ABSTRACT
Research on fungal endophytes has demonstrated the ability to improve crop performance and protect host plants against diverse biotic and abiotic stresses. Yet, despite the exponential growth of this topic, a whole outline to reflect the relevance and extent of each study type is missing. Hence, we performed an analysis of all available literature to expose the characteristics and limitations of this research field. Our results suggested that, overall, there is still a tendency to study the most known models in plant-fungal-stress combinations (ascomycetous fungi, grasses, abiotic stress). Fungal endophytes in dicot plants or against biotic stress, though promising, are still quite unexplored. All these data could lead future studies to assess less considered study factors that might help discern the beneficial effects of fungal endophytes with more extent and accuracy.
1. Introduction
Current studies about global food and agriculture revealed that world production may need to be increased by 60%–110% before 2050 to avoid food shortage. However, yields are no longer improving on 24–39% of most important cropland areas (Schmidhuber and Tubiello Citation2007; Edgerton Citation2009). Along with the demand for increased production, we need to preserve the environment and promote biodiversity in agronomic ecosystems, mainly by reducing the use of pesticides and making better use of agricultural inputs and resources: land, water, fertilizers and energy. In this context, the use of beneficial microorganisms to improve crop performance and reduce the need for chemical inputs has become a realistic alternative that is gaining interest among researchers and the industry (Lugtenberg et al. Citation2016; Singh and Trivedi Citation2017; Llorens et al. Citation2019).
Microorganisms are widely reported to be naturally associated with plants. Despite the fact that the presence of living microorganisms inside plants has been known since the beginning of the last century, its attention only increased in the last decades with the discovery of their ecological significance and their ability to produce metabolites that could modulate the physiology of the host plant or be of pharmacological interest (Mattoo and Nonzom Citation2021). Given these circumstances, the term ‘plant microbiome’ (Hardoim et al. Citation2015) is on the rise, and the living microorganisms that are in association with plants are investigated with rising intensity.
In this way, one of the emerging areas of study is around fungal endophytes. Plant-associated fungi are typically classified as either pathogenic, saprotrophic, epiphytic, mycorrhizal or endophytic (Porras-Alfaro and Bayman Citation2011). Yet, most, if not all, plants have symbiotic fungi, either epiphytic, endophytic or mycorrhizal (Rodriguez et al. Citation2009).
With the evolution of research regarding endophytic microorganisms, fungal endophytes have held several definitions (Schulz and Boyle Citation2005; Hyde and Soytong Citation2008). In this case, the most accepted consideration establishes endophytic fungi as those fungi that reside entirely within plant tissues, without causing apparent symptoms of disease (Tan and Zou Citation2001; Rodriguez et al. Citation2009). These endophytes also differ from mycorrhizae in that there’s no localized and specialized hyphae or synchronized plant-fungus development (Brundrett Citation2006).
Despite the broad diversity of the group, fungal endophytes have been conventionally divided into two categories, clavicipitaceous and non-clavicipitaceous, and four classes (Rodriguez et al. Citation2009) based on their symbiotic and ecological patterns. Clavicitipaceous endophytes, also called class 1 endophytes, belong to Clavicipitaceae (Hypocreales; Ascomycota) and are restricted to a narrow range of hosts, but they can colonize the whole plant and transmit vertically and horizontally. On the contrary, non-clavicipitaceous endophytes, which comprise Class 2, Class 3, and Class 4, are characterized by colonizing a broad range of hosts, which include both monocot and dicot plants. Class 2 is formed by ascomycetes or basidiomycetes fungi and can be found in any part of the plant and transmitted both vertically and horizontally. Opposed to Class 2, endophytes in Class 3 and Class 4 are restricted to shoots or roots, respectively. Class 3 includes a diverse group of fungi that is transmitted horizontally, whereas Class 4 includes a specific group of sterile fungi also known as dark septate endophytes, which manifest melanized septa (Rodriguez et al. Citation2009).
In addition, the diversity of microbial symbionts varies on a host plant and environmental conditions including biogeography, as seen in Kivlin et al. (Citation2017). Yet, endophyte diversity is not the only complex aspect of endophytes, since the relation between the host plant and its fungal endophytes is also intricate (Saikkonen et al. Citation1998). Although their interaction commonly provides nutrients and stress or competition tolerance to a degree, Schulz and Boyle (Citation2005) hypothesized that there is a continuum of antagonistic interactions and no neutral interactions, and each relationship differs from another. Thus, understanding the nature and particularities of the interactions between endophytes and host plants and how they affect the host could be key to improving agricultural management.
Recent studies have shown that the microbiome has an impact on different aspects of the host plant, such as improving tolerance to drought, heat, or saline stress, reducing susceptibility to diseases, and increasing vigor (Weiß et al. Citation2016; Llorens et al. Citation2019). For instance, species that improve the growth of certain types of grass (Panicum virgatum or rod grass) for the production of biofuels or species that protect maize from fungal pathogens have been reported. Moreover, it has also been described that certain species from wild herbs, when transferred to wheat and tomato, are capable of improving the growth of these plants under conditions of heat and salinity stress (Redman et al. Citation1986; Rodriguez et al. Citation2008). The mechanisms by which the endophytic microorganisms improve the performance and the resistance of the plants could be divided into direct and indirect mechanisms. The direct mechanisms include compounds that are directly secreted by the endophyte that have a straightforward effect. These mechanisms comprise secretion of antibiotics, lytic enzymes, phytohormones, indolic compounds or direct competition of the niche. On the other hand, indirect mechanisms include plant responses induced by the presence of the endophyte. These mechanisms include the stimulation of Induced systemic resistance (ISR) and Systemic acquired resistance (SAR) or the stimulation of plant secondary metabolites (Fadiji and Babalola Citation2020).
The ability and feasibility of improving plant performance and resistance to different stressors using fungal endophytes have fomented the interest in this research area. However, the possibilities of research on different fungi, hosts, stressors, and many other variables give almost endless combinations that can lead to the underestimation of some areas. The rising attention given to microbial symbiosis in the scientific community is reflected in the high number of publications regarding this topic, including several reviews (Tan and Zou Citation2001; Gautam and Avasthi Citation2019; Pozo et al. Citation2021) and some meta-analysis (Mayerhofer et al. Citation2013; Dastogeer Citation2018). Yet, many are focused on a specific aspect of symbiosis, such as induction of resistance or production of metabolites, and some don’t focus on the fungal endophytes.
Now, we have at our disposal the data of many types of study about endophytic fungi, but what is the magnitude of each one? What is the relevance of one type of endophyte compared to others? In order to know to what extent a study category is relevant, we would need a clear description of the whole research field. We hereby introduce a precise interpretation of the data observed in fungal endophytes’ studies to demonstrate the state and tendency of the field by analyzing published literature. In this work, the objective was to contrast the main study aspects and find some minor categories of research that are usually overlooked. The following questions were addressed:
− What is the diversity of the studied fungal endophytes and their host plants?
− Which are the potential endophyte effects that are addressed in the studies? Were the results positive?
− What aspects of fungal endophytes are extensively investigated and what ones are scarcely studied?
2. Methodology
2.1. Study design
We conducted a systematic analysis of literature published about the effect of fungal endophytes in plants. The structure of the analysis process was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.2. Literature search
2.2.1. Eligibility criteria and search methodology
To obtain the metadata that would allow analyzing the current status of fungal endophyte studies we performed a literature search on the Scopus database in 2021, including all the articles published up to December 2021.
The following terminology was used for the search:
- Endophyte
- Plant OR grass: This term was included to avoid research with no inoculation in plants.
- Fungi: Fungus, fungal, and variations were included.
- Stress OR tolerance: To find the purpose of the endophytes
- NOT mycorrhiza: Excluded mycorrhizal fungi to find strict endophytic fungus.
The terminology was looked for in title-abstracts and keywords of articles.
A second search was conducted afterward for growth-promoting endophytes. This time, the keyword for ‘stress or tolerance’ was changed to ‘growth.’
Other non-vital roles like phytoremediation, fruiting characteristics and such can be of great interest for specific host plants or environment but are not as globally significant as the main roles previously discussed, hence their absence in our literature search.
2.2.2. Study selection and criteria for inclusion
Results from both search queries were merged, and then examined to ensure the relevance of the publications for our topic concerns. In order to achieve equality of the results, references with the following conditions were removed:
studying endophyte presence and identity without further application,
focusing on the cattle industry,
aiming to particularly identify the mechanism of the host-endophyte relation,
not fulfilling the search requirements due to other unspecific reasons, and
not having its original source available or verifiable.
From the selected manuscripts, the information provided about the fungal endophytes, the host plants, the type of stress studied, and the results were included in the final analysis. In addition, highly similar articles such as in same authors, endophyte and host, were only counted once, including all the results from them.
2.2.3. Data collection
Subsequently, the references were exported to an MS Excel spreadsheet for further analysis. To compare selected articles, the data was broken down into different sets:
The fungal endophytes’ species, or at least their genus. DSE, unidentified fungi, or the use of all microbiome of a plant was recorded as such if appropriate.
Original host plants’ species, from which the endophytes were isolated. Their condition as wild or cultivated plants was distinguished, and endophytes that were not isolated in the current study had this section unfilled to avoid duplicates.
Study host plants’ species, whether the endophyte is inoculated in the same host plant from which it was isolated, or a new host. Their condition as wild or cultivated plants was noted.
Method used to produce endophyte-free plants, if pertinent. This section does not account for surface sterilization, since it’s a widespread procedure that occurs in most, if not all, studies.
Tested effects of the endophytes.
Experimental results, whether the endophytes conferred clearly beneficial effects, had some varying impact, or had no significant or negative effects on the host plant.
When several species were used, they were all registered. Taxonomic synonymy was avoided by assigning only the current taxonomic name, as consulted in the NCBI database at the moment of the analysis.
Furthermore, to simplify results, we recorded some data into groups:
Fungal endophytes’ division. Ascomycota, Basidiomycota, and Mucoromycota. When fungi of different phyla were reported in the same article, it was considered that the article worked with various phyla.
Original host plants’ family.
Study plants’ family.
Function group of the endophyte. The potential effects were classified into one of these categories: abiotic stress tolerance, biotic stress tolerance, growth promotion, other effects, or several of them. When growth promotion was assessed under stress conditions, only the stress tolerance effect was considered.
2.3. Study quality and risk of bias
This meta-analysis aims to perform a qualitative analysis of the currently available literature. In this way, it is necessary to consider that, due to the diversity and complexity of the analyzed studies, comparing experiments with different study parameters is not always feasible. Therefore, for the purpose of this work, the simplest and clearest variables have been compared.
It is also important to address the issue of independence of the analyzed studies since the search results include papers that are part of the same study or research group and only differ on either study parameters or research progression. To avoid inaccuracy caused by counting dependent items as independent ones, a set of papers from the same study or research group was considered as a single independent item with several study parameters.
We are aware of the possible bias committed by considering only those articles that use the word ‘endophyte’ in the title, keywords, or abstract, excluding some potential useful reports. Yet, opposite bias could also happen, since there might be articles that elaborate on endophytic organisms only in the main text, hence remaining out of the current research and analysis. All this said, the only way to correct such bias would be reading all articles with the word ‘endophyte’ in the main text and selecting those that meet the other criteria for inclusion, which effort we considered as unmanageable for the purpose of this work.
3. Results and discussion
Beneficial microorganisms play an important role in plant performance and adaptation to adverse conditions. Among them, the interest in fungal endophytes has raised in the last decades due to their ability to produce secondary metabolites that could protect the host plant against herbivory (Bultman and Bell Citation2003; Shiba and Sugawara Citation2005). More recently, it also has been observed that fungal endophytes could play other roles related to plant protection against stress conditions (Waller et al. Citation2005; Hossain et al. Citation2017), increasing the interest of the researchers in this emerging area.
In order to confirm and analyze the state of fungal endophytes’ studies, we performed two literature searches that retrieved around 1138 online references for the first one, covering stress tolerance, and 418 for the second one, focused on growth promotion. After the pertinent reductions described in the methodology section of this paper, we elaborated a database with a sum of 392 papers, containing scientific publications that range from the year 1988 to December 2021.
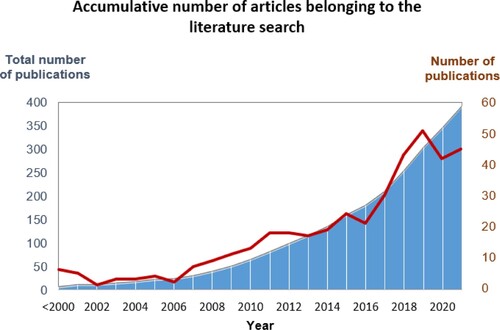
The preliminary analysis of the gathered literature proves the rising tendency of this research area, as observed in the graphic slope of . This figure shows how this study field has been increasing steadily since 2006, and how the number of publications on this topic in the last 5 years is higher than the number of publications before 2017. However, it has been also observed that, in the last year analyzed, the number of publications that fulfill our search criteria has been slightly lower. On the other hand, an enhancement of articles with related topics as the study of plant microbiomes has increased. Thus, this makes clear the outstanding performance of fungal endophytes as a research field and the ongoing attention given to these microorganisms by the research community.
Figure 1. Number and accumulative number of published articles identified in the present literature research until 2021. Right-side axis belong to graphic line; Left-side axis belong to graphic area.
The literature we examined usually had a common way of working, with experiments arranged following next steps: isolation of endophytes from a host of interest or acquisition from a provider, selection of most relevant endophyte or few endophytes, inoculation in the plant, and evaluation of its effects in growth promotion or against some kind of stress. In the following sections, we show our analysis of the relevant aspects and other details of interest of the reviewed papers.
3.1. Fungal endophytes and their diversity
Studies usually state the fungal strain used in their experiments, either specified by the provider or obtained by identifying the isolates of a host plant. Yet, 21% of the literature did not completely identify the endophyte, leaving the determination at the genus level or potential candidates. This might be caused by several reasons: not finding total coincidence with any species on fungal databases, focusing on the role of the endophyte rather than its identity, etc.
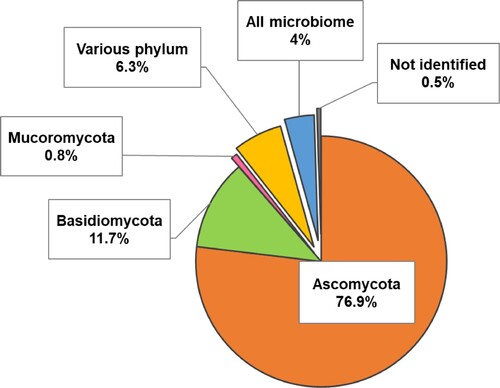
Tested endophytes are categorized at the division level in , and genera frequency is shown in (a). Also, though it was outside the scope of the study, root endophytes seemed to be more commonly studied, followed by leaf and stem endophytes. Our analysis showed that the vast majority of the studies are focused on Ascomycota, which turned out to be almost 80% of the database results, while Basidiomycota covered around 12%, and Mucoromycota barely added up to another 1% of the results.
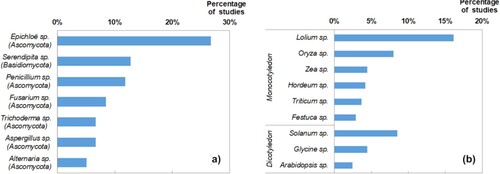
Ascomycota’s prevalence is not surprising, since ascomycetes fungi are the commonest in nature. In this category, we can find genera such as Epichloë, Penicillium, Trichoderma, Fusarium, Aspergillus, and Alternaria.
The relevancy of Epichloë, also known as Neotyphodium or Acremonium in past literature for its asexual morphs, goes back to 1988 when it was discovered. It is considered the first reported endophytic fungi, and it is beyond question the most known fungal endophyte genus since it extensively colonizes widespread forage grasses, such as Lolium spp. (Schardl et al. Citation2004). As seen in reviewed articles, studies about Epichloë are usually focused on plants as forage or turf, and there are no studies about inoculation of this Class 1 endophyte in non-host plants due to its limited host range. West et al. (Citation1988) observed that endophyte-infected Lolium arundinaceum gained tolerance to drought and nematode infection and Elmi and Robbins (Citation2000) and other posterior works confirmed those premises relating both stresses. Lolium perenne as endophyte host has therefore been studied to demonstrate, for instance, tolerance to drought and nitrogen deficit (Ravel et al. Citation1997), to heavy metals like zinc (Monnet et al. Citation2001) or predators like rice leaf bug (Shiba and Sugawara Citation2005).
Another recurrent genus of Ascomycota endophytes is Penicillium for the ability to improve growth and abiotic stress resistance, with species like P. janthinellum (Khan et al. Citation2014) or P. funiculosum (Khan et al. Citation2011). Likewise, Fusarium is not only studied for abiotic resistance ability but to confer biotic resistance, with special regard to pathogenic F. oxysporum strains (Ting et al. Citation2008; Nefzi et al. Citation2019).
Some of the articles refer to the use of dark septate endophytes (DSE). This definition was used for the first time by Read and Haselwandter (Citation1981) to describe a diverse group of ascomycetous endophytes with melanin hyphae that live in roots without causing any visible symptoms. Subsequently, numerous researchers have used this informal term without further classification. Even so, few articles describe the use of DSE and identify the fungi as Alternaria sp. or Penicillium sp.
In contrast to the diversity of ascomycetous endophytes exposed in the publications, basidiomycetes, and mucoromycetes have been less studied according to our literature database. One exception to this is the basidiomycete Serendipita indica, previously known as Piriformospora indica. S. indica was firstly reported in 1998 (Verma et al. Citation1998), discovered in desert soil from India, and found to be similar to arbuscular mycorrhizal fungi (AMF), yet, as opposed to them, it was able to grow in axenic culture (Varma et al. Citation2001). And, unlike Epichloë, it has been since widely tested in both monocots and dicots. The most common benefits of this endophyte have been reported to be growth promotion (Sahay and Varma Citation1999; Rai et al. Citation2001; Bagde et al. Citation2011; Satheesan et al. Citation2012; Noora et al. Citation2017), as well as inducing resistance against abiotic stress such as drought (Sherameti et al. Citation2008; Hosseini et al. Citation2017; Hussin et al. Citation2017; Ahmadvand and Hajinia Citation2018) and salinity (Waller et al. Citation2005; Sinclair et al. Citation2013; Abdelaziz et al. Citation2017; Li et al. Citation2017; Sharma et al. Citation2017). Some studies also report a beneficial role against pathogens (Cosme et al. Citation2016; Lin et al. Citation2019), but this field is less studied.
Not a considerable amount of studies worked with multiple endophytes at once. Some articles referred to various endophytes from different phyla (6%), through the application of various potential endophytes or a selection from the microbiome of the host plant. In this case, studies use a consortium of fungal strains, though using a consortium of both bacterial and fungal strains like done by Varkey et al. (Citation2018) was apparently more common. Furthermore, a scarce number of studies worked with the whole microbiome from a plant (4%). We hypothesize that the scarcity of these types of study is a sign of researchers considering beneficial properties provided by individual endophytes more relevant than their interactions within plant tissue. This, at the same time, is probably due to the complications that involve studying several endophytic species at once since, according to Kivlin et al. (Citation2013), since the interactions depend on not clear conditions, there is the danger of underestimating or overestimating the global effects of a group of endophytes.
3.2. Host plant and their diversity
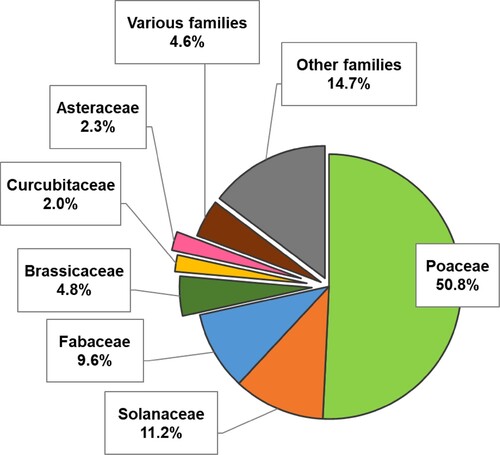
Results of the analysis regarding endophyte host plants are illustrated in . Poaceae family dominates the studies with half of the literature results (51%). The other half refers mostly to a diversity of host plants, usually of economic relevance, and only a few studies refer their experiments to several plant families.
