ABSTRACT
Diazotrophic plant growth-promoting endophytic (DPGPEB) bacterium plays an important role in plant growth and development. However, the molecular mechanism of the interaction between DPGPEB and sugarcane is still less known. In this study, we used the RNA sequencing technology to compare the transcriptome of two sugarcane varieties GT11and B8 inoculated with Enterobacter roggenkampii ED5. The results showed that a total of 1905 differentially expressed genes (DEGs) were obtained in the ED5 inoculated plants as compared to the control for variety GT11, of which 812 were down-regulated and 1093 were up-regulated, respectively. Whereas, for variety B8, 6214 DEGs were detected in the ED5 inoculated plants as compared to the control, of which 1587 and 4627 of down-regulated and up-regulated, respectively. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses showed the DEGs were associated with starch and sucrose, nitrogen metabolism, biosynthesis of phenylpropanoid, phytohormone signal transduction, MAPK signaling pathway, secondary metabolic pathway, cell wall biogenesis, and photosynthesis. Additionally, the results of RNA-seq data were validated with qRT-PCR. In summary, these results provide valuable information about the transcriptomic changes of the two sugarcane varieties in response to E. roggenkampii ED5 for understanding the molecular mechanisms of sugarcane-bacteria interactions.
1. Introduction
Sugarcane (Saccharum officinarum L.) is an important sugar and energy crop, accounting for more than 70% of the global sugar production (Brumbley et al. Citation2009; Li and Yang Citation2015). Sugarcane production requires a large amount of fertilizer, especially nitrogen (N) (Bokhtiar et al. Citation2005). Whereas the continuous application of chemical fertilizers and pesticides leads to contaminated agricultural land and environmental pollution (Srivastav Citation2020; Raheem et al. Citation2020; Sharma and Singhvi Citation2017; Wang et al. Citation2018). Plant growth-promoting bacteria (PGPB) can improve the soil micro-ecological environment and have various plant growth promoting functions such as ammonia production, nitrogen fixation, biocontrol, resistance to abiotic stress, secretion of plant hormones, and siderophore (Jha and Saraf Citation2015; Kuan et al. Citation2016; Mehmood et al. Citation2018; Arora and Verma Citation2017; Mhatre et al. Citation2019; Kumar et al. Citation2018). The application of PGPB in agricultural production can significantly reduce the application of chemical fertilizers and pesticides. Previously, some PGPB were isolated from sugarcane rhizosphere soil and plant tissues, including Bacillus xiamenensis, Klebsiella pneumoniae, Burkholderia anthina, Kosakonia radicincitans, Stenotrophomonas maltophilia, Pseudomonas, Enterobacter cloacae, Streptomyces chartreusis, and Klebsiella variicola (Xia et al. Citation2020; Bhardwaj et al. Citation2017; Malviya et al. Citation2020; Singh et al. Citation2020a; Singh et al. Citation2020b; Li et al. Citation2017; Safirzadeh et al. Citation2019; Wang et al. Citation2019; Lin et al. Citation2015).
Biological nitrogen fixation (BNF) is a vital function of some PGPB to enhance plant growth, which reduces dinitrogen of the atmospheric air to ammonia and provides nitrogen to the plants for proper growth (De Bruijn Citation2015). Using the nitrogen-fixing potential of PGPBs is an effective way to reduce the application rate of chemical nitrogen fertilizer and improve the nitrogen utilization efficiency and yield of sugarcane. In this study, we investigate the molecular mechanism of the interaction between diazotrophic plant growth-promoting endophytic bacterium (DPGPEB) E. roggenkampii ED5 and sugarcane. This reported strain was previously isolated from Saccharum sinense. and showed high nitrogenase activity along with various plant growth-promoting (PGP) and biocontrol properties (Guo et al. Citation2020). Additionally, the nitrogen fixing and PGP genes were predicted by whole gene sequencing (WGS) and proved E. roggenkampii ED5 was able to enhance the plant growth, and improve the agronomic traits and photosynthetic leaf gas exchange capacity in sugarcane under greenhouse experiment (Guo et al. Citation2020). Hence, E. roggenkampii ED5 has significant application potential in sugarcane production. However, the molecular mechanism of the interaction between E. roggenkampii ED5 and sugarcane is unclear.
RNA-seq technology is effective for studying gene functions and expressions at the transcriptional level, and revealing the molecular mechanism of specific biological processes (Kukurba and Montgomery Citation2015; Stark et al. Citation2019; Simoneau et al. Citation2021). Earlier, transcriptomics technology was mainly used to explore the interaction mechanisms between microorganisms and plants in response to water-deficit, root exudates, and biotic and abiotic stresses (Zhao et al. Citation2020; Zafar-ul-Hye et al. Citation2019; Fan et al. Citation2012; Gao et al. Citation2020; Khan et al. Citation2020; Moradi et al. Citation2021). Transcriptome microarray analysis found that some differentially expressed genes (DEGs) were enriched in auxin regulatory metabolism and plant defense response in Arabidopsis thaliana inoculated with Bacillus subtilis FB17, to enhance the plant stress resistance (Lakshmanan et al. Citation2013). Inoculation with Bacillus subtilis RR4 inhibited the transcription of the genes encoding defense response enzymes, cell wall modifying enzymes, transport, and secretion of phytochemicals, indicating that PGPB could regulate plant gene expression to promote its colonization and plant growth in rice (Rekha et al. Citation2018). However, limited information has been found for exploring the molecular interaction mechanism between PGPB and sugarcane using RNA-seq technology.
In the present study, two sugarcane varieties with different nitrogen-fixing abilities were grown and the seedlings were inoculated with E. roggenkampii ED5 at greenhouse conditions. Based on the Illumina Novaseq 6000 sequencing platform, the Illumina PE library was constructed for 2×150 bp sequencing, and all the mRNAs from sugarcane leaves were sequenced after inoculation. The transcriptomic data were analyzed to explore the DEGs and their functions using bioinformatics approaches, to reveal the molecular basis of E. roggenkampii ED5 interaction with sugarcane. To our best knowledge, this is the first study of the molecular mechanism of interaction between E. roggenkampii ED5 and sugarcane-based on transcriptomic analysis, which would provide a reference for the commercial application of Enterobacter roggenkampii ED5 in the near future.
