ABSTRACT
Copper oxide nanoparticles (CuO NPs) are a type of nanomaterial with unique physical and chemical properties that make them useful in various applications. CuO NPs have been studied for their potential agricultural applications, where they can have both positive and negative effects on plants, depending on factors such as concentration and duration of exposure. CuO NPs have been shown to improve plant growth and development by enhancing photosynthesis, nutrient uptake, and root growth. However, high concentrations of CuO NPs can cause oxidative stress and damage to plant cells, resulting in reduced growth and yield. Furthermore, these NPs can be taken up by plants and accumulate in various plant tissues, raising concerns about their potential impact on human health if ingested via the food chain. Further research is needed to determine the safe and effective application method and optimal concentration of CuO NPs in agriculture.
Highlights
CuO NPs can benefit or harm plants, based on concentration and exposure time.
Monocots are more negatively affected by CuO NPs, dicots show diverse response.
CuO NPs impact plants based on species, concentration, and application.
More research needed to understand CuO NPs’ impact on plant growth and health.
KEYWORDS:
1. Introduction
The use of nanoparticles (NPs) in agriculture can be considered a double-edged sword. While they have known positive effects as fertilizers and pesticides (Tapan et al. Citation2010; Madhuban et al. Citation2012; Tiwari et al. Citation2014), plants tend to uptake and translocate them, potentially disrupting their physiological processes (Li et al. Citation2015) and affecting their growth and development (Murali et al. Citation2022). The effects of NPs vary widely, primarily depending on their concentration and chemical composition (El-Moneim et al. Citation2021). NPs composed of essential nutrients can be used as fertilizers as they can provide nutrients to plants and are used in soil remediation projects (Du et al. Citation2017). In these cases, they are preferred because they release their metal content slowly, offering a longer-lasting effect compared to the immediate increase in concentration when using an ionic solution. However, NPs have been shown to inhibit plant growth at lower concentrations than their bulk forms (Song et al. Citation2016). Smaller NPs have demonstrated greater toxicity, penetrating the plant and even the cell wall itself (Du et al. Citation2011; Servin et al. Citation2012), although the exact mechanism of uptake remains incompletely understood (Ma and Yan Citation2018). Due to their variation in size and properties, including chemical composition, surface charge and surface modification, NPs can undergo different internalization processes in plants, leading to their accumulation, migration, and subsequent impact on plant growth (Karami Mehrian and De Lima Citation2016; Gao et al. Citation2023). As recently reviewed by Wang et al. (Citation2023), plants may be able to take up nanoparticles across their entire body surface. Roots uptake nanoparticles through primary roots, pores in the root cell wall, and damaged areas, while leaves uptake nanoparticles using the pores of the periderm and stomata. Once taken up, these nanoparticles are transported within the plant via both the symplastic and apoplastic pathways and can move between different plant tissues through the xylem and phloem. Plants respond differently to NPs depending on their species due to their different physiology and also growth medium (Lin and Xing Citation2008; Dev et al. Citation2018). In general, the mechanism of the effect seems to revolve around the generation of reactive oxygen species (ROS) by the NPs, causing oxidative damage and peroxidation of plant lipids (Kamat et al. Citation2000; Foley et al. Citation2002; Reddy et al. Citation2016).
Copper (Cu), an abundant transition metal in the Earth's crust, is widely used and valued for its good heat and electrical conductivity. On average, the lithosphere contains 60 mg/kg of Cu, while soils typically contain 2–50 mg/kg (Oorts Citation2013). Cu, is an essential microelement for plants, required as a cofactor in many physiological and biochemical processes (Garcia et al. Citation2014). However, Cu can easily become toxic at higher-than-optimal levels. High levels of Cu are well-documented to cause oxidative stress, negative effects on the uptake of other elements, reduced photosynthetic pigment levels, and impairment of cellular components (Shabbir et al. Citation2020). When exposed to high Cu concentrations, plants exhibit various visible toxicity symptoms, including root and shoot growth inhibition or even plant death (Zhang et al. Citation2019).
Copper oxide (CuO) NPs are typically synthesized using various methods, such as chemical precipitation, thermal decomposition, and sol–gel processes (Grigore et al. Citation2016). In addition to these methods, the process of green synthesis has successfully produced a significant quantity of nanoparticles composed of metals or metal oxides via algal, fungal, plant and other biosynthesis routes (Chakraborty et al. Citation2022). These NPs can vary in size, shape, and surface charge depending on the synthesis method and reaction conditions (Naz et al. Citation2020). Furthermore, various studies have demonstrated several interesting properties, including excellent electrical conductivity, catalytic activity, and antimicrobial properties (Grigore et al. Citation2016; Song et al. Citation2019; Shehabeldine et al. Citation2023). These properties make them useful in a wide range of applications, including as catalysts in chemical reactions, solar cells, batteries, nanoelectronics, antibacterial agents in healthcare and environmental remediation (Boboc et al. Citation2017; Rafique et al. Citation2017). Due to their diverse uses, there are multiple pathways through which they can be released into the environment, such as through the use of raw materials, commercial synthesis, or wastewater (Brar et al. Citation2010; Naz et al. Citation2020). However, it is important to consider the potential health and environmental risks associated with the unique properties of CuO NPs. High concentrations of CuO NPs have been shown to be toxic to cells and organisms, and their small size allows them to penetrate cells and tissues, potentially having adverse effects on human health if ingested or inhaled (Hou et al. Citation2017). Therefore, careful assessment of the risks and benefits of CuO NPs to plants is necessary before their widespread use in various applications.
In general, CuO NPs can affect plants in two main ways. Firstly, they can release Cu, which, although an essential element, can become toxic at high doses, or they can cause particle stress by entering the plant itself (Ruttkay-Nedecky et al. Citation2017; Velicogna et al. Citation2020). The primary mechanisms of toxicity are thought to be the generation of excess ROS, leading to oxidative stress and subsequent cytotoxicity, as well as the potential for CuO NPs to cause genetic damage (Atha et al. Citation2012; Naz et al. Citation2020). If the particles release Cu ions, these ions can attach to protein thiol groups, leading to conformational changes in the proteins (Nekrasova and Maleva Citation2007). Another manifestation of toxicity is through the Fenton reaction, where Cu ions convert hydrogen peroxide into hydroxyl radicals, causing damage to nearby macromolecules (Chung et al. Citation2019).
Like many cases, the key to the agricultural application of CuO NPs lies in finding the appropriate dose. Plants experience toxicity and hindered growth when exposed to high concentrations of Cu, while Cu deficiency results in various abnormal conditions, such as distortion of young leaves, necrosis, stem bending, impaired vegetative growth, and reduced grain quality in crop plants (Siddiqi and Husen Citation2020).
Over the last decade, an increasing number of studies have investigated the effects of CuO NPs on various plant species. These studies often employ germination indices and seedling growth parameters to assess the effects of CuO NP exposure. While it is important to study the underlying processes, these measurements provide an excellent basis for comparing CuO NP-induced responses across different plant species. Generally, these changes range from growth promotion to no effect to inhibition of seed germination and root and shoot growth, depending on both the plant species and size, as well as the experimental system employed.
