ABSTRACT
The Psylloidea, >4000 named species known today, are plant-feeding, sap-sucking insects sleeved under the Sternorrhyncha. Most species of Psylloidea are confined to the tropics. They occur as gall-inducing, free-living, and lerp-forming taxa. Lifecycles and generations of gall-inducing Psylloidea vary in temperate and tropical worlds. The Triozidae, Aphalaridae, and Calophyidae include several taxa that induce galls of diverse morphologies, from simple pits and leaf-margin rolls to complex pouches and of two-tier structures. The feeding mechanism and nutritional physiology of the gall-inducing taxa of the Psylloidea differ from those of the free-living and lerp-forming species. A majority of the gall-inducing Psylloidea are associated with the dicotyledons and a small number with the monocotyledons. The gall-inducing Psylloidea are specific to certain plants. Their host specificity is regulated by specific lipids and sterols. The gall-inducing Psylloidea show conservative behavior in terms of geographical distribution. Although the life histories of several gall-inducing Psylloidea are known today, aspects explaining their association with host plants are little known. Details of nutritional physiology of gall-inducing Psylloidea are less known presently compared with that of the free-living species. A better understanding of the association and level of relationship between gall-inducing Psylloidea and their host plants is necessary.
Introduction
The Psylloidea are sap-sucking, exclusively plant-feeding superfamily of the Hemiptera. About 4000 species are currently named and classified under the Aphalaridae, Carsidaridae, Mastigimatidae, Liviidae, Calophyidae, Psyllidae, and Triozidae (Burckhardt et al. Citation2021). The Psylloidea occur as free-living, lerp-forming, and gall-inducing taxa. Most adult Psylloidea measure between 2 and 4 mm in their overall body length. Their hind legs include well-developed coxae that enable them to jump, and therefore, are referred to as the ‘jumping-plant lice’ (louse – singular) in common parlance. Several species of the Psylloidea, e.g. Diaphorina citri (Psyllidae), Trioza erytreae (Triozidae), Cacopsylla pyricola (Psyllidae), Heteropsylla cubana (Psyllidae), and Bactericera cockerelli (Triozidae) damage plants of agricultural and horticultural relevance (Hodkinson Citation2009). By their sucking – feeding habit, they transmit microbial-plant pathogens, similar to their plant-feeding allies of the Auchenorrhyncha. Diaphorina citri and T. erytreae transmit species of Candidatus Liberibacter (Proteobacteria: Rhizobiaceae) thus inducing the citrus-greening disease (the Huánglóngbíing disease) in different species and cultivars of Citrus (Rutaceae) in Asia, Africa, and the Americas (Hall et al. Citation2013; Barnett et al. Citation2019). In contrast, some Psylloidea are useful in the biological management of weedy plants: e.g. Calophya latiforceps (Calophyidae) in managing populations of Schinus terebinthifolia (Anacardiaceae) in the USA (Diaz et al. Citation2015). Some Psylloidea species are invasive. One well-known example is the lerp-forming Glycaspis brimblecombei (Aphalaridae) that lives on various species of Eucalyptus (Myrtaceae) in Australia, and commonly on E. camaldulensis, E. rudis, and E. tereticornis cultivated in many countries for commercial reasons (Brennan et al. Citation1999). Glycaspis brimblecombei spread from Australia to North America in 1998 and to Europe in 2009 (de Queiroz et al. Citation2013), when species of Eucalyptus were introduced in those parts of the world for plantation forestry.
The taxonomy of the Palaearctic Psylloidea is reasonably well clarified presently (Hodkinson and White Citation1981; Hodkinson Citation1986, Citation1989; Burckhardt and Mifsud Citation2003; Burckhardt Citation2005a, Citation2009; Burckhardt et al. Citation2004; Batta and Burckhardt Citation2018; den Bieman et al. Citation2019; Burckhardt et al. Citation2021). Ram Nath Mathur’s Psyllidae of the Indian Subcontinent (1975) is one comprehensive document on the taxonomy and biology of the Psylloidea of the Indian subcontinent. A 2018-checklist of the Indian Psylloidea by Burckhardt et al. is an up-to-date addendum to Mathur (Citation1975). Knowledge of the Psylloidea of the Afrotropical and Neotropical realms is poor, whereas that of the other biogeographical realms is better known (Hodkinson and White Citation1981; Hodkinson Citation1986, Citation1989; Burckhardt et al. Citation2004; Burckhardt and Mifsud Citation2003; Burckhardt Citation2005a; den Bieman et al. Citation2019; Halbert and Burckhardt Citation2020).
Distribution of gall-inducing Psylloidea and the types of galls they induce
Whereas free-living Psylloidea occur almost throughout the world, the lerp-forming Psylloidea – mostly of the Aphalaridae – are especially diverse in the Australian landmass, represented by c. 50% of the known lerp-forming taxa of the world (Hollis Citation2004). The distribution of gall-inducing Psylloidea vis-à-vis that of the lerp-forming taxa does not present any consistency in the pattern. However, what appears consistent among the gall-inducing Psylloidea is that they are species-rich and more diverse in the tropics (Burckhardt et al. Citation2018). For instance, c. 5% of the gall-inducing Psylloidea known globally occur in the Indonesian Islands and the Philippines (Docters van Leeuwen and Docters van Leeuwen-Reijnvann Citation1914; Uichanco Citation1919; Partomihardjo et al. Citation2011). Among the Psylloidea, the Triozidae, Aphalaridae, and Calophyidae include high numbers of gall-inducing taxa, whereas the Carsidaridae, Liviidae, and Psyllidae include relatively fewer numbers (Burckhardt Citation2005a; Yang and Raman Citation2007; Burckhardt et al. Citation2021). Among these, the Triozidae and Aphalaridae include most of the gall-inducing taxa (Burckhardt Citation2005a; Raman Citation2012).
Galls induced by the Psylloidea present highly varied morphologies: from simple leaf rolls to complex two-tier structures (Yang and Raman Citation2007). For example, Lauritrioza alacris (previously Trioza alacris) (Triozidae) induces leaf rolls on Laurus nobilis (Lauraceae) in Europe (Bouyjou and Nguyen Citation1974) ((a)). Psyllopsis fraxini (Liviidae) induces leaf-margin rolls on Fraxinus excelsior (Oleaceae) in Europe (Schindler and Ehrhardt Citation1964). Trioza ocoteae (Triozidae) induces pit galls on Ocotea acutifolia (Lauraceae) in South America (Lizer Trelles and Molle Citation1945) ((b)). Schedotrioza multitudinea (Triozidae) induces urn-shaped galls on Eucalyptus obliqua (Myrtaceae) in Mount Lofty Ranges, South Australia (Taylor Citation1990) ((c)). Pseudophacopteron tuberculatum (Aphalaridae) induces spherical, pouch galls on Alstonia scholaris (Apocynaceae) in India (Mathur Citation1975; Mani Citation2000; Albert et al. Citation2011). Pseudophacopteron aspidospermi and P. longicaudatum (Aphalaridae) induce globular and beutel galls on Aspidosperma macrocarpon and A. tomentosum (Apocynaceae), respectively, in Brazil (Malenovský et al. Citation2015). Trioza bullatae (Triozidae) induces globular galls on Ocotea bullata (Lauraceae) in South Africa (Burckhardt et al. Citation2012). Trioza jambolanae (Triozidae) induces near-spherical, closed galls on Syzygium cumini (Myrtaceae) in India (Mathur Citation1975; Raman Citation1991). Pachypsylla celtidismamma (Carsidaridae) induces closed pouch galls on Celtis occidentalis (Cannabaceae) in North America (Riley Citation1881; Beisler and Baker Citation1992) ((d)); Pauropsylla udei (Triozidae) induces spherical, closed galls on Ficus fulva and F. variegata (Moraceae) in the Indonesian islands, Philippines and in New Guinea (Rübsaamen Citation1899; Uichanco Citation1919; Percy et al. Citation2015) ((e)). Schedotrioza eucalypti (Triozidae) induces spherical galls on Eucalyptus dives in (authors’ unpublished work) ((f)). An unnamed species of Glycaspis (Synglycaspis) (hereafter ‘Glycaspis (Synglycaspis) sp. A’) belonging to the Aphalaridae induces globular, closed, pouch galls on Eucalyptus macrorhyncha (Myrtaceae) in Central-West New South Wales, Australia (Sharma et al. Citation2015a) ((g)). Apsylla cistellata (Aphalaridae) induces fir-cone-like galls on the axillary vegetative buds of Mangifera indica (Anacardiaceae) along the Indo-Gangetic plains of India (Mathur Citation1975; Singh Citation2003) ((a)). Phacopteron lentiginosum (Aphalaridae) induces complex two-tier galls on Garuga pinnata (Burseraceae) in subtropical peninsular India (Mathur Citation1975; Raman Citation1987; Mani Citation2000) ((b)).
Figure 1. Representative gall types induced by the Psylloidea. (a) leaf-roll gall induced by Trioza alacris on Laurus nobilis in Europe (source: Plant Parasites of Europe; bladmineerders.nl/parasites/animalia/arthropoda/insecta/hemiptera/ sternorrhyncha/psylloidea/triozidae/trioza/trioza-alacris/ (accessed on May 20, 2021). (b): pit gall induced by Trioza ocoteae on Ocotea acutifolia in South America (source: Lizer Trelles and Molle Citation1945), (c) urn shaped galls induced by Schedotrioza multidunea on a species of Eucalyptus in Australia (source: Houard Citation1923), (d) closed pouch galls induced by Pachypsylla celtidismamma on Celtis occidentalis in North America (source: Riley Citation1881), (e) and spherical, closed galls as in Pauropsylla udei on a species of Ficus in Indonesian islands (Rübsaamen Citation1899), (f) spherical gall induced by Schedotrioza eucalypti on Eucalyptus dives in Australia (inset–dehisced gall) (bar = 5 mm) (source: unpublished work by authors); (g) spherical closed galls (with sugary accumulation) induced by Glycaspis (Synglycaspis) sp. A on E. macrorhyncha in Australia (bar = 5 mm); the gall on left has been slit open with a razor blade to expose the sugary filaments (source: Sharma et al. Citation2015a). (Scale bars not available for Figures a–e in original texts).
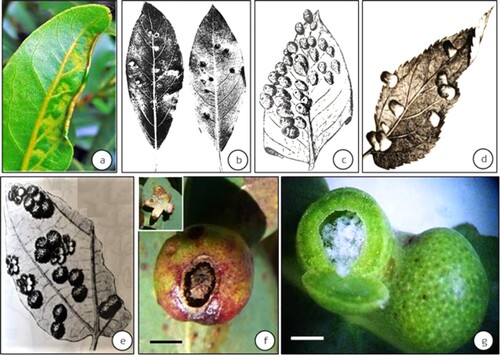
Figure 2. Examples of complex galls induced by the Psylloidea from the Indian subcontinent. (a) fir-cone like galls on Mangifera indica induced by Apsylla cistellata (source: Raman et al. Citation2009 Tropical Zoology) (inset–vertical-sectional view of a gall) (bar = 4 cm), (b) Two-tier saccular galls induced by Phacopteron lentiginosum on Garuga pinnata (courtesy: M. Nasser, University of Calicut, Kerala, India). Inset: Vertically slit gall showing the upper tier of the saccular gall and the supporting lower tier of collar-like growth. A late-stage immature can be seen at 1 o’clock position in the sac-part of this two-tier gall (bar = 5 cm).