2. Materials and methods
2.1. Plant materials and bacterial inoculation
Two sugarcane varieties (GT11, which requires a high concentration of nitrogen for growth; and B8, which requires a low concentration of nitrogen for growth) were used in this study. The seedcane was cut into segments and grown in trays with sand as described by (Li et al. Citation2017a). The bacterial strain E. roggenkampii ED5, isolated from Saccharum sinense roots and exhibited in vitro higher nitrogenase activity (Guo et al. Citation2020), was used for inoculation. The experiment was conducted in the greenhouse of Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences (GXAAS), Nanning, Guangxi, China. The soil was taken from the field and autoclaved for 30 min under 121 oC. The 18 cm high pots with 28 and 25 cm in upper and lower diameter were used for plant culture. The strain E. roggenkampii ED5 was incubated in Luria–bertani (LB) broth for 48 h and centrifuged, and diluted into 1×106 CFU mL−1 with sterilized water. The sugarcane seedlings were soaked in the bacterial solution for 1 h and then transplanted in the pots. The plants soaked in sterile water were used as the control.
2.2. Colonization of E. roggenkampii ED5 in sugarcane variety GT11
Green Fluorescent Protein (GFP) was used to detect the colonization of Enterobacter roggenkampii ED5 in sugarcane plant tissues, i.e. roots, stems, and leaves. The pPROBE-pTetr-TT plasmid having GFP gene and the plantlets of sugarcane variety GT11 were provided by Sugarcane Research Institute, GXAAS. The strain ED5 and plasmid vector were incubated at 32 °C (48 h) in an orbital shaker at 160 rpm, then mixed (1:2 ratio) and continued to culture for 48 h under the same conditions. The cells were centrifuged at 6,000×g for 5 min and the supernatant was discarded, and then added with sterile water. The sugarcane plantlets were put into a flask with the bacterial suspension and kept under light at 30 °C for 72 h. The plant tissues were cut into small pieces by blade and observed under a confocal laser scanning microscope (CLSM). The ED5 colonization in sugarcane tissues was assessed according to Singh et al. (2020).
2.3. Extraction of RNA
Total RNA was isolated from the plant leaves at 30 days after inoculation with strain ED5 using TRIzol® Reagent (plant RNA purification reagent for plant tissue) as per the manufacturer’s guidelines (Invitrogen, Carlsbard, CA, USA), and genomic DNA was removed with TaKara DNase I (Biotechnology, Dalian, China). The integrity and purity of the total RNA quality were detected by 2100 Bioanalyser (Agilent Technologies, Inc., Santa Clara, CA, USA) and measured using the ND-2000 (NanoDrop Thermo Scientific, Wilmington, DE, USA). 15 μg of high-quality RNA samples (RIN value ≥ 8.0, OD260/280 ≥ 1.8, OD260/230 ≥ 1.0, 28S:18S ≥ 1.0) were used to construct the sequencing cDNA library.
2.4. Library preparation, and Illumina NovaSeq 6000 sequencing
The RNA library was established using the TruSeq TM RNA sample preparation kit (Illumina, San Diego, CA, USA). Initially, magnetic beads with Oligo(dT) were used to enrich the mRNA with poly-A tail from 5 µg of total RNA. A six-base random primer (Invitrogen, CA, USA) and the template mRNA were used to reversely synthesize the one-strand cDNA, and the two-strand cDNA synthesis was performed by using SuperScript double-stranded cDNA synthesis kit (Invitrogen, CA, USA) to form a stable double-strand structure. The double-stranded cDNA structure had a sticky end. End Repair Mix was added to make a blunt end, and then a base was added to the 3’ end to connect to the Y-shaped linker. After the cDNA was purified by PCR, a 200–300 bp band was retrieved by electrophoresis using 2% agarose gel. After being quantified by TBS380 (Picogreen, CA, USA), the library was used for Illumina NovaSeq 6000 sequencing platform for high-throughput sequencing. The sequencing read length is PE 150.
2.5. De novo assembly and annotation
The raw paired-end readings were trimmed and the quality was regulated with default parameters using SeqPrep (https://github.com/jstjohn/SeqPrep) and Sickle (https: /github.com/najoshi /sickle). Trinity (Version v2.8.5, http://trinityrnaseq.Sourcefor ge.net/) was used to perform de novo assembly of the clean data of the samples (Grabherr et al. Citation2011). All the transcripts obtained by transcriptomic sequencing were compared with six major databases, Swiss-Prot, Pfam, Non-Redundant Protein Sequence (NR), Cluster of Orthologous Groups of Proteins (COG), Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). The annotation information in each database was obtained, and statistical analysis on the annotation status was performed.
2.6. Differentially expressed genes and functional enrichment analysis
Differentially expressed genes (DEGs) between treatment and control group were identified with DESeq2 (Version v2.8.5, http://bioconductor.Org/packages/stats/bioc/) software after obtaining the read counts of genes. The transcripts per million reads (TPM) method was employed to calculate the expression level of each transcript. The DESeq2 with Q value ≤ 0.05, DEGs with |log2FC|>1 were considered to be significant for differentially expressed genes. In addition, GO and KEGG analyses were performed to analyze the functional enrichment. A Bonferroni-corrected P-value of 0.05 was used to determine which DEGs were significantly enriched in GO terms and the metabolic pathways as compared to the whole-transcriptome background. KEGG pathway and GO KOBAS were carried out for functional enrichment analysis (http://kobas.cbi.pku.edu.cn/home.do) and Goatools (https://github.Com/tanghaibao/Goatools) (Chen et al. Citation2011).
2.7. Quantitative real-time-PCR
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to confirm the transcripts obtained from high-throughput sequencing. The total RNA was isolated by TRIzol1 (Cowin Biosciences, Beijing, China) and NanoDrop 2000 was used to assess the RNA quality. The TAKARA PrimeScriptTM RT reagent kit (Biotechnology, Dalian, China) was used for cDNA synthesis. SYBR Premix Ex TapTM II was used for qRT-PCR with LightCycler1480 II (Roche Applied Science, Germany) following the PCR conditions described by Zhu et al. (Citation2021). The 2−ΔΔCt procedure was performed to analyze the relative expression levels of genes (Livak and Schmittgen Citation2002). The primers for the internal reference gene (Glyceraldehyde-3-phosphate dehydrogenase, GAPDH) and candidate genes were designed and produced by Tsingke Biotechnology (Nanning, China) using Primer 5.0 software (Premier, Canada), they were presented in Table S1.
3. Results
3.1. Colonization of E. roggenkampii ED5 in sugarcane
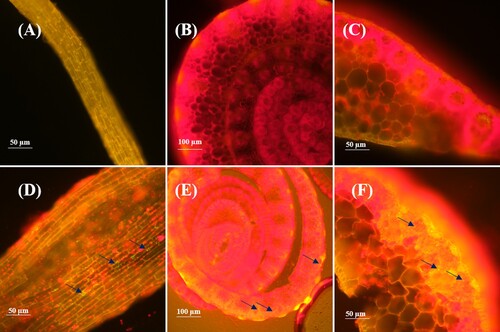
Fluorescent confocal microscopy images showed that strain E. roggenkampii ED5 labeled with GFP had been successfully colonized in sugarcane plant tissues such as roots, stems, and leaves ().