The objective of this review is to collect, organize and evaluate the available studies on the relationship between plants and CuO NPs. By reviewing and organizing existing studies, this review helps consolidate the current understanding of the effects of CuO NPs on plants. It enables researchers and scientists to have a comprehensive understanding of the topic, including the range of responses observed across different plant species and experimental conditions and helps to identify gaps in the existing research, highlighting areas that require further investigation.
2. Responses of monocotyledonous plants to copper oxide nanoparticles
Studies have indicated that the responses of monocotyledonous plants to CuO NPs can vary depending on various factors, such as NP concentration, duration of exposure, and, most importantly, the specific plant species being investigated. In general, monocotyledons have shown a negative response to CuO NP treatment, with only a few exceptions.
Among the monocotyledons studied, a significant number of experiments have focused on investigating the response of rice, wheat, and maize to CuO NPs. Interestingly, the results reveal that wheat is much less sensitive to the presence of CuO NPs compared to rice or maize.
The relationship between rice (Oryza sativa L.) and CuO NPs has been extensively studied across various applied concentrations and experimental systems. The majority of these studies have demonstrated growth inhibition in rice when exposed to CuO NPs (). Oxidative stress induced by CuO NPs has emerged as a common factor contributing to the observed growth inhibition. In the Nipponbare cultivar of rice, the addition of CuO NPs led to increased lipid peroxidation in both root and shoot tissues, and this oxidative stress response was found to be mediated by ethylene (Azhar et al. Citation2023). In the Jyoti cultivar, oxidative and osmotic stress were detected after 30 days of exposure, negatively impacting productivity primarily due to structural damage to the thylakoid membrane (Da Costa et al. Citation2020). Other studies have also suggested that damage to photosynthetic efficiency and apparatus could be a potential cause of growth inhibition (Da Costa and Sharma Citation2016; Yang et al. Citation2020; Rai et al. Citation2021). The outcome of CuO NP stress on rice did not appear to be influenced by the experimental system used, as inhibition of seed germination and growth of the main organs was observed in various setups, including NP suspension on filter paper (Wang et al. Citation2020a), soil (Peng et al. Citation2017), hydroponics (Wang et al. Citation2015), and cotton pads soaked in nano-CuO suspension (Shaw and Hossain Citation2013). In a rare case, while germination and shoot growth of rice seedlings were not inhibited, root length was still significantly reduced in the presence of CuO NPs (Yang et al. Citation2015). In conclusion, extensive studies have shown that the exposure of rice to CuO NPs leads to growth inhibition. Oxidative stress induced by CuO NPs has been identified as a common factor contributing to the growth inhibition. The inhibitory effects of CuO NPs on rice have been observed in different experimental setups, suggesting that the outcome is not significantly influenced by the specific system used.
Table 1. Studies investigating the relationship between rice (Oryza sativa L.) and CuO NPs. ‘-’ means inhibition, while ‘n.d.’ marks parameters not determined.
In contrast, the results of experiments with wheat (Triticum aestivum L.) as the experimental subject are more complex and less consistent (). In some cases, the application of CuO NPs had a positive effect on wheat growth. For instance, when applied as foliar spray or grain pre-treatment, CuO NPs were found to increase the growth parameters of wheat plants compared to the control group (Badawy et al. Citation2021). Low concentrations of CuO NP suspension also showed enhancement of growth and yield of wheat in hydroponic and in vitro systems (Hafeez et al. Citation2015; Yasmeen et al. Citation2018) as well as in soil (Yasmeen et al. Citation2018). Ibrahim et al. (Citation2022) demonstrated concentration-dependent growth responses in wheat in vitro, where low concentration of CuO NPs enhanced plant growth, while higher concentrations resulted in a decrease in plant growth. Conversely, other studies have shown that higher amounts of CuO can inhibit wheat growth (Dimkpa et al. Citation2012; Kacziba et al. Citation2023). When cultivated in soil, wheat growth parameters were not affected by the presence of CuO NPs at a concentration of 50 mg/kg (Guan et al. Citation2020). However, when grown in sand, only a concentration of 3 mg/kg of CuO NPs did not have an inhibitory effect on root growth, while higher concentrations (10, 30, and 300 mg/kg) did have an inhibitory effect (Adams et al. Citation2017). In summary, the response of wheat to CuO NPs is more complex and less consistent compared to rice. The effects of CuO NPs on wheat growth vary depending on various factors such as concentration and experimental conditions. In some cases, the application of CuO NPs has shown a positive impact on wheat growth, leading to enhanced growth parameters and increased yield. This positive effect has been observed when CuO NPs were applied as a foliar spray, grain pre-treatment, or at low concentrations in hydroponic, in vitro, and soil systems. However, it should be noted that the growth response of wheat to CuO NPs is concentration-dependent, with higher concentrations often resulting in inhibitory effects on plant growth. The specific effect of CuO NPs on wheat growth also appears to be influenced by the growth medium, as the inhibitory effect was more pronounced in sand compared to soil. Overall, the response of wheat to CuO NPs is characterized by a complex interplay between concentration, application method, and growth conditions, highlighting the need for further research to better understand the underlying mechanisms and optimize nanoparticle applications in wheat cultivation.
Table 2. Studies investigating the relationship between wheat (Triticum aestivum L.) and CuO NPs. ‘+’ indicates growth induction, ‘-’ represents inhibition, while ‘n.d.’ marks parameters not determined. (RNS – reactive nitrogen species, H2S – hydrogen sulphide).
Although fewer experiments have been conducted using maize (Zea mays L.) as an experimental subject, the results consistently show that both root and shoot growth of maize are inhibited by CuO NPs both in soil, hydroponic culture, and in vitro experiments (). In soil experiments, Pu et al. (Citation2019) found that reducible Cu was the predominant form, suggesting that oxidative stress is at least partially responsible for the observed growth inhibition. In hydroponic systems, CuO NPs were observed to be transported between the root and shoot via the xylem and phloem (Wang et al. Citation2012). It has also been demonstrated that bulk CuO particles are more toxic to maize seedlings compared to nano CuO, primarily due to the higher dissolution of Cu ions in hydroponic environments (Roy et al. Citation2022). Additionally, increasing concentrations of applied CuO NPs significantly and concentration-dependently inhibited maize root elongation when grown of filter paper, unlike ions added in similar quantities. (Yang et al. Citation2015). These findings highlight the consistent negative impact of CuO NPs on maize growth and suggest that oxidative stress and copper ion dissolution are key factors contributing to the observed inhibition.
Table 3. Studies investigating CuO NP-induced changes in maize (Zea mays L.), barley (Hordeum vulgare L. and Hordeum sativum L.), green onion (Allium fistulosum L.), rye (Secale cereale L.), sorghum (Sorghum bicolor L.), triticale (x Triticosecale), perennial ryegrass (Lolium perenne L.) and annual ryegrass (Lolium rigidum L.). ‘+’ indicates growth induction, ‘-’ means inhibition, while ‘n.d.’ marks parameters not determined. (ROS - reactive oxygen species, RNS – reactive nitrogen species, H2S – hydrogen sulphide).