Biology of gall-inducing Psylloidea
Juvenile stages of the Psylloidea (previously ‘nymphs,’ ‘nymphal instars,’ ‘larvae’) will be referred to as ‘immatures’ in this article, following Burckhardt et al. (Citation2014). Immatures of different species of Psylloidea show striking variations in their morphology in accordance with the plant parts they infest (White and Hodkinson Citation1985). For instance, the immatures of those Psylloidea living in pit galls have near-circular outlines with sclerotized dorsal surfaces almost covering the pit: e.g. Calophya clavuligera (Calophyidae) on Lithraea brasiliensis (Anacardiaceae) in Argentina, Bolivia, Brazil, Paraguay, and Uruguay (Burckhardt and Bassett Citation2000). In contrast, those living in closed galls have short legs and weakly sclerotized bodies, compared with those in pit galls. Short legs and weakly sclerotized bodies are considered an adaptation for living in closed galls (e.g. Glycaspis (Synglycaspis) sp. A) (Burckhardt Citation2005a). The lerp-forming taxa, e.g. species of Glycaspis construct dome-shaped lerps using sugary secretions on the leaves of Eucalyptus sideroxylon (Myrtaceae), within which an individual of Glycaspis will reside until becoming adults (). Immatures of both free-living and gall-inducing Psylloidea also secrete sugary filamentous material, but not as intensely as the lerp-forming taxa do (Sharma and Raman Citation2017). Almost all known gall-inducing Psylloidea secrete sugary filaments and retain them within the galls they induce (e.g. Glycaspis (Synglycaspis) sp. A on E. macrorhyncha, (g), Celtisaspis sinica (Carsidaridae) on Celtis sinensis (Cannabaceae) (Yang and Li Citation1982; Yang and Raman Citation2007; Sharma et al. Citation2015a). Pachypsylla cohabitans (Carsidaridae) occupies the galls induced by other species of Pachypsylla (e.g. P. celtidismamma) on different species of Celtis in North America (Yang et al. Citation2001; Burckhardt Citation2005a).
Figure 3. Lerp-constructing Psylloidea. (a) Lerps of the III (α), IV (β), and V (γ) stage immatures [bar = 1 mm], (b) Enlargement of a lerp showing sugary filaments constructed by early immature stages of an unnamed species of Glycaspis on Eucalyptus sideroxylon in Australia [bar = 100 μm], (c) The same Glycaspis (male) adult in Australia [bar = 1 mm] (Source: Sharma et al. Citation2013).
![Figure 3. Lerp-constructing Psylloidea. (a) Lerps of the III (α), IV (β), and V (γ) stage immatures [bar = 1 mm], (b) Enlargement of a lerp showing sugary filaments constructed by early immature stages of an unnamed species of Glycaspis on Eucalyptus sideroxylon in Australia [bar = 100 μm], (c) The same Glycaspis (male) adult in Australia [bar = 1 mm] (Source: Sharma et al. Citation2013).](/cms/asset/c5790a71-9359-468f-acb7-dc510d260a58/tjpi_a_2065371_f0003_oc.jpg)
Life stages of the Psylloidea include an egg, five immature, and adult stages. Females are usually 0.15x larger than males and live 7–10 d longer than males (Sharma et al. Citation2013, Citation2014a, Citation2015a). Life cycles of gall-inducing Psylloidea are univoltine in temperate, subarctic, and subantarctic regions, supplemented by inactivity in winter (e.g. Lauritrioza alacris, Triozidae). In the tropics, in contrast, they are multivoltine, completing several generations in a year (e.g. Trioza jambolanae, Triozidae; Diaphorina truncata Psyllidae) (Balakrishna P. Raman Citation1992). Populations of gall-inducing T. jambolanae on the leaves of S. cumini present varied biologies in different parts of India under different climatic conditions: in humid and warm peninsular India, T. jambolanae completes 6–8 generations in a year (Raman Citation1991), whereas in the cooler Mussoorie Hill Ranges, proximal to the Himalaya, the same species presents only one generation in a year with a long diapause from late autumn (late October–early November) to early spring (late February–early March) (Mathur Citation1975). The gall-inducing Aphalaridae in Central-west New South Wales (e.g. Glycaspis (Synglycasipis) sp. A on leaves of E. macrorrhyncha) complete two generations in a year. In contrast, the lerp-forming Aphalaridae (e.g. unnamed species of Glycaspis on Eucalyptus sideroxylon, Myrtaceae) complete 3–4 generations, and the free-living Aphalaridae (Ctenarytaina eucalypti on E. globulus) complete 5–6 overlapping generations in a year (Sharma et al. Citation2014a). Compared with other major gall-inducing taxa belonging to the Cynipidae and Cecidomyiidae, only a few parasitoids are presently known to be associated with gall-inducing Psylloidea. Psyllaephagus bliteus (Hymenoptera: Encyrtidae) is a non-specific parasitoid that is commonly associated with various species of gall-inducing Psylloidea, irrespective of geographical distances. In recent years, Psyllaephagus ramamurthyi, P. baccharidis, P. garuga, P. phacopteron, P. caillardiae, P. longiventris, P. ogazae, Aprostocetus essugonjaevi (Eulophidae), one unnamed species of Eurytoma (Eurytomidae), one unnamed species of Torymoides (Torymidae), and one unnamed species of Synopeas (Platygastridae) have been described from galls induced by the Psylloidea (Hollis Citation2004; Cuevas-Reyes et al. Citation2007; Singh and Singh Citation2011; Hayat et al. Citation2013; Veenakumari et al. Citation2018; Zhao et al. Citation2021).
Gall development (Cecidogenesis)
Feeding action of the first-instar immatures of Psylloidea triggers gall initiation (Burckhardt Citation2005a; Raman Citation2011) (). Eggs deposited by Trioza flavipennis (Triozidae) on leaf surfaces of Aegopodium podagraria (Apiaceae) induce pit galls in Europe (den Bieman et al. Citation2019). Incitement of a gall by the egg and not by a first-instar immature is rare among the gall-inducing Psylloidea.
Figure 4. Developmental sequence of the two-tier saccular gall induced by Phacopteron lentiginousm on the leaves of Garuga pinnata: from oviposition to dehiscence (not to scale). Arrow pairs: meristematic activity; (●): immature. (source: Raman Citation2011). ‘e’ is egg and part of egg stalk is buried in the leaf tissue.
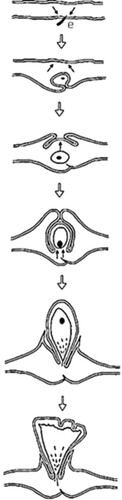
We will use the gall systems induced by T. jambolanae and A. cistellata on S. cumini and M. indica, respectively, as examples in this article. Gall development on the leaves of S. cumini – from initiation to dehiscence – gets completed in c. 40 d in subtropical – tropical southern India. Growing immatures of T. jambolanae shift their feeding sites within galls, synchronizing with gall growth. Mature galls dehisce because of specific physiological changes in cells in the gall wall, which differentially produces starch and polyphenolic materials. Cells that include secondary metabolic products (polyphenolic materials) turn into sclereids (dead cells with thick lignified cell walls) during later stages of gall growth, i.e. when occupied by the usually non-feeding, fifth-stage immatures. Sclereids occur in linear files, alternating with files of living, thin-walled parenchyma. The functionally antagonistic sclereids and parenchyma, supplemented by the tension caused by water deficit, facilitate the mature gall to dehisce from a weak point in the gall’s roof, splitting along the files of thin-walled parenchyma (Raman Citation1991). Feeding action of the first-instar immatures of A. cistellata (‘embryonic stages’ according to Gajendra Singh Citation2000, p. 2003) triggers the gall, which grows for the next c. 120 d. The immatures, partly remaining enclosed in their eggshells, feed on the same leaf where the adult female had oviposited (Singh et al. Citation1975). The feeding action of several first-instar immatures stimulates the gall through the modification of the nearest vegetative axillary bud, usually at a distance of 3–6 mm from the leaf on which the first-instar immatures feed. First-instar immatures crawl and occupy the spaces between the unfurled leaves and stem axis in the shoot bud as it transforms into the gall ((a)) that resembles a fertile cone of Abies (Pinaceae).
In the Psidium myrtoides (Myrtaceae) – Nothotrioza myrtoidis (Triozidae) gall system, an increase in biomass is indicated due to pronounced hyperplasia of the median and outer cortical cell layers (Carneiro and Isaias Citation2015a, Citation2015b). The globoid shape of the gall is the outcome of centrifugal gradients between tissue hyperplasia and cell hypertrophy (Carneiro et al. Citation2014). Demethyl-esterified homo-galacturonans denoted the activity of pectin methylesterases especially during the senescent phase, increasing the stiffness of cell walls, which, which according to Carneiro et al. (Citation2014) triggers gall dehiscence.
Gall initiation
Salivary enzymes (the effector proteins) released by the feeding immatures trigger gall initiation. A 58 kD protein isolated from the saliva of Eurosta solidaginis (Diptera: Tephritidae) is indicated as the key trigger in the induction of ball galls arising on the stems of Solidago altissima (Asteraceae) (Carango Citation1988). The role of a specific protein, viz., cysteine-tyrosine-cysteine residue, has been recently demonstrated as the key trigger factor in gall initiation by Hormaphis cornu (Hemiptera: Aphididae) on Hamamelis virginiana (Hamamelidaceae) (Korgaonkar et al. Citation2021). Although we liberally speak of the role of plant-growth hormones (PGRs) in gall induction, the establishment of either one or a small group of activated cells – the metaplasied cell(s) (sensu Küster Citation1911; Maresquelle and Meyer Citation1965) – is the earliest ≥24 h recognizable response to feeding by any gall-inducing arthropod. The discharge of a protein (or a cluster of proteins), however, remains to be verified in the instance of gall-inducing Psylloidea. The second key trigger is chitin discharged from the mandibles of the feeding arthropod. Chitin – a glucosamine polymer – is a principal constituent of arthropod mouthparts (Brożek et al. Citation2015). Plants include chitinases (Collingel et al. Citation1993) and are capable of recognizing chito-oligosaccharides during attack by an invading organism, be it a plant-pathogenic fungus or a plant-feeding arthropod. The chitin, in principle, acts as an elicitor (Shamshina et al. Citation2019) provoking the first attacked plant cell to recruit a novel pathway negotiating either a susceptible or a resistant response (Rohfritsch Citation1988; Westphal et al. Citation1990; Pusztahelyi Citation2018). Chitin stimulates mitogen-activated protein kinase (MAPK) cascades and a network of transcription factors (Schweitzer et al. Citation2013; Bi et al. Citation2018). Activation of MAPKs provokes osmotic changes in the cytoplasm of the attacked cell, resulting in the establishment of one or more metaplasied cell(s). Wounding of plant cells by the feeding immature stage of the psylloid, accompanied by a chemical ‘shock’ resulting from the action of chitin and salivary proteins (Sharma et al. Citation2014b; Sharma and Raman Citation2017) induces rapid chemical alterations, such as vacuolar alkalinization, in the subcellular environment of host-plant cells (Westphal Citation1982). Proteins specific to the inducing psylloid species interact with the host-plant’s genomic material and dramatically alter the developmental pathway resulting in a gall in a specific timeframe. The dissipation of various, newly synthesized metabolites remains restricted to the gall and its immediate neighborhood in the attacked plant organ, explaining why the altered physiology of the gall – when fully organized – is limited to that part of the plant only.
