumenal chaperones and then shuttled to the Golgi complex by transport vesicles (Hanton et al. Citation2005; Robinson et al. Citation2015; Brandizzi Citation2018). Misfolded, defective, and superfluous proteins are retained in the ER and undergo degradation via the proteasome in an ER-associated process (Asano et al. Citation2003; Mehrtash and Hochstrasser Citation2019; Zhao et al. Citation2020). Alternatively, ER stress can result in the unfolded protein response (Liu and Howell Citation2010; Howell Citation2013; Afrin et al. Citation2020). As ubiquitination is an important post-translational modification that is involved mainly in protein degradation, the close relationship between the UBMP and PPER pathways makes sense.
In addition to co-pathway proteins bound tightly to their associated pathways, some key nodal proteins are associated closely with given pathways (, Table S7). Among these nodal proteins, PHYA (P14712) and KIN10 (Q38997) stand out. PHYA interacts directly with COP1 (P43254, UBMP), NDK2 (O64903, MAPK), IAA26 (Q8LAL2, PHST), and IAA27 (Q9ZSY8, PHST), whereas KIN10 interacts directly with SCE1 (Q42551, UBMP), SKP1A (Q39255, PPER and UBMP), P2C56 (P49592, PHST), EIN3 (O24606, MAPK), P2C37 (P49598, PHST and MAPK), and MYC2 (Q39204, PHST and MAPK). Thus, some pathways that are not linked by co-pathway proteins are bound together by nodal proteins. This result indicates that different biological pathways do not function independently. Upon exposure to biotic and/or abiotic stress, plants quickly transmit signals through the MAPK or PHST pathways, and the stress response is activated through cooperative pathways such as the UBMP and PPER pathways. During this process, co-pathway nuclear proteins and nodal proteins play critical roles; further exploration of this topic is warranted.
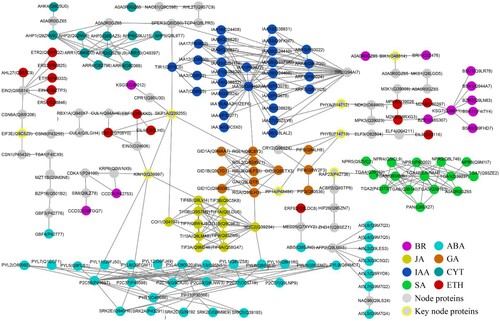
Multi-location proteins are key links among different hormone pathways
Among the pathways involved by Arabidopsis nuclear proteins, PHST pathways rank first. The shortest protein-interaction paths were selected to show the connections among eight hormone pathways (). Overall, most proteins were relatively concentrated in the pathways of ABA, JA, SA, GA, IAA, and CYT. At the protein level, however, some proteins were located far from their own pathways (especially the ETH and BR pathways), due to unknown interactions or loose linkages. Various hormonal pathways are closely linked. For example, the IAA and GA pathways are linked directly by the interaction between IAA16 (O24407) and RGL1 (Q9C8Y3), the GA and JA pathways are linked directly by the interaction between RGA (Q9SLH3) and MYC2 (Q39204), and MYC2 (Q39204) of the JA pathway interacts directly with PYL6 (Q8S8E3) of the ABA pathway. In addition, some key nodal proteins, especially SKP1A, KIN10, PHYA, PHYB, PP14, RAP23, BIK1, and EIF3E, play important roles in communication among hormonal pathways. For example, SKP1A, which interacts directly with GID2 of the GA pathway, COI1 of the JA pathway, TIR1 of the IAA pathway, and EBF1 and EBF2 of the ETH pathway, also interacts indirectly with the BR and ABA pathways. Altogether, SKP1A affects six hormonal pathways directly or indirectly. PHYA and PHYB efficiently connect the IAA, GA, ETH, and BR pathways. Further analysis revealed that all of these key nodal proteins are multi-localized. For instance, KIN10 is multi-localized in the chloroplast, nucleus, Golgi, and ER; SKP1A is multi-localized in the nucleus and cytoskeleton; and PHYA and PHYB are multi-localized in the nucleus and cytoplasm. PHYA is a regulatory photoreceptor that exists in two reversibly interconvertible forms (Pr and Pfr), which exhibit maximal absorption in the red and far-red regions of the light spectrum, respectively. Photoconversion of Pr to Pfr induces an array of morphogenetic responses, whereas the conversion of Pfr to Pr cancels the induction of those responses. Pfr controls the expression of several nuclear genes, including those encoding the small subunit of ribulose-bisphosphate carboxylase, chlorophyll A/B-binding protein, protochlorophyllide reductase, and rRNA. It also controls the expression of its own gene(s) in a negative feedback fashion. PHYA is involved in the regulation of flowering time (Kim et al. Citation2002). It can phosphorylate FHY1, and possibly FHL, under red-light conditions, inactivating the co-shuttling of the proteins into the nucleus (Shen et al. Citation2009). PHYA also regulates phototropic responses in the nucleus (e.g. hypocotyl elongation and cotyledon opening under high-irradiance conditions and seed germination under very-low-fluence conditions) and the cytoplasm (e.g. negative gravitropism in blue light and red-enhanced phototropism) (Rosler et al. Citation2007; Sokolova et al. Citation2012). So far, there have been 557 reports related to Arabidopsis PHYA in TAIR (https://www.arabidopsis.org/servlets/Search?type=publication&search_action=search&locus_tair_object_id=2012300&locus_name=AT1G09570&sort=date). Thus, we hypothesized that multi-location proteins play important roles in plant growth and development. We performed a subcellular localization analysis of 114 high-frequency (AD > 10) proteins, which showed that more than 40% proteins were multi-localized (Table S8). Thus, multi-localized protein screening may be effective for the investigation of critical proteins and genes.