Barley (Hordeum vulgare or Hordeum sativum L.) displayed varied responses to CuO NPs depending on the experimental system, applied concentration, and exposure duration (). In vitro growth experiments showed that the presence of CuO NPs enhanced seed germination and seedling growth parameters (Kadri et al. Citation2022). However, in soil experiments, higher concentrations of slightly smaller CuO NPs significantly decreased plant growth (Burachevskaya et al. Citation2021; Fedorenko et al. Citation2021). Interestingly, in a soil study, CuO NPs at a concentration of 300 mg/kg initially reduced barley biomass over a one-week period, but after a month, the inhibitory effect disappeared completely (Jośko et al. Citation2021). These findings highlight that various factors such as the experimental system, applied concentration, and exposure duration can influence the response of barley to CuO NPs.
For several monocotyledonous plant species, there is limited research available on their response to CuO NPs, making it challenging to draw comprehensive conclusions (). In the case of green onions (Allium fistulosum L.) grown in soil treated with a wide range of CuO NP concentrations, one study found no significant changes in growth parameters (Wang et al. Citation2020c). A recent study investigated the effect of CuO NP treatment on sorghum (Sorghum bicolor L.), rye (Secale cereale L.), and triticale (x Triticosecale), and revealed that sorghum exhibited higher sensitivity compared to the other studied species. The study also identified variations in the homeostasis of reactive molecules as underlying factors related to sensitivity (Kacziba et al. Citation2023). Moreover, in ryegrass species (Lolium perenne and L. rigidum L.) the exposure to CuO NPs resulted in concentration-dependent growth reduction accompanied by DNA damage (Atha et al. Citation2012). These limited studies highlight the need for further research to better understand the response of these monocotyledonous plant species to CuO NPs and the underlying mechanisms involved.
Studies on the response of aquatic monocotyledonous plants to CuO NPs have been conducted, as shown in . In these studies, regardless of the size and concentration of CuO NP applied, the growth of both Landoltia punctata (G. Mey.) (Shi et al. Citation2011) and Lemna minor (L.) (Song et al. Citation2016; Koce Citation2017; Yue et al. Citation2018) was significantly decreased. These studies have also reported the presence of oxidative stress as an underlying symptom of the observed growth inhibition. These findings suggest that CuO NPs can negatively impact the growth and physiology of aquatic monocotyledonous plants and highlight the importance of understanding the potential ecological implications of nanoparticle pollution in aquatic ecosystems.
Table 4. Studies investigating the responses of aquatic monocot common duckweed (Lemna minor L.) and dotted duckmeat (Landoltia punctata G. Mey.) to CuO NPs. ‘-’ represents inhibition, while ‘n.d.’ marks parameters not determined.
3. Responses of dicotyledonous plants to copper oxide nanoparticles
Studies have revealed that dicotyledonous plants can manifest varied responses to CuO NPs, which are contingent upon factors such as nanoparticle concentration, exposure duration, and the specific plant species under investigation. In comparison to monocotyledonous species, a greater number of studies have explored the interaction between dicotyledons and CuO NPs, although there are fewer concurrent and complementary investigations focused on individual species.
Arabidopsis thaliana (L.), being one of the most extensively studied model organisms in plant biology, has been the subject of numerous investigations exploring its interaction with CuO NPs. The majority of these studies indicate a detrimental impact of CuO NPs on Arabidopsis growth (). Several publications highlight the ability CuO NPs to influence the expression of a wide range of genes in A. thaliana. For instance, a recent study demonstrated that CuO NPs caused damage to the root cell walls and down-regulated genes associated in cell wall organization, leading to inhibition of root growth (Nie et al. Citation2020). Similarly, Landa et al. (Citation2017) observed decreased rosette growth in A. thaliana exposed to CuO NPs, accompanied by alterations in the expression of various genes, including the downregulation of metal transporter and aquaporin genes and the up-regulation of metallochaperone-like genes. Another study detected cell damage and oxidative stress as underlying factors contributing CuO NPs-induced growth inhibition, which were further manifested through changes in gene expression levels related to oxidative stress (Tang et al. Citation2016). When grown in agar media, A. thaliana treated with CuO NPs exhibited reduced chlorophyll content and increased anthocyanin content in its leaves This study also revealed lignin deposition and oxidative stress as consequences of altered root growth, as supported by changes in the expression levels of related genes (Nair and Chung Citation2014). In a metabolomics study, it was found that CuO NP stress disrupted the homeostasis of several metabolites involved in the jasmonic acid and glucosinolate pathways, potentially contributing to the stress responses of A. thaliana to CuO NPs (Soria et al. Citation2019). Additionally, the comparison of wild-type and ethylene-insensitive mutant A. thaliana plants suggested the significant role of ethylene in CuO NP-induced oxidative damage in leaves, as the mutant plants exhibited reduced sensitivity to oxidative stress caused by CuO NPs compared to the wild type (Azhar et al. Citation2020). In conclusion, numerous studies have investigated the interaction between A. thaliana and CuO NPs, revealing a detrimental impact on plant growth. Overall, these findings highlight the complex effects of CuO NPs on A. thaliana and provide insights into the molecular mechanisms involved in the plant's response to nanoparticle stress.
Table 5. Studies investigating the relationship between thale cress (Arabidopsis thaliana L.) and CuO NPs. ‘-’ means inhibition, while ‘n.d.’ marks parameters not determined.
In addition to A. thaliana, several economically important plant species from the Brassicaceae family have been studied in relation to CuO NPs (). The response of Indian mustard (Brassica juncea L.) to CuO NPs varied depending on the mode of application CuO NP treatment as a foliar spray resulted in increased growth, higher photosynthetic rate, and enhanced antioxidant capacity in B. juncea plants, while higher concentrations had a neutral effect on growth (Faraz et al. Citation2022). However, when grown in a semi-solid medium, the addition of CuO NPs significantly inhibited root and shoot growth in B. juncea, accompanied by oxidative burst, lipid peroxidation, lignification, and downregulation of catalase and ascorbate peroxidase expression in the roots (Nair and Chung Citation2015). The growth of black mustard (Brassica nigra L.) was also inhibited in the presence of CuO NPs, along with the generation of ROS (Zafar et al. Citation2017). Pre-treating kale (Brassica oleracea L.) seeds with 70 ppm of CuO NPs had a positive effect on the subsequent growth of roots and shoots in the developed plants (Vassell et al. Citation2019). While foliar application of CuO NPs was beneficial in some another cases, it had detrimental effects on cabbage (Brassica oleracea L. var. capitata), leading to reduced biomass, chlorosis, and necrosis on the leaves (Xiong et al. Citation2017). Chinese cabbage (Brassica perkiensis L.) displayed a concentration-dependent response to CuO NPs in hydroponic system, stimulating root and shoot growth at low concentrations but causing growth inhibition at high concentrations, primarily due to oxidative stress (Wang et al. Citation2020b). On the other hand, bok choy (Brassica rapa L.) exhibited clear sensitivity to CuO NPs both in soil and in vitro. Comparing soil-grown Green and Rosie varieties, Rosie was found more susceptible to CuO NP-induced stress, with the nanoform of Cu was more damaging compared to bulk or ionic alternatives (Deng et al. Citation2020). Additionally, an in vitro study on turnip (Brassica rapa L. ssp. rapa), revealed severe changes in the oxidative homeostasis associated with CuO NP-induced growth inhibition of (Chung et al. Citation2019). In summary, the response of Brassicaceae plant species to CuO NPs varies depending on the mode of application and concentration, the studies highlighting the diverse and species-specific responses of Brassicaceae plants to CuO NPs.