As seen in (b), ryegrass (Lolium sp.) stands out among the recurrent host plants. This grass genus, which includes Lolium perenne, Lolium arundinaceum, and Lolium multiflorum, has been included in more than 16% of the total number of articles. We consider that the main reasons for this might be its wide range, importance in the cattle sector, and identity as host of Epichloë endophytes. Another well-used monocot in literature is rice (Oryza sativa). This is one of the most relevant food crops in the world and is inoculated with non-host endophytes in order to obtain growth enhancement and abiotic stress tolerance. Other species from the Poaceae family commonly mentioned are barley (Hordeum vulgare), maize (Zea mays), common wheat (Triticum aestivum), and red fescue (Festuca rubra) in order of recurrence. All of them are extensively cultivated species, and barley and red fescue, along with some close relatives, are also commonly infected with Epichloë sp.
Monocots are surely the prevalent plant group of interest, since they are of great economic and cultural relevance and are relatively easy to work with. However, dicot plants as endophyte hosts have yet a lot of unrevealed potential. Around 30% of the studies covered species that belong to families with relevance as food resources such as Solanaceae, Fabaceae, Brassicaceae, and Cucurbitaceae. Among these, the most used species are tomato (Solanum lycopersicum) and soybean (Glycine max), which take part in around 9% and 5% of the articles, respectively. Both species hold great economic value similar to the previously mentioned monocots, yet are seldom used as biological models in front of biotic stress.
The remaining studies in the database worked with plants that have significance either as ornamental plants, ecological models, or sources for other goods such as medicine, lumber, or resin. In addition to these, a few articles (barely 4%) need to be considered for applying endophytes to several plant species that belong to different families.
Finally, the environment in which plants grow and where the experiments are conducted can also have an impact on the reliability of the results. Although field experiments would be the most realistic approach for the studies (Rai et al. Citation2001; Zhou et al. Citation2018), it is difficult to perform this kind of experiments because of the complexity of all the factors that get involved in it. Instead, most publications referred to growth chambers and greenhouses to carry out their experiments. On the opposite, a couple of studies (5%) performed their experiments only in vitro bioassays (Dovana et al. Citation2015; Khan et al. Citation2017), usually with mutant rice. This last kind of studies has particularly controlled environmental conditions and further experiments would be required to test the effects of the endophytes on the behavior and interactions of the host plant under more realistic conditions.
3.3. Host plant and study plant conditions
Since the original host plant of the studied endophytes may differ from the study’s host plant, the information regarding their conditions was recorded ().
Figure 5. Host plant origin in literature research. (*) Not stated or endophyte is whether from soil, previously isolated or provided by a bank.
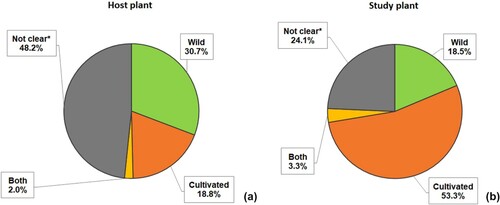
Around 30% of the studies referred to host plants from wild origin (Li et al. Citation2017; Li et al. Citation2018). We consider studying endophytes from nature-adapted plants of great significance due to their biodiversity and wide-ranging microbiome. Those endophytes are also typically acquired from locations with extreme environmental conditions such as deserts to assess their potential role in adverse conditions. On the contrary, only 18% of study plants were clearly traditional or wild plants, against 53% of the studies that inoculated cultivated species. This confirms that finding endophytes from wild origin to use them as means to improve the behavior of crops is more relevant than discerning their role in a natural environment.
In 52% of the articles, isolating the fungal endophyte strains was an integral part of the study. In the other cases, the strains were either isolated in previous work of the research group or provided by microbial banks or another entity.
On another note, we compared the identity of the host and study plant. In this case, we could see that the fungal endophyte was inoculated to the same host plant species from which it was isolated in at least 48% of the articles, while at least 25% of them used a different species of host plants in the experiments. Inoculation of the same plant species from which the endophyte was isolated is, possibly, an indicator of the interest in exploring the effect of the endophyte on its natural host.
There also was a tendency to use some methods in order to produce endophyte-free plants, but this seems to be of decreasing importance in the more recent studies. These methods are specified in , and focus on obtaining plants that are cleared up from any microbiota to avoid interference with the endophyte of interest. Among these treatments, systemic fungicide treatment prevailed (11%), especially in the early years of research. However, these practices have been discontinued since, presumably due to the need of considering interaction within the plant microbiome in order to ascertain the effect of the study endophyte. Likewise, heat treatment use has almost disappeared nowadays (4%). In this case, the reason may be the possible negative impact of heat treatment in seed germination and future plant physiologic aspects and performance. The only method that is still used with the same incidence as before is the individual selection of study material (6%). This is a procedure that usually makes use of microscopy to identify the presence or absence of the study endophyte to classify their plant material. In the end, these treatments are not used anymore on a current basis, and most of the studies (73%) tend to not apply them. This includes the great number of publications where inoculated plants differ from the original host plant of the study endophyte and the other studies that only resort to surface sterilization of the seeds before inoculation.
Table 1. Used method to obtain endophyte-free plants besides surface sterilization.
3.4. Role of fungal endophytes in studied plants
Plants are always exposed to multiple environmental factors and many of them can result in biological stress, an adverse condition that inhibits their normal functioning (Jones and Jones Citation1989). However, fungal endophytes can also affect multiple aspects of the host plant’s life cycle. It has been extensively reported how fungal endophytes can alter host physiology under stress and confer tolerance to host plants by enhancing activation of the host’s defense system (Tidke et al. Citation2017; Shankar Naik Citation2019). The diverse effects that endophytes trigger in their host plants can range from growth improvement to protection against cattle. Thus, the effects are not straightforward and there is a whole range of benefits that could be induced in the plant and play a critical role in their resilience (Rodriguez et al. Citation2009; Mei and Flinn Citation2010; White and Torres Citation2010).
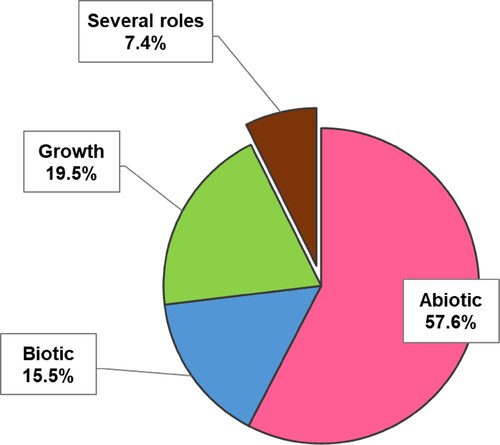
In this study, studied effects of fungal endophytes are sorted as seen in . Resistance against abiotic stress is the most studied condition (57%), followed by growth promotion (20%) and resistance against biotic stress (15%). The remaining articles experimented with several types of plant stress conditions (8%).
In the following sections, we check the specific results for each type of stress, taking into account individual results for the studies.
3.4.1. Effect of fungal endophytes under abiotic stress
With the advance of climate change, plants are severely exposed to abiotic stress, both physical and chemical. Abiotic stress is defined, according to Ben-Ari and Lavi (Citation2012), as the negative impact of non-living factors on living organisms in a specific environment. These conditions, such as water deficit, extreme temperature, soil salinity and heavy metal contamination are common adversities that affect crop productivity worldwide.
In this context, endophytes can play a significant role in conferring stress tolerance to host plants by altering water relations, osmolyte production, and synthesis of hormones and reactive oxygen species (ROS) (Shankar Naik Citation2019). Thus, we believe that countering the extensive consequences of global warming and contamination is one of the main goals of studying fungal endophytes. The interest in the potential induction of resistance against abiotic stress is reflected in a high number of the analyzed articles in the current review (55%). The specific stress conditions tested in each study are classified and presented in (a).
Figure 7. Most studied potential roles of fungal endophytes in front of abiotic (a) and biotic (b) stresses.
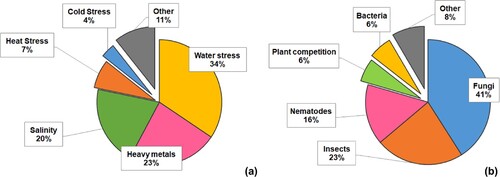
Water stress takes part in 40% of the studies, and this being the most studied condition is expected since drought is the most widespread limiting factor for plant productivity (Kane Citation2011; Ahmadvand and Hajinia Citation2018; Kavroulakis et al. Citation2018; Kuzhuppillymyal-Prabhakarankutty et al. Citation2020). Following water stress, heavy metals (Bilal et al. Citation2018; Ikram et al. Citation2018) and salinity stress (Radhakrishnan et al. Citation2015; Wang et al. Citation2020) were also recurrently studied, with each covering 22% and 18% of the studies about abiotic stress, respectively. These are the commonest source of soil contamination, and both are capable of interfering with vital cellular processes and causing severe injuries to plants (Munns and Tester Citation2008; Yadav Citation2010).
The remaining articles dealt with temperature stress, nutrient availability, cutting and other minor categories. It is known that plants subjected to extreme temperature stress alter processes that critically affect development and growth (Bokszczanin and Fragkostefanakis Citation2013). However, it is surprising there isn’t a considerable amount of studies about the stress caused by temperature change, albeit the importance of its environmental effects. In this way, Acuña-Rodríguez et al. (Citation2020) consider cold stress as a substantial gap in this study field, and according to their analysis, microbial symbionts confer more benefits at low temperatures.
3.4.2. Effect of fungal endophytes under biotic stress
Another relevant condition that can affect plants severely is biotic stress. Biotic stress refers to the negative impact caused by other living organisms, usually plant pathogens like insects, nematodes, pathogenic bacteria, and fungi or competitive plants or weeds (Gull et al. Citation2019). Plants respond to this type of stress with defense mechanisms of their immune system, generating chemical compounds such as salicylic acid (SA) or jasmonic acid (JA), producing proteins and enzymes, increasing cell lignification and other morphological or structural barriers (Madani et al. Citation2018).
In order to confront biotic stress, endophytic symbioses can play an important role, since these associations are reported to affect the performance and behavior of pathogenic organisms (Hartley and Gange Citation2009; Shankar Naik Citation2019). In this way, entomopathogenic fungi (EPF) are common in terrestrial environments and can be important natural regulators of insect and arachnid populations (Chandler Citation2017).
However, the analysis reveals that, compared to abiotic stress, endophyte roles regarding biotic stress are much less studied. Less than 20% of articles studied the role of endophytes for biotic stress resistance. Biotic stressors (b) mainly cover fungal pathogens (Waqas et al. Citation2015), insects (Cosme et al. Citation2016), and soil nematodes (Liarzi et al. Citation2016). Fungi pathogens are present in 41% of studies, while insects add up to 23% and nematodes to 16%. Thus, the main tested effect of fungal endophytes is to confer protection against other fungi, presumably pathogenic, and probably anticipating a competition for the ecological niche (Oliva et al. Citation2021).
Interestingly, the effect of fungal endophytes against bacterial pathogens was rarely evaluated (6%). In spite of that, manuscripts describing the protective effect of fungal endophytes against bacterial pathogens usually report good control of the disease. For instance, Lin et al. (Citation2019) observed that plants treated with Serendipita indica and infected with the bacterial pathogen Ralstonia solanacearum showed an enhanced response of the Jasmonic acid pathway and the expression of VSP, PR1 and PR5 genes.
3.4.3. Effect of fungal endophytes in promoting growth
Aside from detrimental abiotic and biotic stress conditions, the growth properties of plants are the main concern in crops. Fungal endophytes, along with bacterial endophytes and mycorrhizae, are associated with the transfer of nutrients and minerals in the host plant (Shankar Naik Citation2019), so they are biological treatments that can improve different aspects of the plant life cycle. In this way, Plant Growth Promoting Fungi (PGPF) are known as beneficial fungi in close interaction with plants, inducing an enhancement of the plant performance by alteration of physiological pathways and molecular mechanism regulation. They’re also associated with induced systemic resistance (ISR) through the ability to enhance nutrient uptake and phytohormone production (Hossain et al. Citation2017).
Therefore, fungal endophytes have been extensively studied for their beneficial properties and potential to enhance productivity for crop plants. The literature research conducted in this paper reveals that studies focused on PGPF take near 20% of the fungal endophyte investigation, covering both in vitro and in field application on plants. These reports cover their beneficial effects on germination (Vujanovic et al. Citation2016), nutrient uptake (Khayamim et al. Citation2011), host plant vigor, biomass (Al-Hosni et al. Citation2018), and fruit production (Rho et al. Citation2020), all in accordance with an ecological agriculture approach.
Among the studies that assess growth promotion, 80% of the research is done in absence of pathogens in stable environmental conditions, while the other 20% further studied the effects of the same endophytes when the host plants were exposed to abiotic or biotic stress. The latter scenario is especially useful since it allows comparison of effects both on stable and stress conditions. It should be reminded that the effects on growth promotion only under stress conditions are not accounted for in this section since it has no stable conditions to be compared to.
3.5. Results of efficacy
Research results are decisive to know the real impact of the experimental studies. As expected, most analyzed articles in the current review report beneficial roles of the studied endophytes, irrespective of the magnitude of the effects. We categorized these results as good, neutral or variable. We expected to find a high number of positive results since there is a preference for reporting positive results and keeping the negative ones unpublished (Mehta Citation2019).
Around a quarter of the studies agreed that the effects on the host and environmental factors are variable, to the extent that some endophytes could negatively affect the host plant in some way. This potential negative impact is likewise reported in a small number of articles that were not able to provide positive results. This usually occurred in experiments that focus on finding new effects of previously known endophytic fungi, such as Epichloë spp., inoculating it on non-host plants and/or under new conditions (Hall et al. Citation2014; Heineck et al. Citation2018). It is to be noted that the particular conditions of the experiments can affect the reliability of the study comparisons (Arnold Citation2007). For instance, as observed by Singh et al. (Citation2016) with Serendipita indica, growth media can influence the relationship between the fungal endophytes and their host plant. Yokoya et al. (Citation2017) also showed how habitats with suboptimal conditions have more endophytes that prove to be beneficial to their host plant. Regardless, we consider these results to be key in providing knowledge about research limitations since they indicate what can be avoided in future studies.
4. Conclusions and future prospects
This work has analyzed the current structure of fungal endophyte research, focused on the organization and variables of the studies that aim to find beneficial effects of fungal endophytes on a host plant. This field of study has been on the rising trend for 20 years now and has worked mostly on Ascomycota fungi, with ubiquitous and widely known endophytes such as Epichloë spp. We here confirmed that only a few fungal species are recurrently studied as endophytes, while there is a broad spectrum of novel, potentially interesting endophytic fungi that have only been sporadically studied. Moreover, less than 10% of the studies deal with several endophytes, implying a shortage of studies regarding the symbioses interaction and the high difficulty attached to them. Furthermore, other analyzed aspects of the study also denote certain gaps. In particular, host plants are still mainly monocots like Lolium spp., leaving fair room to improve on dicots’ studies. Wild species are frequently used as a source of fungal endophytes but quite less as study plants. The potential effects of fungal endophytes are most commonly tested on cultivated species (between 51% and 80%) and assessed in front of abiotic stress (around 57%), especially on drought conditions. A relevant abiotic scenario that is surprisingly barely studied is stress caused by temperature changes (11%), especially cold stress. Biotic stress experiments, in turn, only appear in 15% of the literature, and among the biotic factors, fungal pathogens are the most studied, while other biotic factors like bacterial pathogens are scarcely covered. On another note, resorting to procedures to ensure endophyte-free plants has decreased lately. The majority of the studies use growth chambers or greenhouse experiments, while field studies are very limited. Lastly, we found that more than 25% of the studies reported neutral, variable, or negative effects from some endophytes, which denotes the difficulty of this experimental research.
The results showed in this work highlight the interest in the field of the use of fungal endophytes to improve the plant performance, but also uncover certain areas are still understudied such as protection against bacterial diseases. Despite that, the use of fungal endophytes is still in its beginning and presents some interesting questions for future studies such as: what is the effect of an introduced endophyte in the plant microbiome?, could it affect other functions besides the studied ones? Could different fungal endophytes be combined in a synthetic community to achieve a broad spectrum of protection? Is the observed effect caused by the presence of the endophyte or by its secreted metabolites? If the latter, could it be possible to mimick the effect using endophyte exudates?
Taken together, fungal endophytes and their interaction with their host plant are still an intricate subject, with effects that vary from mutualist to pathogenic depending on several factors. Nonetheless, their relevance as a potential source of growth promotion and stress tolerance is unquestionably recognized, and their beneficial effects on plant physiology are reported across several endophytes and host plants. The results presented here demonstrate the incidence and direction of fungal endophyte research, and also prove the presence of some unexplored subjects in the field, especially regarding dicot plants and against biotic stress.
Declarations
Data availability statement for basic data sharing policy
All reviewed articles in the present manuscript are accessible through Scopus® database and checked on 30st December 2021. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare there are no conflicts of interest that are relevant to the content of this manuscript.
Author contributions
Conceptualization: LLX, BV, PGA, EL; data collection: LLX, EL; methodology: LLX, EL; formal analysis: LLX; writing—original draft: LLX, EL; writing—review and editing: LLX, BV, PGA, EL; funding acquisition: BV, PGA and EL.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Luisa Liu-Xu
Luisa Liu-Xu is a PhD student at the Departament of Agricultural and Environmental Sciences, Universitat Jaume I.
Begonya Vicedo
Begonya Vicedo is professor at the Departament of Agricultural and Environmental Sciences, Universitat Jaume I.
Pilar García-Agustín
Pilar García-Agustín is professor at the Departament of Agricultural and Environmental Sciences, Universitat Jaume I.
Eugenio Llorens
Eugenio Llorens is Post-doctoral researcher at the Departament of Agricultural and Environmental Sciences, Universitat Jaume I.
References
- Abdelaziz ME, Kim D, Ali S, Fedoroff NV, Al-Babili S. 2017. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 263:107–115.
- Acuña-Rodríguez IS, Newsham KK, Gundel PE, Torres-Díaz C, Molina-Montenegro MA. 2020. Functional roles of microbial symbionts in plant cold tolerance. Ecol Lett. 23(6):1034–1048.
- Ahmadvand G, Hajinia S. 2018. Effect of endophytic fungus Piriformospora indica on yield and some physiological traits of millet (Panicum miliaceum) under water stress. Crop Pasture Sci. 69(6):594–605.
- Al-Hosni K, Shahzad R, Khan AL, Imran QM, Al Harrasi A, Al Rawahi A, Asaf S, Kang SM, Yun BW, Lee IJ. 2018. Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J Plant Interact. 13(1):112–118. https://www.tandfonline.com/doi/full/10.1080/17429145.2018.1432773
- Arnold AE. 2007. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol Rev. 21(2–3):51–66.
- Bagde US, Prasad R, Varma A. 2011. Influence of culture filtrate of Piriformospora indica on growth and yield of seed oil in Helianthus annus. Symbiosis. 53(2):83–88. https://link.springer.com/article/10.1007/s13199-011-0114-6
- Ben-Ari G, Lavi U. 2012. Marker-assisted selection in plant breeding. Plant Biotech Agric. 163–184. doi:10.1016/B978-0-12-381466-1.00011-0
- Bilal S, Shahzad R, Khan AL, Kang S-M, Imran QM, Al-Harrasi A, Yun B-W, Lee I-J. 2018. Endophytic Microbial Consortia of Phytohormones-Producing Fungus Paecilomyces formosus LHL10 and Bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to Attenuate Aluminum and Zinc stresses. Front Plant Sci . 9:1273. https://www.frontiersin.org/article/10.3389/fpls.2018.01273/full
- Bokszczanin KL, Fragkostefanakis S. 2013. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front Plant Sci. 4(AUG):315. www.frontiersin.org
- Brundrett M. 2006. Understanding the Roles of Multifunctional Mycorrhizal and Endophytic Fungi. 281–298. https://research-repository.uwa.edu.au/en/publications/understanding-the-roles-of-multifunctional-mycorrhizal-and-endoph
- Bultman TL, Bell GD. 2003. Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos. 103(1):182–190. http://doi.wiley.com/10.1034/j.1600-0706.2003.11574.x
- Chandler D. 2017. Basic and applied research on Entomopathogenic fungi. In: Microb control insect mite pests from theory to pract. [place unknown]: Elsevier Inc; p. 69–89.