Application of what is known about PHST pathway in Arabidopsis to crop research
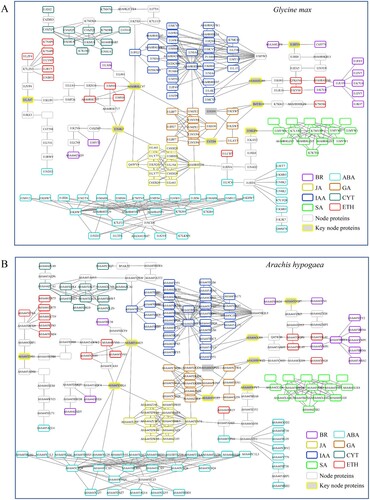
Among the pathways involved by Arabidopsis nuclear proteins, PHST pathways rank first. Homologous proteins related to PHST pathways were screened and identified from dicotyledonous peanut (Arachis hypogaea) and soybean (Glycine max) (), and monocotyledonous crop plants rice (Oryza sativa, Figure S2), wheat (Triticum aestivum, Figure S3), and maize (Zea mays, Figure S4). According to the reports, less information about verified PPI was obtained from the above crops, especially in peanut and soybean (data not shown). The comparison of known protein interactions between crops and Arabidopsis revealed that much remains unknown about this topic in crop. Here, the establishment of PHST pathway interactome through homologous comparison could provide foundational information for the study of protein interactions in crops.
Conclusion and perspective
The reviewed nuclear proteins of Arabidopsis and their interaction partners are mainly involved mainly in the PHST, PPAI, UBMP, PPER, MAPK, and metabolic pathways. Importantly, these pathways also interact with one another. Proteins, such as KIN10 and PHYA, function as key nodes or link other proteins through the pathways. Most of these proteins are localized in multiple organelles and/or cellular compartments and play key roles in organelle communication and plant growth and development. Thus, the screening and study of multi-location proteins seem important in the near future. In addition, in PHST pathways in Arabidopsis, ABA and JA, JA and GA, and BR and ETH are connected directly, whereas ABA, IAA, BR, and GA are connected through ubiquitination. Finally, we compared pathway proteins in monocotyledonous and dicotyledonous crops to derive basic information and generate ideas for further research in this field. In this study, we constructed the nuclear-protein interaction network of Arabidopsis and determined the relationships between the PHST, PPAI, UBMP, PPER, and MAPK pathways through step-by-step analysis. Our results indicate the importance of co-pathway proteins and multi-organelle-localized proteins and could provide new directions for plant proteomics research.
Supplemental Material
Download Zip (29.3 MB)Acknowledgements
XEH and GFP conceived the idea of this research. GFP and XEH wrote the original manuscript. XEH, CD and GFP processed and analyzed the data, and CD participated in the proofreading of all data in the revised draft. YDM and ZQZ revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Fangping Gong
Fangping Gong is a doctor at College of Agronomy, Henan Agricultural University (HAU). Her research interests lie in plant proteomics and bioinformatics.
Di Cao
Di Cao is a postdoctoral fellow at College of Agronomy, HAU, China. He conducts proteomics experiments.
Chengxin Qu
Chengxin Qu is a master student at College of Agronomy, HAU. His research interests lie in plant molecular genetics and bioinformatics.
Dongmei Yin
Dongmei Yin is professor at HAU. Her research focuses on elucidating the mechanisms of the adaptations of peanut to environmental stresses and yield characters using molecular, omics and bioinformatics approaches. She has published many articles in the top magazine, such as Advanced Science and Sci Rep.
Quanzhi Zhao
Quanzhi Zhao is a professor at HAU. His research focuses on elucidating the mechanisms of the adaptations of rice to environmental stresses and yield characters using molecular, omics and bioinformatics approaches. He has published many articles in the top magazine, such as Briefings in Bioinformatics and Molecular plant.
Erhui Xiong
Erhui Xiong is a doctor at College of Agronomy, HAU, China. He investigates specific protein functions and interactions involved in rice leaf senescence using genetics, proteomics and bioinformatics.
References
- Afrin T, Diwan D, Sahawneh K, Pajerowska-Mukhtar K. 2020. Multilevel regulation of endoplasmic reticulum stress responses in plants: where old roads and new paths meet. J Exp Bot. 71(5):1659–1667. doi:10.1093/jxb/erz487.
- Altmann M, Altmann S, Rodriguez PA, Weller B, Vergara LE, Palme J, la Rosa NMD, Sauer M, Wenig M, Villaecija-Aguilar JA, et al. 2020. Extensive signal integration by the phytohormone protein network. Nature. 12:631. doi:10.1038/s41586-020-2585-1.
- Asano S, Kimura T, Ishizuka H, Morii M, Takeguchi N. 2003. Quality control of gastric proton pump in the endoplasmic reticulum by ubiquitin/proteasome system. Ann N Y Acad Sci. 986:655–657. doi:10.1111/j.1749-6632.2003.tb07278.x.