Table 6. Studies investigating the relationship between the members of Brassicaceae family and CuO NPs. ‘+’ indicates growth induction, ‘-’ represents inhibition, while ‘n.d.’ marks parameters not determined (ROS - reactive oxygen species).
Lettuce (Lactuca sativa L.) is another plant species commonly studied in in the context of the relationship between CuO NPs and plants (). Among the reports available, low concentration of CuO NP (10 mg/plant) applied as foliar spray had a positive effect on lettuce, while a higher quantity in the same form became harmful (Xiong et al. Citation2017). In another study, supplementing L. sativa plants with 20 mg of CuO NPs through foliar spray increased leaf number and dry weight, while irrigation with CuO NPs enhanced macro- and microelement content in the roots (Kohatsu et al. Citation2021). Conversely, higher concentrations of CuO NPs applied as foliar spray significantly inhibited lettuce growth and induced changes in oxidative status (Xiong et al. Citation2021b). In a different study, lower amounts of CuO NPs slightly improved germination and root growth of lettuce seedlings in vitro, but higher concentrations disrupted nitric oxide signaling, resulting in reduced antioxidant capacity, germination, and root elongation (Pelegrino et al. Citation2020). Previous in vitro research demonstrated that CuO NPs were more toxic than Cu ions and inhibited the root growth in lettuce seedlings (Liu et al. Citation2016). Using a hydroponic growth system, both low (Hong et al. Citation2015) and high (Xiong et al. Citation2021a) concentrations of CuO NPs decreased root growth in lettuce, attributed to changes in oxidative homeostasis and an increased antioxidant response. In summary, the effect of CuO NPs on lettuce growth is influenced by the concentration and method of application, highlighting the concentration-dependent and method-specific effects of CuO NPs on lettuce growth and oxidative status.
Table 7. Studies investigating the relationship between lettuce (Lactuca sativa L.) and CuO NPs. ‘+’ indicates growth induction, ‘-’ means inhibition, while ‘n.d.’ marks parameters not determined.
The response of soybean (Glycine max L.) to CuO NPs has been relatively understudied, and the available results show mixed outcomes (). In hydroponic conditions, CuO NP inhibited soybean root growth in a concentration- and exposure time-dependent manner, accompanied by increased Cu levels and oxidative stress (Liu et al. Citation2021). Conversely, in an in vitro study conducted on filter paper, low levels of CuO exposure had a positive impact on soybean root elongation. However, as the concentration increased, both root and shoot growth were significantly inhibited (Adhikari et al. Citation2012).
Table 8. Studies investigating CuO NP-induced changes in soybean (Glycine max L.), spinach (Spinacia oleracea L.), and mung bean (Vigna radiata L.). ‘+’ indicates growth induction, ‘-’ represents inhibition, while ‘n.d.’ marks parameters not determined.
To date, only a limited number of studies have examined the interaction between spinach (Spinacia oleracea L.) and CuO NPs, and notably, all of these studies focused on soil-grown plants. (). The findings of these studies suggest that, except at high concentrations (1000 mg/L) where CuO NPs inhibited plant growth (Singh and Kumar Citation2016), spinach plants generally exhibited no or moderate growth responses to CuO NPs (Singh and Kumar Citation2016, Citation2020; Rawat et al. Citation2021).
Based on the very limited available information, it appears that mung bean (Vigna radiata L.) is relatively sensitive to exposure to CuO NP (). In a recent study, when grown in soil supplemented with low amounts of CuO NPs, seed germination, root growth, and shoot growth were not affected, but at a higher concentration (100 mg/kg), it significantly inhibited germination and shoot development while not impacting root elongation (Subpiramaniyam et al. Citation2021). In a previous study, CuO NP were found to inhibit root growth across a wide range of concentrations in vitro, accompanied by increased lipid peroxidation and lignification, while shoot biomass was only reduced at high concentrations of CuO NPs (Gopalakrishnan Nair et al. Citation2014).
Among several dicotyledonous plant species, only isolated studies are available thus far, which will be discussed below, grouped according to the effects of CuO NPs.
Some dicotyledonous plant species have shown a clear concentration-dependent response to the presence of CuO NPs, with low amounts of CuO NPs having a positive effect but higher concentrations inhibiting plant growth in the same experiment (). For example, in vitro treatment of chickpea (Cicer arietinum L.) with CuO NPs at concentrations of 1–60 mg/L improved root and shoot growth, while higher concentrations in the range of 100–600 mg/L severely inhibited growth parameters (Adhikari et al. Citation2012). Similarly, in an another in vitro study, high concentrations of CuO NPs significantly decreased buckwheat (Fagopyrum esculentum Moench.) root elongation, while lower amounts had no effect on this organ (Lee et al. Citation2013). In the case of tomatoes (Solanum lycopersicon L.) grown in various media, CuO NPs exerted a concentration-dependent effect on plant growth, promoting growth at low concentrations, but either have no effect or a negative effect on plant biomass at higher concentrations, regardless of the growing conditions (soil, agar, or hydroponics), (Ahmed et al. Citation2018). The observed growth inhibition was associated with oxidative stress and changes in photosynthetic properties. Furthermore, in a recent study, a similar concentration-dependent effect was observed in candyleaf (Stevia rebaudiana Bertoni) regenerant plants, where supplementation of agar medium with 2–20 mg/L CuO NPs increased root formation, plant length, and steviol glycoside content, while higher concentrations between 200 and 2000mg/L significantly decreased these parameters (Ahmad et al. Citation2020).
Table 9. Concentration-dependent effect of CuO NPs. Studies investigating CuO NP-induced changes in chickpea (Cicer arietinum L.), buckwheat (Fagopyrum esculentum Moench.), tomato (Solanum lycopersicon L.) and candyleaf (Stevia rebaudiana Bertoni.). ‘+’ indicates growth induction, ‘-’ means inhibition, while ‘n.d.’ marks parameters not determined.
In some cases, research has shown that CuO NPs have had a positive effect on plant growth, as summarized in . For instance, when pigeon pea (Cajanus cajan L.) seedlings were cultivated in soil containing a relatively low concentration of CuO NPs (20 ppm), their growth performance showed a significant increase (Shende et al. Citation2017). In the case of sweet potato (Ipomoea batatas L.), two varieties treated with CuO exhibited longer roots in response to low concentrations of nanoparticles, particularly in the Covington variety, which also showed higher lignin content (Bonilla-Bird et al. Citation2020). Similarly, in a previous study conducted by Ochoa et al. (Citation2017), it was found that supplementing the soil of green pea (Pisum sativum L.) plants with CuO NPs positively influenced their root elongation.