- Cosme M, Lu J, Erb M, Stout MJ, Franken P, Wurst S. 2016. A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling. New Phytol. 211(3):1065–1076. https://onlinelibrary.wiley.com/doi/abs/10.1111/nph.13957
- Dastogeer KMG. 2018. Influence of fungal endophytes on plant physiology is more pronounced under stress than well-watered conditions: a meta-analysis. Planta. 248(6):1403–1416. doi:10.1007/s00425-018-2982-y.
- Dovana F, Mucciarelli M, Mascarello M, Fusconi A. 2015. In Vitro Morphogenesis of Arabidopsis to Search for Novel Endophytic Fungi Modulating Plant growth.Labra M, editor. PLoS One. 10(12):e0143353. https://dx.plos.org/10.1371/journal.pone.0143353
- Edgerton MD. 2009. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 149(1):7–13. www.plantphysiol.org/cgi/doi/10.1104/pp.108.130195
- Elmi W, Robbins K. 2000. Endophyte effects on reproduction of a root-knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. Grass Forage Sci. 55(2):166–172. http://doi.wiley.com/10.1046/j.1365-2494.2000.00210.x
- Fadiji AE, Babalola OO. 2020. Elucidating mechanisms of endophytes used in plant protection and other bioactivities With Multifunctional prospects. Front Bioeng Biotechnol. 8:467. www.frontiersin.org
- Gautam AK, Avasthi S. 2019. Fungal endophytes: potential biocontrol agents in agriculture. [place unknown]: Elsevier Inc. doi:10.1016/B978-0-12-817004-5.00014-2
- Gull A, Ahmad Lone A, Ul Islam Wani N. 2019. Biotic and Abiotic Stresses in Plants. In: Abiotic Biot Stress Plants. www.intechopen.com
- Hall SL, McCulley RL, Barney RJ, Phillips TD. 2014. Does Fungal Endophyte Infection Improve Tall Fescue’s Growth Response to Fire and Water limitation?Heil M, editor. PLoS One. 9(1):e86904. https://dx.plos.org/10.1371/journal.pone.0086904
- Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A. 2015. The Hidden World within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 79(3):293–320.
- Hartley SE, Gange AC. 2009. Impacts of plant symbiotic fungi on insect herbivores: Mutualism in a multitrophic context. Annu Rev Entomol. 54:323–342.
- Heineck GC, Watkins E, Ehlke NJ. 2018. The fungal endophyte Epichloë festucae var. lolii Does Not Improve the Freezing Tolerance of Perennial ryegrass. Crop Sci. 58(4):1788–1800. http://doi.wiley.com/10.2135/cropsci2017.12.0731
- Hossain MM, Sultana F, Islam S. 2017. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In: Plant-Microbe Interact Agro-Ecological perspect. p. 135–191. https://link.springer.com/chapter/10.1007/978-981-10-6593-4_6
- Hosseini F, Mosaddeghi MR, Dexter AR. 2017. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Biochem. 118:107–120.
- Hussin S, Khalifa W, Geissler N, Koyro H-W. 2017. Influence of the root endophyte Piriformospora indica on the plant water relations, gas exchange and growth of Chenopodium quinoa at limited water availability. J Agron Crop Sci. 203(5):373–384. http://doi.wiley.com/10.1111/jac.12199
- Hyde KD, Soytong K. 2008. The fungal endophyte dilemma.
- Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. 2018. IAA producing fungal endophyte Penicillium roqueforti thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One. 13(11.
- Jones HG, Jones MB. 1989. Introduction: some terminology and common mechanisms. [place unknown]: Plants under Stress. p. 1–10.
- Kane KH. 2011. Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the Mediterranean region. Environ Exp Bot. 71(3):337–344.
- Kavroulakis N, Doupis G, Papadakis IE, Ehaliotis C. 2018. Tolerance of tomato plants to water stress is improved by the root endophyte Fusarium solani FsK. Rhizosphere. 6(March):77–85. doi:10.1016/j.rhisph.2018.04.003.
- Khan AL, Gilani SA, Waqas M, Al-Hosni K, Al-Khiziri S, Kim Y-H, Ali L, Kang S-M, Asaf S, Shahzad R, et al. 2017. Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J Zhejiang Univ B. 18(2):125–137. http://link.springer.com/10.1631/jzus.B1500271
- Khan AL, Hamayun M, Kim YH, Kang SM, Lee IJ. 2011. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol Biochem. 49(8):852–861.
- Khan AL, Waqas M, Hussain J, Al-Harrasi A, Lee IJ. 2014. Fungal endophyte Penicillium janthinellum LK5 can reduce cadmium toxicity in Solanum lycopersicum (Sitiens and Rhe). Biol Fertil Soils. 50(1):75–85.
- Khayamim F, Khademi H, Sabzalian MR. 2011. Effect of Neotyphodium endophyte-tall fescue symbiosis on mineralogical changes in clay-sized phlogopite and muscovite. Plant Soil. 341(1–2):473–484. https://link.springer.com/article/10.1007/s11104-010-0659-9
- Kivlin SN, Emery SM, Rudgers JA. 2013. Fungal symbionts alter plant responses to global change. Am J Bot. 100(7):1445–1457.
- Kivlin SN, Lynn JS, Kazenel MR, Beals KK, Rudgers JA. 2017. Biogeography of plant-associated fungal symbionts in mountain ecosystems: A meta-analysis. Divers Distrib. 23(9):1067–1077.
- Kuzhuppillymyal-Prabhakarankutty L, Tamez-Guerra P, Gomez-Flores R, Rodriguez-Padilla MC, Ek-Ramos MJ. 2020. Endophytic Beauveria bassiana promotes drought tolerance and early flowering in corn. World J Microbiol Biotechnol. 36(3):47. doi:10.1007/s11274-020-02823-4.
- Li L, Lei L, Wang X, Zhu P, Wu H, Qi S. 2017. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol Biochem. 119:211–223.
- Li X, He X, Hou L, Ren Y, Wang S, Su F. 2018. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci Rep. 8(1):7896. www.nature.com/scientificreports/
- Li X-Z, Song M-L, Yao X, Chai Q, Simpson WR, Li C-J, Nan Z-B. 2017. The Effect of Seed-Borne Fungi and Epichloë Endophyte on Seed Germination and Biomass of Elymus sibiricus. Front Microbiol. 8(DEC):2488. http://journal.frontiersin.org/article/10.3389/fmicb.2017.02488/full
- Liarzi O, Bucki P, Braun Miyara S, Ezra D. 2016. Bioactive Volatiles from an Endophytic Daldinia cf. concentrica Isolate Affect the Viability of the Plant Parasitic Nematode Meloidogyne javanica.Jones J, editor. PLoS One. 11(12):e0168437. https://dx.plos.org/10.1371/journal.pone.0168437
- Lin HF, Xiong J, Zhou HM, Chen CM, Lin FZ, Xu XM, Oelmüller R, Xu WF, Yeh KW. 2019. Growth promotion and disease resistance induced in Anthurium colonized by the beneficial root endophyte Piriformospora indica. BMC Plant Biol. 19(1):40. https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-019-1649-6
- Llorens E, Sharon O, Camañes G, García-Agustín P, Sharon A. 2019. Endophytes from wild cereals protect wheat plants from drought by alteration of physiological responses of the plants to water stress. Environ Microbiol. 21:3299–3312.
- Lugtenberg BJJ, Caradus JR, Johnson LJ. 2016. Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol. 92(12):fiw194.
- Madani B, Shekari AM, Imahori Y. 2018. Physiological responses to stress. In: Postharvest physiol Biochem fruits Veg. Elsevier; p. 405–425.
- Mattoo AJ, Nonzom S. 2021. Endophytic fungi: understanding complex cross-talks. Symbiosis. 1–28. doi:10.1007/s13199-020-00744-2.
- Mayerhofer MS, Kernaghan G, Harper KA. 2013. The effects of fungal root endophytes on plant growth: A meta-analysis. Mycorrhiza. 23(2):119–128.
- Mehta D. 2019. Highlight negative results to improve science. Nature.
- Mei C, Flinn BS. 2010. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol. 4(1):81.
- Monnet F, Vaillant N, Hitmi A, Coudret A, Sallanon H. 2001. Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne. Physiol Plant. 113(4):557–563. http://doi.wiley.com/10.1034/j.1399-3054.2001.1130415.x
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 59(1):651–681. http://www.annualreviews.org/doi/10.1146/annurev.arplant.59.032607.092911
- Nefzi A, Ben AR, Jabnoun-Khiareddine H, Ammar N, Daami-Remadi M. 2019. Ability of endophytic fungi associated with Withania somnifera L. to control Fusarium Crown and Root Rot and to promote growth in tomato. Brazilian J Microbiol. 50(2):481–494. doi:10.1007/s42770-019-00062-w.
- Noora H, Shahabivand S, Karimi F, Aghaee A, Aliloo AA. 2017. Piriformospora indica affects growth, tropane alkaloids production and gene expression in Atropa belladonna L. plantlets. Med Plants. 9(1):55–62.
- Oliva J, Ridley M, Redondo MA, Caballol M. 2021. Competitive exclusion amongst endophytes determines shoot blight severity on pine.morriën E, editor. Funct Ecol . 35(1):239–254. https://onlinelibrary.wiley.com/doi/10.1111/1365-2435.13692
- Porras-Alfaro A, Bayman P. 2011. Hidden fungi, emergent properties: endophytes and microbiomes. Annu Rev Phytopathol. 49(1):291–315.
- Pozo MJ, Zabalgogeazcoa I, Vazquez de Aldana BR, Martinez-Medina A. 2021. Untapping the potential of plant mycobiomes for applications in agriculture. Curr Opin Plant Biol. 60:102034.
- Radhakrishnan R, Khan AL, Kang SM, Lee IJ. 2015. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann Microbiol. 65(1):585–593.
- Rai M, Acharya D, Singh A, Varma A. 2001. Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza. 11(3):123–128.
- Ravel C, Courty C, Coudret A, Charmet G. 1997. Beneficial effects of Neotyphodium lolii on the growth and the water status in perennial ryegrass cultivated under nitrogen deficiency or drought stress. Agronomie. 17(3):173–181. http://www.agronomy-journal.org/10.1051/agro:19970304
- Read DJ, Haselwandter K. 1981. Observations on the mycorrhizal status of some alpine plant communities. New Phytol. 88(2):341–352. http://doi.wiley.com/10.1111/j.1469-8137.1981.tb01729.x
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM, Bateson M, Kuhn E, Litvintseva A, Duda J. 1986. Thermotolerance generated by plant/fungal symbiosis. Proc Natl Acad Sci USA . 65(November. www.sciencemag.org/cgi/content/full/298/5598/1581/
- Rho H, Van Epps V, Kim S-H, Doty SL. 2020. Endophytes Increased Fruit Quality with Higher Soluble Sugar Production in Honeycrisp Apple (Malus pumila). Microorganisms. 8(5):699. https://www.mdpi.com/2076-2607/8/5/699
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS. 2008. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2(4):404–416. https://pubmed.ncbi.nlm.nih.gov/18256707/
- Rodriguez RJ, White JF, Arnold AE, Redman RS. 2009. Fungal endophytes: diversity and functional roles: tansley review. New Phytol. 182(2):314–330.
- Sahay N, Varma A. 1999. Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol Lett. 181(2):297–302. https://academic.oup.com/femsle/article-lookup/doi/10.1111/j.1574-6968.1999.tb08858.x
- Saikkonen K, Faeth SH, Helander M, Sullivan TJ. 1998. Fungal endophytes: A continuum of interactions with host plants. Annu Rev Ecol Syst.
- Satheesan J, Narayanan AK, Sakunthala M. 2012. Induction of root colonization by Piriformospora indica leads to enhanced asiaticoside production in Centella asiatica. Mycorrhiza. 22(3):195–202. https://link.springer.com/article/10.1007/s00572-011-0394-y
- Schardl CL, Leuchtmann A, Spiering MJ. 2004. Symbioses off grasses with seedborne fungal endophytes. Annu Rev Plant Biol. 2004(55):315–340.
- Schmidhuber J, Tubiello FN. 2007. Global food security under climate change. Proc Natl Acad Sci U S A. 104(50):19703–19708. www.pnas.orgcgi doi:10.1073/pnas.0701976104
- Schulz B, Boyle C. 2005. The endophytic continuum. Mycol Res. 109(6):661–686.
- Shankar Naik B. 2019. Functional roles of fungal endophytes in host fitness during stress conditions. Symbiosis.
- Sharma P, Kharkwal AC, Abdin MZ, Varma A. 2017. Piriformospora indica-mediated salinity tolerance in Aloe vera plantlets. Symbiosis. 72(2):103–115. https://link.springer.com/article/10.1007/s13199-016-0449-0
- Sherameti I, Tripathi S, Varma A, Oelmüller R. 2008. The Root-Colonizing Endophyte Pirifomospora indica Confers Drought Tolerance in Arabidopsis by Stimulating the Expression of Drought Stress-Related Genes in leaves. / 799 MPMI. 21(6):799–807.
- Shiba T, Sugawara K. 2005. Resistance to the rice leaf bug, Trigonotylus caelestialium, is conferred by Neotyphodium endophyte infection of perennial ryegrass, Lolium perenne. Entomol Exp Appl. 115(3):387–392.
- Sinclair G, Charest C, Dalpé Y, Khanizadeh S. 2013. Influence of arbuscular mycorrhizal fungi and a root endophyte on the biomass and root morphology of selected strawberry cultivars under salt conditions. Can J Plant Sci. 93(6):997–999.
- Singh BK, Trivedi P. 2017. Microbiome and the future for food and nutrient security. Microb Biotechnol. 10(1):50–53. /pmc/articles/PMC5270726/.
- Singh G, Sharma P, Sharma S. 2016. Role of growth media on the phytopromotional potential of symbiotic fungus Piriformospora indica. J Environ Biol. 37(July):565–571.
- Tan RX, Zou WX. 2001. Endophytes: A rich source of functional metabolites. Nat Prod Rep. 18(4):448–459.
- Tidke SA, Kumar Kl R, Ramakrishna D, Kiran S, Kosturkova G, Gokare RA. 2017. Current understanding of endophytes: their relevance, importance, and industrial potentials. IOSR J Biotechnol Biochem (IOSR-JBB. 3(3):43–59. www.iosrjournals.org
- Ting ASY, Meon S, Kadir J, Radu S, Singh G. 2008. Endophytic microorganisms as potential growth promoters of banana. BioControl. 53(3):541–553. https://link.springer.com/article/10.1007/s10526-007-9093-1
- Varkey S, Anith KN, Narayana R, Aswini S. 2018. A consortium of rhizobacteria and fungal endophyte suppress the root-knot nematode parasite in tomato. Rhizosphere. 5:38–42.
- Varma A, Singh A, Sudha Sahay NS, Sharma J, Roy A, Kumari M, Rana D, Thakran S, Deka D, et al. 2001. Piriformospora indica: An axenically Culturable mycorrhiza-like endosymbiotic fungus. In: Fungal assoc . Berlin, Heidelberg: Springer Berlin Heidelberg; p. 125–150. http://link.springer.com/10.1007/978-3-662-07334-6_8
- Verma S, Varma A, Rexer K-H, Hassel A, Kost G, Sarbhoy A, Bisen P, Bütehorn B, Franken P. 1998. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus . Mycologia. 90(5):896–903. https://www.tandfonline.com/doi/abs/10.1080/00275514.1998.12026983
- Vujanovic V, Yuan X, Daida P, Milunovic B, Germida J. 2016. Manipulation of cold stratification and endophytic effects on expression patterns of RSG and KAO genes in coleorhiza of wheat seeds. Plant Growth Regul. 79(2):219–227. https://link.springer.com/article/10.1007/s10725-015-0127-x
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, Von Wettstein D, et al. 2005. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U S A. 102(38):13386–13391. www.pnas.orgcgi doi:10.1073/pnas.0504423102
- Wang Z, Li C, White J. 2020. Effects of Epichloë endophyte infection on growth, physiological properties and seed germination of wild barley under saline conditions. J Agron Crop Sci. 206(1):43–51. https://onlinelibrary.wiley.com/doi/abs/10.1111/jac.12366
- Waqas M, Khan AL, Hamayun M, Shahzad R, Kim YH, Choi KS, Lee IJ. 2015. Endophytic infection alleviates biotic stress in sunflower through regulation of defence hormones, antioxidants and functional amino acids. Eur J Plant Pathol. 141(4):803–824.
- Weiß M, Waller F, Zuccaro A, Selosse MA. 2016. Sebacinales - one thousand and one interactions with land plants. New Phytol. 211(1):20–40. https://pubmed.ncbi.nlm.nih.gov/27193559/
- West CP, Izekor E, Oosterhuis DM, Robbins RT. 1988. The effect of Acremonium coenophialum on the growth and nematode infestation of tall fescue. Plant Soil. 112(1):3–6.
- White JF, Torres MS. 2010. Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol Plant. 138(4):440–446. http://doi.wiley.com/10.1111/j.1399-3054.2009.01332.x
- Yadav SK. 2010. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African J Bot. 76(2):167–179.
- Yokoya K, Postel S, Fang R, Sarasan V. 2017. Endophytic fungal diversity of Fragaria vesca, a crop wild relative of strawberry,along environmental gradients within a small geographical area. PeerJ. 2017(1):e2860. https://peerj.com/articles/2860
- Zhou L, Tang K, Guo S. 2018. The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field conditions. Int J Mol Sci. 19(1):270. http://www.mdpi.com/1422-0067/19/1/270
REFERENCES OF THE ANALYSIS
- Abadi VAJM, Sepehri M. 2016. Effect of Piriformospora indica and azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.). Symbiosis. 69(1):9–19.
- Abdelaziz ME, Kim D, Ali S, Fedoroff NV, Al-Babili S. 2017. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 263:107–115.
- Abou Alhamed MF, Shebany YM. 2012. Endophytic Chaetomium globosum enhances maize seedling copper stress tolerance. Plant Biol. 14(5):859–863. http://doi.wiley.com/10.1111/j.1438-8677.2012.00608.x
- Acuña-Rodríguez IS, Galán A, Torres-Díaz C, Atala C, Molina-Montenegro MA. 2020. Fungal symbionts enhance N-uptake for Antarctic plants even in Non-N limited soils. Front Microbiol. 11.
- Ahmadvand G, Hajinia S. 2018. Effect of endophytic fungus Piriformospora indica on yield and some physiological traits of millet (Panicum miliaceum) under water stress. Crop Pasture Sci. 69(6):594–605.
- Al Khoury C. 2021. Molecular insight into the endophytic growth of Beauveria bassiana within Phaseolus vulgaris in the presence or absence of tetranychus urticae. Mol Biol Rep. 48(3):2485–2496.
- Al-Hosni K, Shahzad R, Khan AL, Imran QM, Al Harrasi A, Al Rawahi A, Asaf S, Kang SM, Yun BW, Lee IJ. 2018. Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J Plant Interact . 13(1):112–118. https://www.tandfonline.com/doi/full/10.1080/17429145.2018.1432773
- Aletaha R, Sinegani AAS. 2020. Water availability in Soil affect performance of different Root Fungal colonizers on Metabolism of wheat. Iran J Sci Technol Trans A Sci. 44(4):919–931.
- Ali AH, Abdelrahman M, Radwan U, El-Zayat S, El-Sayed MA. 2018. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl Soil Ecol. 124:155–162.
- Ali AH, Radwan U, El-Zayat S, El-Sayed MA. 2019. The role of the endophytic fungus, Thermomyces lanuginosus, on mitigation of heat stress to its host desert plant cullen plicata. Biol Futur. 70(1.
- Ali R, Gul H, Hamayun M, Rauf M, Iqbal A, Shah M, Hussain A, Bibi H, Lee IJ. 2021. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem Biol Technol Agric. 8(1.