- Assenov Y, Ramirez F, Schelhorn SE, Lengauer T, Albrecht M. 2008a. Computing topological parameters of biological networks. Bioinformatics. 24(2):282–284. doi:10.1093/bioinformatics/btm554.
- Assenov Y, Ramirez F, Schelhorn SE, Lengauer T, Albrecht M. 2008b. Computing topological parameters of biological networks. Bioinformatics. 24(2):282–284. doi:10.1093/bioinformatics/btm554.
- Balasubramanian S, Schneitz K. 2000. NOZZLE regulates proximal-distal pattern formation, cell proliferation and early sporogenesis during ovule development in Arabidopsis thaliana. Development. 127(19):4227–4238.
- Balasubramanian S, Schneitz K. 2002. NOZZLE links proximal-distal and adaxial-abaxial pattern formation during ovule development in Arabidopsis thaliana. Development. 129(18):4291–4300.
- Batada NN, Hurst LD, Tyers M. 2006. Evolutionary and physiological importance of hub proteins. Plos Comput Biol. 2(7):e88. doi:10.1371/journal.pcbi.0020088.
- Bencivenga S, Simonini S, Benkova E, Colombo L. 2012. The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in arabidopsis. Plant Cell. 24(7):2886–2897. doi:10.1105/tpc.112.100164.
- Blazquez MA, Nelson DC, Weijers D. 2020. Evolution of plant hormone response pathways. Annu Rev Plant Biol. 71:327–353. doi:10.1146/annurev-arplant-050718-100309.
- Boon R, Kumar M, Tricot T, Elia I, Ordovas L, Jacobs F, One J, De Smedt J, Eelen G, Bird M, et al. 2020a. Amino acid levels determine metabolism and CYP450 function of hepatocytes and hepatoma cell lines. Nat Commun. 11(1):1393. doi:10.1038/s41467-020-15058-6.
- Boon R, Silveira GG, Mostoslavsky R. 2020b. Nuclear metabolism and the regulation of the epigenome. Nat Metab. 2(11):1190–1203. doi:10.1038/s42255-020-00285-4.
- Brandizzi F. 2018. Transport from the endoplasmic reticulum to the Golgi in plants: where are we now? Semin Cell Dev Biol. 80:94–105. doi:10.1016/j.semcdb.2017.06.024.
- Chen L, Su ZZ, Huang L, Xia FN, Qi H, Xie LJ, Xiao S, Chen QF. 2017. The AMP-activated protein kinase KIN10 is involved in the regulation of autophagy in arabidopsis. Front Plant Sci. 8. doi:ARTN 120110.3389/fpls.2017.01201.
- Chen XW, Liu M. 2005. Prediction of protein-protein interactions using random decision forest framework. Bioinformatics. 21(24):4394–4400. doi:10.1093/bioinformatics/bti721.
- Choi SW, Lee SB, Na YJ, Jeung SG, Kim SY. 2017. Arabidopsis MAP3K16 and other salt-inducible MAP3Ks regulate ABA response redundantly. Mol Cells. 40(3):230–242. doi:10.14348/molcells.2017.0002.
- Chong LP, Wang Y, Gad N, Anderson N, Shah B, Zhao R. 2015. A highly charged region in the middle domain of plant endoplasmic reticulum (ER)-localized heat-shock protein 90 is required for resistance to tunicamycin or high calcium-induced ER stresses. J Exp Bot. 66(1):113–124. doi:10.1093/jxb/eru403.
- Danquah A, de Zelicourt A, Boudsocq M, Neubauer J, Frei Dit Frey N, Leonhardt N, Pateyron S, Gwinner F, Tamby JP, Ortiz-Masia D, et al. 2015. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 82(2):232–244. doi:10.1111/tpj.12808.
- Danquah A, de Zelicourt A, Colcombet J, Hirt H. 2014. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv. 32(1):40–52. doi:10.1016/j.biotechadv.2013.09.006.
- de Silva E, Thorne T, Ingram P, Agrafioti I, Swire J, Wiuf C, Stumpf MP. 2006. The effects of incomplete protein interaction data on structural and evolutionary inferences. BMC Biol. 4:39. doi:10.1186/1741-7007-4-39.
- Dezfulian MH, Jalili E, Roberto DK, Moss BL, Khoo K, Nemhauser JL, Crosby WL. 2016. Oligomerization of SCFTIR1 Is essential for Aux/IAA degradation and auxin signaling in arabidopsis. PLoS Genet. 12(9):e1006301. doi:10.1371/journal.pgen.1006301.
- Dong J, Ni W, Yu R, Deng XW, Chen H, Wei N. 2017. Light-dependent degradation of PIF3 by SCF(EBF1/2) promotes a photomorphogenic response in arabidopsis. Curr Biol. 27(16):2420–2430. e2426. doi:10.1016/j.cub.2017.06.062.
- Evlampiev K, Isambert H. 2008. Conservation and topology of protein interaction networks under duplication-divergence evolution. Proc Natl Acad Sci U S A. 105(29):9863–9868. doi:10.1073/pnas.0804119105.
- Farras R, Ferrando A, Jasik J, Kleinow T, Okresz L, Tiburcio A, Salchert K, del Pozo C, Schell J, Koncz C. 2001. SKP1-SnRK protein kinase interactions mediate proteasomal binding of a plant SCF ubiquitin ligase. EMBO J. 20(11):2742–2756. doi:10.1093/emboj/20.11.2742.