Table 10. CuO NPs with positive/no/negative effect. Studies investigating CuO NP-induced changes in pigeon pea (Cajanus cajan L.), sweet potato (Ipomoea batatas L.), green pea (Pisum sativum L.), bell pepper (Capsicum annum L.), cucumber (Cucumis sativus L.), alfalfa (Medicago sativa L.) and white mustard (Sinapis alba L.). ‘+’ indicates growth induction, ‘-’ represents inhibition, while ‘n.d.’ marks parameters not determined.
In contrast to other studies, the application of CuO (NPs) on bell pepper (Capsicum annuum L.) did not have any significant effect on plant growth or productivity () (Rawat et al. Citation2018).
For certain plant species, only negative effects were observed in response to CuO NPs exposure, as mentioned in . For instance, when cucumber (Cucumis sativus L.) was treated with CuO NPs, both seed germination and root growth were severely inhibited This treatment also resulted in a change in protein expression pattern compared to the control group (Moon et al. Citation2014). Similarly, in an in vitro study with white mustard (Sinapis alba L.) seeds, their germination and subsequent root elongation were dose-dependently inhibited by CuO NPs (Landa et al. Citation2016). Moreover, hydroponically grown alfalfa (Medicago sativa L.) plants treated with CuO NPs showed a significant reduction in root elongation. This was accompanied by downregulated catalase and increased ascorbate peroxidase activity (Hong et al. Citation2015).
In some cases, mixed effects of CuO NPs on plants have been reported, where it is not possible to clearly determine the direction of the change caused by the treatment (). Such a mixed response was observed when CuO NPs were added to the growth media used for cilantro (Coriandrum sativum L.) germination. The addition of CuO nanoparticles to the artificial soil negatively influenced germination, while root elongation of the germinated seedlings remained unaffected. However, shoot growth was decreased in the presence of the 20 mg/kg CuO NP treatment (Zuverza-Mena et al. Citation2015). In another study, it was found that the germination of shiny elsholtzia (Elsholtzia splendens Willd.) was not affected by CuO NPs. However, the root growth of shiny elsholtzia was significantly inhibited by the NPs (Shi et al. Citation2014). In a recent study, willow (Salix integra Thumb., ‘Yizhibi’) exposed to 100 and 500 mg/kg CuO NP in soil showed contrasting effects. Lower concentrations of CuO NPs increased root growth and reduced shoot length, while high concentrations significantly inhibited the growth of willow (Qu et al. Citation2022). Similarly, in an in vitro study, root growth of eggplant (Solanum melongena L.) was affected by CuO NPs in a concentration-dependent manner, ranging from promotion to inhibition. However, shoot growth of eggplant was decreased by all treatments, accompanied by oxidative stress, DNA damage, and decreased total chlorophyll content (Baskar et al. Citation2018).
Table 11. Mixed effect of CuO NPs. Studies investigating CuO NP-induced changes in cilantro (Coriandrum sativum L.), shiny elsholtzia (Elsholtzia splendens willd.), willow (Salix integra Thunb.) and eggplant (Solanum melonegra L.). ‘+’ indicates growth induction, ‘-’ means inhibition, while ‘n.d.’ marks parameters not determined.
4. Conclusions
In conclusion, the studies discussed in this review highlight the complex and varied growth responses of different plant species to CuO NP exposure. The growth response depends on several factors, including the plant species, concentration of CuO NPs, method of application (foliar spray, soil supplementation, hydroponics), and duration of exposure.
Considering higher taxonomic categories, the overall available evidence suggests that most monocotyledonous plants were negatively affected by exposure to CuO NPs, although the extent of damage depends on several factors. Among the species studied so far, only two, namely wheat and barley, have shown evidence that CuO NPs can have a positive effect on monocot growth. On the other hand, the response of dicotyledonous plant species to CuO NPs is much more diverse, with both positive and negative outcomes depending on the species, concentration, and exposure time being examined. For certain plant species such as spinach, mung bean, chickpea, buckwheat, and tomatoes, the growth response to CuO NPs exhibited a concentration-dependent pattern. Generally, lower concentrations of CuO NPs had a positive or neutral effect on root and shoot growth, while higher concentrations inhibited growth parameters (). This concentration-dependent response suggests that there might be an optimal range of CuO NP concentration for promoting plant growth, beyond which toxicity effects become more prominent.
Figure 1. Influence of CuO nanoparticles on the growth responses of monocotyledonous and dicotyledonous plant species based on available literature.
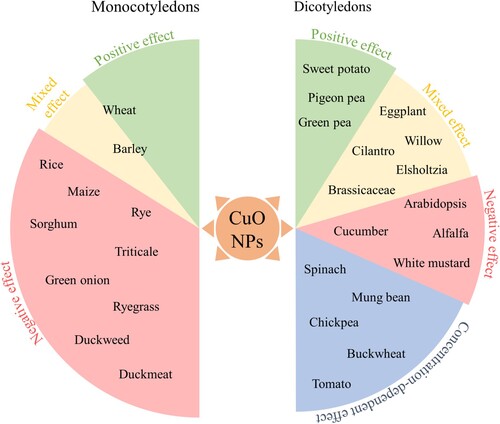
Different plant species within the same family, such as A. thaliana, B. juncea, B. nigra, and B. oleracea, exhibited varying growth responses to CuO NPs. A. thaliana generally displayed detrimental effects on growth, including root growth inhibition, altered gene expression, oxidative stress, and changes in metabolite profiles. Brassica species showed mixed responses, with some varieties showing increased growth, while others experienced growth inhibition, oxidative burst, and altered gene expression. This suggests that genetic variation and species-specific traits play a role in determining the response to CuO NPs.
According to the available data, the effect of CuO NPs on plant growth is influenced by several key factors, including:
Concentration of CuO NPs: Different concentrations of CuO NPs have been found to have varying effects on plant growth, ranging from promotion to inhibition.
Mode of application: The method of CuO NP application, such as foliar spray, soil amendment, hydroponics, or in vitro studies, can affect plant growth responses.
Plant species and genotypes: Different plant species and even different genotypes within the same species can exhibit different sensitivities to CuO NPs. Available results suggest that wheat is relatively tolerant among the monocotyledons, whereas no specific species can be identified among the dicotyledons with a similar general higher tolerance.
Although a growing body of research on the relationship between plants and CuO NPs is becoming available each year and the existing research provides valuable insights into the effects of CuO NPs on plant growth, there are several gaps that require further research fully understand the potential impact of CuO NPs on plant growth and health.
In future studies:
Mechanistic understanding: Further research is needed to fully understand how CuO NPs interact with plants at the cellular and molecular levels, including the specific pathways and signaling mechanisms involved in growth responses and oxidative stress induction.
Standardized experimental protocols: Developing standardized protocols for assessing the effects of CuO NPs on plant growth will improve result reproducibility and enable better comparisons between studies, enhancing our understanding of the overall impacts of CuO NPs on plants.
Ecological implications: Extending research to diverse plant communities and natural ecosystems is necessary to fully grasp the ecological implications of CuO NP exposure, including long-term effects on plant growth, biodiversity, and ecosystem functioning.
Interactions with other stressors: Investigating the interactive effects of CuO NPs with other environmental stressors, such as drought, temperature extremes, and chemical pollutants, provides a more realistic assessment of the potential risks posed by CuO NPs in complex environmental scenarios.