Figure 1. The endophytic E. roggenkampii ED5 strain colonization in sugarcane variety GT11. (a)-(c), root, stem, and leaf tissues of the non-inoculated plants (control); and (d)-(f), GFP-tagged E. roggenkampii ED5 colonization in root, stem and leaf tissues of the inoculated plants observed under confocal laser scanning microscope (CLSM).
3.2. De novo assembly and transcriptome sequencing
Illumina NovaSeq 6000 platform with pair-end sequencing techniques were used to examine the response of two sugarcane varieties GT11 and B8 to E. roggenkampii ED5 inoculation at the transcriptome level. We performed RNA-Seq for the RNA isolated from leaves of both inoculated and non-inoculated sugarcane plants. In this study, a total of 113125765 bp of unigenes were obtained from de novo assembly of clean reads, including 129781 unigenes. The unigenes ranged in length from 201 to 17710 bp with an average of 871.67 bp. The N50 and E90N50 lengths of the unigenes were 1574 and 2798 bp, respectively. The fragments mapped and GC percent of unigenes was 64.68 and 48.01%, respectively (Table S2).
Based on the sequence length, the unigenes were categorized into various groups, those with 200–500 bp were 69526 (53.60%), and 29407 (22.66%) of them fell in a range from 501 to 1000 bp in length. The unigenes ranging from 1001–1500, 1501–200, 2001–2500, 2501–3000, 3001–3500, 3501–4000, 4001–4500 bp and more than 4500 bp were 10050 (7.74%), 6390 (4.92%), 4612 (3.55%), 3161 (2.44%), 2231 (1.72%), 1442 (1.11%), 957 (0.74%) and 1979 (1.52%), respectively, as shown in Figure S1. The raw data of the transcriptome sequences have been submitted to the NCBI SRA database with the accession number PRJNA735861.
3.3. Functional annotation of unigenes
Sugarcane cultivars lack a reference genome. Unigene annotation in GO, KEGG, COG, NR, Swiss-prot, and Pfam public databases indicated that 47991 unigenes (36.98%) were annotated in GO, 19820 (15.27%) in KEGG, 53781 (41.44%) in COG, 32742 (25.23%) in SwissProt, 34311 (26.44%) in pfam, and 59916 (46.17%) in NR (Figure S2).
3.4. Species homology distribution
BlastX search was performed to analyze the species homology for all the assembled unigenes in the NR public database. The results showed that 29788 (49.29%), 10579 (17.50%), 3011 (4.98%), 2761 (4.57%), 2189 (3.62%), 1982 (3.28%), 1642 (2.72%), 1569 (2.60%), 1310 (2.17%), 1107 (1.83%), 423 (0.70%), 331 (0.55%), 297 (0.49%), 251 (0.42%), and 3198 (5.29%) unigenes matched to Sorghum bicolor, Zea mays, Panicum miliaceum, Oryza sativa, Setaria italica, Panicum hallii, Saccharum hybrid cultivar, Quercus suber, Dichanthelium oligosanthes, Setaria viridis, Aegilops tauschii, Brachypodium distachyon, Hordeum vulgare, Oryza brachyantha, and other species, respectively (Figure S3). Sorghum bicolor and Zea mays had closer homology with sugarcane, which further attested to the reliability of the assembly.
3.5. GO, COG, and KEGG annotation
The functions of all the assembled unigenes were predicted by the GO database, and the results showed that the unigenes functions were categorized into three parts, that is, biological process (BP), molecular function (MF), and cellular component (CC). In this study, A total of 198,593 GO terms with 52 subfunctional groups were annotated in the three functional categories, including BP (terms-61846, 31.14%), CC (terms-74608, 37.57%), and MF (terms-62139, 31.29%) (Figure S4).
The entire assembled unigenes from sugarcane leaves were re-edited and labeled with COG database. The results showed that all the unigenes were grouped into 23 subfunctional categories in COG. Most of the unigenes were found to have unknown functions (32565 unigenes, 55.36%) whereas the least annotation was in nuclear structure (1 unigene) (Figure S5).
To investigate the metabolic pathways, the unigenes were predicted by using the KEGG database. The top five pathways were found to be metabolism (with 11-second category), genetic information processing (with 4-second category), environmental information processing (with 2-second category), cellular processes (with 2-second category), and organismal systems (with 2-second category). Among them, the carbohydrate metabolism pathway included the most unigenes (1619) (Figure S6).
3.6. DEGs analysis
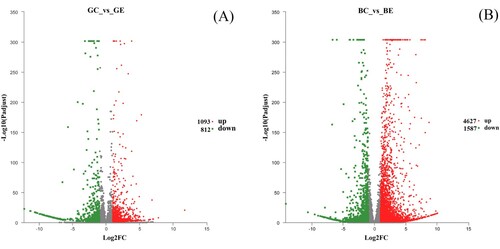
The DEGs in sugarcane leaves in response to strain ED5 inoculation were identified with DESeq2 using a gathering cut-off value of fold change. The DEGs between the treatment inoculated with strain ED5 and the non-inoculated control in the two sugarcane varieties GT11 (GE, GC) and B18 (BE, BC) were compared. The volcano plot showed that a total of 1905 DEGs (1093 up-regulated and 812 down-regulated) were identified in GT11, and 6214 (4627 up-regulated and 1587 down-regulated) were found in B8 ().
Figure 2. The volcano plots showed the DEGs in the sugarcane variety GT11 (a) and B8 (b). Each data point in the chart represents a specific unigenes. The red point indicates the unigenes that were significantly up-regulated, the blue point represents the significantly down-regulated unigenes, and the black points were the non-significant unigenes. GE, the treatment inoculated with strain ED5 in GT11; GC: the non-inoculated control in GT11; BE, the treatment inoculated with strain ED5 in B8; BC: the non-inoculated control in B8.