- Fernandez-Calvo P, Chini A, Fernandez-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, et al. 2011. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell. 23(2):701–715. doi:10.1105/tpc.110.080788.
- Freitas N, Cunha C. 2009. Mechanisms and signals for the nuclear import of proteins. Curr Genomics. 10(8):550–557. doi:10.2174/138920209789503941.
- Friedel CC, Zimmer R. 2007. Influence of degree correlations on network structure and stability in protein-protein interaction networks. BMC Bioinformatics. 8:297. doi:10.1186/1471-2105-8-297.
- Geisler-Lee J, O'Toole N, Ammar R, Provart NJ, Millar AH, Geisler M. 2007. A predicted interactome for arabidopsis. Plant Physiol. 145(2):317–329. doi:10.1104/pp.107.103465.
- Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, et al. 2003. A protein interaction map of Drosophila melanogaster. Science. 302(5651):1727–1736. doi:10.1126/science.1090289.
- Gu H, Zhu P, Jiao Y, Meng Y, Chen M. 2011. PRIN: a predicted rice interactome network. BMC Bioinformatics. 12:161. doi:10.1186/1471-2105-12-161.
- Guo J, Lu C, Zhao F, Gao S, Wang B. 2020. Improved reproductive growth of euhalophyte suaeda salsa under salinity is correlated with altered phytohormone biosynthesis and signal transduction. Funct Plant Biol. 47(2):170–183. doi:10.1071/FP19215.
- Habibi M, Eslahchi C, Wong L. 2010. Protein complex prediction based on k-connected subgraphs in protein interaction network. BMC Syst Biol. 4:129. doi:10.1186/1752-0509-4-129.
- Hanton SL, Bortolotti LE, Renna L, Stefano G, Brandizzi F. 2005. Crossing the divide–transport between the endoplasmic reticulum and Golgi apparatus in plants. Traffic. 6(4):267–277. doi:10.1111/j.1600-0854.2005.00278.x.
- Harmon FG, Kay SA. 2003. The F box protein AFR is a positive regulator of phytochrome A-mediated light signaling. Curr Biol. 13(23):2091–2096. doi:10.1016/j.cub.2003.11.019.
- Hawkins T, Kihara D. 2007. Function prediction of uncharacterized proteins. J Bioinform Comput Biol. 5(1):1–30. doi:10.1142/s0219720007002503.
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. 2007. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol. 17(13):1109–1115. doi:10.1016/j.cub.2007.05.036.
- Hemerly AS, Ferreira PC, Van Montagu M, Engler G, Inze D. 2000. Cell division events are essential for embryo patterning and morphogenesis: studies on dominant-negative cdc2aAt mutants of arabidopsis. Plant J. 23(1):123–130. doi:10.1046/j.1365-313x.2000.00800.x.
- Horak H. 2020. Defense, fast and slow: activation of different MAPK pathways in response to wounding. Plant Cell. 32(6):1788–1789. doi:10.1105/tpc.20.00282.
- Howell SH. 2013. Endoplasmic reticulum stress responses in plants. Annu Rev Plant Biol. 64:477–499. doi:10.1146/annurev-arplant-050312-120053.
- Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, et al. 2009. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci U S A. 106(48):20520–20525. doi:10.1073/pnas.0907205106.
- Janota CS, Calero-Cuenca FJ, Gomes ER. 2020. The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol. 63:204–211. doi:10.1016/j.ceb.2020.03.001.
- Jing D, Chen W, Xia Y, Shi M, Wang P, Wang S, Wu D, He Q, Liang G, Guo Q. 2020. Homeotic transformation from stamen to petal in Eriobotrya japonica is associated with hormone signal transduction and reduction of the transcriptional activity of EjAG. Physiol Plant. 168(4):893–908. doi:10.1111/ppl.13029.
- Johnson LM, Du J, Hale CJ, Bischof S, Feng S, Chodavarapu RK, Zhong X, Marson G, Pellegrini M, Segal DJ, et al. 2014. SRA- and SET-domain-containing proteins link RNA polymerase V occupancy to DNA methylation. Nature. 507(7490):124–128. doi:10.1038/nature12931.
- Johnson LM, Law JA, Khattar A, Henderson IR, Jacobsen SE. 2008. SRA-domain proteins required for DRM2-mediated de novo DNA methylation. PLoS Genet. 4(11):e1000280. doi:10.1371/journal.pgen.1000280.
- Joseph T, Lee TL, Ning C, Nishiuchi Y, Kimura T, Jikuya H, Ou K, Chin YC, Tachibana S. 2006. Identification of mature nocistatin and nociceptin in human brain and cerebrospinal fluid by mass spectrometry combined with affinity chromatography and HPLC. Peptides. 27(1):122–130. doi:10.1016/j.peptides.2005.06.013.
- Juan D, Pazos F, Valencia A. 2008. High-confidence prediction of global interactomes based on genome-wide coevolutionary networks. P Natl Acad Sci USA. 105(3):934–939. doi:10.1073/pnas.0709671105.
- Juhlen R, Fahrenkrog B. 2018. Moonlighting nuclear pore proteins: tissue-specific nucleoporin function in health and disease. Histochem Cell Biol. 150(6):593–605. doi:10.1007/s00418-018-1748-8.
- Kanehisa M. 2002. The KEGG database. Novartis Found Symp. 247:91–101. discussion 101–103, 119–128, 244–152.