Acknowledgements
This work was supported by the National Research, Development and Innovation Office (NKFIH PD 131589). Open Access has been made possible by the University of Szeged Open Access Fund (grant number 6245).
Icons in the Graphical abstract by dDara, Freepik, zky.icon.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Gábor Feigl
Gábor Feigl obtained his Ph.D. degree in 2015, with a thesis focusing on the response of crucifers to heavy metal stress. Currently, he is dedicated to researching the effects of anthropogenic stressors, such as nanoparticles and plastics, on plants. He serves as the leader of the Environmental Plant Biology and Protein Biochemistry Group at the Department of Plant Biology, University of Szeged, Hungary.
References
- Adams J, Wright M, Wagner H, Valiente J, Britt D, Anderson A. 2017. Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol Biochem. 110:108–117. doi:10.1016/j.plaphy.2016.08.005.
- Adhikari T, Kundu S, Biswas AK, Tarafdar JC, Rao AS. 2012. Effect of copper oxide nano particle on seed germination of selected crops. J Agr Sci Technol. A. 2(6A):815.
- Ahmad MA, Javed R, Adeel M, Rizwan M, Ao Q, Yang Y. 2020. Engineered ZnO and CuO nanoparticles ameliorate morphological and biochemical response in tissue culture regenerants of candyleaf (Stevia rebaudiana). Molecules. 25(6):1356. doi:10.3390/molecules25061356.
- Ahmed B, Khan MS, Musarrat J. 2018. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): a study on growth dynamics and plant cell death. Environ Pollut. 240:802–816. doi:10.1016/j.envpol.2018.05.015.
- Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, … Nelson BC. 2012. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol. 46(3):1819–1827. doi:10.1021/es202660k.
- Azhar W, Khan AR, Muhammad N, Liu B, Song G, Hussain A, … Gan Y. 2020. Ethylene mediates CuO NP-induced ultrastructural changes and oxidative stress in Arabidopsis thaliana leaves. Environmental Science: Nano. 7(3):938–953. doi:10.1039/C9EN01302D.
- Azhar W, Khan AR, Salam A, Ulhassan Z, Qi J, Shah G, … Gan Y. 2023. Ethylene accelerates copper oxide nanoparticle-induced toxicity at physiological, biochemical, and ultrastructural levels in rice seedlings. Env Sci Pollut Res. 30(10):26137–26149. doi:10.1007/s11356-022-23915-8.
- Badawy AA, Abdelfattah NA, Salem SS, Awad MF, Fouda A. 2021. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology. 10(3):233. doi:10.3390/biology10030233.
- Baskar V, Nayeem S, Kuppuraj SP, Muthu T, Ramalingam S. 2018. Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in in vitro grown eggplant (Solanum melongena). 3 Biotech. 8:1–12. doi:10.1007/s13205-018-1386-9.
- Boboc M, Curti F, Fleacă AM, Jianu ML, Roşu AM, Curutiu C, … Grumezescu AM. 2017. Preparation and antimicrobial activity of inorganic nanoparticles: promising solutions to fight antibiotic resistance. In: Ficai Anton, Grumezescu Alexandru Mihai, editors. Nanostructures for antimicrobial therapy. Elsevier; p. 325–340. doi:10.1016/B978-0-323-46152-8.00014-7.
- Bonilla-Bird NJ, Ye Y, Akter T, Valdes-Bracamontes C, Darrouzet-Nardi AJ, Saupe GB, … Gardea-Torresdey JL. 2020. Effect of copper oxide nanoparticles on two varieties of sweetpotato plants. Plant Physiol Biochem. 154:277–286. doi:10.1016/j.plaphy.2020.06.009.
- Brar SK, Verma M, Tyagi RD, Surampalli RY. 2010. Engineered nanoparticles in wastewater and wastewater sludge–evidence and impacts. Waste Manage. 30(3):504–520. doi:10.1016/j.wasman.2009.10.012.
- Burachevskaya M, Minkina T, Mandzhieva S, Bauer T, Nevidomskaya D, Shuvaeva V, … Rajput V. 2021. Transformation of copper oxide and copper oxide nanoparticles in the soil and their accumulation by Hordeum sativum. Environ Geochem Health. 43:1655–1672. doi:10.1007/s10653-021-00857-7.
- Chakraborty N, Banerjee J, Chakraborty P, Banerjee A, Chanda S, Ray K, … Sarkar J. 2022. Green synthesis of copper/copper oxide nanoparticles and their applications: a review. Green Chem Lett Rev. 15(1):187–215. doi:10.1080/17518253.2022.2025916.
- Chung IM, Rekha K, Venkidasamy B, Thiruvengadam M. 2019. Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. rapa seedlings. Water Air Soil Pollut. 230:1–14. doi:10.1007/s11270-018-4051-3.
- Da Costa MVJ, Kevat N, Sharma PK. 2020. Copper oxide nanoparticle and copper (II) ion exposure in Oryza sativa reveals two different mechanisms of toxicity. Water Air Soil Pollut. 231:1–16. doi:10.1007/s11270-019-4368-6.
- Da Costa MVJ, Sharma PK. 2016. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica. 54:110–119. doi:10.1007/s11099-015-0167-5.
- Deng C, Wang Y, Cota-Ruiz K, Reyes A, Sun Y, Peralta-Videa J, … Gardea-Torresdey J. 2020. Bok choy (Brassica rapa) grown in copper oxide nanoparticles-amended soils exhibits toxicity in a phenotype-dependent manner: translocation, biodistribution and nutritional disturbance. J Hazard Mater. 398:122978. doi:10.1016/j.jhazmat.2020.122978.
- Dev A, Srivastava AK, Karmakar S. 2018. Nanomaterial toxicity for plants. Environ Chem Lett. 16:85–100. doi:10.1007/s10311-017-0667-6.
- Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, … Anderson AJ. 2012. Cuo and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res. 14:1–15. doi:10.1007/s11051-012-1125-9.
- Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H. 2011. Tio 2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit. 13(4):822–828. doi:10.1039/c0em00611d.
- Du W, Tan W, Peralta-Videa JR, Gardea-Torresdey JL, Ji R, Yin Y, Guo H. 2017. Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol Biochem. 110:210–225. doi:10.1016/j.plaphy.2016.04.024.
- El-Moneim DA, Dawood MF, Moursi YS, Farghaly AA, Afifi M, Sallam A. 2021. Positive and negative effects of nanoparticles on agricultural crops. Nanotechnol Env Eng. 6(2):21. doi:10.1007/s41204-021-00117-0.
- Faraz A, Faizan M, Hayat S, Alam P. 2022. Foliar application of copper oxide nanoparticles increases the photosynthetic efficiency and antioxidant activity in Brassica juncea. J Food Qual. 2022. doi:10.1155/2022/5535100.
- Fedorenko AG, Minkina TM, Chernikova NP, Fedorenko GM, Mandzhieva SS, Rajput VD, … Soldatov AV. 2021. The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum). Environ Geochem Health. 43:1673–1687. doi:10.1007/s10653-020-00530-5.
- Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C. 2002. Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun. 294(1):116–119. doi:10.1016/S0006-291X(02)00445-X.