- Alikhani M, Khatabi B, Sepehri M, Nekouei MK, Mardi M, Salekdeh GH. 2013. A proteomics approach to study the molecular basis of enhanced salt tolerance in barley (Hordeum vulgare L.) conferred by the root mutualistic fungus Piriformospora indica. Mol Biosyst. 9(6):1498–1510. https://pubs.rsc.org/en/content/articlehtml/2013/mb/c3mb70069k
- Amalric C, Sallanon H, Monnet F, Hitmi A, Coudret A. 1999. Gas exchange and chlorophyll fluorescence in symbiotic and non-symbiotic ryegrass under water stress. Photosynthetica. 37(1):107–112. https://ps.ueb.cas.cz/pdfs/phs/1999/01/14.pdf
- Arora P, Wani ZA, Ahmad T, Sultan P, Gupta S, Riyaz-Ul-Hassan S. 2019. Community structure, spatial distribution, diversity and functional characterization of culturable endophytic fungi associated with glycyrrhiza glabra L. Fungal Biol. 123(5):373–383.
- Asay KH, Jensen KB, Waldron BL. 2001. Responses of tall fescue cultivars to an irrigation gradient. Crop Sci. 41(2):350–357. https://onlinelibrary.wiley.com/doi/abs/10.2135/cropsci2001.412350x
- Ashrafi J, Rahnama K, Babaeizad V, Ramezanpour SS, Keel C. 2021. Induction of wheat resistance to stb by the endophytic fungus serendipita indica and pseudomonas protegens. Iran J Biotechnol. 19(2):30–39.
- Assuero SG, Tognetti JA, Colabelli MR, Agnusdei MG, Petroni EC, Posse MA. 2006. Endophyte infection accelerates morpho-physiological responses to water deficit in tall fescue. New Zeal J Agric Res. 49(4):359–370. https://www.tandfonline.com/action/journalInformation?journalCode=tnza20
- Attitalla IH, Latiffah Z, Salleh B, Brishammar S. 2011. Biology and partial sequencing of an endophytic Fusarium oxysporum and plant defense complex. Am J Biochem Mol Biol. 1(2):121–144.
- Azad K, Kaminskyj S. 2016. A fungal endophyte strategy for mitigating the effect of salt and drought stress on plant growth. Symbiosis. 68(1–3):73–78.
- Aziz L, Hamayun M, Rauf M, Iqbal A, Arif M, Husssin A, Khan SA. 2021. Endophytic Aspergillus Niger reprograms the physicochemical traits of tomato under cadmium and chromium stress. Environ Exp Bot. 186.
- Aziz L, Hamayun M, Rauf M, Iqbal A, Husssin A, Khan SA, Irshad M, Jung LI. 2021. Aspergillus flavus reprogrammed morphological and chemical attributes of Solanum lycopersicum through SlGSH1 and SlPCS1 genes modulation under heavy metal stress. J Plant Interact. 16(1):104–115.
- Bacetty AA, Snook ME, Glenn AE, Noe JP, Hill N, Culbreath A, Timper P, Nagabhyru P, Bacon CW. 2009. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on pratylenchus scribneri. Phytopathology. 99(12):1336–1345. https://apsjournals.apsnet.org/doi/abs/10.1094/PHYTO-99-12-1336
- Badawy AA, Alotaibi MO, Abdelaziz AM, Osman MS, Khalil AMA, Saleh AM, Mohammed AE, Hashem AH. 2021. Enhancement of seawater stress tolerance in barley by the endophytic fungus aspergillus ochraceus. Metabolites. 11(7.
- Bae H, Sicher RC, Kim MS, Kim S-H, Strem MD, Melnick RL, Bailey BA. 2009. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in theobroma cacao. J Exp Bot. 60(11):3279–3295. https://academic.oup.com/jxb/article-lookup/doi/10.1093/jxb/erp165
- Bagde US, Prasad R, Varma A. 2011. Influence of culture filtrate of Piriformospora indica on growth and yield of seed oil in Helianthus annus. Symbiosis. 53(2):83–88. https://link.springer.com/article/10.1007/s13199-011-0114-6
- Bajaj R, Hu W, Huang YY, Chen S, Prasad R, Varma A, Bushley KE. 2015. The beneficial root endophyte Piriformospora indica reduces egg density of the soybean cyst nematode. Biol Control. 90:193–199.
- Ban Y, Xu Z, Yang Y, Zhang H, Chen H, Tang M. 2017. Effect of Dark septate endophytic fungus gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.). Pedosphere. 27(2):283–292.
- Bao G, Song M, Wang Y, Saikkonen K, Wang H. 2019. Interactive effects of Epichloë fungal and host origins on the seed germination of Achnatherum inebrians. Symbiosis. 79(1):49–58. doi:10.1007/s13199-019-00636-0.
- Barrera A, Hereme R, Ruiz-Lara S, Larrondo LF, Gundel PE, Pollmann S, Molina-Montenegro MA, Ramos P. 2020. Fungal Endophytes Enhance the photoprotective mechanisms and photochemical efficiency in the Antarctic Colobanthus quitensis (kunth) bartl. exposed to UV-B radiation. Front Ecol Evol. 8:122. https://www.frontiersin.org/article/10.3389/fevo.2020.00122/full
- Bayat F, Mirlohi A, Khodambashi M. 2009. Effects of endophytic fungi on some drought tolerance mechanisms of tall fescue in a hydroponics culture. Russ J Plant Physiol. 56(4):510–516. https://link.springer.com/article/10.1134/S1021443709040104
- Belesky DP, Burner DM, Ruckle JM. 2008. Does endophyte influence resource acquisition and allocation in defoliated tall fescue as a function of microsite conditions? Environ Exp Bot. 63(1–3):368–377.
- Berthelot C, Leyval C, Foulon J, Chalot M, Blaudez D. 2016. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal-polluted poplar phytomanagement sites. FEMS Microbiol Ecol. 92(10. https://academic.oup.com/femsec/article/92/10/fiw144/2197742
- Bhattacharjee S, Roy Das A, Saha AK, Das P. 2019. Fungal endophytes from medicinal plants: growth promotion in Oryza sativa L. and Cicer arietinum L. Vegetos. 32(3):381–386.
- Bibi N, Jan G, Jan FG, Hamayun M, Iqbal A, Hussain A, Rehman H, Tawab A, Khushdil F. 2019. Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant Physiol Biochem. 139:459–469.
- Bibi S, Hussain A, Hamayun M, Rahman H, Iqbal A, Shah M, Irshad M, Qasim M, Islam B. 2018. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of lactuca sativa L. Chemosphere. 211:653–663.
- Bilal L, Asaf S, Hamayun M, Gul H, Iqbal A, Ullah I, Lee IJ, Hussain A. 2018. Plant growth promoting endophytic fungi asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 76(2):117–127. doi:10.1007/s13199-018-0545-4.
- Bilal S, Khan AL, Shahzad R, Asaf S, Kang SM, Lee IJ. 2017. Endophytic paecilomyces formosus LHL10 augments glycine max L. Adaptation to ni-contamination through affecting endogenous phytohormones and oxidative stress. Front Plant Sci. 8:870. www.frontiersin.org
- Bilal S, Shahzad R, Imran M, Jan R, Kim KM, Lee IJ. 2020. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: heavy metals, high temperature and drought stress. Ind Crops Prod. 143:111931.
- Bilal S, Shahzad R, Khan AL, Al-Harrassi A, Kim CK, Lee IJ. 2019. Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stress-responsive proteins modulation. J Hazard Mater. 379:120824.
- Bilal S, Shahzad R, Khan AL, Kang S-M, Imran QM, Al-Harrasi A, Yun B-W, Lee I-J. 2018. Endophytic Microbial Consortia of Phytohormones-Producing Fungus Paecilomyces formosus LHL10 and Bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to Attenuate Aluminum and Zinc stresses. Front Plant Sci. 9:1273. https://www.frontiersin.org/article/10.3389/fpls.2018.01273/full
- Bilal S, Shahzad R, Lee IJ. 2021. Synergistic interaction of fungal endophytes, Paecilomyces formosus LHL10 and Penicillium funiculosum LHL06, in alleviating multi-metal toxicity stress in Glycine max L. Environ Sci Pollut Res. 28(47):67429–67444.
- Bouzouina M, Kouadria R, Lotmani B. 2021. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J Appl Microbiol. 130(3):913–925.
- Bu Y, Guo P, Ji Y, Zhang S, Yu H, Wang Z. 2019. Effects of Epichloë sinica on roegneria kamoji seedling physiology under PEG-6000 simulated drought stress. Symbiosis. 77(2):123–132. doi:10.1007/s13199-018-0570-3.
- Bultman TL, Bell G, Martin WD. 2004. A fungal endophyte mediates reversal of wound-induced resistance and constrains tolerance in a grass. Ecology. 85(3):679–685. http://doi.wiley.com/10.1890/03-0073
- Bultman TL, Bell GD. 2003. Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos. 103(1):182–190. http://doi.wiley.com/10.1034/j.1600-0706.2003.11574.x
- Casler MD, Van Santen E. 2008. Fungal endophyte removal does not reduce cold tolerance of tall fescue. Crop Sci. 48(5):2033–2039.
- Chand K, Shah S, Sharma J, Paudel MR, Pant B. 2020. Isolation, characterization, and plant growth-promoting activities of endophytic fungi from a wild orchid vanda cristata. Plant Signal Behav. 15(5.
- Chen N, He R, Chai Q, Li C, Nan Z. 2016. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 80(3):367–375. http://www.ebi.ac.uk/arrayexpress/
- Chen T, Johnson R, Chen S, Lv H, Zhou J, Li C. 2018. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil. 428(1–2):353–370. doi:10.1007/s11104-018-3643-4.
- Chen T, Li C, White JF, Nan Z. 2019. Effect of the fungal endophyte Epichloë bromicola on polyamines in wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil. 436(1–2):29–48. doi:10.1007/s11104-018-03913-x.
- Chen T, White JF, Li C. 2021. Fungal endophyte Epichloë bromicola infection regulates anatomical changes to account for salt stress tolerance in wild barley (Hordeum brevisubulatum). Plant Soil. 461(1–2):533–546.
- Chen XM, Dong HL, Hu KX, Sun ZR, Chen J, Guo SX. 2010. Diversity and antimicrobial and plant-growth-promoting activities of endophytic fungi in Dendrobium loddigesii rolfe. J Plant Growth Regul. 29(3):328–337. https://link.springer.com/article/10.1007/s00344-010-9139-y
- Chen Z, Jin Y, Yao X, Chen T, Wei X, Li C, White JF, Nan Z. 2020. Fungal endophyte improves survival of Lolium perenne in Low fertility soils by increasing root growth, Metabolic activity and absorption of nutrients. Plant Soil. 452(1–2):185–206. doi:10.1007/s11104-020-04556-7.
- Cheong SL, Cheow YL, Ting ASY. 2017. Characterizing antagonistic activities and host compatibility (via simple endophyte-calli test) of endophytes as biocontrol agents of ganoderma boninense. Biol Control. 105:86–92.
- Cheplick GP. 2004. Recovery from drought stress in Lolium perenne (poaceae): Are fungal endophytes detrimental? Am J Bot. 91(12):1960–1968. http://doi.wiley.com/10.3732/ajb.91.12.1960
- Cheplick GP, Harrichandra AP, Liu A. 2014. Competitive outcomes depend on host genotype, but not clavicipitaceous fungal endophytes, in Lolium perenne (poaceae). Am J Bot. 101(12):2068–2078.
- Cheplick GP, Perera A, Koulouris K. 2000. Effect of drought on the growth of Lolium perenne genotypes with and without fungal endophytes. Funct Ecol. 14(6):657–667. http://doi.wiley.com/10.1046/j.1365-2435.2000.00466.x
- Cong GQ, Yin CL, He BI, Li L, Gao KX. 2015. Effect of the endophytic fungus chaetomium globosum ND35 on the growth and resistance to drought of winter wheat at the seedling stage under water stress. Shengtai Xuebao/ Acta Ecol Sin. 35(18):6120–6128.
- Connor EW, Sandy M, Hawkes CV. 2017. Microbial tools in agriculture require an ecological context: stress-dependent Non-additive symbiont interactions. Agron J. 109(3):917–926. http://doi.wiley.com/10.2134/agronj2016.10.0568
- Cosme M, Lu J, Erb M, Stout MJ, Franken P, Wurst S. 2016. A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling. New Phytol. 211(3):1065–1076. https://onlinelibrary.wiley.com/doi/abs/10.1111/nph.13957
- Craig S, Kannadan S, Flory SL, Seifert EK, Whitney KD, Rudgers JA. 2011. Potential for endophyte symbiosis to increase resistance of the native grass Poa alsodes to invasion by the non-native grass microstegium vimineum. Symbiosis. 53(1):17–28. https://link.springer.com/article/10.1007/s13199-010-0102-2
- Cruz C, Martins-Loução MA, Varma A. 2010. The influence of plant co-culture of tomato plants with Piriformospora indica on biomass accumulation and stress tolerance. In: Acta Hortic. Vol. 868. [place unknown]: International Society for Horticultural Science; p. 123–127.
- Dastogeer KMG, Li H, Sivasithamparam K, Jones MGK, Wylie SJ. 2018. Fungal endophytes and a virus confer drought tolerance to Nicotiana benthamiana plants through modulating osmolytes, antioxidant enzymes and expression of host drought responsive genes. Environ Exp Bot. 149:95–108.
- de Siqueira KA, Senabio JA, Pietro-Souza W, de Oliveira Mendes TA, Soares MA. 2021. Aspergillus sp. A31 and Curvularia geniculata P1 mitigate mercury toxicity to Oryza sativa L. Arch Microbiol. 203(9):5345–5361.
- Deng Z, Wang W, Tan H, Cao L. 2012. Characterization of heavy metal-resistant endophytic yeast cryptococcus sp. CBSB78 from rapes (brassica chinensis) and its potential in promoting the growth of brassica spp. in metal-contaminated soils. Water Air Soil Pollut . 223(8):5321–5329. https://link.springer.com/article/10.1007/s11270-012-1282-6
- Deng Z, Zhang R, Shi Y, Hu L, Tan H, Cao L. 2014. Characterization of Cd-, Pb-, Zn-resistant endophytic lasiodiplodia sp. MXSF31 from metal accumulating portulaca oleracea and its potential in promoting the growth of rape in metal-contaminated soils. Environ Sci Pollut Res. 21(3):2346–2357.
- Deshmukh SD, Kogel KH. 2007. Piriformospora indica protects barley from root rot caused by Fusarium graminearum. J Plant Dis Prot. 114(6):263–268.
- Diene O, Takahashi T, Yonekura A, Nitta Y, Narisawa K. 2010. A new fungal endophyte, helminthosporium velutinum, promoting growth of a bioalcohol plant, sweet sorghum. Microbes Environ. 25(3):216–219.
- Dinkins RD, Nagabhyru P, Young CA, West CP, Schardl CL. 2019. Transcriptome analysis and differential expression in tall fescue harboring different endophyte strains in response to water deficit. Plant Genome. 12(2):180071. https://onlinelibrary.wiley.com/doi/10.3835/plantgenome2018.09.0071
- Doan TT, Jäschke D, Ludwig-Müller J. 2010. An endophytic fungus induces tolerance against the clubroot pathogen plasmodiophora brassicae in Arabidopsis thaliana and Brassica rapa roots. In: Acta Hortic. Vol. 867. [place unknown]: International Society for Horticultural Science; p. 173–180.
- Domka A, Rozpądek P, Ważny R, Turnau K. 2019. Mucor sp.-An endophyte of Brassicaceae capable of surviving in toxic metal-rich sites. J Basic Microbiol. 59(1):24–37. http://doi.wiley.com/10.1002/jobm.201800406
- Dovana F, Mucciarelli M, Mascarello M, Fusconi A. 2015. In Vitro Morphogenesis of Arabidopsis to Search for Novel Endophytic Fungi Modulating Plant growth.Labra M, editor. PLoS One. 10(12):e0143353. https://dx.plos.org/10.1371/journal.pone.0143353
- Druege U, Baltruschat H, Franken P. 2007. Piriformospora indica promotes adventitious root formation in cuttings. Sci Hortic (Amsterdam). 112(4):422–426.
- Elmi W, Robbins K. 2000. Endophyte effects on reproduction of a root-knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. Grass Forage Sci. 55(2):166–172. http://doi.wiley.com/10.1046/j.1365-2494.2000.00210.x
- Escudero N, Lopez-Llorca LV. 2012. Effects on plant growth and root-knot nematode infection of an endophytic GFP transformant of the nematophagous fungus Pochonia chlamydosporia. Symbiosis. 57(1):33–42. https://link.springer.com/article/10.1007/s13199-012-0173-3
- Faeth SH. 2009. Asexual fungal symbionts alter reproductive allocation and herbivory over time in their native perennial grass hosts. Am Nat. 173(5):554–565. https://www.journals.uchicago.edu/doi/10.1086/597376
- Fakhro A, Andrade-Linares DR, von Bargen S, Bandte M, Büttner C, Grosch R, Schwarz D, Franken P. 2010. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza. 20(3):191–200. https://link.springer.com/article/10.1007/s00572-009-0279-5
- Ferus P, Barta M, Konôpková J. 2019. Endophytic fungus Beauveria bassiana can enhance drought tolerance in red oak seedlings. Trees - Struct Funct. 33(4):1179–1186. doi:10.1007/s00468-019-01854-1.
- Fiorini L, Guglielminetti L, Mariotti L, Curadi M, Picciarelli P, Scartazza A, Sarrocco S, Vannacci G. 2016. Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. micro-Tom growth. Plant Soil. 400(1–2):351–366. https://link.springer.com/article/10.1007/s11104-015-2736-6
- Formenti L, Caggìa V, Puissant J, Goodall T, Glauser G, Griffiths R, Rasmann S. 2021. The effect of root-associated microbes on plant growth and chemical defence traits across two contrasted elevations. J Ecol. 109(1):38–50. https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/1365-2745.13440
- Forouzi A, Ghasemnezhad A, Nasrabad RG. 2020. Phytochemical response of stevia plant to growth promoting microorganisms under salinity stress. South African J Bot. 134:109–118.
- Fu W, Xu M, Sun K, Hu L, Cao W, Dai C, Jia Y. 2018. Biodegradation of phenanthrene by endophytic fungus Phomopsis liquidambari in vitro and in vivo. Chemosphere. 203:160–169.
- Furtado BU, Szymańska S, Hrynkiewicz K. 2019. A window into fungal endophytism in Salicornia europaea: deciphering fungal characteristics as plant growth promoting agents. Plant Soil. 445(1–2):577–594. doi:10.1007/s11104-019-04315-3.
- Gan H, Churchill ACL, Wickings K. 2017. Invisible but consequential: root endophytic fungi have variable effects on belowground plant-insect interactions. Ecosphere. 8(3):e01710. http://doi.wiley.com/10.1002/ecs2.1710
- Gao Y, Li GP, Shi H, Liu H, Ren AZ, Gao YB. 2017. Allelopathic effect of endophyte-infected Achnatherum sibiricum on stipa grandis. Shengtai Xuebao/ Acta Ecol Sin. 37(4):1063–1073.
- García-Latorre C, Rodrigo S, Santamaria O. 2021. Effect of fungal endophytes on plant growth and nutrient uptake in trifolium subterraneum and Poa pratensis as affected by plant host specificity. Mycol Prog. 20(9):1217–1231.
- Geddes-Mcalister J, Sukumaran A, Patchett A, Hager HA, Dale JCM, Roloson JL, Prudhomme N, Bolton K, Muselius B, Powers J, Newman JA. 2020. Examining the impacts of co2 concentration and genetic compatibility on perennial ryegrass—epichloë festucae var lolii interactions. J Fungi. 6(4):1–38.
- Gera Hol WH, de la Peña E, Moens M, Cook R. 2007. Interaction between a fungal endophyte and root herbivores of ammophila arenaria. Basic Appl Ecol. 8(6):500–509.