3.7. GO enrichment of DEGs
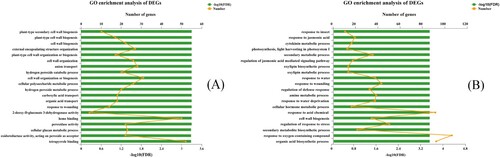
After E. roggenkampii ED5 inoculation, the DEGs (FPKM>10) were enriched in GO terms and KEGG pathways for functional analysis. Among the significant GO terms in GT11, the DEGs mainly involved in plant resistance to biotic or abiotic stress, for example, 20 were found in ‘hydrogen peroxide metabolic process,’ 22 in ‘peroxidase activity (GO:0004601),’ 20 in ‘hydrogen peroxide catabolic process (GO:0042744),’ and 22 in ‘oxidoreductase activity, acting on peroxide as acceptor (GO:0016684),’ respectively. Some DEGs were enriched in plant growth-related metabolism, for example, 10 in ‘plant-type secondary cell wall biogenesis (GO: 0009834),’ 17 in ‘plant-type cell wall biogenesis (GO:0009832),’ 22 in ‘cell wall biogenesis (GO:0042546),’ and 31 in ‘cell wall organization or biogenesis (GO:0071554),’ respectively ((a)). For the DEGs in B8, some DEGs were found to be related to the plant disease resistance metabolic pathway, for example, 19 to ‘response to jasmonic acid (GO:0009753)’ and 17 to ‘regulation of jasmonic acid-mediated signaling pathway (GO:2000022).’ For Plant drought resistance-related terms,‘response to water (GO:0009415)’ had 37 DEGs, and ‘response to water scarcity (GO:0009414)’ had 37 DEGs in GO enrichment. Additionally, some plant growth related DEGs were also found, for example, 16 DEGs were associated with ‘cytokinin metabolic process (GO:0009690),’ 12 with ‘photosynthesis, light harvesting in photosystem I (PS-I) (GO:0009768).’ For other important GO terms, ‘secondary metabolic process (GO:0019748)’ had 35 DEGs, ‘cellular hormone metabolic process (GO:0034754)’ had 16 DEGs, and ‘secondary metabolite biosynthetic mechanisms (GO:0044550)’ had 22 DEGs ((b)).
Figure 3. The GO enrichment of DEGs in two sugarcane varieties GT11 (a) and B8 (b). The ordinate represents the GO term; the upper abscissa reflects the number of unigenes of the GO term on the comparison, which corresponds to different points on the polyline; the lower abscissa indicates the significance level of enrichment, which correlates to the column height. For FDR, the higher the -log10 (FDR) value, the more significantly enriched the GO term (p-value <.5) among them. This figure shows the enrichment result of the top 20.
3.8. KEGG enrichment of DEGs
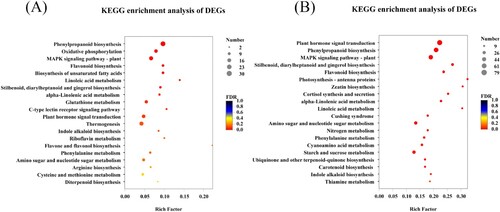
Bubble plots were used to show the significance of the DEGs enriched KEGG pathways in two sugarcane varieties (). It showed that the 30 DEGs were involved in ‘phenylpropanoid biosynthesis (map00940),’ 21 in ‘MAPK signaling pathway-plant (map04016),’ 14 in ‘glutathione metabolism (map00480),’ and 17 in ‘Plant hormone signal transduction (map04075),’ respectively ((a)). Similarly, some key pathways for DEGs enrichment were found in B8, for example, 15 DEGs were involved in ‘nitrogen metabolism (map00910),’ 35 in ‘starch and sucrose metabolism (map00500),’ 79 in ‘plant hormone signal transduction (map04075),’ 65 in ‘phenylpropanoid biosynthesis (map00940),’ and 60 in ‘MAPK signaling pathway-plant (map04016),’ respectively. In addition, ‘photosynthesis-antenna proteins (map00196)’ had 13 DEGs, ‘amino sugar and nucleotide sugar metabolism (map00520)’ had 34 DEGs, ‘phenylalanine metabolism (map00360)’ had 18 DEGs, and ‘carotenoid biosynthesis (map00906)’ had 14 DEGs, respectively ((b)).
Figure 4. The KEGG enrichment of DEGs in sugarcane varieties GT11 (a) and B8 (b). The rich factor is the ratio of the number of DEGs enriched in the pathway to the number of annotated DEGs. The vertical axis shows the pathway's name, while the horizontal axis represents the rich factor. The degree of enrichment increases as the rich factor rises. The color of the point correlates to different Q value ranges, and the size of the point represents the number of genes in the pathway. This figure shows the enrichment result of the top 20 (p-value <.5).
3.9. Nitrogen metabolism-related DEGs
Nitrogen utilization is essential for plant growth and development in sugarcane. In this study, it was found that, after E. roggenkampii ED5 inoculation, 15 DEGs (seven up-regulated, eigtht down-regulated) related to nitrogen metabolism were expressed in B8 while only one DEG was upregulated in GT11. Further analysis showed that, out of these nitrogen metabolism-related DEGs, five were involved in ‘glutamine synthetase,’ five in ‘nitrate reductase,’ five in ‘alpha carbonic anhydrase,’ and another gene was related to ‘hypothetical protein’ and ‘uncharacterized protein’ (Table S3.)
3.10. DEGs related to metabolism of starch and sucrose
Metabolism of starch and sucrose is the fundamental way to accumulate sugar in sugarcane. KEGG enrichment analysis found that there were 11 and 35 DEGs associated with the ‘starch and sucrose metabolism’ pathway (map00500) in GT11 and B8, respectively, after ED5 inoculation. The functions mainly included alpha-amylase isozyme, probable trehalose-phosphate phosphatase, probable trehalose-phosphate phosphatase, soluble starch synthase, glucose-1-phosphate adenyltransferase small subunit, granule-bound starch synthase, beta-glucosidase, starch branching enzyme, phosphate phosphatase, glucan endo-1,3 beta-glucosidase, and beta-amylase (Table S4).
3.11. Validation by qRT-PCR
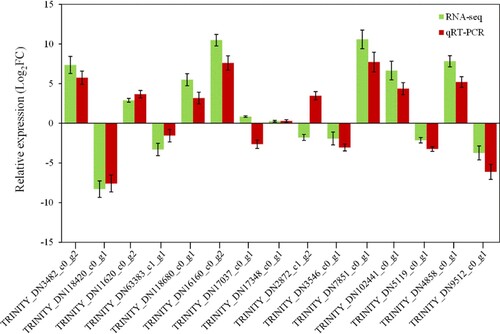
qRT-PCR was employed to validate the accuracy of the RNA-Seq results. A total of 15 sugarcane growth-related genes were randomly selected for the analysis, including seven up-regulated, three down-regulated, and two non-changed genes. The results showed that 13 of the selected genes had similar expression patterns as identified by RNA-Seq, 2 genes (TRINITY_DN17037_c0_g1, TRINITY_DN2872_c1_g2) did not (), reflecting the results of RNA-seq are generally reliable.