- Gao M, Chang J, Wang Z, Zhang H, Wang T. 2023. Advances in transport and toxicity of nanoparticles in plants. J Nanobiotechnol. 21(1):75. doi:10.1186/s12951-023-01830-5.
- Garcia L, Welchen E, Gonzalez DH. 2014. Mitochondria and copper homeostasis in plants. Mitochondrion. 19:269–274. doi:10.1016/j.mito.2014.02.011.
- Gopalakrishnan Nair PM, Kim SH, Chung IM. 2014. Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant. 36:2947–2958. doi:10.1007/s11738-014-1667-9.
- Grigore ME, Biscu ER, Holban AM, Gestal MC, Grumezescu AM. 2016. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals. 9(4):75. doi:10.3390/ph9040075.
- Guan X, Gao X, Avellan A, Spielman-Sun E, Xu J, Laughton S, … Lowry GV. 2020. Cuo nanoparticles alter the rhizospheric bacterial community and local nitrogen cycling for wheat grown in a calcareous soil. Environ Sci Technol. 54(14):8699–8709. doi:10.1021/acs.est.0c00036.
- Hafeez A, Razzaq A, Mahmood T, Jhanzab HM. 2015. Potential of copper nanoparticles to increase growth and yield of wheat. J Nanosci Adv Technol. 1(1):6–11. doi:10.24218/jnat.2015.02.
- Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL. 2015. Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Env Sci: Proc Impacts. 17(1):177–185. doi:10.1039/C4EM00551A.
- Hou J, Wang X, Hayat T, Wang X. 2017. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ Pollut. 221:209–217. doi:10.1016/j.envpol.2016.11.066.
- Ibrahim AS, Ali GA, Hassanein A, Attia AM, Marzouk ER. 2022. Toxicity and uptake of CuO nanoparticles: evaluation of an emerging nanofertilizer on wheat (Triticum aestivum L.) plant. Sustainability. 14(9):4914. doi:10.3390/su14094914.
- Jośko I, Kusiak M, Xing B, Oleszczuk P. 2021. Combined effect of nano-CuO and nano-ZnO in plant-related system: from bioavailability in soil to transcriptional regulation of metal homeostasis in barley. J Hazard Mater. 416:126230. doi:10.1016/j.jhazmat.2021.126230.
- Kacziba B, Szierer Á, Mészáros E, Rónavári A, Kónya Z, Feigl G. 2023. Exploration the homeostasis of signaling molecules in monocotyledonous crops with different CuO nanoparticle tolerance. Plant Stress. 7:100145. doi:10.1016/j.stress.2023.100145.
- Kadri O, Karmous I, Kharbech O, Arfaoui H, Chaoui A. 2022. Cu and CuO nanoparticles affected the germination and the growth of barley (Hordeum vulgare L.) seedling. Bull Environ Contam Toxicol. 108(3):585–593. doi:10.1007/s00128-021-03425-y.
- Kamat JP, Devasagayam TPA, Priyadarsini KI, Mohan H. 2000. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology. 155(1–3):55–61. doi:10.1016/S0300-483X(00)00277-8.
- Karami Mehrian S, De Lima R. 2016. Nanoparticles cyto and genotoxicity in plants: mechanisms and abnormalities. Environ Nanotechnol Monit Manag. 6:184–193. doi:10.1016/j.enmm.2016.08.003.
- Koce JD. 2017. Effects of exposure to nano and bulk sized TiO2 and CuO in Lemna minor. Plant Physiol Biochem. 119:43–49. doi:10.1016/j.plaphy.2017.08.014.
- Kohatsu MY, Pelegrino MT, Monteiro LR, Freire BM, Pereira RM, Fincheira P, … Lange CN. 2021. Comparison of foliar spray and soil irrigation of biogenic CuO nanoparticles (NPs) on elemental uptake and accumulation in lettuce. Env Sci Pollut Res. 28:16350–16367. doi:10.1007/s11356-020-12169-x.
- Landa P, Cyrusova T, Jerabkova J, Drabek O, Vanek T, Podlipna R. 2016. Effect of metal oxides on plant germination: phytotoxicity of nanoparticles, bulk materials, and metal ions. Water Air Soil Pollut. 227:1–10. doi:10.1007/s11270-016-3156-9.
- Landa P, Dytrych P, Prerostova S, Petrova S, Vankova R, Vanek T. 2017. Transcriptomic response of Arabidopsis thaliana exposed to CuO nanoparticles, bulk material, and ionic copper. Environ Sci Technol. 51(18):10814–10824. doi:10.1021/acs.est.7b02265.
- Lee S, Chung H, Kim S, Lee I. 2013. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut. 224:1–11. doi:10.1007/s11270-013-1668-0.
- Li KE, Chang ZY, Shen CX, Yao N.. 2015. Toxicity of nanomaterials to plants. In: Manzer H. Siddiqui, Firoz Mohammad, Mohamed H. Al-Whaibi, editors. Nanotechnology and Plant Sciences - Nanoparticles and Their Impact on Plants. Springer; p. 101–123. doi:10.1007/978-3-319-14502-0_6.
- Lin D, Xing B. 2008. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol. 42(15):5580–5585. doi:10.1021/es800422x.
- Liu C, Yu Y, Liu H, Xin H. 2021. Effect of different copper oxide particles on cell division and related genes of soybean roots. Plant Physiol Biochem. 163:205–214. doi:10.1016/j.plaphy.2021.03.051.
- Liu R, Zhang H, Lal R. 2016. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water Air Soil Pollut. 227:1–14. doi:10.1007/s11270-015-2689-7.
- Ma X, Yan J. 2018. Plant uptake and accumulation of engineered metallic nanoparticles from lab to field conditions. Curr Opin Env Sci Health. 6:16–20. doi:10.1016/j.coesh.2018.07.008.
- Madhuban G, Rajesh K, Arunava G. 2012. Nano-pesticides-A recent approach for pest control. J Plant Prot Sci. 4(2):1–7.
- Moon YS, Park ES, Kim TO, Lee HS, Lee SE. 2014. SELDI-TOF MS-based discovery of a biomarker in Cucumis sativus seeds exposed to CuO nanoparticles. Environ Toxicol Pharmacol. 38(3):922–931. doi:10.1016/j.etap.2014.10.002.
- Murali M, Gowtham HG, Singh SB, Shilpa N, Aiyaz M, Alomary MN, … Amruthesh KN. 2022. Fate, bioaccumulation and toxicity of engineered nanomaterials in plants: current challenges and future prospects. Sci Total Environ. 811:152249. doi:10.1016/j.scitotenv.2021.152249.
- Nair PMG, Chung IM. 2014. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Env Sci Pollut Res. 21:12709–12722. doi:10.1007/s11356-014-3210-3.
- Nair PMG, Chung IM. 2015. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol Environ Saf. 113:302–313. doi:10.1016/j.ecoenv.2014.12.013.
- Naz S, Gul A, Zia M. 2020. Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol. 14(1):1–13. doi:10.1049/iet-nbt.2019.0176.