PGRs (especially the auxins) have been demonstrated in mature galls of Metrosideros polymorpha (Myrtaceae) induced by an unnamed Triozidae in Hawaii (Bailey et al. Citation2015). Bailey et al. suggest significant enrichment of auxin-response gene action during gall induction. During later stages of gall growth, i.e. when inhabited by the third-, fourth-, and fifth-stage immatures, PGRs kick in enabling the realization of the final shape of the gall (Miller and Raman Citation2019; Raman Citation2021). Other secondary metabolites, such as volatile phenols, especially the ortho-dihydric phenols, as shown in the galls of Pauropsylla depressa (Triozidae) on the leaves of Ficus glomerata (Moraceae) influence the lignification, anti-auxin activities, and IAA-oxidase inhibition (Dsouza and Ravishankar Citation2014). During gall induction by Glycaspis (Synglycaspis) sp. A on the leaves of E. macrorhyncha, membrane-bound galactolipids (mono- and di-galactosyl-diacyl-glycerols: MGDG, DGDG) occur in greater levels than storage triglycerides, which point to substantially changed physiology of cell membranes (Sharma et al. Citation2016). The DGDG level increases during the inhabitation of the first- and second-stage immatures of Glycaspis (Synglycaspis) sp. A reinforcing that physiological compatibility materializes between Glycaspis (Synglycaspis) sp. A and the host E. macrorhyncha.
Plants hosting gall-inducing insects employ diverse strategies to mitigate the stress (Hirano et al. Citation2020) that materializes during gall induction and ensuing growth-and-differentiation phase. These stress-neutralizing strategies are necessarily dictated by the genetic constitution of host plants. Plant responses are inevitably mediated by molecular changes, but those responses vary dramatically with the kinds of protein triggers provided by the psylloids. During gall induction, susceptible plants use a flexible, short-term strategy responding to the stress inflicted by the psylloid. That short-term strategy involves mobilization of energy and other metabolites to the injured site as a reparative effort to heal the injury, which the inducing insect exploits the plant for its nourishment. This becomes clear when we recognize that the plant returns to its normal physiology the moment the insect ceases to feed (Rohfritsch Citation1971, p. 1992). Genetic factors play a role in controlling the shape of the gall, coordinated by the innate correlating morphogenetic factors that normally operate in the plant (Miller and Raman Citation2019; Raman Citation2021).
Mouthparts, feeding biology, and nutritional physiology of the Psylloidea
Psylloidea have long, at least 500 μm, piercing-and-sucking mouthparts, made of two pairs of interlocking stylets (also referred as ‘stylet,’‘stylet bundle’), which are the modified mandibles and maxillae (). Their mouthparts can function for both salivation and ingestion of plant sap. The salivary glands consist of one principal and one accessory gland (Sharma et al. Citation2014b, Citation2015b). Psylloid saliva includes cell-digesting enzymes: cellulases, amylases, and pectinases, which facilitate delicate stylet movement within plant tissue, inflicting minimal physical damage. Salivary dehydrogenases polymerize phenolics sequestered in the plant-cell apoplast and evoke induced-defence responses (Sharma et al. Citation2013, Citation2014b, Citation2015b). The known gall-inducing Psylloidea possess longer stylet bundles than those of the free-living and lerp-forming species. For example, the stylet bundle of a gall-inducing species G. (Synglycaspis) sp. A is c. 950 μm long, whereas its adult body length is 4.6–4.8 mm. In a free-living species, viz., Ctenarytaina eucalypti, the stylet bundle is 600 μm, whereas the body length is 2.5–2.8 mm. In a lerp-forming, unnamed species of Glycaspis, it is 850 μm, whereas the body length is 3.2–3.6 mm. During the early stages of gall development, the inhabiting first- and second-stage immatures, and occasionally the third-stage immature feed on undifferentiated parenchyma made of thin cell walls and rich, dynamic cytoplasm. Feeding activity of the third- and fourth-stage immatures usually shifts to phloem ducts (Sharma et al. Citation2015a; Miller and Raman Citation2019).
Figure 5. Sensilla on the mouthparts of an unnamed species of Glycaspis on Eucalyptus sideroxylon (sensilla on extended labium, lb; stylet bundle, sb) [bar = 1 μm] (Source: Sharma et al. Citation2013).
![Figure 5. Sensilla on the mouthparts of an unnamed species of Glycaspis on Eucalyptus sideroxylon (sensilla on extended labium, lb; stylet bundle, sb) [bar = 1 μm] (Source: Sharma et al. Citation2013).](/cms/asset/0325ce12-6626-4988-a59a-b607c3b7736c/tjpi_a_2065371_f0005_ob.jpg)
Among the biologies of different gall-inducing Psylloidea known today, gall induction by A. cistellata is noticeably different from the better known Triozidae and other Aphalaridae, although finer details of the feeding biology of A. cistellata are yet to be clarified. Simultaneous feeding by several first-instar immatures of A. cistellata stimulates gall induction by translocating a ‘stimulus’ of unknown chemistry, invariably over a distance of 3–6 mm (Burckhardt Citation2005a). Gall induction by A. cistellata is vaguely similar to the gall-induction behavior of Adelges cooleyi (Aphidoidea: Adelgidae) on the vegetative shoot buds of Picea glauca × P. engelmannii hybrids (Pinaceae) in North America (Havill and Foottit Citation2007; Holman Citation2009). In the A. cooleyi – Picea glauca × P. engelmannii system, a dose-dependent chemical stimulus translocates over a ‘long’ distance from the point where the gall-founding female first settles and commences feeding (Sopow et al. Citation2003). The similarity between the shoot galls induced by A. cistellata on M. indica and A. cooleyi on P. glauca × P. engelmannii needs to be treated as ‘apparent,’ because, in the galls arising on the vegetative axillary shoot buds of M. indica, the first-instar immatures of A. cistellata induce the gall, whereas, in the vegetative axillary shoot-bud galls of Picea glauca × P. engelmannii hybrids, adult females of A. cooleyi induce galls. The distance between the site of gall-inducing agent(s) and the site where galls manifest is greater in A. cooleyi – P. glauca × P. engelmannii system than what is known in A. cistellata – M. indica system.
Feeding action of the first-instar immatures of gall-inducing Aphalaridae (e.g. Glycaspis (Synglycaspis) sp. A) plasmolyzes the cytoplasm of the attacked epidermal and palisade cells in plant tissues in less than the first six h of attack (). The cells around the stylet rapidly respond (i.e. within the first six h of attack) to the psylloid by presenting hyaline cytoplasm and unusually thick horizontal walls. Apoplastic nutrient movement occurs in these cells (Sharma et al. Citation2015a, Citation2015b), which include large intercellular spaces, reinforcing the production of reactive oxygen species (Sattelmacher Citation2001). The feeding action of gall-inducing Psylloidea induces subcellular alterations of relatively low intensity (Sharma et al. Citation2015a, Citation2015b) when compared with those cells attacked by free-living taxa. In addition to the mechanical damage caused by the insertion of the stylet, the discharged salivary chemicals (especially the proteins) inflict a chemical injury, activating a cascade of transcriptomic changes as shown in the feeding sites of D. citri (De Vos et al. Citation2005). In the Psylloidea, the salivary-enzyme profiles are complex. For instance, in the free-living species C. eucalypti, 60–65 kD proteins such as oxidoreductases, dehydrogenases, and esterases and 58 kD proteins such as trehalases, amylases, lipases, and cytochrome P–450 occur (Sharma et al. Citation2015b). Enabled with such a wide range of enzymatic proteins, the amylases help the feeding Psylloidea to pierce host-plant tissues; the oxidoreductases, dehydrogenases, and esterases detoxify host-plant allelochemicals (Sharma et al. Citation2015b). In the gall-inducing Psylloidea, the role of detoxifying enzymes is not yet adequately clarified: however, cell-degrading enzymes, amylases, proteases, and lipases have been demonstrated in the saliva of the gall-inducing T. jambolanae (Rajadurai et al. Citation1990). On the other hand, the galls induced by P. depressa on F. glomerata show the intense activity of amylases and invertases compared with normal leaves. Although the saliva of gall-inducing Psylloidea include both degrading and detoxifying enzymes, the association of the spherical pouch gall-inducing P. tuberculatum on a latex-producing A. scholaris is intriguing because A. scholaris includes secondary metabolites such as picrinine, schloaricine, and many triterpenes that are generally toxic to any feeding arthropod (Waliwitiya et al. Citation2012), leaving the question how the immatures of these Psylloidea are able to detoxify such secondary metabolites and feed on these plant materials. Similar questions arise in the interactions between gall-inducing psylloids on various Anacardiaceae (e.g. Calophya on Schinus, Apsylla on Mangifera) that usually include a wide suite of secondary metabolic compounds, such as alkyl- and alkenyl phenols.
Figure 6. (a) Camera-lucida drawing of a portion of the nymphal bed of an early immature of the gall-inducing Glycaspis (Synglycaspis) sp. A, on Eucalyptus macrorhyncha. (nutritive cells, nc; vascular trace, vt; stylet track, st; stomatal aperture, s; proliferating epidermal cells, pe; neoformed meristem, nm; dividing cells, circled area) [bar = 100 μm]. (b) submicroscopic changes in E. macrorhyncha leaf (cross-sectional view) due to feeding by Glycaspis (Synglycaspis) A: (endosome, es; chloroplast, ch; secondary metabolic inclusion, sm; autophagic vacuole, av; cell junctions, cj; mitochondrion, m; desmosomal condensation, dc; oil inclusion, oi; dissolved middle lamella, ⇔; stylet track, circled area) [bar = 1 μm] (Source: Sharma et al. Citation2015a).