4. Discussion
In the present study, we used the RNA-seq method to analyze the molecular basis of interaction between two sugarcane varieties in response to N-fixing endophytic strain ED5 inoculation at the transcriptome level. A total of 1905 DEGs were found between treatment and control group in sugarcane variety GT11, and 6214 DEGs were identified in B8. Both sugarcane varieties showed a significant difference in DEGs expression profiles after E. roggenkampii ED5 inoculation, which may be due to genetic differences between them (Yang et al. Citation2019). There were 15 DEGs linked to the nitrogen metabolism processes in B8, however, only one was in GT11 in this study. The nitrogen-fixing effect of PGPB on sugarcane has been confirmed by some researchers (Dong et al. Citation1994; Lin et al. Citation2009; Li et al. Citation2017). Additionally, it has been reported that the PGPB have different responses to different sugarcane varieties (Hari and Srinivasan Citation2005). In previous study, 15N dilution method was used to detect the nitrogen utilization rate of both sugarcane varieties and found that variety B8 had a higher nitrogen utilization rate and showed better nitrogen fixation capacity than variety GT11 (Ting et al. Citation2010), which supported the finding in this study. We found three NR-related DEGs (TRINITY_DN1933_c0_g1, TRINITY_DN2372_c0_g1, TRINITY_DN35828_c0_g1) were up-regulated after E. roggenkampii ED5 inoculation in variety B8. Nitrate is the main nitrogen source in plants, and nitrate assimilation is a highly regulated process (Ferrario-Méry et al. Citation1998). The regulation of NR activity in plants plays a vital role in regulating primary nitrogen assimilation and significantly affects the growth and development of plants (Reyes et al. Citation2018; Yu et al. Citation2017). The reduction of NR activity in plant roots will change the distribution of degraded nitrogen compounds and carbohydrates (Hänsch et al. Citation2001), and over-expression of the NR gene can increase the mRNA level (Vincentz and Caboche Citation1991). Similarly, over-expression of the NR genes can increase the mRNA level and enhance nitrogen absorption in the plant system (Rosales et al. Citation2012). After inoculation of E. roggenkampii ED5, NR-related genes were up-regulated in variety B8, indicating the strain ED5 might stimulate the activity of sugarcane nitrate reductase, which indirectly affects the sugarcane nitrogen metabolism pathway. Additionally, the present research showed that there were five DEGs related to glutamine synthetase (GS) in both sugarcane varieties GT11 and B8. Among them, two genes (TRINITY_DN118680_c0_g1, TRINITY_DN91009_c0_g1) were up-regulated and three genes (TRINITY_DN101512_c1_g1, TRINITY_DN1 9631_c0_g1, TRINITY_ DN2872_c1_g2) were down-regulated. NO3- and NH4+ in the soil are the main sources of plant nitrogen, and among them, NO3- must be converted into NH4+ before being assimilated. In biological nitrogen fixation, nitrogenase in nitrogen-fixing microorganisms reduces N2 to form NH4+ which is further assimilated into glutamine (Gln) (Hirel et al. Citation1987). Previous studies showed that the over-expression of glutamine synthetase-related genes could promote plant nitrogen utilization and growth (Oliveira et al. Citation2002; Fuentes et al. Citation2001). The results in this study confirmed that the strain ED5 has the potential for biological nitrogen fixation in sugarcane.
Additionally, this study showed that the some DEGs in two sugarcane varieties were enriched in starch and sucrose metabolism in the KEGG database. Starch mainly functions as the unit of energy storage and the products of photosynthetic carbon assimilation in most crops; however, sucrose is the unit of energy storage (Ma et al. Citation2019). Eleven DEGs related to starch and sucrose metabolism pathway were involved in GT11 (four up-regulated and seven down-regulated) in our study. At the same time, 35 DEGs were enriched in B8 (25 up-regulated and 10 down-regulated). Soluble starch synthase (SSS) is a starch synthase family and plays a key role in starch biosynthesis. The non-reducing ends of glucose chains in starch synthesis are elongated at starch synthases actions (Zeeman et al. Citation2010; Leterrier et al. Citation2008). This study observed that all the four soluble starch synthase-related DEGs were up-regulated in sugarcane B8, however, only one down-regulated DEG was detected in GT11 after inoculated strain ED5, suggesting that strain ED5 has different effects on the soluble starch synthase pathway in different sugarcane varieties. For β-amylase related DEGs, we found another type of starch and sucrose metabolism genes in sugarcane variety B8 but none in variety GT11. Previous studies reported that β-amylase is related to sugar production of sugarcane and abiotic stresses (Ukoskit et al. Citation2019; Nawae et al. Citation2020). The active center of β-amylase contains at least three special gene groups X, A, and B. These gene groups participate in the combination of enzyme and substrate and involve in the reaction process of enzyme–substrate complex conversion into a product (Dicko et al. Citation1999; Kaplan et al. Citation2006). The results in this study showed that the strain ED5 had an impact on sugarcane starch and sucrose metabolism.
Additionally, the DEGs related to trehalose-phosphate phosphatase, granule-bound starch synthase, β-glucosidase, and glucan endo-1,3 β-glucosidase were also found between the two sugarcane varieties. These enzymes are vital in sugarcane starch and sucrose metabolism, and they have an indirect impact on sugarcane growth (Du et al. Citation2000). Using PGPB to promote plant resistance to biotic or abiotic stress is an effective way to develop ecological agriculture. The present study showed that the E. roggenkampii ED5 could induce sugarcane to produce these hydrolytic enzymes which are helpful to resist various abiotic stresses in vitro conditions. Whole-genome analysis of this strain confirmed the presence of different coding genes (CDS) related to biotic or abiotic tolerance (Guo et al. Citation2020).
Some DEGs between the two sugarcane varieties were observed to be linked to the MAPK signaling pathway-plant, phytohormone signaling, biosynthesis of phenylpropanoid, and photosynthesis. Some reports showed that these metabolic pathways would be changed in plants under biotic or abiotic stresses (Danquah et al. Citation2014; Vogt Citation2010; Zhu et al. Citation2021). However, E. roggenkampii ED5 was isolated from healthy sugarcane plants, and DEGs were enriched in these pathways, indicating strain ED5 enhanced the sugarcane immune response.
In this study, phenylpropanoid biosynthesis (KEGG, map00940) related DEGs were enriched in both sugarcane varieties. The biosynthesis of the phenylpropanoid pathway will be changed under biotic or abiotic stresses (Geng et al. Citation2020). The phenylpropanoid biosynthesis pathway plays an important role in plant development and in response to adverse conditions such as improving plant disease resistance, avoiding plant damage from stresses, and serving as a signal transduction molecule (Xu et al. Citation2014; Colquhoun et al. Citation2011). For different varieties, however, 30 DEGs of the phenylpropanoid biosynthesis pathway were enriched in GT11 and 65 in B8, which reflected the genetic variance in different sugarcane varieties.