- Ghezel Sefloo N, Wieczorek K, Steinkellner S, Hage-Ahmed K. 2019. Serendipita species trigger cultivar-specific responses to Fusarium wilt in tomato. Agronomy. 9(10):595. https://www.mdpi.com/2073-4395/9/10/595
- Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H. 2018. Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Franken P, editor. Plant Biol. 20(4):729–736. http://doi.wiley.com/10.1111/plb.12717
- Giauque H, Connor EW, Hawkes CV. 2019. Endophyte traits relevant to stress tolerance, resource use and habitat of origin predict effects on host plants. New Phytol. 221(4):2239–2249. https://onlinelibrary.wiley.com/doi/abs/10.1111/nph.15504
- Gibert A, Hazard L. 2011. Endophyte infection of Festuca eskia enhances seedling survival to drought and cutting at the expense of clonal expansion. J Plant Ecol. 4(4):201–208. https://academic.oup.com/jpe/article-lookup/doi/10.1093/jpe/rtr009
- Gibert A, Volaire F, Barre P, Hazard L. 2012. A fungal endophyte reinforces population adaptive differentiation in its host grass species. New Phytol. 194(2):561–571.
- Gonçalves DR, Pena R, Zotz G, Albach DC. 2021. Effects of fungal inoculation on the growth of Salicornia (amaranthaceae) under different salinity conditions. Symbiosis. 84(2):195–208.
- Gong B, Liu G, Liao R, Song J, Zhang H. 2017. Endophytic fungus purpureocillium sp. A5 protect mangrove plant kandelia candel under copper stress. Brazilian J Microbiol. 48(3):530–536.
- Gonzalez Mateu M, Baldwin AH, Maul JE, Yarwood SA. 2020. Dark septate endophyte improves salt tolerance of native and invasive lineages of phragmites australis. ISME J. 14(8):1943–1954. doi:10.1038/s41396-020-0654-y.
- González-Mas N, Sánchez-Ortiz A, Valverde-García P, Quesada-Moraga E. 2019. Effects of endophytic Entomopathogenic ascomycetes on the life-history traits of aphis gossypii glover and Its interactions with melon plants. Insects. 10(6):165. https://www.mdpi.com/2075-4450/10/6/165
- González-Teuber M, Urzúa A, Plaza P, Bascuñán-Godoy L. 2018. Effects of root endophytic fungi on response of Chenopodium quinoa to drought stress. Plant Ecol. 219(3):231–240. doi:10.1007/s11258-017-0791-1.
- Gul Jan F, Hamayun M, Hussain A, Jan G, Iqbal A, Khan A, Lee IJ. 2019. An endophytic isolate of the fungus yarrowia lipolytica produces metabolites that ameliorate the negative impact of salt stress on the physiology of maize 06 biological Sciences 0607 plant biology 07 agricultural and veterinary Sciences 0703 crop and past. BMC Microbiol. 19(1):3. https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-018-1374-6
- Gundel PE, Maseda PH, Vila-Aiub MM, Ghersa CM, Benech-Arnold R. 2006. Effects of Neotyphodium fungi on Lolium multiflorum seed germination in relation to water availability. Ann Bot. 97(4):571–577. http://academic.oup.com/aob/article/97/4/571/186410/Effects-of-Neotyphodium-Fungi-on-Lolium
- Gundel PE, Sorzoli N, Ueno AC, Ghersa CM, Seal CE, Bastías DA, Martínez-Ghersa MA. 2015. Impact of ozone on the viability and antioxidant content of grass seeds is affected by a vertically transmitted symbiotic fungus. Environ Exp Bot. 113:40–46.
- Guo J, McCulley RL, McNear DH. 2015. Tall fescue cultivar and fungal endophyte combinations influence plant growth and root exudate composition. Front Plant Sci. 6(APR):183. www.frontiersin.org
- Guxdel PE, Bigaxzoli F, Freitas PP, Landesmaxn JB, Martínez-Ghersa MA, Ghersa CM. 2020. Plant damage, seed production and persistence of the fungal endophyte Epichloë occultans in Lolium multiflorum plants under an herbivore lepidopteran attack and ozone pollution. Ecol Austral. 30(2):321–330.
- Hahn H, McManus MT, Warnstorff K, Monahan BJ, Young CA, Davies E, Tapper BA, Scott B. 2008. Neotyphodium fungal endophytes confer physiological protection to perennial ryegrass (Lolium perenne L.) subjected to a water deficit. Environ Exp Bot. 63(1–3):183–199.
- Hall SL, McCulley RL, Barney RJ, Phillips TD. 2014. Does Fungal Endophyte Infection Improve Tall Fescue’s Growth Response to Fire and Water limitation? Heil M, editor. PLoS One. 9(1):e86904. https://dx.plos.org/10.1371/journal.pone.0086904
- Halo BA, Al-Yahyai RA, Al-Sadi AM. 2020. An endophytic talaromyces omanensis enhances reproductive, physiological and anatomical characteristics of drought-stressed tomato. J Plant Physiol. 249.
- Hamayun M, Afzal Khan S, Ahmad N, Tang DS, Kang SM, Na CI, Sohn EY, Hwang YH, Shin DH, Lee BH, et al. 2009. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) merr. World J Microbiol Biotechnol. 25(4):627–632. https://link.springer.com/article/10.1007/s11274-009-9982-9
- Hamayun M, Khan SA, Iqbal I, Ahmad B, Lee IJ. 2010. Isolation of a gibberellin-producing fungus (Penicillium sp. MH7) and growth promotion of crown daisy (chrysanthemum coronarium). J Microbiol Biotechnol . 20(1):202–207. http://www.jmb.or.kr/journal/view.html?doi=10.4014/jmb.0905.05040
- Hamayun M, Khan SA, Khan AL, Ahmad N, Nawaz Y, Sher H, Lee IJ. 2011. Gibberellin producing neosartorya sp. CC8 reprograms Chinese cabbage to higher growth. Sci Hortic (Amsterdam). 129(3):347–352.
- Hamayun M, Khan SA, Khan AL, Rehman G, Kim YH, Iqbal I, Hussain J, Lee IJ, Sohn EY. 2010. Gibberellin production and plant growth promotion from pure cultures of Cladosporium sp. MH-6 isolated from cucumber (Cucumis sativus L.). Mycologia. 102(5):989–995.
- Hamayun M, Khan SA, Khan AL, Tang DS, Hussain J, Ahmad B, Anwar Y, Lee IJ. 2010. Growth promotion of cucumber by pure cultures of gibberellin-producing Phoma sp. GAH7. World J Microbiol Biotechnol . 26(5):889–894. https://link.springer.com/article/10.1007/s11274-009-0248-3
- Haruma T, Yamaji K, Masuya H. 2021. Phialocephala fortinii increases aluminum tolerance in Miscanthus sinensis growing in acidic mine soil. Lett Appl Microbiol. 73(3):300–307.
- Haruma T, Yamaji K, Masuya H, Hanyu K. 2018. Root endophytic Chaetomium cupreum promotes plant growth and detoxifies aluminum in Miscanthus sinensis andersson growing at the acidic mine site. Plant Species Biol. 33(2):109–122. http://doi.wiley.com/10.1111/1442-1984.12197
- Hassan SED. 2017. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of teucrium polium L. J Adv Res. 8(6):687–695. doi:10.1016/j.jare.2017.09.001.
- He C, Wang W, Hou J. 2019. Characterization of Dark septate endophytic fungi and improve the performance of liquorice under organic residue treatment. Front Microbiol. 10(JUN):1364. https://www.frontiersin.org/article/10.3389/fmicb.2019.01364/full
- He C, Wang W, Hou J. 2019. Plant growth and Soil microbial Impacts of enhancing Licorice With inoculating Dark septate endophytes under drought stress. Front Microbiol. 10:2277. https://www.frontiersin.org/article/10.3389/fmicb.2019.02277/full
- He C, Wang W, Hou J, Li X. 2021. Dark septate endophytes isolated from wild Licorice roots grown in the desert regions of northwest China enhance the growth of host plants under water deficit stress. Front Microbiol. 12.
- Heineck GC, Watkins E, Ehlke NJ. 2018. The fungal endophyte Epichloë festucae var. lolii Does Not Improve the Freezing Tolerance of Perennial ryegrass. Crop Sci. 58(4):1788–1800. http://doi.wiley.com/10.2135/cropsci2017.12.0731
- Heinz KM, Harding PA, Ek-Ramos MJ, Hernandez H, Krauter PC, Sword GA. 2018. Fungal endophytes in knock out® rose and performance effects of entomopathogens on marigold and zinnia. HortScience. 53(12):1791–1798. doi:10.21273/HORTSCI13370-18.
- Hereme R, Morales-Navarro S, Ballesteros G, Barrera A, Ramos P, Gundel PE, Molina-Montenegro MA. 2020. Fungal endophytes exert positive effects on Colobanthus quitensis under water stress but neutral under a projected climate change scenario in Antarctica. Front Microbiol. 11:264. https://www.frontiersin.org/article/10.3389/fmicb.2020.00264/full
- Hesse U, Schöberlein W, Wittenmayer L, Förster K, Warnstorff K, Diepenbrock W, Merbach W. 2003. Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass Forage Sci. 58(4):407–415. http://doi.wiley.com/10.1111/j.1365-2494.2003.00393.x
- Hossain MM, Sultana F, Islam S. 2017. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In: Plant-Microbe Interact Agro-Ecological Perspect . Vol. 2. [place unknown]: Springer Singapore; p. 135–191. https://link.springer.com/chapter/10.1007/978-981-10-6593-4_6
- Hosseini F, Mosaddeghi MR, Dexter AR. 2017. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Biochem. 118:107–120.
- Hosseini F, Mosaddeghi MR, Hajabbasi MA, Sabzalian MR. 2016. Role of fungal endophyte of tall fescue (Epichloë coenophiala) on water availability, wilting point and integral energy in texturally-different soils. Agric Water Manag. 163:197–211.
- Hosseyni Moghaddam MS, Safaie N, Soltani J, Hagh-Doust N. 2021. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol Biochem. 160:225–238.
- Hou L, Li X, He X, Zuo Y, Zhang D, Zhao L. 2021. Effect of dark septate endophytes on plant performance of artemisia ordosica and associated soil microbial functional group abundance under salt stress. Appl Soil Ecol. 165.
- Hou L, Yu J, Zhao L, He X. 2020. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front Microbiol. 10:3061. https://www.frontiersin.org/article/10.3389/fmicb.2019.03061/full
- Hoyos-Carvajal L, Orduz S, Bissett J. 2009. Growth stimulation in bean (Phaseolus vulgaris L.) by trichoderma. Biol Control. 51(3):409–416.
- Hu LY, Li D, Sun K, Cao W, Fu WQ, Zhang W, Dai CC. 2018. Mutualistic fungus Phomopsis liquidambari increases root aerenchyma formation through auxin-mediated ethylene accumulation in rice (Oryza sativa L.). Plant Physiol Biochem. 130:367–376.
- Huang G, Jin Q, Peng H, Zhu T, Ye H. 2019. Effect of a fungus, hypoxylon spp., on endophytes in the roots of asparagus. FEMS Microbiol Lett. 366(16.
- Huang LQ, Niu YC, Su L, Deng H, Lyu H. 2020. The potential of endophytic fungi isolated from cucurbit plants for biocontrol of soilborne fungal diseases of cucumber. Microbiol Res. 231:126369.
- Hubbard M, Germida J, Vujanovic V. 2012. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany. 90(2):137–149. www.nrcresearchpress.com/cjb
- Hubbard M, Germida JJ, Vujanovic V. 2014. Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J Appl Microbiol. 116(1):109–122.
- Hughes AR, Moore AFP, Gehring C. 2020. Plant response to fungal root endophytes varies by host genotype in the foundation species spartina alterniflora. Am J Bot. 107(12):1645–1653.
- Hui F, Liu J, Gao Q, Lou B. 2015. Piriformospora indica confers cadmium tolerance in Nicotiana tabacum. J Environ Sci (China). 37:184–191.
- Hussin S, Khalifa W, Geissler N, Koyro H-W. 2017. Influence of the root endophyte Piriformospora indica on the plant water relations, gas exchange and growth of Chenopodium quinoa at limited water availability. J Agron Crop Sci. 203(5):373–384. http://doi.wiley.com/10.1111/jac.12199
- Hwang J-S, You Y-H, Bae J-J, Khan SA, Kim J-G, Choo Y-S. 2011. Effects of endophytic fungal secondary metabolites on the growth and physiological response of carex kobomugi ohwi. J Coast Res. 27(3):544–548. http://www.bioone.org/doi/abs/10.2112/JCOASTRES-D-10-00090.1
- Iannone LJ, Pinget AD, Nagabhyru P, Schardl CL, De Battista JP. 2012. Beneficial effects of Neotyphodium tembladerae and Neotyphodium pampeanum on a wild forage grass. Grass Forage Sci. 67(3):382–390.
- Ibiang SR, Sakamoto K, Kuwahara N. 2020. Performance of tomato and lettuce to arbuscular mycorrhizal fungi and Penicillium pinophilum EU0013 inoculation varies with soil, culture media of inoculum, and fungal consortium composition. Rhizosphere. 16: Article ID 100246.
- Ikram M, Ali N, Jan G, Iqbal A, Hamayun M, Jan FG, Hussain A, Lee I-J. 2019. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J Plant Interact. 14(1):590–602. https://www.tandfonline.com/doi/full/10.1080/17429145.2019.1684582
- Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. 2018. IAA producing fungal endophyte Penicillium roqueforti thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils.berta G, editor. PLoS One. 13(11):e0208150. https://dx.plos.org/10.1371/journal.pone.0208150
- Isabel Miranda M, Omacini M, Chaneton EJ. 2011. Environmental context of endophyte symbioses: interacting effects of water stress and insect herbivory. Int J Plant Sci. 172(4):499–508.
- Ismail HA, Mehmood A, Qadir M, Husna IA, Hamayun M, Khan N. 2020. Thermal stress alleviating potential of endophytic fungus rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (glycine max L.). Pakistan J Bot. 52(5):1857–1865. http://pakbs.org/pjbot/paper_details.php?id=8656
- Ismail HM, Hussain A, Afzal Khan S, Iqbal A, Lee IJ. 2019. Aspergillus flavus promoted the growth of soybean and sunflower seedlings at elevated temperature. Biomed Res Int. 2019: Article ID 1295457.
- Ismail HM, Hussain A, Iqbal A, Khan SA, Ahmad A, Gul S, Kim HY, Lee IJ. 2021a. Aspergillus foetidus regulated the biochemical characteristics of soybean and sunflower under heat stress condition: role in sustainability. Sustain. 13(13): Article ID 7159.
- Ismail HM, Hussain A, Iqbal A, Khan SA, Gul S, Khan H, Rehman KU, Bibi H, Lee IJ. 2021b. Penicillium glabrum acted as a heat stress relieving endophyte in soybean and sunflower. Polish J Environ Stud. 30(4):3099–3110.
- Ismail HM, Hussain A, Iqbal A, Khan SA, Lee IJ. 2018. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. Biomed Res Int. 2018: Article ID 7696831.
- Jan FG, Hamayun M, Hussain A, Iqbal A, Jan G, Khan SA, Khan H, Lee IJ. 2019. A promising growth promoting meyerozyma caribbica from Solanum xanthocarpum alleviated stress in maize plants. Biosci Rep. 39(10):20190290. doi:10.1042/BSR20190290.
- Javed A, Shah AH, Hussain A, Shinwari ZK, Khan SA, Khan W, Jan SA. 2020. Potential of endophytic fungus aspergillus terreus as potent plant growth promoter. Pakistan J Bot. 52(3):1083–1086. http://pakbs.org/pjbot/paper_details.php?id=8287
- Jiang W, Pan R, Buitrago S, Wu C, Abdelaziz ME, Oelmüller R, Zhang W. 2021. Transcriptome analysis of Arabidopsis reveals freezing-tolerance related genes induced by root endophytic fungus Piriformospora indica. Physiol Mol Biol Plants. 27(2):189–201.
- Jin HQ, Liu HB, Xie YY, Zhang YG, Xu QQ, Mao LJ, Li XJ, Chen J, Lin FC, Zhang CL. 2018. Effect of the dark septate endophytic fungus acrocalymma vagum on heavy metal content in tobacco leaves. Symbiosis. 74(2):89–95. https://link.springer.com/article/10.1007/s13199-017-0485-4
- Jin W, Peng L, Zhang X, Sun H, Yuan Z. 2019. Effects of endophytic and ectomycorrhizal basidiomycetes on quercus virginiana seedling growth and nutrient absorption. J Sustain For. 38(5):457–470.
- Kane KH. 2011. Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the Mediterranean region. Environ Exp Bot. 71(3):337–344.
- Kannadan S, Rudgers JA. 2008. Endophyte symbiosis benefits a rare grass under low water availability. Funct Ecol. 22(4):706–713. http://doi.wiley.com/10.1111/j.1365-2435.2008.01395.x
- Kauppinen M, Helander M, Anttila N, Saloniemi I, Saikkonen K. 2018. Epichloë endophyte effects on leaf blotch pathogen (rhynchosporium sp.) of tall fescue (schedonorus phoenix) vary among grass origin and environmental conditions. Plant Ecol Divers. 11(5–6):625–635.
- Kavroulakis N, Doupis G, Papadakis IE, Ehaliotis C, Papadopoulou KK. 2018. Tolerance of tomato plants to water stress is improved by the root endophyte Fusarium solani FsK. Rhizosphere. 6(March):77–85. doi:10.1016/j.rhisph.2018.04.003.
- Khalmuratova I, Kim H, Nam YJ, Oh Y, Jeong MJ, Choi HR, You YH, Choo YS, Lee IJ, Shin JH, et al. 2015. Diversity and plant growth promoting capacity of endophytic fungi associated with halophytic plants from the west coast of korea. Mycobiology. 43(4):373–383.
- Khan A, Hamayun M, Kang SM, Kim YH, Jung HY, Lee JH, Lee IJ. 2012. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 12(1):3. http://bmcmicrobiol.biomedcentral.com/articles/10.1186/1471-2180-12-3
- Khan AL, Gilani SA, Waqas M, Al-Hosni K, Al-Khiziri S, Kim Y-H, Ali L, Kang S-M, Asaf S, Shahzad R, et al. 2017. Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J Zhejiang Univ B. 18(2):125–137. http://link.springer.com/10.1631/jzus.B1500271
- Khan AL, Hamayun M, Ahmad N, Hussain J, Kang SM, Kim YH, Adnan M, Tang DS, Waqas M, Radhakrishnan R, et al. 2011. Salinity stress resistance offered by endophytic fungal interaction between penicillium minioluteum LHL09 and glycine max. L. J Microbiol Biotechnol. 21(9):893–902. http://www.jmb.or.kr/journal/view.html?doi=10.4014/jmb.1103.03012
- Khan AL, Hamayun M, Ahmad N, Waqas M, Kang SM, Kim YH, Lee IJ. 2011. Exophiala sp. LHL08 reprograms Cucumis sativus to higher growth under abiotic stresses. Physiol Plant. 143(4):329–343.
- Khan AL, Hamayun M, Khan SA, Kang SM, Shinwari ZK, Kamran M, ur Rehman S, Kim JG, Lee IJ. 2012. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J Microbiol Biotechnol . 28(4):1483–1494. https://link.springer.com/article/10.1007/s11274-011-0950-9
- Khan AL, Hamayun M, Kim YH, Kang SM, Lee IJ. 2011. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol Biochem. 49(8):852–861.
- Khan AL, Hamayun M, Radhakrishnan R, Waqas M, Kang SM, Kim YH, Shin JH, Choo YS, Kim JG, Lee IJ. 2012. Mutualistic association of Paecilomyces formosus LHL10 offers thermotolerance to Cucumis sativus. antonie van leeuwenhoek. Int J Gen Mol Microbiol. 101(2):267–279. https://link.springer.com/article/10.1007/s10482-011-9630-x
- Khan AL, Hamayun M, Waqas M, Kang SM, Kim YH, Kim DH, Lee IJ. 2012. Exophiala sp.LHL08 association gives heat stress tolerance by avoiding oxidative damage to cucumber plants. Biol Fertil Soils . 48(5):519–529. https://link.springer.com/article/10.1007/s00374-011-0649-y
- Khan AL, Kang SM, Dhakal KH, Hussain J, Adnan M, Kim JG, Lee IJ. 2013. Flavonoids and amino acid regulation in Capsicum annuum L. by endophytic fungi under different heat stress regimes. Sci Hortic (Amsterdam). 155:1–7.