![Figure 6. (a) Camera-lucida drawing of a portion of the nymphal bed of an early immature of the gall-inducing Glycaspis (Synglycaspis) sp. A, on Eucalyptus macrorhyncha. (nutritive cells, nc; vascular trace, vt; stylet track, st; stomatal aperture, s; proliferating epidermal cells, pe; neoformed meristem, nm; dividing cells, circled area) [bar = 100 μm]. (b) submicroscopic changes in E. macrorhyncha leaf (cross-sectional view) due to feeding by Glycaspis (Synglycaspis) A: (endosome, es; chloroplast, ch; secondary metabolic inclusion, sm; autophagic vacuole, av; cell junctions, cj; mitochondrion, m; desmosomal condensation, dc; oil inclusion, oi; dissolved middle lamella, ⇔; stylet track, circled area) [bar = 1 μm] (Source: Sharma et al. Citation2015a).](/cms/asset/f112c2a9-6a4f-4743-912d-6ea109b0fa7b/tjpi_a_2065371_f0006_ob.jpg)
Leaves of E. macrorhyncha hosting Glycaspis (Synglycaspis) sp. A show a high δ13C–δ15N ratio. An increase in δ13C and a decline in δ15N occurs consequent to Glycaspis (Synglycaspis) sp. A’s feeding action indicating that the populations of this taxon utilize carbon-based materials and the mobile nitrogenous material that occur in relatively high concentrations at the feeding sites (Sharma et al. Citation2015c). During gall induction, rapid mobilization of nutrients occurs from the normally biosynthesizing plant parts to the injured – feeding – sites. Consistent mechanical wounding by the feeding immature is restricted to gall sites only, but the stress caused by the mechanical injury spreads centrifugally into tissues in the vicinity (Sharma et al. Citation2015a). Infestation by the Hemiptera, in general, intensifies amino-acid biosynthesis in plant tissues via decarboxylation of amino acids as an effort to ward off other invading plant-feeding insects (Raman Citation2012; Dubey et al. Citation2013). High levels of essential amino acids, viz., leucine, isoleucine, and threonine are utilized by the first- and second-stage immatures of Glycaspis (Synglycaspis) sp. A and these essential amino acids decline in levels as the immatures grow into later stages (Sharma et al. Citation2015c). A positive correlation between total non-structural carbohydrates (TNC), amino acids, and growth rates of the inhabiting immatures have been demonstrated, which indicates greater utilization of TNCs and amino acids by Glycaspis (Synglycaspis) sp. A (Sharma et al. Citation2015c) (). Young and mature leaves of E. macrorhyncha hosting Glycaspis (Synglycaspis) sp. A show extensive variations in the levels of complex lipids and sterols. Further, the detected high levels of invertase and amylase in galls induced by P. depressa on the leaves of F. glomerata reinforce the gall’s function as a nutrient sink (Sharma et al. Citation2015c) due to the established role of hydrolyzing enzymes in plant cell-wall expansion and subcellular carbon partitioning (Rehill and Schultz Citation2003).
Figure 7. Isotopic carbon and nitrogen values in the normal and gall-bearing leaves of Eucalyptus macrorhyncha hosting Glycaspis (Synglycaspis) sp. A in Australia. (a) δ13C values in uninfested (U) and infested [1C–5C: five developmental stages of Glycaspis (Synglycaspis)]. (b) δ15N values in uninfested (U) and infested [1N–5N: five developmental stages of Glycaspis (Synglycaspis)]. Error bars are shown along the y axes of the graphs (source: Sharma et al. Citation2015c).
![Figure 7. Isotopic carbon and nitrogen values in the normal and gall-bearing leaves of Eucalyptus macrorhyncha hosting Glycaspis (Synglycaspis) sp. A in Australia. (a) δ13C values in uninfested (U) and infested [1C–5C: five developmental stages of Glycaspis (Synglycaspis)]. (b) δ15N values in uninfested (U) and infested [1N–5N: five developmental stages of Glycaspis (Synglycaspis)]. Error bars are shown along the y axes of the graphs (source: Sharma et al. Citation2015c).](/cms/asset/af920c24-ed31-45b5-bdaf-8cc78837aaf6/tjpi_a_2065371_f0007_ob.jpg)
A majority of the free-living Psylloidea extract carbohydrates and fatty acids from the phloem sap, although the phloem diet is known to be generally deficient in essential amino acids, vitamins, and lipids. Symbiotic microorganisms (e.g. Carsonella rudii, and other Enterobacteriaceae [Proteobacteria]) occurring in the bacteriome of the Psylloidea compensate for the deficiency of nitrogenous materials (Nakabachi et al. Citation2006). The nutritional ecology of gall-inducing Psylloidea is strikingly different from that of the free-living Psylloidea. For instance, the feeding pressure imposed by a free-living and gregariously feeding, unnamed species of Cardiaspina on a species of Eucalyptus results in the rapid mobilization of nutrients from uninfested to infested parts of the host plant (White Citation1970). In contrast, the feeding pressure imposed by solitarily occurring, gall-inducing T. jambolanae stresses leaf tissues of S. cumini creating ‘nutrient sinks’ in galls to extract concentrated levels of nutrients (Raman Citation1991; Mani and Raman Citation1994). Galls are ‘nutrient sinks’ for carbon-based and nitrogenous nutrients translocated from normal plant parts to galls (Kirst and Rapp Citation1974; Raman et al. Citation2006). Once the gall is initiated, the hosting plant pumps minerals and nutrients to repair and heal the injured site. The nutrients, however, are utilized by the inhabiting immature psylloid and the gall turns into a site supplying nutrients to the actively feeding immature (or, immatures, as the case may be) (Raman et al. Citation2006).
Galls as the site of concentrated nutrients also attract non-gall-inducing Psylloidea, which occupy the space in galls induced by other arthropods during certain portions of their life cycles to utilize the readily available nutrients for a longer period than what will be available in ungalled leaves. For example, a few unnamed species of the Psylloidea and Aphidoidea feed on leaves of Rhamnus cathartica (Rhamnaceae) that bear galls induced by Trichochermes walkeri (Triozidae) (McLean Citation1994; Burckhardt Citation2005a). Populations of Pachypsylla cohabitans inhabit galls induced by P. celtidismamma arising on the leaves of Celtis occidentalis, utilize the nutrients available in galls, and in the process negatively affect the performance of the immatures of the inducing P. celtidismamma (Yang et al. Citation2001). Occupation by inquilinous Hymenoptera and consequent modification of gall tissues by their feeding are amply demonstrated in the galls induced by Cynipidae in North America (Brooks and Shorthouse Citation1998).
Association between gall-inducing Psylloidea and plants
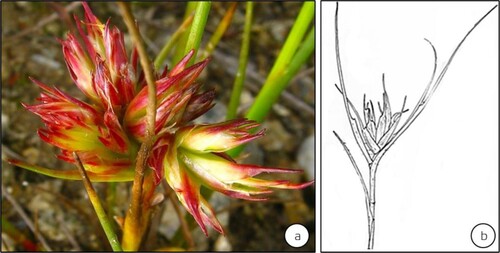
A majority of the Psylloidea are associated with dicotyledons. Very few gymnosperms are known hosting the Psylloidea (Burckhardt Citation2005b; Raman et al. Citation2005; Yang and Raman Citation2007): e.g. two species of Ehrendorferiana on Austrocedrus and Fitzroya (both Cupressaceae) in Chile and Trioza colorata and T. dacrydii on (Podocarpaceae) in New Zealand (Burckhardt Citation2005b). Fewer than 2% of gall-inducing Psylloidea live on monocotyledons: e.g. Juncaceae, Cyperaceae (Ouvrard et al. Citation2015). Particularly the species of Livia (Liviidae) are associated with species of Juncaceae (Hodkinson and Bird Citation2000; Burckhardt Citation2005a). For instance, L. junci induces organoid galls on the shoots of Juncus articulatus (Juncaceae)] (Schmidt Citation1966; Schmidt and Meyer Citation1966; Hodkinson Citation1986; Meyer Citation1987) (). A study in Portugal suggests that L. junci vectors a phytoplasma that induces the witches-broom disease on Juncus fontanesii (Juncaceae), previously indicated as ‘L. junci-induced galls’ (Jarzembowski et al. Citation2013). A vast majority of gall-inducing Psylloidea are associated with species of Myrtaceae, and Asteraceae (Burckhardt Citation2005a; Ouvrard et al. Citation2015). Gall-inducing taxa belonging to the Triozidae, Aphalaridae, and Calophyidae are generally associated with species of Magnoliales, Myrtales, Sapindales, and Malvales (Burckhardt Citation2005a). The Asteraceae (Campanulid/Asterid II: Asterales) host more of gall-inducing Aphalaridae and a few of the gall-inducing Triozidae (Burckhardt and Mifsud Citation2003; Burckhardt Citation2005a). The Asteraceae-associated Triozidae induce galls on the floral capitula, in addition to inducing leaf rolls and bean-shaped galls, whereas the members of monophyletic Calophyidae induce pit, cone-like, disk-shaped, spherical, and beutel galls on various species of Anacardiaceae (Malvids/Rosid II: Sapindales) (Burckhardt Citation2005a). A majority of the Oriental gall-inducing Psylloidea are tied to tree taxa of the families classified under the Eurosids (Yang and Raman Citation2007; Yang et al. Citation2009, Citation2013).
Figure 8. Galls modifying the vegetative shoot buds of Juncus articulatus (Juncaceae) induced by Livia junci distributed in Continental Europe (sensu Epstein Citation2014). Gall development inhibits leaf expansion and the elongation of internodal segments. (Source: Jiří Kameníček: Biolib.cz) (b) A line sketch of the gall (Source: Schmidt and Meyer Citation1966). (No scale bars are indicated by respective authors).
The gall-inducing Psylloidea and those of the lerp-forming and free-living guilds are narrowly specific to plants. A vast majority of the gall-inducing Psylloidea, similar to the gall-inducing Cecidomyiidae (Diptera) and Cynipidae (Hymenoptera), are highly selective of host plants and remain committed to them (Raman Citation2009). Different species of Schinus (Anacardiaceae) host more than one species of different genera of gall-inducing Psylloidea, such as Tainarys schini and T. maculipectus (Aphalaridae), Calophya clausa, C. hermicitae, and C. mammifex (Calophyidae) on different species of Schinus (Burckhardt and Bassett Citation2000). Trioza fletcheri minor (Triozidae) induce galls of nearly identical morphologies on different species of Terminalia (T. tomentosa, T. arjuna, T. catappa, T. paniculata, and T. tomentosa x T. arjuna, Combretaceae) in the Indian subcontinent (Mathur Citation1975; Raman et al. Citation1996; Burckhardt et al. Citation2018). Several species of the same genus of gall-inducing Psylloidea also, although infrequently, occur on the same host. For example, three gall-inducing Calophya, viz., C. mammifex, C. rubra, and C. scrobicola occur on Schinus polygamus (Anacardiaceae) in Central Chile; similarly, C. catillicola, C. gallifex, and C. orbicola on S. fasciculatus in Argentina (Burckhardt and Bassett Citation2000; Burckhardt Citation2005a). Burckhardt (Citation2005) attributes this pattern to efficient utilization of food resources and indicates that this is an adaptive strategy to evade predators and parasitoids by co-existing in and utilizing the same space. Such a behavior suggests a coevolutionary thread between the Psylloidea and their host plants (Hodkinson Citation2009; Burckhardt et al. Citation2014; Ouvrard et al. Citation2015). An apparent discontinuity of the association of the Psylloidea and their host plant is the probable central reason for the patchy distribution of the Psylloidea.