Mitogen-activated protein kinase (MAPK) signaling pathway-plant (map04016) was found to be enriched in both sugarcane varieties in this study. MAPK signaling pathway is an important pathway for plants to respond to stresses (Xu and Zhang Citation2015). The MAPK cascade pathway transmits signals through MAPKKK→MAPKK→MAPK phosphorylation step by step. Then the downstream products (cytoskeleton proteins, protein kinases, apoptosis factors, nuclear receptors, phospholipases, and transcription factors) are phosphorylated by MAPK. They regulate the expression of corresponding genes and prompt plants to respond to adversity (Zhang et al. Citation2018). In addition, MAPK has also transmitted signals through interaction with ethylene, auxin, jasmonic acid, abscisic acid, and phospholipid signaling pathways (Zhang et al. Citation2018). In this study, 21 DEGs related to the MAPK signaling pathway in sugarcane variety GT11 and 60 in B8 were detected after ED5 inoculation, which suggested that ED5 would open the immune response system of sugarcane against stresses.
In our previous study, whole-genome analysis of E. roggenkampii ED5 showed the presence of some key plant growth-promoting genes such as nitrogen metabolism, siderophore, plant hormones, synthesis of resistance inducers, root colonization, biofilm formation, oxidoreductase, cold-shock protein, heat shock proteins, heavy metal resistance, and drought resistance in its genome. In addition, it was found that all growth parameters in GT11 were significantly enhanced after ED5 inoculation (Guo et al. Citation2020). We speculated that strain ED5 promotes sugarcane growth in multiple ways. Previous studies also showed that PGPB promotes crop growth in various ways (Goswami et al. Citation2016; Basu et al. Citation2021). Our findings also confirmed that strain ED5 has multiple pathways in interaction with sugarcane at the transcriptome level. The transcriptomic DEGs enrichment pathways in sugarcane variety B8 were significantly more than GT11 after E. roggenkampii ED5 inoculation might be due to the genetic differences between the two sugarcane varieties.
5. Conclusions
This study used RNA-seq method to compare the key gene expressions in two sugarcane varieties GT11 and B8 at transcriptome level after DPGPEB E. roggenkampii ED5 inoculation. Some DEGs were found in the ED5 inoculation treatment as compared to control. These DEGs were enriched in starch, sucrose, and nitrogen metabolism, phenylpropanoid biosynthesis, phytohormone signaling transduction, MAPK signaling pathway-plant, secondary metabolic process, cell wall biogenesis, and photosynthesis in GO and KEGG databases. These findings provided a reference for further research in the interactions between E. roggenkampii ED5 and sugarcane.
Author contributions
DJG, RS, PS, and YRL designed the experiments. DJG, DPL, RS, PS accomplished the experiments. KKV, YQ, AS, QK, XPS, and MM analyzed the data. DJG, DPL, RS and PS drafted the manuscript. YRL and YXX critically revised the article. All authors reviewed the article and approved it for publication.
Availability of data and material
All the data supporting the findings of this study are available within the article and its supplementary materials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Dao-Jun Guo
Dao-Jun Guo is a Ph.D. student in College of Agriculture, Guangxi University, Nanning, China.
Dong-Ping Li
Dong-Ping Li is a Research Associate in Microbiology Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China.
Rajesh Kumar Singh
Rajesh Kumar Singh, is Postdoctoral researcher in Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China.
Pratiksha Singh
Pratiksha Singh is Postdoctoral researcher in Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning,Guangxi, China.
Krishan K. Verma
Krishan K. Verma is Postdoctoral researcher in Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China.
Anjney Sharma
Anjney Sharma is Postdoctoral researcher in Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China.
Ying Qin
Ying Qin is a Ph.D. student in College of Agriculture, Guangxi University, Nanning, China.
Qaisar Khan
Qaisar Khan is a Ph.D. student in College of Agriculture, Guangxi University, Nanning, China.
Xiu-Peng Song
Xiu-Peng Song is a Research Associate in Guangxi Key Laboratory of Crop Genetic Improvement and Biotechnology, Nanning, Guangxi, China.
Mukesh K. Malviya
Mukesh K. Malviya is Postdoctoral researcher in Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China.
Yong-Xiu Xing
Yong-Xiu Xing is an Associate Professor in College of Agriculture, Guangxi University, Nanning, China.
Yang-Rui Li
Yang-Rui Li is a Professor and Head of Guangxi Key Laboratory of Sugarcane Genetic Improvement, Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China.
References
- Arora NK, Verma M. 2017. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech. 7:1–9.
- Basu A, Prasad P, Das SN, Kalam S, Sayyed R, Reddy M, El Enshasy H. 2021. Plant growth promoting rhizobacteria (PGPR) as Green bioinoculants: recent developments, constraints, and prospects. Sustainability. 13:1140.
- Bhardwaj G, Shah R, Joshi B, Patel P. 2017. Klebsiella pneumoniae VRE36 as a PGPR isolated from Saccharum officinarum cultivar Co99004. J. Appl. Biol. Biotechnol. 5:047–052.
- Bokhtiar S, Sakurai K, Science S. 2005. Effects of organic manure and chemical fertilizer on soil fertility and productivity of plant and ratoon crops of sugarcane. Archives of Agronomy. 51:325–334.
- Brumbley SM, Snyman SJ, Gnanasambandam A, Joyce P, Hermann SR, da Silva JA, McQualter RB, Wang ML, Egan BT, Paterson AH, et al. 2009. Sugarcane. In: Kole C, Hall TC, editors. Compendium of transgenic crop plants: transgenic sugar, tuber and fiber crops; pp. 1–58.
- Chen X, Mao X, Huang J, Yang D, Wu J, Dong S, Lei K, Ge G, Li CY, Wei L. 2011. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39:316–322.
- Colquhoun TA, Kim JY, Wedde AE, Levin LA, Schmitt KC, Schuurink RC, Clark DG. 2011. PhMYB4 fine-tunes the floral volatile signature of petunia× hybrida through PhC4H. J Exp Bot. 62:1133–1143.
- Danquah A, de Zelicourt A, Colcombet J, Hirt H. 2014. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv. 32:40–52.
- De Bruijn FJ. 2015. Biological nitrogen fixation. In: Lugtenberg B, editor. Principles of plant-microbe interactions. Switzerland: Springer; pp. 215–224.
- Dicko MH, Searle-van Leeuwen M, Beldman G, Ouedraogo O, Hilhorst R, Traore A. 1999. Purification and characterization of β-amylase from curculigo pilosa. Applied Microbiology Biotechnology. 52:802–805.