- Kelley DR, Estelle M. 2012. Ubiquitin-mediated control of plant hormone signaling. Plant Physiol. 160(1):47–55. doi:10.1104/pp.112.200527.
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Mussig C, et al. 2007. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol. 17(13):1116–1122. doi:10.1016/j.cub.2007.05.046.
- Kenworthy AK. 2001. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 24(3):289–296. doi:10.1006/meth.2001.1189.
- Khan IK, Kihara D. 2016. Genome-scale prediction of moonlighting proteins using diverse protein association information. Bioinformatics. 32(15):2281–2288. doi:10.1093/bioinformatics/btw166.
- Kim DH, Kang JG, Yang SS, Chung KS, Song PS, Park CM. 2002. A phytochrome-associated protein phosphatase 2A modulates light signals in flowering time control in arabidopsis. Plant Cell. 14(12):3043–3056. doi:10.1105/tpc.005306.
- King AD, Przulj N, Jurisica I. 2004. Protein complex prediction via cost-based clustering. Bioinformatics. 20(17):3013–3020. doi:10.1093/bioinformatics/bth351.
- Klingstrom T, Plewczynski D. 2011. Protein-protein interaction and pathway databases, a graphical review. Brief Bioinform. 12(6):702–713. doi:10.1093/bib/bbq064.
- Kohler B, Hills A, Blatt MR. 2003. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol. 131(2):385–388. doi:10.1104/pp.016014.
- Kumari M, Pandey S, Mishra SK, Giri VP, Agarwal L, Dwivedi S, Pandey AK, Nautiyal CS, Mishra A. 2020. Omics-based mechanistic insight into the role of bioengineered nanoparticles for biotic stress amelioration by modulating plant metabolic pathways. Front Bioeng Biotechnol. 8:242. doi:10.3389/fbioe.2020.00242.
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in arabidopsis. EMBO J. 22(11):2623–2633. doi:10.1093/emboj/cdg277.
- Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, Zipfel C, Jones JD. 2009. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci U S A. 106(37):15973–15978. doi:10.1073/pnas.0905532106.
- Li Q, Zhang L, Pan F, Guo W, Chen B, Yang H, Wang G, Li X. 2020. Transcriptomic analysis reveals ethylene signal transduction genes involved in pistil development of pumpkin. PeerJ. 8:e9677. doi:10.7717/peerj.9677.
- Li S, Armstrong CM, Bertin N, Ge H, Milstein S, Boxem M, Vidalain PO, Han JD, Chesneau A, Hao T, et al. 2004. A map of the interactome network of the metazoan C. elegans. Science. 303(5657):540–543. doi:10.1126/science.1091403.
- Liscum E, Reed JW. 2002. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol. 49(3-4):387–400.
- Liu JX, Howell SH. 2010. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell. 22(9):2930–2942. doi:10.1105/tpc.110.078154.
- Liu ZW, Shao CR, Zhang CJ, Zhou JX, Zhang SW, Li L, Chen S, Huang HW, Cai T, He XJ. 2014. The SET domain proteins SUVH2 and SUVH9 are required for Pol V occupancy at RNA-directed DNA methylation loci. PLoS Genet. 10(1):e1003948. doi:10.1371/journal.pgen.1003948.
- Liu ZW, Zhou JX, Huang HW, Li YQ, Shao CR, Li L, Cai T, Chen S, He XJ. 2016. Two components of the RNA-directed DNA methylation pathway associate with MORC6 and silence loci targeted by MORC6 in arabidopsis. PLoS Genet. 12(5):e1006026. doi:10.1371/journal.pgen.1006026.
- Long JA, Ohno C, Smith ZR, Meyerowitz EM. 2006. TOPLESS regulates apical embryonic fate in arabidopsis. Science. 312(5779):1520–1523. doi:10.1126/science.1123841.
- Ma X, Claus LAN, Leslie ME, Tao K, Wu Z, Liu J, Yu X, Li B, Zhou J, Savatin DV, et al. 2020. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature. 581(7807):199–203. doi:10.1038/s41586-020-2210-3.
- Marrocco K, Bergdoll M, Achard P, Criqui MC, Genschik P. 2010. Selective proteolysis sets the tempo of the cell cycle. Curr Opin Plant Biol. 13(6):631–639. doi:10.1016/j.pbi.2010.07.004.
- Marrocco K, Zhou Y, Bury E, Dieterle M, Funk M, Genschik P, Krenz M, Stolpe T, Kretsch T. 2006. Functional analysis of EID1, an F-box protein involved in phytochrome A-dependent light signal transduction. Plant J. 45(3):423–438. doi:10.1111/j.1365-313X.2005.02635.x.
- McWhite CD, Papoulas O, Drew K, Cox RM, June V, Dong OX, Kwon T, Wan C, Salmi ML, Roux SJ, et al. 2020. A Pan-plant protein complex Map reveals deep Conservation and novel assemblies. Cell. 181(2):460–474. e414. doi:10.1016/j.cell.2020.02.049.
- Mehrtash AB, Hochstrasser M. 2019. Ubiquitin-dependent protein degradation at the endoplasmic reticulum and nuclear envelope. Semin Cell Dev Biol. 93:111–124. doi:10.1016/j.semcdb.2018.09.013.
- Meier I, Richards EJ, Evans DE. 2017. Cell biology of the plant nucleus. Annu Rev Plant Biol. 68:139–172. doi:10.1146/annurev-arplant-042916-041115.