Psylloidea-induced galls occur mostly on leaves, although a small number develops on flowers and stems. A specificity to sites usually manifests among the gall-inducing Psylloidea. For example, more than 20 gall-inducing species of Glycaspis (Synglycaspis) in Australia induce galls only on leaves of different species of Eucalyptus (Sharma et al. Citation2015a), whereas the species of Pachypsylla induce galls on buds, twigs, and leaf blades of species of Celtis in North America (Hodkinson Citation1984; Yang Citation1995; Burckhardt Citation2005a). Pseudophacopteron tuberculatum (earlier Pauropsylla tuberculata) traditionally known to induce galls on the leaves of A. scholaris are being presently found to infest and induce galls on the flowers of A. scholaris (Chauhan et al. Citation2020). Reports of Trioza fletcheri minori (Triozidae) inducing galls both on the leaves (Mathur Citation1975) and flowers (Sokhi and Kapil Citation1984, p. 1985) of T. arjuna exist. One widely indicated generalization with gall-inducing insects is that they are highly host and site-specific (Raman Citation1996). Therefore, it is highly likely that gall-inducing psylloids infesting two plant organs of the same plant belong to a cryptic species complex rather than being the same species. Based on ecological, behavioral, and molecular studies, cryptic species have been indicated (Dhileepan et al. Citation2017) and determined among the Cecidomyiidae (Dorchin et al. Citation2015; Fitzpatrick et al. Citation2013). An approach integrating morphological and molecular tools with ecological and behavioral studies is currently necessary to determine the taxonomy of the Psylloidea that infest different plant organs simultaneously. Yang (Citation1995) suggests that Pachypsylla species that attack the same plant organ form a monophyletic group, whereas the phylogenetic relationships of Psylloidea inducing galls on different plant organs follow an evolutionary sequence of gall position shifting from leaf to petiole to bud to the branch.
How and why the gall-inducing Psylloidea are tied to specific plants remains to be clarified. Gall-inducing Hemiptera bear specialized sensory appendages, that can be different from non-gall inducing Hemiptera, on their antennae, mouthparts, legs, and abdomen enabling them to select the right site on the right plant for oviposition and resulting in gall induction (Sharma et al. Citation2015a). For example, the immatures of Glycaspis (Synglycaspis) sp. A bear unequal apical antennal bristles that are olfactory in function. Besides them, specialized terminal antennal bristles enable the gall-inducing Psylloidea to select the oviposition site. The gall-inducing species of G. (Synglycaspis) include unique, cupola-shaped rhinaria on each of the flagellar segments 2, 4, 6, and 7 in subapical positions. These rhinaria act as chemoreceptors and facilitate the adults to determine and choose the most-suitable host in a heterogeneous natural environment (Sharma et al. Citation2015a) (). Further, gall induction necessarily requires specific molecular signals that can be activated only by a specific species of Psylloidea endowed with specific salivary proteins. The natural habitat of E. macrorhyncha – the preferred host plant of Glycaspis (Synglycaspis) sp. A – includes closely co-occurring populations of E. rossii and E. dives. Ian Brooker (Citation2000) treats these three taxa under ‘Eucalyptus subgen. Eucalyptus + Primitiva.’ The pouch gall-inducing Glycaspis (Synglycaspis) sp. A never occurs on either E. rossii or E. dives. Significant levels of sitosterol, ergosterol, and stigmasterol were detected in young leaves of E. macrorhyncha, susceptible to gall induction by Glycaspis (Synglycaspis) sp. A. Moreover, sitosterol and three other undetermined sterols of molecular weights 354, 382, and 440 g mol–1 occurred maximally only in the young leaves of E. macrorhyncha (), that were distinctly absent in E. dives and E. rossii leaves of comparable age. A unique ‘440 g mol–1 sterol’ was found as the key in the selection of E. macrorhyncha by Glycaspis (Synglycaspis) sp. A, because of its high levels in the young, gall-susceptible leaves of E. macrorhyncha () (Sharma et al. Citation2016). The level of the sterol of 440.3 µg molecular weight was high in young leaves of E. macrorhyncha, the colonization site by the gall-inducing first-instar immatures of Glycaspis (Synglycaspis) sp. A (). Moreover, the levels of ergosterol, a sterol with a Δ5,7 nucleus (Festucci-Buselli et al. Citation2008) occurred maximally in young galls of E. macrorhyncha occupied by the first- and second-stage immatures of Glycaspis (Synglycaspis) sp. A reinforcing that ergosterol plays a key role in the successful metamorphosis of subsequent immature stages of Glycaspis (Synglycaspis) sp. A (Sharma et al. Citation2016). This clarifies the choice of E. macrorhyncha by Glycaspis (Synglycaspis) sp. A. in a mixed community of E. macrorhyncha, E. dives, and E. rossii, underpinning that the gall-inducing Psylloidea choose specific plants to meet their sterol requirements (Sharma and Raman Citation2017). Variation in the nature and types of lipids and sterols is one strong indicator of the host fidelity in interactions between gall-inducing arthropods in general, applicable to the gall-inducing Psylloidea as well and their host plants (Behmer and Nes Citation2003; Miller and Raman Citation2019).
Figure 9. Antennae on adult female of gall-inducing Glycaspis (Synglycaspis) sp. A that specifically oviposit on E. macrorhyncha leaves. (a) close up of the antennae (trichoid sensilla on adult antenna, ts) [bar = 50 μm]. (b) close up of cupola shaped rhinarium (basiconic sensillum, bs; rhinarium, rh) [bar = 10 μm]. (Source: Sharma et al. Citation2015a).
![Figure 9. Antennae on adult female of gall-inducing Glycaspis (Synglycaspis) sp. A that specifically oviposit on E. macrorhyncha leaves. (a) close up of the antennae (trichoid sensilla on adult antenna, ts) [bar = 50 μm]. (b) close up of cupola shaped rhinarium (basiconic sensillum, bs; rhinarium, rh) [bar = 10 μm]. (Source: Sharma et al. Citation2015a).](/cms/asset/393b4701-b9b2-426d-ba54-2cdce6a85869/tjpi_a_2065371_f0009_ob.jpg)
Figure 10. Sterols in young (▪) and mature (
 ) predominant in young leaves of E. macrorhyncha, is the potential sterol that regulates the specificity of the Glycaspis (Synglycaspis) sp. A. x-axis – molecular weights of sterols; y-axis – mole % of sterols. Vertical bar represents the SE value. (Source: Sharma et al. Citation2016).
) predominant in young leaves of E. macrorhyncha, is the potential sterol that regulates the specificity of the Glycaspis (Synglycaspis) sp. A. x-axis – molecular weights of sterols; y-axis – mole % of sterols. Vertical bar represents the SE value. (Source: Sharma et al. Citation2016).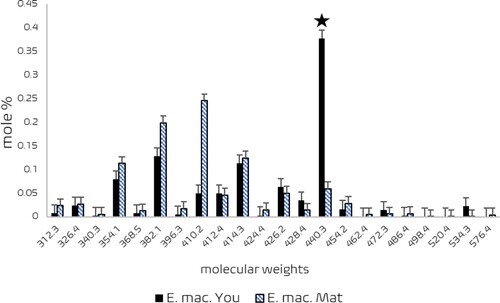
Table 1. Sterols in normal and developing stages of galls induced by Glycaspis (Synglycaspis) sp. A on the leaves of Eucalyptus macrorhyncha (adapted from Sharma et al. Citation2015c).
Concluding remarks
With more than 4000 described species, the Psylloidea include diverse gall-inducing species. Unlike the Hymenoptera, which induce galls by inserting eggs into plant tissues, the known Psylloidea predominately induce galls by the feeding action of the first-instar immatures, except T. flavipennis in Western Europe. Feeding biology of the Psylloidea is closely similar to that of the gall-inducing Cecidomyiidae (Rohfritsch Citation1992). The role of PGRs (plant growth regulators) and consequent gene manipulation due to their action have been widely indicated in galls of arthropod origin, including those by the Psylloidea (Bailey et al. Citation2015). Greater levels of secondary metabolites such as the phenols and terpenes have been determined in the interactions between gall-inducing Psylloidea and flowering plants as critical physiological alterations (Dsouza and Ravishankar Citation2014; Agudelo et al. Citation2018). The role and effect of effector proteins released from the salivary glands are currently widely considered in gall induction by the Cecidomyiidae suggesting the function of a novel class of effector proteins in disabling the innate defence mechanism of the host plants (Zhao et al. Citation2015; Shih et al. Citation2018). However, this aspect has not been clarified in the specific context of gall-inducing Psylloidea.
Another challenging issue in the context of gall induction by the Psylloidea (for that matter by every other known gall-inducing arthropod) is how galls are induced. The critical stage will be to determine the metabolic changes that occur during early two phases of gall induction, viz., initiation and triggering of new differentiation pathways. These phases necessarily involve the activation of molecular messengers responding to the signals by the host plant arising due to insect action. The widely promoted idea of the key role of PGRs in gall induction needs to be viewed with extreme caution, since the endogenous PGRs are synthesized during the occupation of a gall by either the late second or the third stage immature only. The elementary reason being the production of PGRs at a greater intensity in galls necessarily requires a trigger, which should be either one or a cluster of high-molecular-weight proteins. An interplay of abscisic acid and ethylene along with auxins and cytokinins manifests in about-to-senesce galls (Raman Citation2021).
From the available, but disjointed, literature on gall-inducing Psylloidea and extrapolating what we know in other gall-inducing insect groups, e.g. the Cecidomyiidae and the Cynipidae (Favery et al. Citation2020), we can propose that, in general, during gall initiation, the gall-inducing psylloids overcome the innate immunity of the plant and provoke a susceptible response. The tricky question is whether the gall is a defence response of the plant to limit the localized irritation and damage by the psylloid or whether a symbiotic relationship occurs between the Psylloidea and chosen plants. Presently, no firm answer to this question is available, since different studies have supplied answers in support of both contentions. Osmotic change-related metabolic pressure builds up when gall-inducing psylloids attack plant cells activating a train of events in the immediate environment of those plant cells. The following sequence of events includes alterations in gas exchange and synthesis of PGRs. Gall induction involves the vigorous uptake of oxygen from the reactive-oxygen species generated consequent to insect attack, which in turn stimulates auxin synthesis and activity. Osmotic stress alters the electrical properties of the plasma membrane and impacts on IAA synthesis and activity, which, alters the H+ transport. From what we know thus far, it is possible to infer that the plant actively mobilizes nutrients to mitigate the stress and repair the injury right from the time of attack by the psylloid. The gall-inducing psylloid, incidentally, utilizes the nutrients mobilized at this site to its advantage. A summary, nonetheless, would be that the metabolic changes consequent to changes in the vacuolar pH in the metaplasied cell produces certain ‘novel’ chemicals (of unknown details presently), which diffuse from the dedifferentiated metaplasied cell into its immediate neighborhood. But we need to recognize here that the dissipation of these novel chemicals is localized to a narrow window in the plant organ, viz., the gall, because of their obviously weak nature. This necessarily means that the effect of these novel chemicals does not spread throughout either the involved organ or the plant, explaining why galls and their effects are highly localized.