- Dong Z, Canny MJ, McCully ME, Roboredo MR, Cabadilla CF, Ortega E, Rodes R. 1994. A nitrogen-fixing endophyte of sugarcane stems (a new role for the apoplast). Plant Physiol. 105:1139–1147.
- Du Y-C, Nose A, Kondo A, Wasano K. 2000. Diurnal changes in photosynthesis in sugarcane leaves: II. enzyme activities and metabolite levels relating to sucrose and starch metabolism. Plant Prod Sci. 3:9–16.
- Fan B, Carvalhais LC, Becker A, Fedoseyenko D, von Wirén N, Borriss R. 2012. Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates. BMC Microbiol. 12:1–13.
- Ferrario-Méry S, Valadier M-H, Foyer CH. 1998. Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol. 117:293–302.
- Fuentes SI, Allen DJ, Ortiz-Lopez A, Hernández G. 2001. Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J Exp Bot. 52:1071–1081.
- Gao J, Wu S, Liu Y, Wu S, Jiang C, Li X, Wang R, Bai Z, Zhuang G, Zhuang X. 2020. Characterization and transcriptomic analysis of a highly Cr (VI)-resistant and-reductive plant-growth-promoting rhizobacterium Stenotrophomonas rhizophila DSM14405 T. Environ Pollut. 263:114622.
- Geng D, Shen X, Xie Y, Yang Y, Bian R, Gao Y, Li P, Sun L, Feng H, Ma F. 2020. Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Hortic Res. 7:1–11.
- Goswami D, Thakker JN, Dhandhukia PC. 2016. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food and Agriculture. 2.
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 29:644–652.
- Guo D-J, Singh RK, Singh P, Li D-P, Sharma A, Xing Y-X, Song X-P, Yang L-T, Li Y-R. 2020. Complete genome sequence of Enterobacter roggenkampii ED5, a nitrogen fixing plant growth promoting endophytic bacterium With biocontrol and stress tolerance properties, isolated from sugarcane root. Front Microbiol. 11:2270.
- Hänsch R, Fessel DG, Witt C, Hesberg C, Hoffmann G, Walch-Liu P, Engels C, Kruse J, Rennenberg H, Kaiser WM. 2001. Tobacco plants that lack expression of functional nitrate reductase in roots show changes in growth rates and metabolite accumulation. J Exp Bot. 52:1251–1258.
- Hari K, Srinivasan T. 2005. Response of sugarcane varieties to application of nitrogen fixing bacteria under different nitrogen levels. Sugar Tech. 7:28–31.
- Hirel B, Bouet C, King B, Layzell D, Jacobs F, Verma DPS. 1987. Glutamine synthetase genes are regulated by ammonia provided externally or by symbiotic nitrogen fixation. EMBO J. 6:1167–1171.
- Jha CK, Saraf MD. 2015. Plant growth promoting rhizobacteria (PGPR): a review. J Agric Res. 5:108–119.
- Kaplan F, Sung DY, Guy CL. 2006. Roles of β-amylase and starch breakdown during temperatures stress. Physiol Plant. 126:120–128.
- Khan N, Bano A, Ali S, Babar MA. 2020. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 90:189–203.
- Kuan KB, Othman R, Abdul Rahim K, Shamsuddin ZH. 2016. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PloS one. 11:e0152478.
- Kukurba KR, Montgomery SB. 2015. RNA sequencing and analysis. Cold Spring Harb Protoc. 11:951.
- Kumar A, Singh VK, Tripathi V, Singh PP, Singh AK. 2018. Plant growth-promoting rhizobacteria (PGPR): perspective in agriculture under biotic and abiotic stress. In: Ram Prasad, Sarvajeet S. Gill, Narendra Tuteja, editors. Crop improvement through microbial biotechnology. Elsevier; p. 333–342.
- Leterrier M, Holappa LD, Broglie KE, Beckles DM. 2008. Cloning, characterisation and comparative analysis of a starch synthase IV gene in wheat: functional and evolutionary implications. BMC Plant Biol. 8:1–21.
- Li HB, Singh RK, Singh P, Song QQ, Xing YX, Yang LT, Li YR. 2017. Genetic Diversity of nitrogen-fixing and plant growth promoting Pseudomonas species isolated from sugarcane rhizosphere. Front Microbiol. 8:1268.
- Li Y-R, Yang L-T. 2015. Sugarcane agriculture and sugar industry in China. Sugar Tech. 17:1–8.
- Lin L, Li Z, Hu C, Zhang X, Chang S, Yang L, Li Y, An Q. 2009. Plant growth-promoting nitrogen-fixing enterobacteria are in association with sugarcane plants growing in guangxi, China. Microbes Environments. 27:391–398.
- Lin L, Wei C, Chen M, Wang H, Li Y, Li Y, Yang L, An Q. 2015. Complete genome sequence of endophytic nitrogen-fixing Klebsiella variicola strain DX120E. Stand Genomic Sci. 27:391–398.
- Livak KJ, Schmittgen TD. 2002. Analysis of relative gene expression data using real-time Quantitative PCR. Methods. 25:402–408.
- Ma P, Yuan Y, Shen Q, Jiang Q, Hua X, Zhang Q, Zhang M, Ming R, Zhang J. 2019. Evolution and expression analysis of starch synthase gene families in Saccharum spontaneum. Trop Plant Biol. 12:158–173.
- Malviya MK, Li C-N, Solanki MK, Singh RK, Htun R, Singh P, Verma KK, Yang L-T, Li Y-R. 2020. Comparative analysis of sugarcane root transcriptome in response to the plant growth-promoting Burkholderia anthina MYSP113. PloS one. 15:e0231206.
- Mehmood U, Inam-ul-Haq M, Saeed M, Altaf A, Azam F, Hayat S. 2018. A brief review on plant growth promoting rhizobacteria (PGPR): a key role in plant growth promotion. Plant Protection. 2:77–82.
- Mhatre PH, Karthik C, Kadirvelu K, Divya K, Venkatasalam E, Srinivasan S, Ramkumar G, Saranya C, Shanmuganathan R. 2019. Plant growth promoting rhizobacteria (PGPR): a potential alternative tool for nematodes bio-control. Biocatalysis Agricultural Biotechnology. 17:119–128.
- Moradi S, Khoshru B, Mitra D, Mahakur B, Mohapatra PKD, Lajayer BA, Ghorbanpour M. 2021. Transcriptomics analyses and the relationship between plant and plant growth-promoting rhizobacteria (PGPR). In: Pudake RN, Sahu BB, Kumari M, Sharma AK, editors. Omics Science for rhizosphere biology. Singapore: Springer; p. 89–111.