- Mersmann S, Bourdais G, Rietz S, Robatzek S. 2010. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154(1):391–400. doi:10.1104/pp.110.154567.
- Musungu B, Bhatnagar D, Brown RL, Fakhoury AM, Geisler M. 2015. A predicted protein interactome identifies conserved global networks and disease resistance subnetworks in maize. Front Genet. 6:201. doi:10.3389/fgene.2015.00201.
- Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GK, Weijers D. 2018. Origin and evolution of the nuclear auxin response system. Elife. 7. doi:10.7554/eLife.33399.
- Najafabadi HS, Salavati R. 2008. Sequence-based prediction of protein-protein interactions by means of codon usage. Genome Biol. 9(5):R87. doi:10.1186/gb-2008-9-5-r87.
- Naumann K, Fischer A, Hofmann I, Krauss V, Phalke S, Irmler K, Hause G, Aurich AC, Dorn R, Jenuwein T, Reuter G. 2005. Pivotal role of AtSUVH2 in heterochromatic histone methylation and gene silencing in arabidopsis. EMBO J. 24(7):1418–1429. doi:10.1038/sj.emboj.7600604.
- Nawaz I, Tariq R, Nazir T, Khan I, Basit A, Gul H, Anwar T, Awan SA, Bacha SAS, Zhang L, et al. 2020. RNA-Seq profiling reveals the plant hormones and molecular mechanisms stimulating the early ripening in apple. Genomics. doi:10.1016/j.ygeno.2020.09.040.
- Ning K, Ng HK, Srihari S, Leong HW, Nesvizhskii AI. 2010. Examination of the relationship between essential genes in PPI network and hub proteins in reverse nearest neighbor topology. BMC Bioinformatics. 11:505. doi:10.1186/1471-2105-11-505.
- Noriane MLS, Jelena K, Fernandez-Lopez A, Chembath A, Belbin FE, Dodd AN. 2018. The energy-signaling hub SnRK1 is important for sucrose-induced hypocotyl elongation. Plant Physiol. 176(2):1299–1310. doi:10.1104/pp.17.01395.
- Ohtaka K, Hori K, Kanno Y, Seo M, Ohta H. 2017. Primitive Auxin response without TIR1 and Aux/IAA in the Charophyte Alga Klebsormidium nitens. Plant Physiol. 174(3):1621–1632. doi:10.1104/pp.17.00274.
- Ortigosa A, Fonseca S, Franco-Zorrilla JM, Fernandez-Calvo P, Zander M, Lewsey MG, Garcia-Casado G, Fernandez-Barbero G, Ecker JR, Solano R. 2020. The JA-pathway MYC transcription factors regulate photomorphogenic responses by targeting HY5 gene expression. Plant J. 102(1):138–152. doi:10.1111/tpj.14618.
- Ota M, Gonja H, Koike R, Fukuchi S. 2016. Multiple-localization and hub proteins. PLoS One. 11(6):e0156455. doi:10.1371/journal.pone.0156455.
- Park CJ, Seo YS. 2015. Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J. 31(4):323–333. doi:10.5423/PPJ.RW.08.2015.0150.
- Petrovska B, Sebela M, Dolezel J. 2015. Inside a plant nucleus: discovering the proteins. J Exp Bot. 66(6):1627–1640. doi:10.1093/jxb/erv041.
- Pham VN, Kathare PK, Huq E. 2018. Phytochromes and phytochrome interacting factors. Plant Physiol. 176(2):1025–1038. doi:10.1104/pp.17.01384.
- Phee BK, Shin DH, Cho JH, Kim SH, Kim JI, Lee YH, Jeon JS, Bhoo SH, Hahn TR. 2006. Identification of phytochrome-interacting protein candidates in Arabidopsis thaliana by co-immunoprecipitation coupled with MALDI-TOF MS. Proteomics. 6(12):3671–3680. doi:10.1002/pmic.200500222.
- Robinson DG, Brandizzi F, Hawes C, Nakano A. 2015. Vesicles versus tubes: Is endoplasmic reticulum-Golgi transport in plants fundamentally different from other eukaryotes? Plant Physiol. 168(2):393–406. doi:10.1104/pp.15.00124.
- Rodriguez-Negrete E, Bejarano ER, Castillo AG. 2014. Using the yeast two-hybrid system to identify protein-protein interactions. Methods Mol Biol. 1072:241–258. doi:10.1007/978-1-62703-631-3_18.
- Roelfsema MR, Levchenko V, Hedrich R. 2004. ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J. 37(4):578–588. doi:10.1111/j.1365-313x.2003.01985.x.
- Rosler J, Klein I, Zeidler M. 2007. Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc Natl Acad Sci U S A. 104(25):10737–10742. doi:10.1073/pnas.0703855104.
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tor M, de Vries S, Zipfel C. 2011. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 23(6):2440–2455. doi:10.1105/tpc.111.084301.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, et al. 2005. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 437(7062):1173–1178. doi:10.1038/nature04209.
- Ruan SL, Ma HS, Wang SH, Xin Y, Qian LH, Tong JX, Zhao HP, Wang J. 2006. Advances in plant proteomics. II. Application of proteome techniques to plant biology research. Yi Chuan. 28(12):1633–1648. doi:10.1360/yc-006-1633.