The inhabiting immatures of the Psylloidea are fascinatingly adapted to the design of galls they provoke. How the development of the gall synchronizes both morphologically and physiologically with that of the inducing agent is clearly illustrated in the study of P. lentiginosum that induces two-tier galls on the leaflets of G. pinnata (Raman Citation1987). The shape of galls is generally consistent in some of the families of the Psylloidea, for instance, Baccharis (Asteraceae) hosts c. 25 species of Triozidae that induce similar galls on the floral capitula in the Americas, whereas that behavior varies considerably in other taxa; for example, species of Calophya induce galls of varied morphologies on different species of Schinus, also in the Americas: e.g. Calophya schini (Calophyidae) induce open-pit gall, C. andina induce nipple or conical galls, C. orbicola induce disc-shaped galls. Similar to the gall-inducing Cecidomyiidae and Cynipidae, the gall-inducing Psylloidea are host and site-specific, although a few exceptions exist. The fidelity of gall induction in the Psylloidea is determined by sterols, as demonstrated in Glycaspis (Synglycaspis) sp. A–E. macrorhyncha interactions.
The free-living Psylloidea are better adapted to different climatic regions, whereas the gall-inducing Psylloidea show conservative behavior in terms of geographical distribution. For instance, in Australia, various species of Eucalyptus host several species of free-living, lerp-forming, and gall-inducing Psylloidea. However, within the same biogeographical realm, the free-living Psylloidea display a greater number of generations than the gall-inducing taxa. Life-history performances are known of gall-inducing Psylloidea inducing a variety of galls, however, aspects of their association with host plants are less known. Several factors of nutritional physiology of gall-inducing Psylloidea are also less known presently compared with that of the free-living, species. Since most of the gall-inducing Psylloidea are solitary inhabitants within a gall, their nutritional requirement varies starkly from the free-living Psylloidea. The gall-inducing Psylloidea manipulate the synthesis of a greater quantity of sugars in the galls and utilize them, as against the free-living Psylloidea. A better comprehension of the association and commitment between gall-inducing Psylloidea and their host plants is needed presently. Moreover, several aspects of the nutritional physiology of Psylloidea and especially that of the gall-inducing Psylloidea remain to be clarified.
Author contributions
AR and AS developed the concept and wrote the paper cooperatively making equal contributions.
Acknowledgement
We thank Igor Malenowský (Masaryk University, Brno, Czech Republic) and S. Raghu (CSIRO, Brisbane, Australia) for insightful comments on the pre-final draft of this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Anamika Sharma
Anamika Sharma, assistant professor of entomology at Florida A & M University, Florida, USA, studies the aspects of insect-plant interactions, biological control, and integrated pest management. Her publications include peer-reviewed and outreach articles on these subjects.
Anantanarayanan Raman
Anantanarayanan Raman, is a Senior Visiting Scientist with CSIRO (Floreat Park, Perth) and an adjunct professor of ecological agriculture and sustainable land management sociology at Charles Sturt University (Orange, NSW). He writes on both the chemical and molecular ecology of arthropod--plant interactions with special reference to the interactions between gall-inducing arthropods and their host plants. His published books and professional-journal articles on this subject are many.
References
- Agudelo I, Cogoi L, Filip R, Kuzmanich N, Wagner ML, Ricco RA. 2018. Anatomy, histochemistry, and comparative analysis of hydroxycinnamic derivatives in healthy leaves and galls induced by Baccharopelma spp. (Hemiptera: Psyllidae) in Baccharis spicata (Lam) Baill. (Asteraceae). Biochem Syst Ecol. 77:22–30.
- Albert S, Padhar A, Gandhi D, Nityanand P. 2011. Morphological, anatomical, and biochemical studies on the foliar galls of Alstonia scholaris (Apocynaceae). Rev Bras Bot. 34:343–358.
- Bailey S, Percy DM, Hefer CA, Cronk QCB. 2015. The transcriptional landscape of insect galls: psyllid (Hemiptera) gall formation in Hawaiian Metrosideros polymorpha (Myrtaceae). BMC Genom. 16:943. doi:10.1186/s12864-015-2109-9.
- Balakrishna P. Raman A. 1992. Cecidogenesis of leaf galls of Strychnos nux-vomica (Loganiaceae) induced by the jumping plant louse species Diaphorina truncata (Homoptera: Psylloidea: Psyllidae). Entomol Gener. 17:285–295.
- Barnett MJ, Solow-Cordero DE, Long SR. 2019. A high-throughput system to identify inhibitors of Candidatus Liberibacter asiaticus transcription regulators. Proc Nat Acad Sci. USA. 116:18009–18014.
- Batta Y, Burckhardt D. 2018. Taxonomy and biology of Pauropsylla buxtoni comb. nov. (Hemiptera: Psylloidea) on Ficus carica (Moraceae). J Entomol Res Soc. 20:39–52.
- Behmer ST, Nes WD. 2003. Insect sterol nutrition and physiology: a global overview. Adv Insect Physiol. 31:1–72.
- Beisler JM, Baker GT. 1992. Pachypsylla celtidismamma (Fletcher) (Homoptera: Psyllidae): morphology and histology of its gall and ultrastructure of its adult and nymphal sensilla. Missis Agric Forest Exp Sta (USA). Tech Bull. 179:1–27.
- Bi G, Zhou Z, Wang W, Li L, Rao S, Wu Y, Zhang X, Menke FLH, Chen S, Zhou J-M. 2018. Receptor-like cytoplasmic kinases directly link diverse pattern recognition receptors to the activation of mitogen-activated protein kinase cascades in Arabidopsis. Plant Cell. 30:1543–1561.
- Bouyjou B, Nguyen T-X. 1974. Observations sur la morphogenèse et la structure de la galle de Trioza alacris Flor (Homoptera—Psyllidae) sur Laurus nobilis L. Marcellia. 38:49–56.
- Brennan EB, Gill RJ, Hrusa GF, Weinbaum SA. 1999. First record of Glycaspis brimblecombei (Moore) (Homoptera: Psyllidae) in North America: initial observations and predator associations of a potentially serious new pest of Eucalyptus in California. Pan-Pac Entomol. 75:55–57.
- Brooker MIH. 2000. A new classification of the genus Eucalyptus L’Hér. (Myrtaceae). Aust Syst Bot. 13:79–148.
- Brooks SE, Shorthouse JD. 1998. Developmental morphology of stem galls of Diplolepis nodulosa (Hymenoptera: Cynipidae) and those modified by the inquiline Periclistus pirata (Hymenoptera: Cynipidae) on Rosa blanda (Rosaceae). Can J Bot. 76:365–381.
- Brożek J, Mróz E, Wylężek D, Depa L, Węgierek P. 2015. The structure of extremely long mouthparts in the aphid genus Stomaphis Walker (Hemiptera: Sternorrhyncha: Aphididae). Zoomorph. 134:431–445.
- Burckhardt D. 2005a. Biology, ecology, and evolution of gall-inducing psyllids (Hemiptera: Psylloidea). In: Raman A, Schaefer CW, Withers TM, editor. Biology, ecology, and evolution of gall-inducing arthropods. Enfield: Science Publishers, Inc.; p. 143–157.
- Burckhardt D. 2005b. Ehrendorferiana, a new genus of Neotropical jumping plant lice (Insecta: Hemiptera: Psylloidea) associated with conifers (Cupressaceae). Org Divers Evol. 4:317–319.
- Burckhardt D. 2009. Fauna Europaea: Psylloidea; [accessed 26 July 2020]. http://www.fauna-eu.org.
- Burckhardt D, Bassett Y. 2000. The jumping plant-lice (Hemiptera, Psylloidea) associated with Schinus (Anacardiaceae): systematics, biogeography and host plant relationships. J Nat Hist. 34:57–155.
- Burckhardt D, Drohojowska J, Giliomee JH. 2012. Trioza bullatae sp. n. (Hemiptera: Psylloidea), a new gall-inducing pest on black stinkwood (Ocotea bullata, Lauraceae) in South Africa. Afr Entomol. 20:144–149.
- Burckhardt D, Espirito-Santo MM, Fernandes GW, Malénovsky I. 2004. Gall-inducing jumping plant-lice of the Neotropical genus Baccharopelma (Hemiptera, Psylloidea) associated with Baccharis (Asteraceae). J Nat Hist. 38:2051–2071.
- Burckhardt D, Mifsud D. 2003. Jumping plant-lice of the Paurocephalinae (Insecta, Hemiptera, Psylloidea): systematics and phylogeny. Contrib Nat Hist, Bern. 2:3–34.
- Burckhardt D, Ouvrard D, de Queiroz DL, Percy D. 2014. Psyllid host-plants (Hemiptera: Psylloidea): resolving a semantic problem. Fla Entomol. 97:242–246.
- Burckhardt D, Ouvrard D, Percy DM. 2021. An updated classification of the jumping plant-lice (Hemiptera: Psylloidea) integrating molecular and morphological evidence. Eur J Taxon. 736:137–182.
- Burckhardt D, Sharma A, Raman A. 2018. Checklist and comments on the jumping plant-lice (Hemiptera: Psylloidea) from the Indian subcontinent. Zootaxa. 4457:1–38.
- Carango P. 1988. Induction of a 58,000 Dalton protein during goldenrod gall formation. Biochem Biophys Res Comm. 152:1348–1358.
- Carneiro RGS, Isaias RMS. 2015a. Cytological cycles and fates in Psidium myrtoides are altered towards new cell metabolism and functionalities by the galling activity of Nothotrioza myrtoidis. Protoplasma. 252:637–646.
- Carneiro RGS, Oliveira DC, Isaias RMS. 2014. Developmental anatomy and immunocytochemistry reveal the neo-ontogenesis of the leaf tissues of Psidium myrtoides (Myrtaceae) towards the globoid galls of Nothotrioza myrtoidis (Triozidae). Plant Cell Rep. 33:2093–2106.
- Carneiro RGS, Pacheco P, Isaias RMS. 2015b. Could the extended phenotype extend to the cellular and subcellular levels in insect-induced galls? Plos ONE. 10. doi:10.1371/journal.pone.0129331.
- Chauhan S, Singh N, Chauhan SVS. 2020. Morphological studies of insect-induced galls in flowers and fruits of Alstonia scholaris (L.) R. Br. Proc Natl Acad Sci, India, B. 90:705–712.
- Collingel DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. 1993. Plant chitinases. Plant J. 3:31–40.
- Cuevas-Reyes P, Quesada M, Hanson P, Oyama K. 2007. Interactions among three trophic levels and diversity of parasitoids: a case of top-down processes in Mexican tropical dry forest. Environ Entomol. 36:792–800.
- de Queiroz DL, Majer J, Burckhardt D, Zanetti R, Fernandez JIR, de Queiroz EC, Garrastazu M, Fernandes BV, dos Anjos N. 2013. Predicting the geographical distribution of Glycaspis brimblecombei (Hemiptera: Psylloidea) in Brazil. Aust J Entomol. 52:20–30.
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux J-P, Van Loon LC, Dicke M, Pieterse CMJ. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant-Microbe Interact. 18:923–937.
- den Bieman K, Malenovský I, Burckhardt D, Heijerman T. 2019. First checklist of the Dutch jumping plant lice since 93 years (Hemiptera: Psylloidea. Nederl Faunist Mededel. 53:55–118.