- Nawae W, Shearman JR, Tangphatsornruang S, Punpee P, Yoocha T, Sangsrakru D, Naktang C, Sonthirod C, Wirojsirasak W, Ukoskit K. 2020. Differential expression between drought-tolerant and drought-sensitive sugarcane under mild and moderate water stress as revealed by a comparative analysis of leaf transcriptome. PeerJ. 8:e9608.
- Oliveira IC, Brears T, Knight TJ, Clark A, Coruzzi GM. 2002. Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiol. 129:1170–1180.
- Raheem SM, Rasul HI, Harun R. 2020. Farmer's behavior and attitude in using chemical fertilizers and pesticide in Rural areas. Journal of Plant Production. 11:1077–1081.
- Rekha K, Kumar RM, Ilango K, Rex A, Usha B. 2018. Transcriptome profiling of rice roots in early response to Bacillus subtilis (RR4) colonization. Botany. 96:749–765.
- Reyes TH, Scartazza A, Pompeiano A, Ciurli A, Lu Y, Guglielminetti L, Yamaguchi J. 2018. Nitrate reductase modulation in response to changes in C/N balance and nitrogen source in arabidopsis. Plant Cell Physiology. 59:1248–1254.
- Rosales EP, Iannone MF, Groppa MD, Benavides MP. 2012. Polyamines modulate nitrate reductase activity in wheat leaves: involvement of nitric oxide. Amino Acids. 42:857–865.
- Safirzadeh S, Chorom M, Enayatizamir N. 2019. Effect of phosphate solubilising bacteria (Enterobacter cloacae) on phosphorus uptake efficiency in sugarcane (Saccharum officinarum L. Soil Research. 57:333–341.
- Sharma N, Singhvi R. 2017. Effects of chemical fertilizers and pesticides on human health and environment: a review. International Journal of Agriculture, Environment Biotechnology. 10:675–679.
- Simoneau J, Dumontier S, Gosselin R, Scott MS. 2021. Current RNA-seq methodology reporting limits reproducibility. Brief Bioinform. 22:140–145.
- Singh RK, Singh P, Li H-B, Guo D-J, Song Q-Q, Yang L-T, Malviya MK, Song X-P, Li Y-R. 2020a. Plant-PGPR interaction study of plant growth-promoting diazotrophs Kosakonia radicincitans BA1 and Stenotrophomonas maltophilia COA2 to enhance growth and stress-related gene expression in Saccharum spp. Journal of Plant Interactions. 15:427–445.
- Singh RK, Singh P, Li H-B, Song Q-Q, Guo D-J, Solanki MK, Verma KK, Malviya MK, Song X-P, Lakshmanan P. 2020b. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: a comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 20:1–21.
- Srivastav AL. 2020. Chemical fertilizers and pesticides: role in ground water contamination. In: Majeti Narasimha Vara Prasad, editor. Agrochemicals detection, treatment and remediation. Elsevier; p. 143–159.
- Stark R, Grzelak M, Hadfield J. 2019. RNA sequencing: the teenage years. Nat Rev Genet. 20:631–656.
- Ting L, Improvement GCG, Xueqing O, Litao Y, Yangrui L. 2010. Effect of nitrogen-fixing bacteria inoculation on biological nitrogen fixation in sugarcane by 15 N isotope dilution technique. Journal of Nuclear Agricultural Sciences. 24:1026–1031.
- Ukoskit K, Posudsavang G, Pongsiripat N, Chatwachirawong P, Klomsa-Ard P, Poomipant P, Tragoonrung S. 2019. Detection and validation of EST-SSR markers associated with sugar-related traits in sugarcane using linkage and association mapping. Genomics. 111:1–9.
- Venkatachalam Lakshmanan, Rafael Castaneda, Thimmaraju Rudrappa. 2013. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta. 238:657–668.
- Vincentz M, Caboche M. 1991. Constitutive expression of nitrate reductase allows normal growth and development of nicotiana plumbaginifolia plants. EMBO J. 10:1027–1035.
- Vogt T. 2010. Phenylpropanoid biosynthesis. Mol Plant. 3:2–20.
- Wang Z, Solanki MK, Yu Z-X, Yang L-T, An Q-L, Dong D-F, Li Y-R. 2019. Draft genome analysis offers insights into the mechanism by which Streptomyces chartreusis WZS021 increases drought tolerance in sugarcane. Front Microbiol. 9(9):3262.
- Wang Z, Zhang T, Tan C, Vadas P, Qi Z, Wellen C. 2018. Modeling phosphorus losses from soils amended with cattle manures and chemical fertilizers. Sci Total Environ. 639:580–587.
- Xia Y, Farooq MA, Javed MT, Kamran MA, Mukhtar T, Ali J, Tabassum T, ur Rehman S, Munis MFH, Sultan T. 2020. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiology Biochemistry. 151:640–649.
- Xu J, Zhang S. 2015. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20:56–64.
- Xu Q, Yin X-r, Zeng J-k, Ge H, Song M, Xu C-j, Li X, Ferguson IB, Chen K-s. 2014. Activator-and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J Exp Bot. 65:4349–4359.
- Yang Y, Gao S, Jiang Y, Lin Z, Luo J, Li M, Guo J, Su Y, Xu L, Que Y. 2019. The physiological and agronomic responses to nitrogen dosage in different sugarcane varieties. Frontiers in Palnt Science. 10:406.
- Yu X-Z, Zhang F-F, Liu W. 2017. Chromium-induced depression of 15 N content and nitrate reductase activity in rice seedlings. International Journal of Environmental Science Technology. 14:29–36.
- Zafar-ul-Hye M, Danish S, Abbas M, Ahmad M, Munir TMJA. 2019. ACC deaminase producing PGPR Bacillus amyloliquefaciens and agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy. 9:343.
- Zeeman SC, Kossmann J, Smith AM. 2010. Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol. 61:209–234.
- Zhang M, Su J, Zhang Y, Xu J, Zhang S. 2018. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr Opin Plant Biol. 45:1–10.
- Zhao T, Wu T, Zhang J, Wang Z, Pei T, Yang H, Li J, Xu X. 2020. Genome-Wide analyses of the Genetic screening of C2H2-type zinc finger transcription factors and abiotic and biotic stress responses in tomato (solanum lycopersicum) Based on RNA-Seq data. Front Genet. 11:540.
- Zhu K, Yang LT, Li CX, Lakshmanan P, Xing YX, Li YR. 2021. A transcriptomic analysis of sugarcane response to leifsonia xyli subsp. xyli infection. PLoS One. 16:e0245613.