- Salehin M, Bagchi R, Estelle M. 2015. SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell. 27(1):9–19. doi:10.1105/tpc.114.133744.
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11):2498–2504. doi:10.1101/gr.1239303.
- Shen J, Zhang J, Luo X, Zhu W, Yu K, Chen K, Li Y, Jiang H. 2007. Predicting protein-protein interactions based only on sequences information. Proc Natl Acad Sci U S A. 104(11):4337–4341. doi:10.1073/pnas.0607879104.
- Shen Y, Zhou Z, Feng S, Li J, Tan-Wilson A, Qu LJ, Wang H, Deng XW. 2009. Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell. 21(2):494–506. doi:10.1105/tpc.108.061259.
- Sokolova V, Bindics J, Kircher S, Adam E, Schafer E, Nagy F, Viczian A. 2012. Missense mutation in the amino terminus of phytochrome A disrupts the nuclear import of the photoreceptor. Plant Physiol. 158(1):107–118. doi:10.1104/pp.111.186288.
- Soong TT, Wrzeszczynski KO, Rost B. 2008. Physical protein-protein interactions predicted from microarrays. Bioinformatics. 24(22):2608–2614. doi:10.1093/bioinformatics/btn498.
- Soto-Burgos J, Bassham DC. 2017. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS One. 12(8):e0182591. doi:10.1371/journal.pone.0182591.
- Subba P, Narayana Kotimoole C, Prasad TSK. 2019. Plant proteome databases and Bioinformatic tools: an expert review and comparative insights. OMICS. 23(4):190–206. doi:10.1089/omi.2019.0024.
- Sun K, Xue X, Liu N, Zhu Z, Li H. 2020. A point-to-point protein-protein interaction assay reveals the signaling interplays among plant hormones and environmental cues. Plant Direct. 4(5):e00228. doi:10.1002/pld3.228.
- Szemenyei H, Hannon M, Long JA. 2008. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science. 319(5868):1384–1386. doi:10.1126/science.1151461.
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. 2019. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47(D1):D607–D613. doi:10.1093/nar/gky1131.
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. 2017. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 45(D1):D362–D368. doi:10.1093/nar/gkw937.
- Takatsuka H, Umeda-Hara C, Umeda M. 2015. Cyclin-dependent kinase-activating kinases CDKD;1 and CDKD;3 are essential for preserving mitotic activity in Arabidopsis thaliana. Plant J. 82(6):1004–1017. doi:10.1111/tpj.12872.
- Tatsis EC, O'Connor SE. 2016. New developments in engineering plant metabolic pathways. Curr Opin Biotechnol. 42:126–132. doi:10.1016/j.copbio.2016.04.012.
- Tsai AY, Gazzarrini S. 2012. Overlapping and distinct roles of AKIN10 and FUSCA3 in ABA and sugar signaling during seed germination. Plant Signal Behav. 7(10):1238–1242. doi:10.4161/psb.21549.
- Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, Goossens A, Korbes AP, Memelink J, Ritsema T, et al. 2013. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell. 25(2):744–761. doi:10.1105/tpc.112.108548.
- Vandereyken K, Van Leene J, De Coninck B, Cammue BPA. 2018. Hub protein controversy: taking a closer look at plant stress response hubs. Front Plant Sci. 9:694. doi:10.3389/fpls.2018.00694.
- Volkening JD, Stecker KE, Sussman MR. 2019. Proteome-wide analysis of protein thermal stability in the model higher plant Arabidopsis thaliana. Mol Cell Proteomics. 18(2):308–319. doi:10.1074/mcp.RA118.001124.
- Walhout AJ, Sordella R, Lu X, Hartley JL, Temple GF, Brasch MA, Thierry-Mieg N, Vidal M. 2000. Protein interaction mapping in C. elegans using proteins involved in vulval development. Science. 287(5450):116–122. doi:10.1126/science.287.5450.116.
- Wang H, Li Y, Pan J, Lou D, Hu Y, Yu D. 2017. The bHLH transcription factors MYC2, MYC3, and MYC4 Are required for jasmonate-mediated inhibition of flowering in arabidopsis. Mol Plant. 10(11):1461–1464. doi:10.1016/j.molp.2017.08.007.
- Wang K, Ndathe RW, Kumar N, Zeringue EA, Kato N, Larkin JC. 2020. The CDK inhibitor SIAMESE targets both CDKA;1 and CDKB1 complexes to establish endoreplication in trichomes. Plant Physiol. 184(1):165–175. doi:10.1104/pp.20.00271.
- Wang X, Feng S, Nakayama N, Crosby WL, Irish V, Deng XW, Wei N. 2003. The COP9 signalosome interacts with SCF UFO and participates in Arabidopsis flower development. Plant Cell. 15(5):1071–1082. doi:10.1105/tpc.009936.
- Watts DJ, Strogatz SH. 1998. Collective dynamics of ‘small-world’ networks. Nature. 393(6684):440–442. doi:10.1038/30918.
- Wei B, Zhang J, Pang C, Yu H, Guo D, Jiang H, Ding M, Chen Z, Tao Q, Gu H, et al. 2015. The molecular mechanism of sporocyteless/nozzle in controlling Arabidopsis ovule development. Cell Res. 25(1):121–134. doi:10.1038/cr.2014.145.