- Khan AL, Lee IJ. 2013. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 13(1):86. http://bmcplantbiol.biomedcentral.com/articles/10.1186/1471-2229-13-86
- Khan AL, Waqas M, Hamayun M, Al-Harrasi A, Al-Rawahi A, Lee IJ. 2013. Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuum in biomass recovery and osmotic stress mitigation. BMC Microbiol. 13(1):51. http://bmcmicrobiol.biomedcentral.com/articles/10.1186/1471-2180-13-51
- Khan AL, Waqas M, Hussain J, Al-Harrasi A, Al-Rawahi A, Al-Hosni K, Kim MJ, Adnan M, Lee IJ. 2014. Endophytes Aspergillus caespitosus LK12 and Phoma sp. LK13 of moringa peregrina produce gibberellins and improve rice plant growth. J Plant Interact. 9(1):731–737. https://www.tandfonline.com/doi/full/10.1080/17429145.2014.917384
- Khan AL, Waqas M, Hussain J, Al-Harrasi A, Hamayun M, Lee IJ. 2015. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: An examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J Hazard Mater. 295:70–78.
- Khan AL, Waqas M, Hussain J, Al-Harrasi A, Lee IJ. 2014. Fungal endophyte Penicillium janthinellum LK5 can reduce cadmium toxicity in Solanum lycopersicum (Sitiens and Rhe). Biol Fertil Soils. 50(1):75–85.
- Khan AL, Waqas M, Khan AR, Hussain J, Kang SM, Gilani SA, Hamayun M, Shin JH, Kamran M, Al-Harrasi A, et al. 2013. Fungal endophyte Penicillium janthinellum LK5 improves growth of ABA-deficient tomato under salinity. World J Microbiol Biotechnol. 29(11):2133–2144. https://link.springer.com/article/10.1007/s11274-013-1378-1
- Khan AL, Waqas M, Lee IJ. 2015. Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J Plant Res. 128(2):259–268.
- Khan AR, Ullah I, Waqas M, Park GS, Khan AL, Hong SJ, Ullah R, Jung BK, Park CE, Ur-Rehman S, et al. 2017. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol Environ Saf. 136:180–188.
- Khan AR, Ullah I, Waqas M, Shahzad R, Hong SJ, Park GS, Jung BK, Lee IJ, Shin JH. 2015. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J Microbiol Biotechnol. 31(9):1461–1466.
- Khan AR, Waqas M, Ullah I, Khan AL, Khan MA, Lee IJ, Shin JH. 2017. Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ Exp Bot. 135:126–135.
- Khayamim F, Khademi H, Sabzalian MR. 2011. Effect of Neotyphodium endophyte-tall fescue symbiosis on mineralogical changes in clay-sized phlogopite and muscovite. Plant Soil. 341(1–2):473–484. https://link.springer.com/article/10.1007/s11104-010-0659-9
- Khushdil F, Jan FG, Jan G, Hamayun M, Iqbal A, Hussain A, Bibi N. 2019. Salt stress alleviation in pennisetum glaucum through secondary metabolites modulation by Aspergillus terreus L. Plant Physiol Biochem. 144:127–134.
- Kifle MH, Yobo KS, Laing MD. 2017. Biocontrol of Aspergillus flavus in groundnut using Trichoderma harzianum stain kd. J Plant Dis Prot. 124(1):51–56. https://link.springer.com/article/10.1007/s41348-016-0066-4
- Kleczewski NM, Bauer JT, Bever JD, Clay K, Reynolds HL. 2012. A survey of endophytic fungi of switchgrass (Panicum virgatum) in the midwest, and their putative roles in plant growth. Fungal Ecol. 5(5):521–529.
- Klypina N, Pinch M, Schutte BJ, Maruthavanan J, Sterling TM. 2017. Water-deficit stress tolerance differs between two locoweed genera (Astragalus and oxytropis) with fungal endophytes. Weed Sci. 65(5):626–638. doi:10.1017/wsc.2017.21.
- Krell V, Unger S, Jakobs-Schoenwandt D, Patel AV. 2018. Endophytic Metarhizium brunneum mitigates nutrient deficits in potato and improves plant productivity and vitality. Fungal Ecol. 34:43–49.
- Kumari V, Germida J, Vujanovic V. 2018. Legume endosymbionts: drought stress tolerance in second-generation chickpea (Cicer arietinum) seeds. J Agron Crop Sci. 204(6):529–540. http://doi.wiley.com/10.1111/jac.12283
- Kumari V, Vujanovic V. 2020. Transgenerational benefits of endophytes on resilience and antioxidant genes expressions in pea (pisum sativum L.) under osmotic stress. Acta Physiol Plant. 42(4):49. doi:10.1007/s11738-020-03042-y.
- Kuzhuppillymyal-Prabhakarankutty L, Tamez-Guerra P, Gomez-Flores R, Rodriguez-Padilla MC, Ek-Ramos MJ. 2020. Endophytic Beauveria bassiana promotes drought tolerance and early flowering in corn. World J Microbiol Biotechnol. 36(3):47. doi:10.1007/s11274-020-02823-4.
- Laitinen RK, Hellström KO, Wäli PR. 2016. Context-dependent outcomes of subarctic grass-endophyte symbiosis. Fungal Ecol. 23:66–74.
- Lalancette S, Lerat S, Roy S, Beaulieu C. 2019. Fungal endophytes of Alnus incana ssp. rugosa and Alnus alnobetula ssp. crispa and their potential to tolerate heavy metals and to promote plant growth. Mycobiology. 47(4):415–429. https://www.tandfonline.com/doi/full/10.1080/12298093.2019.1660297
- Lang M, Zhou J, Chen T, Chen Z, Malik K, Li C. 2021. Influence of interactions between nitrogen, phosphorus supply and epichloë bromicola on growth of wild barley (Hordeum brevisubulatum). J Fungi. 7(8): Article ID 615.
- Lanza M, Haro R, Conchillo LB, Benito B. 2019. The endophyte Serendipita indica reduces the sodium content of Arabidopsis plants exposed to salt stress: fungal ENA ATPases are expressed and regulated at high pH and during plant co-cultivation in salinity. Environ Microbiol. 21(9):3364–3378. https://onlinelibrary.wiley.com/doi/abs/10.1111/1462-2920.14619
- Lewis GC. 1992. Effect of ryegrass endophyte in mixed swards of perennial ryegrass and white clover under two levels of irrigation and pesticide treatment. Grass Forage Sci. 47(3):302–305. http://doi.wiley.com/10.1111/j.1365-2494.1992.tb02274.x
- Lewis GC. 2004. Effects of biotic and abiotic stress on the growth of three genotypes of Lolium perenne with and without infection by the fungal endophyte Neotyphodium lolii. Ann Appl Biol. 144(1):53–63.
- Li D, Bodjrenou DM, Zhang S, Wang B, Pan H, Yeh KW, Lai Z, Cheng C. 2021. The endophytic fungus piriformospora indica reprograms banana to cold resistance. Int J Mol Sci. 22(9):4973. doi:10.1007/s13199-021-00813-0Growth-promoting.
- Li K, Shi C, He FY, Li HY. 2020. Effects of endophyte infection on growth and physiological characteristics of melica transsilvanica under Pb stress. Acta Prataculturae Sin. 29(3):112–120. http://gb.oversea.cnki.net/KCMS/detail/detail.aspx?filename=CYXB202003012&dbcode=CJFD&dbname=CJFDTEMP
- Li L, Li L, Wang X, Zhu P, Wu H, Qi S. 2017. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol Biochem. 119:211–223.
- Li M, Hou L, Liu J, Yang J, Zuo Y, Zhao L, He X. 2021. Growth-promoting effects of dark septate endophytes on the non-mycorrhizal plant isatis indigotica under different water conditions. Symbiosis.
- Li Q, Kuo YW, Lin KH, Huang W, Deng C, Yeh KW, Chen SP. 2021. Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (ipomoea batatas L.). Plant Cell Rep. 40(2):339–350.
- Li T, Liu MJ, Zhang XT, Zhang HB, Sha T, Zhao ZW. 2011. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci Total Environ. 409(6):1069–1074.
- Li X, Bu N, Li Y, Ma L, Xin S, Zhang L. 2012. Growth, photosynthesis and antioxidant responses of endophyte infected and non-infected rice under lead stress conditions. J Hazard Mater. 213–214:55–61.
- Li X, He C, He X, Su F, Hou L, Ren Y, Hou Y. 2019. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil. 439(1–2):259–272. doi:10.1007/s11104-019-04057-2.
- Li X, He X, Hou L, Ren Y, Wang S, Su F. 2018. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci Rep. 8(1):7896. www.nature.com/scientificreports/
- Li X, He X-L, Zhou Y, Hou Y-T, Zuo Y-L. 2019. Effects of Dark septate endophytes on the performance of hedysarum scoparium Under Water Deficit stress. Front Plant Sci. 10:903. https://www.frontiersin.org/article/10.3389/fpls.2019.00903/full
- Li X, Ma L, Li Y, Wang L, Zhang L. 2019. Endophyte infection enhances accumulation of organic acids and minerals in rice under Pb 2+ stress conditions. Ecotoxicol Environ Saf. 174:255–262.
- Li X-Z, Song M-L, Yao X, Chai Q, Simpson WR, Li C-J, Nan Z-B. 2017. The Effect of Seed-Borne Fungi and Epichloë Endophyte on Seed Germination and Biomass of Elymus sibiricus. Front Microbiol. 8(DEC):2488. http://journal.frontiersin.org/article/10.3389/fmicb.2017.02488/full
- Liarzi O, Bucki P, Braun Miyara S, Ezra D. 2016. Bioactive Volatiles from an Endophytic Daldinia cf. concentrica Isolate Affect the Viability of the Plant Parasitic Nematode Meloidogyne javanica.Jones J, editor. PLoS One. 11(12):e0168437. https://dx.plos.org/10.1371/journal.pone.0168437
- Likar M, Regvar M. 2013. Isolates of dark septate endophytes reduce metal uptake and improve physiology of salix caprea L. Plant Soil. 370(1–2):593–604. https://link.springer.com/article/10.1007/s11104-013-1656-6
- Lin HF, Xiong J, Zhou HM, Chen CM, Lin FZ, Xu XM, Oelmüller R, Xu WF, Yeh KW. 2019. Growth promotion and disease resistance induced in Anthurium colonized by the beneficial root endophyte Piriformospora indica. BMC Plant Biol. 19(1):40. https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-019-1649-6
- Lindblom SD, Valdez-Barillas JR, Fakra SC, Marcus MA, Wangeline AL, Pilon-Smits EAH. 2013. Influence of microbial associations on selenium localization and speciation in roots of Astragalus and stanleya hyperaccumulators. Environ Exp Bot. 88:33–42.
- Lindblom SD, Wangeline AL, Valdez Barillas JR, Devilbiss B, Fakra SC, Pilon-Smits EAH. 2018. Fungal endophyte Alternaria tenuissima Can affect growth and selenium accumulation in Its hyperaccumulator host Astragalus bisulcatus. Front Plant Sci. 9:1213. https://www.frontiersin.org/article/10.3389/fpls.2018.01213/full
- Liu D, Zhu L, Li T, Zhao Z. 2021. Mutualism between Dark septate endophytes (DSEs) and their host plants under metal stress: a case study. All Life. 14(1):667–677.
- López AC, Alvarenga AE, Vereschuk ML, Barua RC, Zapata PD, Luna MF, Villaba LL. 2020. Trichoderma strains isolated from ilex paraguariensis ST. HIL: promising biocontrol agents with chitinolytic activity and plant growth promoter on lycopersicum esculentum. Arab J Basic Appl Sci. 27(1):105–113. https://www.tandfonline.com/doi/full/10.1080/25765299.2020.1732033
- Lubna AS, Hamayun M, Gul H, Lee IJ, Hussain A. 2018. Aspergillus Niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J Plant Interact. 13(1):100–111. https://www.tandfonline.com/doi/full/10.1080/17429145.2018.1436199
- Lubna AS, Hamayun M, Khan AL, Waqas M, Khan MA, Jan R, Lee IJ, Hussain A. 2018. Salt tolerance of Glycine max.L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol Biochem. 128:13–23.
- Ma BH, Lin WH, Gao M, Di WX, Tian P. 2020. Effects of salicylic acid and epichloё on perennial ryegrass (Lolium perenne) under drought stress. Acta Prataculturae Sin. 29(1):135–144.
- Ma L, Li X, Wang L, Li Y, Bu N, Yu C. 2019. Endophytic infection modulates ROS-scavenging systems and modifies cadmium distribution in rice seedlings exposed to cadmium stress. Theor Exp Plant Physiol. 31(4):463–474. doi:10.1007/s40626-019-00159-5.
- Ma M, Christensen MJ, Nan Z. 2015. Effects of the endophyte Epichloë festucae var. lolii of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. Eur J Plant Pathol. 141(3):571–583.
- MacIá-Vicente JG, Rosso LC, Ciancio A, Jansson HB, Lopez-Llorca LV. 2009. Colonisation of barley roots by endophytic Fusarium equiseti and Pochonia chlamydosporia: effects on plant growth and disease. Ann Appl Biol. 155(3):391–401.
- Malinowski DP, Belesky DP. 1999. Tall fescue aluminum tolerance is affected by Neotyphodium coenophialum endophyte. J Plant Nutr. 22(8):1335–1349.
- Malinowski DP, Butler TJ, Belesky DP. 2011. Competitive ability of tall fescue against alfalfa as a function of summer dormancy, endophyte infection, and Soil moisture availability. Crop Sci. 51(3):1282–1290. http://doi.wiley.com/10.2135/cropsci2010.08.0456
- Marks S, Clay K. 2007. Low resource availability differentially affects the growth of host grasses infected by fungal endophytes. Int J Plant Sci. 168(9):1269–1277. https://www.journals.uchicago.edu/doi/abs/10.1086/521834
- Martínez-Arias C, Macaya-Sanz D, Witzell J, Martín JA. 2019. Enhancement of populus alba tolerance to venturia tremulae upon inoculation with endophytes showing in vitro biocontrol potential. Eur J Plant Pathol. 153(4):1031–1042. doi:10.1007/s10658-018-01618-6.
- Martínez-arias C, Sobrino-plata J, Gil L, Rodríguez-calcerrada J, Martín JA. 2021. Priming of plant defenses against ophiostoma novo-ulmi by elm (ulmus minor mill.) fungal endophytes. J Fungi 7(9):687. doi:10.3390/jof7090687.
- Mastan A, Bharadwaj R, Kushwaha RK, Vivek Babu CS. 2019. Functional fungal endophytes in coleus forskohlii regulate labdane diterpene biosynthesis for elevated forskolin accumulation in roots. Microb Ecol. 78(4):914–926. doi:10.1007/s00248-019-01376-w.
- Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. 2007. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science (80-). 315(5811):513–515. https://pubmed.ncbi.nlm.nih.gov/17255511/
- Mehmood A, Hussain A, Irshad M, Hamayun M, Iqbal A, Khan N. 2019. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis. 77(3):225–235. doi:10.1007/s13199-018-0583-y.
- Mendarte-Alquisira C, Gutiérrez-Rojas M, González-Márquez H, Volke-Sepúlveda T. 2017. Improved growth and control of oxidative stress in plants of Festuca arundinacea exposed to hydrocarbons by the endophytic fungus lewia sp. Plant Soil. 411(1–2):347–358. http://www.ncbi.nlm.nih.gov/blast
- Mendarte-Alquisira C, Gutiérrez-Rojasy M, Volke-Sepúlveda T. 2020. The fungus lewia sp. alleviates the oxidative stress in f. arundinacea during the endophyte-assisted phytoremediation of hydrocarbons. Rev Mex Ing Quim. 19:69–80. http://rmiq.org/ojs311/index.php/rmiq/article/view/1547/933
- Ming Q, Su C, Zheng C, Jia M, Zhang Q, Zhang H, Rahman K, Han T, Qin L. 2013. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J Exp Bot. 64(18):5687–5694. https://academic.oup.com/jxb/article-lookup/doi/10.1093/jxb/ert342
- Mirzahosseini Z, Shabani L, Sabzalian MR, Sharifi-Tehrani M. 2014. Neotyphodium endophytes may increase tolerance to Ni in tall fescue. Eur J Soil Biol. 63:33–40.
- Molina-Montenegro MA, Acuña-Rodríguez IS, Torres-Díaz C, Gundel PE, Dreyer I. 2020. Antarctic root endophytes improve physiological performance and yield in crops under salt stress by enhanced energy production and Na+ sequestration. Sci Rep. 10(1):1–10. doi:10.1038/s41598-020-62544-4.
- Molina-Montenegro MA, Oses R, Torres-Díaz C, Atala C, Zurita-Silva A, Ruiz-Lara S. 2016. Root-endophytes improve the ecophysiological performance and production of an agricultural species under drought condition. AoB Plants. 8:plw062. https://academic.oup.com/aobpla/article-lookup/doi/10.1093/aobpla/plw062
- Monnet F, Vaillant N, Hitmi A, Coudret A, Sallanon H. 2001. Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne. Physiol Plant. 113(4):557–563. http://doi.wiley.com/10.1034/j.1399-3054.2001.1130415.x
- Monnet F, Vaillant N, Hitmi A, Sallanon H. 2005. Photosynthetic activity of Lolium perenne as a function of endophyte status and zinc nutrition. Funct Plant Biol. 32(2):131–139.
- Morse LJ, Day TA, Faeth SH. 2002. Effect of Neotyphodium endophyte infection on growth and leaf gas exchange of arizona fescue under contrasting water availability regimes. Environ Exp Bot. 48(3):257–268.
- Murphy BR, Doohan FM, Hodkinson TR. 2014. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis. 62(1):29–39.
- Murphy BR, Doohan FM, Hodkinson TR. 2015. Fungal root endophytes of a wild barley species increase yield in a nutrient-stressed barley cultivar. Symbiosis. 65:1–7. doi:10.1007/s13199-015-0314-6.
- Murphy BR, Martin Nieto L, Doohan FM, Hodkinson TR. 2015. Fungal Endophytes Enhance agronomically important traits in severely drought-stressed barley. J Agron Crop Sci. 201(6):419–427.
- Muvea AM, Meyhöfer R, Maniania NK, Poehling HM, Ekesi S, Subramanian S. 2015. Behavioral responses of thrips tabaci lindeman to endophyte-inoculated onion plants. J Pest Sci (2004). 88(3):555–562.
- Mwamburi LA. 2021. Endophytic fungi, Beauveria bassiana and Metarhizium anisopliae, confer control of the fall armyworm, spodoptera frugiperda (J. E. Smith) (lepidoptera: noctuidae), in two tomato varieties. Egypt J Biol Pest Control. 31: Article number 7.
- Nagabhyru P, Dinkins RD, Wood CL, Bacon CW, Schardl CL. 2013. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol. 13(1):127. http://bmcplantbiol.biomedcentral.com/articles/10.1186/1471-2229-13-127
- Nandhini M, Rajini SB, Udayashankar AC, Niranjana SR, Lund OS, Shetty HS, Prakash HS. 2018. Diversity, plant growth promoting and downy mildew disease suppression potential of cultivable endophytic fungal communities associated with pearl millet. Biol Control. 127:127–138.
- Neelipally RTKR, Anoruo AO, Nelson S. 2020. Effect of co-inoculation of bradyrhizobium and trichoderma on growth, development, and yield of arachis hypogaea L. (peanut). Agronomy. 10(9):1415. doi:10.3390/agronomy10091415.
- Nefzi A, Abdallah RB, Jabnoun-Khiareddine H, Ammar N, Daami-Remadi M. 2019. Ability of endophytic fungi associated with Withania somnifera L. to control Fusarium Crown and Root Rot and to promote growth in tomato. Brazilian J Microbiol. 50(2):481–494. doi:10.1007/s42770-019-00062-w.
- Nieva AS, Romero FM, Erban A, Carrasco P, Ruiz OA, Kopka J. 2021. Metabolic profiling and metabolite correlation network analysis reveal that fusarium solani induces differential metabolic responses in lotus japonicus and lotus tenuis against severe phosphate starvation. J Fungi. 7(9):765. doi:10.3390/jof7090765.