- Dhileepan K, Neser S, Rumiz D, Raman A, Sharma A. 2017. Host associations of gall-inducing Prodiplosis longifila (Diptera: Cecidomyiidae) from Bolivia: implications for Its use as a biological control agent for Jatropha gossypiifolia (Euphorbiaceae). Fla Entomol. 100:777–786.
- Diaz R, Manrique V, Munyaneza JE, Sengoda VG, Adkins S, Hendricks K, Roberts PD, Overholt WA. 2015. Host specificity testing and examination for plant pathogens reveals that the gall-inducing psyllid Calophya latiforceps is safe to release for biological control of Brazilian peppertree. Entomol Exp Appl. 154:1–14.
- Docters van Leeuwen WM, Docters van Leeuwen-Reijnvann J. 1914. Einige Gallen aus Java (Siebenter Beitrag). Bull Jard Bot Buitenz. 16:1–68.
- Dorchin N, Joy JB, Hilke LK, Wise MJ, Abrahamson WG. 2015. Taxonomy and phylogeny of the Asphondylia species (Diptera: Cecidomyiidae) of North American goldenrods: challenging morphology, complex host associations, and cryptic speciation. Zool J Linn Soc. 174:265–304.
- Dsouza MR, Ravishankar BE. 2014. Nutritional sink formation in galls of Ficus glomerata Roxb. (Moraceae) by the insect Pauropsylla depressa (Psyllidae, Hemiptera). Trop Ecol. 55:129–136.
- Dubey NK, Goel R, Ranjan A, Idris A, Singh SK, Bag SK, Chandrashekar K, Pandey KD, Singh PK, Sawant SV. 2013. Comparative transcriptome analysis of Gossypium hirsutum L. in response to sap sucking insects: aphid and whitefly. BMC Genom. DOI: 10.1186/1471-2164-14-241.
- Epstein Y. 2014. The habitats directive and Bern Convention: synergy and dysfunction in public international and EU law. Georgetown International Environmental Law Review (G. I. E. L. R). 26:139–173.
- Favery B, Dubreuil G, Chen M-S, Giron D, Abad P. 2020. Gall-Inducing parasites: convergent and conserved strategies of plant manipulation by insects and nematodes. Annu Rev Phytopath. 58:1–22.
- Festucci-Buselli RA, Contim LAS, Barbosa LCA, Stuart J, Otoni WC. 2008. Biosynthesis and potential functions of the ecdysteroid 20-hydroxyecdysone — a review. Botany (Can Sci Publ.). 978–987. doi:10.1139/B08-049.
- Fitzpatrick SM, Gries R, Khaskin G, Peach DA, Iwanski J, Gries G. 2013. Populations of the gall midge Dasineura oxycoccana on cranberry and blueberry produce and respond to different sex pheromones. Jour Chem Ecol. 39:37–49.
- Halbert SE, Burckhardt D. 2020. The psyllids (Hemiptera: Psylloidea) of Florida: newly established and rarely collected taxa and checklist. Insecta Mundi. 0788:1–88.
- Hall DG, Richardson ML, Ammar ED, Halbert SE. 2013. Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), vector of citrus huanglongbing disease. Entomol Exp Appl. 146:207–223.
- Havill NP, Foottit RC. 2007. The biology and evolution of the Adelgidae. Annu Rev Entomol. 52:325–349.
- Hayat M, Longjam R, Kumar R. 2013. A new species of encyrtid (Hymenoptera) on gall-inducing psyllids (Hemiptera) from India. Orient Ins. 47:150–154.
- Hirano T, Kimura S, Sakamoto T, Okamoto A, Nakayama T, Matsuura T, Ikeda Y, Takeda S, Suzuki Y, Ohshima I, Sato MH. 2020. Reprogramming of the developmental program of Rhus javanica during initial stage of gall induction by Schlechtendalia chinensis. Front Plant Sci. 1–13. doi:10.3389/fpls.2020.00471.
- Hodkinson ID. 1984. The biology and ecology of the gall-forming psylloidea. In: Ananthakrishnan TN, editor. The biology of gall forming insects. London: Edward Arnold. p. 59–77.
- Hodkinson ID. 1986. The psyllids (Homoptera: Psylloidea) of the Oriental zoogeographical region: an annotated checklist. J Nat Hist. 20:299–357.
- Hodkinson ID. 1989. The biogeography of the Neotropical jumping plant-lice (Insecta: Homoptera: Psylloidea). J Biogeogr. 16:203–217.
- Hodkinson ID. 2009. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): a global synthesis. J Nat Hist. 43:65–179.
- Hodkinson ID, Bird J. 2000. Sedge and rush-feeding psyllids of the subfamily Liviinae (Insecta: Hemiptera: Psylloidea): a review. Zool. J. Linn. Soc. 128:1–49.
- Hodkinson ID, White IM. 1981. The Neotropical Psylloidea (Homoptera, Insecta) — an annotated check list. J Nat Hist. 15:491–523.
- Hollis D. 2004. Australian Psylloidea: jumping plant lice and lerp insects. Australian Biological Resources Study, CSIRO, Canberra, Australia. p. 216.
- Holman J. 2009. Host plant catalog of aphids, Palaearctic region. Dordrecht: Springer Netherlands: Springer Science + Business Media B.V. p. 1216.
- Houard C. 1923. Les zoocécidies des plantes d'Afrique, d'Asie et d'Océanie: description des galles, illustration, bibliographie détaillée, répartition géographique, index bibliographique, Tome 2: Dicotylédones (2e partie), Index bibliographique, Nos. 1807 à 3293. (Paris: J. Hermann, 1922–1923). 2 vols. pp. 497–1056.
- Jarzembowski P, Faltyn A, Jakubska-Busse A, Proćków J. 2013. First report of the occurrence of Livia junci (Schrank, 1789) (Hemiptera: Psyllidae) on Juncus fontanesii J. Gay ex Laharpe (Juncaceae) from Portugal. Arch Biol Sci. Belgrade. 65:1521–1524.
- Kirst GO, Rapp H. 1974. Zur Physiologie der Galle von Mikiola fagi Htg. auf Blättern von Fagus silvatica L. 2. Transport 14C markitiert Assimilate aus dem befallenen Blatt und aus Nachbarblättern in die Galle. Biochem Physiol Pflanzen. 165:445–455.
- Korgaonkar A, Han C, Lemire AL, Siwanowicz I, Bennouna D, Kopec RE, Andolfatto P, Shigenobu S, Stern DL. 2021. A novel family of secreted insect proteins linked to plant gall development. Curr Biol. 31:1836–1849.
- Küster E. 1911. Die Gallen der Pflanzen: ein Lehrbuch für Botaniker und Entomologen. Leipzig: S. Hirzel. p. 437.
- Lizer Trelles CA, Molle CC. 1945. Estructura anatómica de filocecidias neotrópicas. Lilloa. 11:153–207.
- Malenovský I, Burckhardt D, Queiroz DL, Isaias RMS, Oliveira DC. 2015. Descriptions of two new Pseudophacopteron species (Hemiptera: Psylloidea: Phacopteronidae) inducing galls on Aspidosperma (Apocynaceae). Acta Entomol Mus Natl Pragae. 55:513–538.
- Mani MS. 2000. Plant Galls of India. New Hampshire: Science Publishers.
- Mani T, Raman A. 1994. Biochemical changes in relation to growth in two leaf gall systems induced by Trioza jambolanae and Microceropsylla longispiculata (Homoptera: Psylloidea). Phytophaga. 6:59–64.
- Maresquelle H-J, Meyer J. 1965. Physiologie et morphogenèse des galls d’origine animale (Zoocécidies). Handb. Pflanzenphysiol. 15:280–329.
- Mathur RN. 1975. Psyllidae of the Indian subcontinent. New Delhi: Indian Council of Agricultural Research. p. 429.
- McLean IFG. 1994. Interactions between Trichochermes walkeri (Homoptera: Psylloidea) and other Homoptera on Rhamnus catharticus. In: Williams MAJ, editor. Plant galls. Systematics association special volume, 49. Oxford: Clarendon Press; p. 151–160.
- Meyer J. 1987. Plant galls and gall inducers. Stuttgart: Gebrüder Bornträger. p. 291.
- Miller DG, Raman A. 2019. Host–plant relations of gall-inducing insects. Ann Entomol Soc Am. 112:1–19.
- Nakabachi A, Yamashita A, Toh H, Ishikawa H, Dunbar HE, Moran NA, Hattori M. 2006. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science. 314:267. doi:10.1126/science.1134196.
- Ouvrard D, Chalise P, Percy DM. 2015. Host-plant leaps versus host-plant shuffle: a global survey reveals contrasting patterns in an oligophagous insect group (Hemiptera, Psylloidea). Syst Biodivers. 13:434–454.
- Partomihardjo T, Yukawa J, Uechi N, Abe J. 2011. Arthropod galls found on the Krakatau Islands and in adjacent areas of Indonesia, with reference to faunistic disharmony between the Islands and the whole of Indonesia. Esakia. 50:9–21.
- Percy DM, Butterill PT, Malenovský I. 2015. Three new species of gall-forming psyllids (Hemiptera: Psylloidea) from Papua New Guinea, with new records and notes on related species. J Nat Hist. 50:1073–1101.
- Pusztahelyi T. 2018. Chitin and chitin-related compounds in plant–fungal interactions. Mycology. 9:189–201.
- Rajadurai S, Mani T, Balakrishna P, Raman A. 1990. On the digestive enzymes and soluble proteins of the nymphal salivary glands of Trioza jambolanae Crawford (Triozinae: Psyllidae: Homoptera), the gall maker of the leaves of Syzygium cumini (L.) Skeels (Myrtaceae). Phytophaga. 3:47–53.
- Raman A. 1987. On the cecidogenesis and nutritive tissues of the leaf galls of Garuga pinnata Roxburgh (Burseraceae) induced by Phacopteron lentiginosum Buckton (Pauropsyllinae: Psyllidae: Homoptera). Phytophaga. 1:121–140.
- Raman A. 1991. Cecidogenesis of leaf galls on Syzygium cumini (L.) Skeels (Myrtaceae) induced by Trioza jambolanae Crawford (Homoptera: Psylloidea). Jour Nat Hist. 25:653–663.
- Raman A. 1996. Nutritional diversity in gall-inducing insects and their evolutionary relationships with flowering plants. Internat Jour Ecol Environ Sci. 22:133–143.
- Raman A. 2009. Insect—plant interactions: the gall dimension. In: Seckbach J, Dubinsky Z, editor. All flesh is grass: plant-animal interactions, a love—hate affair. springer, Berlin, Germany. p. 119–146.
- Raman A. 2011. Morphogenesis of insect-induced plant galls: facts and questions. Flora. 206:517–533.
- Raman A. 2012. Gall induction by hemipteroid insects. J Plant Interact. 7:29–44.
- Raman A. 2021. Gall-inducing insects and plants: the induction conundrum. Curr Sci. 120:66–78.
- Raman A, Burckhardt D, Harris KM. 2009. Biology and adaptive radiation in the gall inducing Cecidomyiidae (Insecta: Diptera) and Calophyidae (Insecta: Hemiptera) on Mangifera indica (Anacardiaceae) in the Indian subcontinent. Trop Zool. 22:27–56.