- Wijnker E, Harashima H, Muller K, Parra-Nunez P, de Snoo CB, van de Belt J, Dissmeyer N, Bayer M, Pradillo M, Schnittger A. 2019. The Cdk1/Cdk2 homolog CDKA;1 controls the recombination landscape in arabidopsis. Proc Natl Acad Sci U S A. 116(25):12534–12539. doi:10.1073/pnas.1820753116.
- Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG. 2001. ORE9, an F-box protein that regulates leaf senescence in arabidopsis. Plant Cell. 13(8):1779–1790. doi:10.1105/tpc.010061.
- Wu R, Citovsky V. 2017. Adaptor proteins GIR1 and GIR2. II. interaction with the co-repressor TOPLESS and promotion of histone deacetylation of target chromatin. Biochem Biophys Res Commun. 488(4):609–613. doi:10.1016/j.bbrc.2017.05.085.
- Wu X, Xiong E, Wang W, Scali M, Cresti M. 2014. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nat Protoc. 9(2):362–374. doi:10.1038/nprot.2014.022.
- Xing Y, Jia W, Zhang J. 2008. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in arabidopsis. Plant J. 54(3):440–451. doi:10.1111/j.1365-313X.2008.03433.x.
- Xiong E, Li Z, Zhang C, Zhang J, Liu Y, Peng T, Chen Z, Zhao Q. 2020. A study of leaf-senescence genes in rice based on a combination of genomics, proteomics and bioinformatics. Brief Bioinform. doi:10.1093/bib/bbaa305.
- Yan J, Li H, Li S, Yao R, Deng H, Xie Q, Xie D. 2013. The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell. 25(2):486–498. doi:10.1105/tpc.112.105486.
- Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. 2010. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 6(6):411–417. doi:10.1038/nchembio.364.
- Yang C, Sofroni K, Wijnker E, Hamamura Y, Carstens L, Harashima H, Stolze SC, Vezon D, Chelysheva L, Orban-Nemeth Z, et al. 2020. The Arabidopsis Cdk1/Cdk2 homolog CDKA;1 controls chromosome axis assembly during plant meiosis. EMBO J. 39(3):e101625. doi:10.15252/embj.2019101625.
- Zhai Z, Liu H, Shanklin J. 2017. Phosphorylation of WRINKLED1 by KIN10 results in its proteasomal degradation, providing a link between energy homeostasis and lipid biosynthesis. Plant Cell. 29(4):871–889. doi:10.1105/tpc.17.00019.
- Zhang H, Zhang S, Wang W, Wang Q, Kuang H, Wang Q. 2020. Characterizing metabolites and potential metabolic pathways changes to understanding the mechanism of medicinal plant phellodendri amurensis cortex against doxorubicin-induced nephritis rats using UPLC-Q/TOF-MS metabolomics. J Pharm Biomed Anal. 188:113336. doi:10.1016/j.jpba.2020.113336.
- Zhang L, Du LQ, Shen CJ, Yang YJ, Poovaiah BW. 2014. Regulation of plant immunity through ubiquitin-mediated modulation of Ca2+-calmodulin-AtSR1/CAMTA3 signaling. Plant J. 78(2):269–281. doi:10.1111/tpj.12473.
- Zhang QC, Petrey D, Deng L, Qiang L, Shi Y, Thu CA, Bisikirska B, Lefebvre C, Accili D, Hunter T, et al. 2012. Structure-based prediction of protein-protein interactions on a genome-wide scale. Nature. 490(7421):556–560. doi:10.1038/nature11503.
- Zhang SB, Tang QR. 2016. Protein-protein interaction inference based on semantic similarity of Gene Ontology terms. J Theor Biol. 401:30–37. doi:10.1016/j.jtbi.2016.04.020.
- Zhao X, Harashima H, Dissmeyer N, Pusch S, Weimer AK, Bramsiepe J, Bouyer D, Rademacher S, Nowack MK, Novak B, et al. 2012. A general G1/S-phase cell-cycle control module in the flowering plant Arabidopsis thaliana. PLoS Genet. 8(8):e1002847. doi:10.1371/journal.pgen.1002847.
- Zhao Y, Feng Z, Zou Y, Liu Y. 2020. The E3 ubiquitin ligase SYVN1 ubiquitinates atlastins to remodel the endoplasmic reticulum network. iScience. 23(9):101494. doi:10.1016/j.isci.2020.101494.
- Zhou X, Xiang Y, Li C, Yu G. 2020. Modulatory role of reactive oxygen species in Root development in model plant of Arabidopsis thaliana. Front Plant Sci. 11:485932. doi:10.3389/fpls.2020.485932.
- Zhu P, Gu H, Jiao Y, Huang D, Chen M. 2011. Computational identification of protein-protein interactions in rice based on the predicted rice interactome network. Genomics Proteomics Bioinformatics. 9(4-5):128–137. doi:10.1016/S1672-0229(11)60016-8.
- Zobel RW. 2016. Arabidopsis: an adequate model for dicot root systems? Front Plant Sci. 7:58. doi:10.3389/fpls.2016.00058.
- Zong XJ, Li DP, Gu LK, Li DQ, Liu LX, Hu XL. 2009. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta. 229(3):485–495. doi:10.1007/s00425-008-0848-4.
- Zotenko E, Mestre J, O'Leary DP, Przytycka TM. 2008. Why do hubs in the yeast protein interaction network tend to be essential: reexamining the connection between the network topology and essentiality. Plos Comput Biol. 4(8):e1000140. doi:10.1371/journal.pcbi.1000140.