- Noora H, Shahabivand S, Karimi F, Aghaee A, Aliloo AAAA. 2017. Piriformospora indica affects growth, tropane alkaloids production and gene expression in Atropa belladonna L. plantlets. Med Plants. 9(1):55–62.
- Nuangmek W, Aiduang W, Kumla J, Lumyong S, Suwannarach N. 2021. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Front Microbiol. 12. doi:10.3389/fmicb.2021.634772.
- Oberhofer M, Güsewell S, Leuchtmann A. 2014. Effects of natural hybrid and non-hybrid Epichloë endophytes on the response of hordelymus europaeus to drought stress. New Phytol. 201(1):242–253.
- Odokonyero K, Acuña TB, Cardoso JA, de la Cruz Jimenez J, Rao IM. 2016. Fungal endophyte association with brachiaria grasses and its influence on plant water status, total non-structural carbohydrates and biomass production under drought stress. Plant Soil. 409(1–2):273–282. https://link.springer.com/article/10.1007/s11104-016-2947-5
- Ortiz J, Soto J, Fuentes A, Herrera H, Meneses C, Arriagada C. 2019. The endophytic fungus Chaetomium cupreum regulates expression of genes involved in the tolerance to metals and plant growth promotion in eucalyptus globulus roots. Microorganisms. 7(11):490. https://www.mdpi.com/2076-2607/7/11/490
- Pan X, Qin Y, Yuan Z. 2018. Potential of a halophyte-associated endophytic fungus for sustaining Chinese white poplar growth under salinity. Symbiosis. 76(2):109–116. doi:10.1007/s13199-018-0541-8.
- Pandey R, Mishra AK, Tiwari S, Singh HN, Kalra A. 2011. Enhanced tolerance of mentha arvensis against Meloidogyne incognita (kofoid and White) chitwood through mutualistic endophytes and PGPRs. J Plant Interact. 6(4):247–253. http://www.tandfonline.com/doi/abs/10.1080/17429145.2011.554892
- Paparu P, Dubois T, Coyne D, Viljoen A. 2010. Effect of Fusarium oxysporum endophyte inoculation on the activities of phenylpropanoid pathway enzymes and radopholus similis numbers in susceptible and tolerant east African highland bananas. Nematology. 12(3):469–480.
- Pecetti L, Romani M, Carroni AM, Annicchiarico P, Piano E. 2007. The effect of endophyte infection on persistence of tall fescue (Festuca arundinacea schreb.) populations in two climatically contrasting Italian locations. Aust J Agric Res. 58(9):893–899.
- Pedrero-Méndez A, Insuasti HC, Neagu T, Illescas M, Rubio MB, Monte E, Hermosa R. 2021. Why Is the correct selection of Trichoderma strains important? The case of wheat endophytic strains of T. harzianum and T. simmonsii. J Fungi. 7(12):1087. https://www.mdpi.com/2309-608X/7/12/1087
- Pereira EC, Vazquez de Aldana BR, Arellano JB, Zabalgogeazcoa I. 2021. The role of fungal microbiome components on the Adaptation to salinity of Festuca rubra subsp. pruinosa. Front Plant Sci. 12. doi:10.3389/fpls.2021.695717.
- Pérez LI, Gundel PE, Ghersa CM, Omacini M. 2013. Family issues: fungal endophyte protects host grass from the closely related pathogen claviceps purpurea. Fungal Ecol. 6(5):379–386.
- Pinto C, Custódio V, Nunes M, Songy A, Rabenoelina F, Courteaux B, Clément C, Gomes AC, Fontaine F. 2018. Understand the potential role of aureobasidium pullulans, a resident microorganism from grapevine, to prevent the infection caused by diplodia seriata. Front Microbiol. 9(DEC):3047. https://www.frontiersin.org/article/10.3389/fmicb.2018.03047/full
- Poveda J, Zabalgogeazcoa I, Soengas P, Rodríguez VM, Cartea ME, Abilleira R, Velasco P. 2020. Brassica oleracea var. acephala (kale) improvement by biological activity of root endophytic fungi. Sci Rep. 10(1):20224. doi:10.1038/s41598-020-77215-7.
- Priyadharsini P, Muthukumar T. 2017. The root endophytic fungus Curvularia geniculata from parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 27:69–77.
- Qiang X, Ding J, Lin W, Li Q, Xu C, Zheng Q, Li Y. 2019. Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil. 439(1–2):373–391. doi:10.1007/s11104-019-04028-7.
- Qin J, Wu M, Liu H, Gao Y, Ren A. 2019. Endophyte infection and methyl jasmonate treatment increased the resistance of achnatherum sibiricum to insect herbivores independently. Toxins (Basel). 11(1):7. www.mdpi.com/journal/toxins
- Qin J, Yuan G, Liu H, Zhou Y, Ren A, Yubao G. 2016. Effect of endophyte infection and clipping treatment on resistance and tolerance of Achnatherum sibiricum. Front Microbiol. 7(DEC):1988. http://journal.frontiersin.org/article/10.3389/fmicb.2016.01988/full
- Qin W, Liu C, Jiang W, Xue Y, Wang G, Liu S. 2019. A coumarin analogue NFA from endophytic Aspergillus fumigatus improves drought resistance in rice as an antioxidant. BMC Microbiol. 19(1):50. https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-019-1419-5
- Qin X, Zhao X, Huang S, Deng J, Li X, Luo Z, Zhang Y. 2021. Pest management via endophytic colonization of tobacco seedlings by the insect fungal pathogen Beauveria bassiana. Pest Manag Sci. 77(4):2007–2018.
- Qin Y, Pan X, Kubicek C, Druzhinina I, Chenthamara K, Labbé J, Yuan Z. 2017. Diverse plant-associated pleosporalean fungi from saline areas: ecological tolerance and nitrogen-status dependent effects on plant growth. Front Microbiol. 8(FEB):158. https://blast.ncbi.nlm.nih.gov/Blast.cgi
- Qu Z, Zhao H, Zhang H, Wang Q, Yao Y, Cheng J, Lin Y, Xie J, Fu Y, Jiang D. 2020. Bio-priming with a hypovirulent phytopathogenic fungus enhances the connection and strength of microbial interaction network in rapeseed. npj Biofilms Microbiomes. 6(1):45. doi:10.1038/s41522-020-00157-5.
- Quiring D, Adams G, Flaherty L, McCartney A, Miller JD, Edwards S. 2019. Influence of a Foliar endophyte and budburst phenology on survival of wild and laboratory-reared eastern Spruce budworm, choristoneura fumiferana on White Spruce (picea glauca). Forests. 10(6):503. https://www.mdpi.com/1999-4907/10/6/503
- Radhakrishnan R, Khan AL, Kang SM, Lee IJ. 2015. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann Microbiol. 65(1):585–593.
- Radhakrishnan R, Khan AL, Lee IJ. 2013. Endophytic fungal pre-treatments of seeds alleviates salinity stress effects in soybean plants. J Microbiol. 51(6):850–857. https://link.springer.com/article/10.1007/s12275-013-3168-8
- Rahman MH, Simpson WR, Matthew C, Sabreen S, Okubo A, Islam KR. 2015. Response of diploid perennial ryegrass to fungal endophyte AR29 infections under water stress. Commun Soil Sci Plant Anal. 46(7):845–860.
- Rai M, Acharya D, Singh A, Varma A. 2001. Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza. 11(3):123–128. http://link.springer.com/10.1007/s005720100115
- Rajak J, Bawaskar M, Rathod D, Agarkar G, Nagaonkar D, Gade A, Rai M. 2017. Interaction of copper nanoparticles and an endophytic growth promoter Piriformospora indica with cajanus cajan. J Sci Food Agric. 97(13):4562–4570.
- Rajani P, Aiswarya H, Vasanthakumari MM, Jain SK, Bharate SB, Rajasekaran C, Ravikanth G, Uma Shaanker R. 2019. Inhibition of the collar rot fungus, sclerotium rolfsii sacc. by an endophytic fungus Alternaria sp.: implications for biocontrol. Plant Physiol Reports. 24(4):521–532. doi:10.1007/s40502-019-00484-6.
- Rajini SB, Nandhini M, Udayashankar AC, Niranjana SR, Lund OS, Prakash HS. 2020. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of sorghum bicolor. Plant Pathol. 69(4):642–654. https://onlinelibrary.wiley.com/doi/abs/10.1111/ppa.13151
- Rauf M, Awais M, Ud-Din A, Ali K, Gul H, Rahman MM, Hamayun M, Arif M. 2021. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front Plant Sci. 11. doi:10.3389/fpls.2020.614971.
- Ravel C, Courty C, Coudret A, Charmet G. 1997. Beneficial effects of Neotyphodium lolii on the growth and the water status in perennial ryegrass cultivated under nitrogen deficiency or drought stress. Agronomie. 17(3):173–181. http://www.agronomy-journal.org/10.1051/agro:19970304
- Razinger J, Praprotnik E, Schroers HJ. 2020. Bioaugmentation of Entomopathogenic fungi for sustainable agriotes larvae (wireworms) management in maize. Front Plant Sci. 11. doi:10.3389/fpls.2020.535005.
- Redman RS, Kim YO, Woodward CJDA, Greer C, Espino L, Doty SL, Rodriguez RJ. 2011. Increased fitness of rice plants to abiotic stress Via habitat adapted symbiosis: A strategy for mitigating Impacts of climate change.El-shemy HA, editor. PLoS One. 6(7):e14823. https://dx.plos.org/10.1371/journal.pone.0014823
- Ren A, Li C, Gao Y. 2011. Endophytic fungus improves growth and metal uptake of Lolium arundinaceum darbyshire ex. schreb. Int J Phytoremediation. 13(3):233–243. https://www.tandfonline.com/doi/abs/10.1080/15226511003671387
- Ren A, Wei M, Yin L, Wu L, Zhou Y, Li X, Gao Y. 2014. Benefits of a fungal endophyte in Leymus chinensis depend more on water than on nutrient availability. Environ Exp Bot. 108:71–78.
- Ren AZ, Gao YB, Wang W, Wang JL. 2005. Photosynthetic pigments and photosynthetic products of endophyte-infection and endophyte-free Lolium perenne L. under drought stress conditions. Acta Ecol Sin. 25(2):225–231.
- Ren AZ, Li X, Han R, Yin LJ, Wei MY, Gao YB. 2011. Benefits of a symbiotic association with endophytic fungi are subject to water and nutrient availability in Achnatherum sibiricum. Plant Soil. 346(1):363–373. https://link.springer.com/article/10.1007/s11104-011-0824-9
- Ren XN, Shan Y, Li X, Wang LL, Li YY, Ma LJ, Li XM. 2021. Endophytic infection programs the ascorbateglutathione cycle in rice (Oryza sativa l.) under na2co3 stress. Appl Ecol Environ Res. 19(3):1895–1907.
- Repas TS, Gillis DM, Boubakir Z, Bao X, Samuels GJ, Kaminskyj SGW. 2017. Growing plants on oily, nutrient-poor soil using a native symbiotic fungus.bradley R, editor. PLoS One. 12(10):e0186704. https://dx.plos.org/10.1371/journal.pone.0186704
- Reza Sabzalian M, Mirlohi A. 2010. Neotyphodium endophytes trigger salt resistance in tall and meadow fescues. J Plant Nutr Soil Sci. 173(6):952–957.
- Rho H, Van Epps V, Kim S-H, Doty SL. 2020. Endophytes Increased Fruit Quality with Higher Soluble Sugar Production in Honeycrisp Apple (Malus pumila). Microorganisms. 8(5):699. https://www.mdpi.com/2076-2607/8/5/699
- Rinu K, Sati P, Pandey A. 2014. Trichoderma gamsii (NFCCI 2177): A newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J Basic Microbiol. 54(5):408–417.
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS. 2008. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2(4):404–416. www.nature.com/ismej
- Rudgers JA, Swafford AL. 2009. Benefits of a fungal endophyte in Elymus virginicus decline under drought stress. Basic Appl Ecol. 10(1):43–51.
- Ryszka P, Lichtscheidl I, Tylko G, Turnau K. 2019. Symbiotic microbes of saxifraga stellaris ssp. alpigena from the copper creek of schwarzwand (Austrian Alps) enhance plant tolerance to copper. Chemosphere. 228:183–194.
- Saari S, Faeth SH. 2012. Hybridization of Neotyphodium endophytes enhances competitive ability of the host grass. New Phytol. 195(1):231–236.
- Sabzalian MR, Mirlohi A, Sharifnabi B. 2012. Reaction to powdery mildew fungus, Blumeria graminis in endophyte-infected and endophyte-free tall and meadow fescues. Australas Plant Pathol. 41(5):565–572.
- Saddique MAB, Ali Z, Khan AS, Rana IA, Shamsi IH. 2018. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice. 11(1):34. https://thericejournal.springeropen.com/articles/10.1186/s12284-018-0226-1
- Sadeghi F, Samsampour D, Askari Seyahooei M, Bagheri A, Soltani J. 2020. Fungal endophytes alleviate drought-induced oxidative stress in mandarin (citrus reticulata L.): toward regulating the ascorbate–glutathione cycle. Sci Hortic (Amsterdam). 261:108991.
- Saedi T, Mosaddeghi MR, Sabzalian MR, Zarebanadkouki M. 2021. Effect of Epichloë fungal endophyte symbiosis on tall fescue to cope with flooding-derived oxygen-limited conditions depends on the host genotype. Plant Soil. 468(1–2):353–373.
- Sahay N, Varma A. 1999. Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol Lett. 181(2):297–302. https://academic.oup.com/femsle/article-lookup/doi/10.1111/j.1574-6968.1999.tb08858.x
- Saleem K, Iqbal A, Mirza CR, Butt TA, Toqeer M, Yousaf S, Zafar MI, Iqbal M. 2021. Role of trametes hirsuta on petunia hybrida vilm. in the presence of cadmium and lead. Russ J Plant Physiol. 68:S116–S130.
- Sampangi-Ramaiah MH, Jagadheesh S, Dey P, Jambagi S, Vasantha Kumari MM, Oelmüller R, Nataraja KN, Venkataramana Ravishankar K, Ravikanth G, Uma Shaanker R. 2020. An endophyte from salt-adapted pokkali rice confers salt-tolerance to a salt-sensitive rice variety and targets a unique pattern of genes in its new host. Sci Rep. 10(1):1–14. https://www.nature.com/articles/s41598-020-59998-x
- Sangamesh MB, Jambagi S, Vasanthakumari MM, Shetty NJ, Kolte H, Ravikanth G, Nataraja KN, Uma Shaanker R. 2018. Thermotolerance of fungal endophytes isolated from plants adapted to the thar desert, India. Symbiosis. 75(2):135–147. doi:10.1007/s13199-017-0527-y.
- Santangelo JS, Kotanen PM. 2016. Nonsystemic fungal endophytes increase survival but reduce tolerance to simulated herbivory in subarctic Festuca rubra.peters DPC, editor. Ecosphere. 7(5):e01260. https://onlinelibrary.wiley.com/doi/10.1002/ecs2.1260
- Santos S, Silva P, Garcia AC, Zilli JÉ, Berbara RLL. 2017. Dark septate endophyte decreases stress on rice plants. Brazilian J Microbiol. 48(2):333–341.
- Sarkar D, Rovenich H, Jeena G, Nizam S, Tissier A, Balcke GU, Mahdi LK, Bonkowski M, Langen G, Zuccaro A. 2019. The inconspicuous gatekeeper: endophytic Serendipita vermifera acts as extended plant protection barrier in the rhizosphere. New Phytol. 224(2):886–901. https://onlinelibrary.wiley.com/doi/abs/10.1111/nph.15904
- Satheesan J, Narayanan AK, Sakunthala M. 2012. Induction of root colonization by Piriformospora indica leads to enhanced asiaticoside production in Centella asiatica. Mycorrhiza. 22(3):195–202. https://link.springer.com/article/10.1007/s00572-011-0394-y
- Sánchez-Rodríguez AR, Del Campillo MC, Quesada-Moraga E. 2015. Beauveria bassiana: An entomopathogenic fungus alleviates Fe chlorosis symptoms in plants grown on calcareous substrates. Sci Hortic (Amsterdam). 197:193–202.
- Schmidt CS, Mrnka L, Frantík T, Lovecká P, Vosátka M. 2018. Plant growth promotion of miscanthus × giganteus by endophytic bacteria and fungi on non-polluted and polluted soils. World J Microbiol Biotechnol. 34(3):48. doi:10.1007/s11274-018-2426-7.
- Sepehri M, Ghaffari MR, Khayam Nekoui M, Sarhadi E, Moghadam A, Khatabi B, Hosseini Salekdeh G. 2021. Root endophytic fungus Serendipita indica modulates barley leaf blade proteome by increasing the abundance of photosynthetic proteins in response to salinity. J Appl Microbiol. 131(4):1870–1889.
- Shadmani L, Jamali S, Fatemi A. 2021. Effects of root endophytic fungus, microdochium bolleyi on cadmium uptake, translocation and tolerance by Hordeum vulgare L. Biologia (Bratisl). 76(2):711–719.
- Shah S, Shrestha R, Maharjan S, Selosse M-A, Pant B. 2018. Isolation and Characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants. 8(1):5. http://www.mdpi.com/2223-7747/8/1/5
- Sharma P, Kharkwal AC, Abdin MZ, Varma A. 2017. Piriformospora indica-mediated salinity tolerance in Aloe vera plantlets. Symbiosis. 72(2):103–115. https://link.springer.com/article/10.1007/s13199-016-0449-0
- Sharma VK, Li X, Wu G, Bai W, Parmar S, White JF, Li H. 2019. Endophytic community of Pb-Zn hyperaccumulator arabis alpina and its role in host plants metal tolerance. Plant Soil. 437(1–2):397–411. doi:10.1007/s11104-019-03988-0.
- Shen M, Liu L, Li DW, Zhou WN, Zhou ZP, Zhang CF, Luo YY, Wang HB, Li HY. 2013. The effect of endophytic peyronellaea from heavy metal-contaminated and uncontaminated sites on maize growth, heavy metal absorption and accumulation. Fungal Ecol. 6(6):539–545.
- Sherameti I, Tripathi S, Varma A, Oelmüller R. 2008. The Root-Colonizing Endophyte Pirifomospora indica Confers Drought Tolerance in Arabidopsis by Stimulating the Expression of Drought Stress-Related Genes in leaves. / 799 MPMI. 21(6):799–807. https://pubmed.ncbi.nlm.nih.gov/18624643/
- Shiba T, Sugawara K. 2005. Resistance to the rice leaf bug, Trigonotylus caelestialium, is conferred by Neotyphodium endophyte infection of perennial ryegrass, Lolium perenne. Entomol Exp Appl. 115(3):387–392.
- Shittu HO, Shakir AS, Nazar RN, Robb J. 2009. Endophyte-induced verticillium protection in tomato is range-restricted. Plant Signal Behav. 4(2):160–161.
- Shukla N, Awasthi RP, Rawat L, Kumar J. 2012. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem. 54:78–88.
- Sinclair G, Charest C, Dalpé Y, Khanizadeh S. 2013. Influence of arbuscular mycorrhizal fungi and a root endophyte on the biomass and root morphology of selected strawberry cultivars under salt conditions. Can J Plant Sci. 93(6):997–999.
- Singh G, Sharma P, Sharma S. 2016. Role of growth media on the phytopromotional potential of symbiotic fungus Piriformospora indica. J Environ Biol. 37(July):565–571.
- Singh UB, Sahu A, Sahu N, Singh BP, Singh RK, Renu Singh DP, Jaiswal RK, Sarma BK, Singh HB, et al. 2013. Can endophytic arthrobotrys oligospora modulate accumulation of defence related biomolecules and induced systemic resistance in tomato (lycopersicon esculentum mill.) against root knot disease caused by Meloidogyne incognita. Appl Soil Ecol. 63:45–56.