- Raman A, Madhavan S, Florentine SK, Dhileepan K. 2006. Metabolite mobilization in the stem galls of Parthenium hysterophorus induced by Epiblema strenuana inferred from the signatures of isotopic carbon and nitrogen and concentrations of total non-structural carbohydrates. Entomol Experimental Applic. 119:101–107.
- Raman A, Schaefer CW, Withers TM. 2005. Biology, ecology and evolution of gall-inducing arthropods. New Hampshire: Enfield: Science Publishers. p. 817.
- Raman A, Singh RN, Maryanska-Nadachowska A. 1996. Biology and karyology of a cecidogenous psylloid, Trioza fletcheri minor (Homoptera: Psylloidea) and morphogenesis of galls on the leaves of Terminalia tomentosa and T. arjuna (Combretaceae). Ins Matsum (New Series). 53:117–134.
- Rehill BJ, Schultz JC. 2003. Enhanced invertase activities in the galls of Hormaphis hamamelidis. J Chem Ecol. 29:2703–2720.
- Riley CV. 1881. Gall insects. Johns New Univers Cyclop. 2:412–416.
- Rohfritsch O. 1971. Développement cécidien et rôle du parasite dans quelques galles d'arthropodes. Marcellia. 37:233–339.
- Rohfritsch O. 1988. A resistance response of Picea excelsa to the aphid, Adelges abietes (Homoptera: Aphidoidea). In: Mattson WJ, Lévieux J, Bernard-Dagan C, editor. Mechanisms of woody plant defenses against insects: Search for Pattern. New York: Springer Verlag; p. 253–266.
- Rohfritsch O. 1992. Patterns in gall development. In: Shorthouse JD, Rohfritsch O, editor. Biology of insect-induced galls. New York: Oxford University Press; p. 60–86.
- Rübsaamen EH. 1899. Mitteilungen über neue und bekannte Gallen aus Europa, Asien, Afrika und Amerika. Entomol Nachricht. 25:225–282.
- Sattelmacher B. 2001. The apoplast and its significance for plant mineral nutrition. New Phytol. 149:167–192.
- Schindler U, Ehrhardt W. 1964. Beobachtungen über Psyllopsis fraxini L. (Psyllidae, Blattflöhe), einen Schädling an Jungeschen. Zeit Angew Entomol. 63:313–319.
- Schmidt E. 1966. Cycle biologique et phases cécidogènes de Livia juncorum sur Juncus articulatus. Marcellia. 33:223–235.
- Schmidt E, Meyer J. 1966. Observations sur la structure des galles de Livia juncorum sur Juncus articulatus. Marcellia. 33:237–253.
- Schweitzer F, Bodenhausen N, Lassueur S, Masclaux FG, Reymond P. 2013. Differential contribution of transcription factors to Arabidopsis thaliana defense against Spodoptera littoralis. Front Plant Sci. 4(13). doi:10.3389/fpls.2013.00013. [accessed on 14 October 2021].
- Shamshina JL, Oldham T, Rogers RD. 2019. Applications of chitin in agriculture. In: Crini G, Lichtfouse E, editor. Sustainable Agriculture reviews, chitin and chitosan in food, Agriculture, pharmacy, medicine, and waste Water treatment. Cham: Springer Nature; p. 125–146.
- Sharma A, Allen J, Madhavan S, Raman A, Taylor G, Fletcher M. 2015c. How do free-living, lerp-forming, and gall-inducing Aphalaridae (Hemiptera: Psylloidea) affect the nutritional quality of Eucalyptus leaves? Ann Entomol Soc Am. 109:127–135.
- Sharma A, Allen J, Madhavan S, Raman A, Taylor G, Fletcher M. 2016. Complex lipids and sterols in the leaves of three species of Eucalyptus (Myrtaceae) hosting three species of Aphalaridae (Hemiptera: Psylloidea): do they have a role in regulating host fidelity? Ann Entomol Soc Am. 109:890–898.
- Sharma A, Khan AN, Subrahmanyam S, Raman A, Taylor GS, Fletcher MJ. 2014b. Salivary proteins of plant-feeding hemipteroids—implication in phytophagy. Bull Entomol Res. 104:117–136.
- Sharma A, Madhavan S, Raman A, Taylor G, Fletcher M. 2015b. Salivary gland structure of Ctenarytaina eucalypti (Hemiptera: Aphalaridae) and interaction of salivary proteins with Eucalyptus globulus. Pol J Entomol. 84:21–32.
- Sharma A, Raman A. 2017. Feeding biology and nutritional physiology of Psylloidea (Insecta: Hemiptera): implications in host-plant relations. Curr Sci. 113:1543–1552.
- Sharma A, Raman A, Taylor G, Fletcher M. 2013. Nymphal development and lerp construction of Glycaspis sp. (Hemiptera: Psylloidea) on Eucalyptus sideroxylon (Myrtaceae) in central-west New South Wales, Australia. Arthropod Struct Dev. 42:551–564.
- Sharma A, Raman A, Taylor GS, Fletcher MJ, Nicol H. 2014a. Bionomics and feeding impact of Ctenarytaina eucalypti (Hemiptera: Psylloidea: Aphalaridae) on Eucalyptus globulus (Myrtaceae) in the central tablelands of New South Wales. Aust Entomol. 54:117–136.
- Sharma A, Raman A, Taylor GS, Fletcher MJ, Nicol H. 2015a. Feeding and oviposition behaviour of a gall-inducing species of Glycaspis (Synglycaspis) (Hemiptera: Psylloidea: Aphalaridae) and development of galls on the leaves of Eucalyptus macrorhyncha (Myrtaceae) in central western New South Wales. Australia. Eur J Entomol. 112:75–90.
- Shih TH, Lin SH, Huang MY, Sun CW, Yang CM. 2018. Transcriptome profile of cup-shaped galls in Litsea acuminata leaves. PloS One. 13(10):e0205265. DOI: 10.1371/journal.pone.0205265.
- Singh G. 2000. Physiology of shoot gall formation and its relationship with juvenility and flowering in mango. Acta Hortic. (ISHS. 509:803–810.
- Singh G. 2003. Mango shoot gall: its causal organism and control measures. In: Ramamurthy V. V., Singh V.S., Gupta G. P., Paul A. V. N., editor. Gleanings in entomology. New Delhi: Indian Agricultural Research Institute; p. 56–77.
- Singh G, Kumar A, Everett TR. 1975. Biological observations and control of Apsylla cistellata Buckton (Psyllidae: Homoptera). Indian J Entomol. 37:46–50.
- Singh S, Singh KP. 2011. Description of two new species of Psyllaephagus Ashmead (Hymenoptera: Encyrtidae) parasitizing Phacopteron lentiginosum Buckton (Hemiptera: Psyllidae), a leaf gall-former of Garuga pinnata Roxburgh (Burseraceae). Zootaxa. 2885:33–43.
- Sokhi J, Kapil RN. 1984. Morphogenetic changes induced by Trioza in flowers of Terminalia arjuna – I. Androecium. Phytomorph. 34:117–128.
- Sokhi J, Kapil RN. 1985. Morphogenetic changes induced by Trioza in flowers of Terminalia arjuna – II. Gynoecium. Phytomorph. 35:69–82.
- Sopow SL, Shorthouse JD, Strong W, Quiring DT. 2003. Evidence for long-distance, chemical gall induction by an insect. Ecol Lett. 6:102–105.
- Taylor GT. 1990. Revision of the genus Schedotrioza Tuthill & Taylor (Homoptera: Psylloidea: Triozidae). Invert Taxon. 4:721–751.
- Uichanco HR. 1919. A biological and systematic study of Philippine plant galls. Philipp J Sci. 14:527–554.
- Veenakumari K, Buhl PN, Mohanraj P. 2018. A new species of Synopeas (Hymenoptera: Platygastridae) parasitizing Pauropsylla cf. depressa (Psylloidea: Triozidae) in India. Acta Entomol Mus Natl Pragae. 58:137–141.
- Waliwitiya R, Belton P, Nicholson RA, Lowenberger CA. 2012. Plant terpenoids: acute toxicities and effects on flight motor activity and wing beat frequency in the blow fly Phaenicia sericata. J Econ Entomol. 105:72–84.
- Westphal E. 1982. Modification du pH vacuolaire des cellules épidermiques foliares de Solanum dulcamara soumises à l’action d’un acarian cécidogène. Can J Bot. 60:2882–2888.
- Westphal E, Dreger F, Bronner R. 1990. The gall mite Aceria cladophthirus (Nalepa). I. Life cycle, survival outside the galls and symptoms expression on susceptible and resistant Solanum dulcamara L. plants. Exp. Appl. Acarol. 9:183–200.
- White IM, Hodkinson ID. 1985. Nymphal taxonomy and systematics of the Psylloidea. Bull Br Nat Hist Mus (Ent. 50:153–301.
- White TCR. 1970. The nymphal stage of Cardiaspina densitexta (Homoptera, Psyllidae) on leaves of Eucalyptus fasciculosa. Aust J Zool. 18:273–293.
- Yang CK, Li F. 1982. Description of the new genus Celtisaspsis and five new species of China (Homoptera: Psyllidae). Entomotaxon. 4:183–198.
- Yang M-M. 1995. Biosystematics and the evolution of gall formation in hackberry psyllids Pachypsylla (Insecta: Homoptera: Psylloidea: Psyllidae), PhD Thesis, University of Maryland. p. 221.
- Yang M-M, Burckhardt D, Fang S. 2013. Psylloidea of Taiwan. Volume II. family triozidae. Taichung: National Chung Hsing University. p. 160.
- Yang M-M, Burckhardt D, Fang SJ. 2009. Psylloidea of Taiwan (Vol. I). Families Calophyidae, Carsidaridae, Homotomidae and Phacopteronidae, with overview and keys to families and genera of Taiwanese Psylloidea (Insecta: Hemiptera). Taichung: National Chung Hsing University. p. 96.
- Yang M-M, Mitter C, Miller DR. 2001. First incidence of inquilinism in gall-forming psyllids, with a description of the new inquiline species (Insecta, Hemiptera, Psylloidea, Psyllidae, Spondyliaspidinae). Zool Scr. 30:97–113.
- Yang M-M, Raman A. 2007. Diversity, richness, and patterns of radiation among gall-inducing psyllids (Hemiptera: Psylloidea) in the Orient and Eastern Palearctic. Orient Ins. 41:55–65.
- Zhao C, Escalante LN, Chan H, Benatti TR, Qu J, Chellapilla S, Waterhouse RM, Wheeler D, Andersson MN, Bao R, et al. 2015. A massive expansion of effector genes underlies gall formation in the wheat pest Mayetiola destructor. Curr Biol. 25:613–620.
- Zhao Q, Ling-Ling J, Jie G, Dong-Kang Z, Hong-Ying H. 2021. Differences in gall induction of flower-like galls on haloxylon by psyllids (hemiptera: aphalaridae), and the emergence of corresponding parasitoids. Insects. 12:861. doi:10.3390/insects12100861.