- Soleimani M, Hajabbasi MA, Afyuni M, Mirlohi A, Borggaard OK, Holm PE. 2010. Effect of endophytic fungi on cadmium tolerance and bioaccumulation by Festuca arundinacea and Festuca pratensis. Int J Phytoremediation. 12(6):535–549. https://www.tandfonline.com/doi/abs/10.1080/15226510903353187
- Su CL, Zhang FM, Sun K, Zhang W, Dai CC. 2019. Fungal endophyte Phomopsis liquidambari improves iron and molybdenum nutrition uptake of Peanut in consecutive monoculture soil. J Soil Sci Plant Nutr. 19(1):71–80.
- Su ZZ, Dai MD, Zhu JN, Liu XH, Li L, Zhu XM, Wang JY, Yuan ZL, Lin FC. 2021. Dark septate endophyte falciphora oryzae-assisted alleviation of cadmium in rice. J Hazard Mater. 419. doi:10.1016/j.jhazmat.2021.126435.
- Sun BT, Akutse KS, Xia XF, Chen JH, Ai X, Tang Y, Wang Q, Feng BW, Goettel MS, You MS. 2018. Endophytic effects of Aspergillus oryzae on radish (raphanus sativus) and its herbivore, plutella xylostella. Planta. 248(3):705–714.
- Sutton JC, Liu W, Ma J, Brown WG, Stewart JF, Walker GD. 2008. Evaluation of the fungal endophyte clonostachys rosea as an inoculant to enhance growth, fitness and productivity of crop plants. In: Acta Hortic. Vol. 782. [place unknown]: International Society for Horticultural Science; p. 279–286. doi:10.17660/ActaHortic.2008.782.34.
- Suwannarach N, Kumla J, Matsui K, Lumyong S. 2015. Characterization and efficacy of muscodor cinnamomi in promoting plant growth and controlling Rhizoctonia root rot in tomatoes. Biol Control. 90:25–33.
- Swarthout D, Harper E, Judd S, Gonthier D, Shyne R, Stowe T, Bultman T. 2009. Measures of leaf-level water-use efficiency in drought stressed endophyte infected and non-infected tall fescue grasses. Environ Exp Bot. 66(1):88–93.
- Tabande L, Sepehri M, Yasrebi J, Zarei M, Ghasemi-Fasaei R, Khatabi B. 2021. A comparison between the function of Serendipita indica and sinorhizobium meliloti in modulating the toxicity of zinc oxide nanoparticles in alfalfa (Medicago sativa L.). Environ Sci Pollut Res. 29(6):8790–8803.
- Thangavelu R, Gopi M. 2015. Combined application of native Trichoderma isolates possessing multiple functions for the control of Fusarium wilt disease in banana cv. grand naine. Biocontrol Sci Technol. 25(10):1147–1164.
- Tian B, Xie J, Fu Y, Cheng J, Li B, Chen T, Zhao Y, Gao Z, Yang P, Barbetti MJ, et al. 2020. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 14(12):3120–3135.
- Tian Z, Huang B, Belanger FC. 2015. Effects of Epichloë festucae fungal endophyte infection on drought and heat stress responses of strong creeping red fescue. J Am Soc Hortic Sci. 140(3):257–264. https://journals.ashs.org/jashs/view/journals/jashs/140/3/article-p257.xml
- Ting ASY, Meon S, Kadir J, Radu S, Singh G. 2008. Endophytic microorganisms as potential growth promoters of banana. BioControl. 53(3):541–553. https://link.springer.com/article/10.1007/s10526-007-9093-1
- Tolba SRT, Moustafa MMA, Elshawaf IIS, Rosso LC, Pentimone I, Colagiero M, Bubici G, Prigigallo MI, Ciancio A. 2021. Root endophytism by pochonia chlamydosporia affects defense-gene expression in leaves of monocot and dicot hosts under multiple biotic interactions. Plants. 10(4):718. doi:10.3390/plants10040718.
- Torres-Díaz C, Valladares MA, Acuña-Rodríguez IS, Ballesteros GI, Barrera A, Atala C, Molina-Montenegro MA. 2021. Symbiotic interaction enhances the recovery of endangered Tree species in the fragmented maulino forest. Front Plant Sci. 12:663017.
- Turbat A, Rakk D, Vigneshwari A, Kocsubé S, Thu H, Szepesi Á, Bakacsy L, Škrbić B D, Jigjiddorj E-A, Vágvölgyi C, Szekeres A. 2020. Characterization of the plant growth-promoting activities of endophytic fungi isolated from sophora flavescens. Microorganisms. 8(5):683. https://www.mdpi.com/2076-2607/8/5/683
- Ueno AC, Gundel PE, Ghersa CM, Agathokleous E, Martínez-Ghersa MA. 2021. Seed-borne fungal endophytes constrain reproductive success of host plants under ozone pollution. Environ Res. 202:111773.
- Ueno AC, Gundel PE, Omacini M, Ghersa CM, Bush LP, Martínez-Ghersa MA. 2016. Mutualism effectiveness of a fungal endophyte in an annual grass is impaired by ozone.bennett A, editor. Funct Ecol. 30(2):226–234. https://onlinelibrary.wiley.com/doi/abs/10.1111/1365-2435.12519
- Varkey S, Anith KN, Narayana R, Aswini S. 2018. A consortium of rhizobacteria and fungal endophyte suppress the root-knot nematode parasite in tomato. Rhizosphere. 5:38–42.
- Vázquez de Aldana BR, Gundel PE, García Criado B, García Ciudad A, García Sánchez A, Zabalgogeazcoa I. 2014. Germination response of endophytic Festuca rubra seeds in the presence of arsenic. Grass Forage Sci. 69(3):462–469.
- Vázquez-de-Aldana BR, García-Ciudad A, García-Criado B, Vicente-Tavera S, Zabalgogeazcoa I. 2013. Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS One. 8(12):e84539. doi:10.1371/journal.pone.0084539.
- Vergara C, Araujo KEC, Sperandio MVL, Santos LA, Urquiaga S, Zilli JÉ. 2019. Dark septate endophytic fungi increase the activity of proton pumps, efficiency of 15N recovery from ammonium sulphate, N content, and micronutrient levels in rice plants. Brazilian J Microbiol. 50(3):825–838. doi:10.1007/s42770-019-00092-4.
- Vila Aiub MM, Ghersa CM. 2001. The role of fungal endophyte infection in the evolution of Lolium multiflorum resistance to diclofop-methyl. Weed Res. 41(3):265–274. http://doi.wiley.com/10.1046/j.1365-3180.2001.00236.x
- Vila-Aiub MM, Ghersa CM, Carceller M. 2003. Effect of herbicide diclofop-methyl on proton extrusion from Lolium multiflorum seedlings differing in resistance and in fungal endophyte (Neotyphodium sp.) infection. Physiol Plant. 119(3):429–439. http://doi.wiley.com/10.1034/j.1399-3054.2003.00188.x
- Vinayarani G, Prakash HS. 2018. Fungal endophytes of turmeric (curcuma longa L.) and their biocontrol potential against pathogens pythium aphanidermatum and Rhizoctonia solani. World J Microbiol Biotechnol. 34(3):49. doi:10.1007/s11274-018-2431-x.
- Vujanovic V, Islam MN, Daida P. 2019. Transgenerational role of seed mycobiome – an endosymbiotic fungal composition as a prerequisite to stress resilience and adaptive phenotypes in triticum. Sci Rep. 9(1):1–13. doi:10.1038/s41598-019-54328-2.
- Vujanovic V, Yuan X, Daida P, Milunovic B, Germida J. 2016. Manipulation of cold stratification and endophytic effects on expression patterns of RSG and KAO genes in coleorhiza of wheat seeds. Plant Growth Regul. 79(2):219–227. https://link.springer.com/article/10.1007/s10725-015-0127-x
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, Von Wettstein D, et al. 2005. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U S A. 102(38):13386–13391. www.pnas.orgcgi doi:10.1073/pnas.0504423102
- Wang D, Wang H, Li J, Zhang W, Pan Y, Liu X. 2018. Investigating the role of endophytic fungi in gentiana scabra bge. by cross-growth period inoculation. Indian J Microbiol. 58(3):319–325. doi:10.1007/s12088-018-0725-1.
- Wang F, Zhang R, Yuan Z, Chen P. 2021. Biological prevention and control of pitaya fruit canker disease using endophytic fungi isolated from papaya. Arch Microbiol. 203(7):4033–4040.
- Wang J, Hou W, Christensen MJ, Xia C, Chen T, Zhang Z, Nan Z. 2020. The fungal endophyte Epichloë gansuensis increases NaCl-tolerance in Achnatherum inebrians through enhancing the activity of plasma membrane H+-ATPase and glucose-6-phosphate dehydrogenase. Sci China Life Sci. 64(3):452–465.
- Wang J, Nan Z, Christensen MJ, Li C. 2018. Glucose-6-phosphate dehydrogenase plays a vital role in Achnatherum inebrians plants host to Epichloë gansuensis by improving growth under nitrogen deficiency. Plant Soil. 430(1–2):37–48. doi:10.1007/s11104-018-3710-x.
- Wang J, Nan Z, Christensen MJ, Zhang X, Tian P, Zhang Z, Niu X, Gao P, Chen T, Ma L. 2018. Effect of Epichloë gansuensis endophyte on the nitrogen metabolism, nitrogen Use efficiency, and stoichiometry of Achnatherum inebrians under nitrogen limitation. J Agric Food Chem. 66(16):4022–4031. https://pubs.acs.org/sharingguidelines
- Wang JL, Li T, Liu GY, Smith JM, Zhao ZW. 2016. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: physiological, cytological and genic aspects. Sci Rep. 6(1):1–12. www.nature.com/scientificreports
- Wang X, Qin J, Chen W, Zhou Y, Ren A, Gao Y. 2016. Pathogen resistant advantage of endophyte-infected over endophyte-free Leymus chinensis is strengthened by pre-drought treatment. Eur J Plant Pathol. 144(3):477–486.
- Wang XY, Zhou Y, Ren AZ, Gao YB. 2014. Effect of endophyte infection on fungal disease resistance of Leymus chinensis. Shengtai Xuebao/ Acta Ecol Sin. 34(23):6789–6796.
- Wang Z, Li C, White J. 2020. Effects of Epichloë endophyte infection on growth, physiological properties and seed germination of wild barley under saline conditions. J Agron Crop Sci. 206(1):43–51. https://onlinelibrary.wiley.com/doi/abs/10.1111/jac.12366
- Wani ZA, Kumar A, Sultan P, Bindu K, Riyaz-Ul-Hassan S, Ashraf N. 2017. Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Sci Rep. 7(1):1–11. www.nature.com/scientificreports
- Wani ZA, Mirza DN, Arora P, Riyaz-Ul-Hassan S. 2016. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: An insight into the microbiome of Crocus sativus linn. Fungal Biol. 120(12):1509–1524.
- Waqas M, Khan AL, Hamayun M, Shahzad R, Kim YH, Choi KS, Lee IJ. 2015. Endophytic infection alleviates biotic stress in sunflower through regulation of defence hormones, antioxidants and functional amino acids. Eur J Plant Pathol. 141(4):803–824.
- Waqas M, Khan AL, Kamran M, Hamayun M, Kang S-M, Kim Y-H, Lee I-J. 2012. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 17(9):10754–10773. http://www.mdpi.com/1420-3049/17/9/10754
- Waqas M, Khan AL, Kang SM, Kim YH, Lee IJ. 2014. Phytohormone-producing fungal endophytes and hardwood-derived biochar interact to ameliorate heavy metal stress in soybeans. Biol Fertil Soils. 50(7):1155–1167.
- Waqas M, Khan AL, Lee IJ. 2014. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. J Plant Interact. 9(1):478–487.
- Waqas M, Khan AL, Shahzad R, Ullah I, Khan AR, Lee IJ. 2015. Mutualistic fungal endophytes produce phytohormones and organic acids that promote japonica rice plant growth under prolonged heat stress. J Zhejiang Univ Sci B. 16(12):1011–1018. http://orcid.org/0000-0001-7154-4820
- Ważny R, Rozpądek P, Domka A, Jędrzejczyk RJ, Nosek M, Hubalewska-Mazgaj M, Lichtscheidl I, Kidd P, Turnau K. 2021. The effect of endophytic fungi on growth and nickel accumulation in noccaea hyperaccumulators. Sci Total Environ. 768:144666.
- Wäli PR, Helander M, Nissinen O, Lehtonen P, Saikkonen K. 2008. Endophyte infection, nutrient status of the soil and duration of snow cover influence the performance of meadow fescue in sub-artic conditions. Grass Forage Sci. 63(3):324–330. http://doi.wiley.com/10.1111/j.1365-2494.2008.00639.x
- Wäli PR, Helander M, Saloniemi I, Ahlholm J, Saikkonen K. 2009. Variable effects of endophytic fungus on seedling establishment of fine fescues. Oecologia. 159(1):49–57. https://link.springer.com/article/10.1007/s00442-008-1202-z
- West CP, Izekor E, Oosterhuis DM, Robbins RT. 1988. The effect of Acremonium coenophialum on the growth and nematode infestation of tall fescue. Plant Soil. 112(1):3–6.
- Wu C, Li B, Wei Q, Pan R, Zhang W. 2019. Endophytic fungus Serendipita indica increased nutrition absorption and biomass accumulation in cunninghamia lanceolata seedlings under low phosphate. Shengtai Xuebao/ Acta Ecol Sin. 39(1):21–29.
- Wu FL, Li Y, Tian W, Sun Y, Chen F, Zhang Y, Zhai Y, Zhang J, Su H, Wang L. 2020. A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol. 40(8):1080–1094.
- Wu LQ, Lv YL, Meng ZX, Chen J, Guo SX. 2010. The promoting role of an isolate of dark-septate fungus on its host plant saussurea involucrata Kar. et Kir. Mycorrhiza. 20(2):127–135. https://link.springer.com/article/10.1007/s00572-009-0268-8
- Wu M, Wei Q, Xu L, Li H, Oelmüller R, Zhang W. 2018. Piriformospora indica enhances phosphorus absorption by stimulating acid phosphatase activities and organic acid accumulation in Brassica napus. Plant Soil. 432(1–2):333–344. doi:10.1007/s11104-018-3795-2.
- Xia C, Zhang X, Christensen MJ, Nan Z, Li C. 2015. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 16:26–33.
- Xu L, Li X, Han L, Li D, Song G. 2017. Epichloe endophyte infection improved drought and heat tolerance of tall fescue through altered antioxidant enzyme activity. Eur J Hortic Sci. 82(2):90–97. doi:10.17660/eJHS.2017/82.2.4.
- Xu R, Li T, Shen M, Yang ZL, Zhao ZW. 2020. Evidence for a Dark septate endophyte (Exophiala pisciphila, H93) enhancing phosphorus absorption by maize seedlings. Plant Soil. 452(1–2):249–266. doi:10.1007/s11104-020-04538-9.
- Yamaji K, Watanabe Y, Masuya H, Shigeto A, Yui H, Haruma T. 2016. Root Fungal Endophytes Enhance Heavy-Metal stress tolerance of clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of Heavy-Metal concentration.aroca R, editor. PLoS One . 11(12):e0169089. https://dx.plos.org/10.1371/journal.pone.0169089
- Yan N, Wang X, Wang Z, Zhang Y, Xue H, Guo D. 2013. Antioxidative system and chlorophyll fluorescence of zizania latifolia turcz. plants are affected by ustilago esculenta infection. Shengtai Xuebao/ Acta Ecol Sin. 33(5):1584–1593.
- Yang M-Z, Ma M-D, Yuan M-Q, Huang Z-Y, Yang W-X, Zhang H-B, Huang L-H, Ren A-Y, Shan H. 2016. Fungal endophytes as a Metabolic fine-tuning regulator for wine Grape.scali M, editor. PLoS One. 11(9):e0163186. https://dx.plos.org/10.1371/journal.pone.0163186
- Yang T, Ma S, Dai CC. 2014. Drought degree constrains the beneficial effects of a fungal endophyte on atractylodes lancea. J Appl Microbiol. 117(5):1435–1449.
- Yokoya K, Postel S, Fang R, Sarasan V. 2017. Endophytic fungal diversity of Fragaria vesca, a crop wild relative of strawberry,along environmental gradients within a small geographical area. PeerJ. 2017(1):e2860. https://peerj.com/articles/2860
- You YH, Yoon H, Kang SM, Shin JH, Choo YS, Lee IJ, Lee JM, Kim JG. 2012. Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in suncheon Bay. J Microbiol Biotechnol. 22(11):1549–1556. http://www.jmb.or.kr/journal/view.html?doi=10.4014/jmb.1205.05010
- Zahn G, Amend AS. 2019. Foliar fungi alter reproductive timing and allocation in Arabidopsis under normal and water-stressed conditions. Fungal Ecol. 41:101–106.
- Zaurov DE, Bonos S, Murphy JA, Richardson M, Belanger FC. 2001. Endophyte infection can contribute to aluminum tolerance in fine fescues. Crop Sci. 41(6):1981–1984. https://onlinelibrary.wiley.com/doi/abs/10.2135/cropsci2001.1981
- Zavala-Gonzalez EA, Escudero N, Lopez-Moya F, Aranda-Martinez A, Exposito A, Ricaño-Rodríguez J, Naranjo-Ortiz MA, Ramírez-Lepe M, Lopez-Llorca LV. 2015. Some isolates of the nematophagous fungus Pochonia chlamydosporia promote root growth and reduce flowering time of tomato. Ann Appl Biol. 166(3):472–483.
- Zhang FS, Lv YL, Zhao Y, Guo SX. 2013. Promoting role of an endophyte on the growth and contents of kinsenosides and flavonoids of anoectochilus formosanus hayata, a rare and threatened medicinal orchidaceae plant. J Zhejiang Univ Sci B. 14(9):785–792. https://link.springer.com/article/10.1631/jzus.B1300056
- Zhang H, Xie J, Fu Y, Cheng J, Qu Z, Zhao Z, Cheng S, Chen T, Li B, Wang Q, et al. 2020. A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for Brassica protection and yield enhancement. Mol Plant. 13(10):1420–1433.
- Zhang Q, Zhang J, Yang L, Zhang L, Jiang D, Chen W, Li G. 2014. Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control. 72:98–108.
- Zhang X, Li C, Nan Z. 2010. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J Hazard Mater. 175(1–3):703–709.
- Zhang Y, Zhou Y, Zhang X, Duan T, Nan Z. 2018. Effects of Epichloë endophyte on antioxidant enzymes activities, photosynthesis and growth of three ecotypes of Elymus dahuricus. Front Agric Sci Eng. 5(1):148–158. doi:10.15302/J-FASE-2017195.
- Zhang YP, Nan ZB. 2007. Growth and anti-oxidative systems changes in Elymus dahuricus is affected by Neotyphodium endophyte under contrasting water availability. J Agron Crop Sci. 193(6):377–386. http://doi.wiley.com/10.1111/j.1439-037X.2007.00279.x
- Zhao J, Xiang D, Peng L, Zou L, Wang Y, Zhao G. 2014. Enhancement of rutin production in fagopyrum tataricum hairy root cultures with its endophytic fungal elicitors. Prep Biochem Biotechnol. 44(8):782–794.
- Zhou L, Li C, White JF, Johnson RD. 2021. Synergism between calcium nitrate applications and fungal endophytes to increase sugar concentration in Festuca sinensis under cold stress. PeerJ. 9:e10568. doi:10.7717/peerj.10568.
- Zhou L, Tang K, Guo S. 2018. The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field conditions. Int J Mol Sci. 19(1):270. http://www.mdpi.com/1422-0067/19/1/270
- Zhou Z, Zhang C, Zhou W, Li W, Chu L, Yan J, Li H. 2014. Diversity and plant growth-promoting ability of endophytic fungi from the five flower plant species collected from yunnan, southwest China. J Plant Interact. 9(1):585–591. https://www.tandfonline.com/doi/full/10.1080/17429145.2013.873959
- Zhu A, Tan H, Cao L. 2019. Isolation of phytase-producing yeasts from rice seedlings for prospective probiotic applications. 3 Biotech. 9(6):216. doi:10.1007/s13205-019-1746-0.
- Zhu L, Li T, Wang C, Zhang X, Xu L, Xu R, Zhao Z. 2018. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ Sci Pollut Res. 25(35):35232–35241. doi:10.1007/s11356-018-3456-2.