- Nekrasova GF, Maleva MG. 2007. Development of Anti oxidant Reactions in Elodea canadensis Leaves upon Short Term Exposure to High Ni2+, Zn2+, and Cu2+ Concentrations, Sovremennaya fiziologiya rastenii: ot molekul do eko sistem: Mat ly mezhdun. konf.(Modern Plant Physiology: From Molecules to Ecosystems. Proc. Int. Conf.). In Modern Plant Physiology: From Molecules to Ecosystems. Proceedings of International Conference (Vol. 278).
- Nie G, Zhao J, He R, Tang Y. 2020. Cuo nanoparticle exposure impairs the root tip cell walls of arabidopsis thaliana seedlings. Water Air Soil Pollut. 231:1–11. doi:10.1007/s11270-019-4368-6.
- Ochoa L, Medina-Velo IA, Barrios AC, Bonilla-Bird NJ, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. 2017. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci Total Environ. 598:513–524. doi:10.1016/j.scitotenv.2017.04.063.
- Oorts K. 2013. Copper. In: B. J. Alloway, editor. Heavy metals in soils. Dordrecht: Springer Science + Business Media; p. 367–394.
- Pelegrino MT, Kohatsu MY, Seabra AB, Monteiro LR, Gomes DG, Oliveira HC, … Lange CN. 2020. Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Environ Monit Assess. 192:1–14. doi:10.1007/s10661-020-8188-3.
- Peng C, Xu C, Liu Q, Sun L, Luo Y, Shi J. 2017. Fate and transformation of CuO nanoparticles in the soil–rice system during the life cycle of rice plants. Environ Sci Technol. 51(9):4907–4917. doi:10.1021/acs.est.6b05882.
- Pu S, Yan C, Huang H, Liu S, Deng D. 2019. Toxicity of nano-CuO particles to maize and microbial community largely depends on its bioavailable fractions. Environ Pollut. 255:113248. doi:10.1016/j.envpol.2019.113248.
- Qu H, Ma C, Xing W, Xue L, Liu H, White JC, … Xing B. 2022. Effects of copper oxide nanoparticles on Salix growth, soil enzyme activity and microbial community composition in a wetland mesocosm. J Hazard Mater. 424:127676. doi:10.1016/j.jhazmat.2021.127676.
- Rafique M, Shaikh AJ, Rasheed R, Tahir MB, Bakhat HF, Rafique MS, Rabbani F. 2017. A review on synthesis, characterization and applications of copper nanoparticles using green method. Nano. 12(04):1750043. doi:10.1142/S1793292017500436.
- Rai P, Singh VP, Peralta-Videa J, Tripathi DK, Sharma S, Corpas FJ. 2021. Hydrogen sulfide (H2S) underpins the beneficial silicon effects against the copper oxide nanoparticles (CuO NPs) phytotoxicity in Oryza sativa seedlings. J Hazard Mater. 415:124907. doi:10.1016/j.jhazmat.2020.124907.
- Rawat S, Cota-Ruiz K, Dou H, Pullagurala VL, Zuverza-Mena N, White JC, … Gardea-Torresdey JL. 2021. Soil-Weathered CuO Nanoparticles compromise foliar health and pigment production in spinach (Spinacia oleracea). Environ Sci Technol. 55(20):13504–13512. doi:10.1021/acs.est.0c06548.
- Rawat S, Pullagurala VL, Hernandez-Molina M, Sun Y, Niu G, Hernandez-Viezcas JA, … Gardea-Torresdey JL. 2018. Impacts of copper oxide nanoparticles on bell pepper (Capsicum annum L.) plants: a full life cycle study. Env Sci Nano. 5(1):83–95. doi:10.1039/C7EN00697G.
- Reddy PVL, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. 2016. Lessons learned: are engineered nanomaterials toxic to terrestrial plants? Sci Total Environ. 568:470–479. doi:10.1016/j.scitotenv.2016.06.042.
- Roy D, Adhikari S, Adhikari A, Ghosh S, Azahar I, Basuli D, Hossain Z. 2022. Impact of CuO nanoparticles on maize: comparison with CuO bulk particles with special reference to oxidative stress damages and antioxidant defense status. Chemosphere. 287:131911. doi:10.1016/j.chemosphere.2021.131911.
- Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam V. 2017. Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnol. 15(1):1–19. doi:10.1186/s12951-017-0268-3.
- Servin AD, Castillo-Michel H, Hernandez-Viezcas JA, Diaz BC, Peralta-Videa JR, Gardea-Torresdey JL. 2012. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ Sci Technol. 46(14):7637–7643. doi:10.1021/es300955b.
- Shabbir Z, Sardar A, Shabbir A, Abbas G, Shamshad S, Khalid S, … Shahid M. 2020. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere. 259:127436. doi:10.1016/j.chemosphere.2020.127436.
- Shaw AK, Hossain Z. 2013. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere. 93(6):906–915. doi:10.1016/j.chemosphere.2013.05.044.
- Shehabeldine AM, Amin BH, Hagras FA, Ramadan AA, Kamel MR, Ahmed MA, … Salem SS. 2023. Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Appl Biochem Biotechnol. 195(1):467–485. doi:10.1007/s12010-022-04120-2.
- Shende S, Rathod D, Gade A, Rai M. 2017. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.). IET Nanobiotechnol. 11(7):773–781. doi:10.1049/iet-nbt.2016.0179.
- Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK. 2011. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut. 159(5):1277–1282. doi:10.1016/j.envpol.2011.01.028.
- Shi J, Peng C, Yang Y, Yang J, Zhang H, Yuan X, … Hu T. 2014. Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology. 8(2):179–188. doi:10.3109/17435390.2013.766768.
- Siddiqi KS, Husen A. 2020. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: a review. Biomater Res. 24(1):1–15. doi:10.1186/s40824-020-00188-1.
- Singh D, Kumar A. 2016. Impact of irrigation using water containing CuO and ZnO nanoparticles on spinach oleracea grown in soil media. Bull Environ Contam Toxicol. 97:548–553. doi:10.1007/s00128-016-1872-x.
- Singh D, Kumar A. 2020. Quantification of metal uptake in Spinacia oleracea irrigated with water containing a mixture of CuO and ZnO nanoparticles. Chemosphere. 243:125239. doi:10.1016/j.chemosphere.2019.125239.
- Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, … Wang H. 2016. Effects of CuO nanoparticles on Lemna minor. Botanical Studies. 57:1–8. doi:10.1186/s40529-016-0118-x.
- Song W, Zhao B, Wang C, Ozaki Y, Lu X. 2019. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J Mater Chem B. 7(6):850–875. doi:10.1039/C8TB02878H.
- Soria NGC, Bisson MA, Atilla-Gokcumen GE, Aga DS. 2019. High-resolution mass spectrometry-based metabolomics reveal the disruption of jasmonic pathway in Arabidopsis thaliana upon copper oxide nanoparticle exposure. Sci Total Environ. 693:133443. doi:10.1016/j.scitotenv.2019.07.249.
- Subpiramaniyam S, Hong SC, Yi PI, Jang SH, Suh JM, Jung ES, … Cho LH. 2021. Influence of sawdust addition on the toxic effects of cadmium and copper oxide nanoparticles on Vigna radiata seeds. Environ Pollut. 289:117311. doi:10.1016/j.envpol.2021.117311.
- Tang Y, He R, Zhao J, Nie G, Xu L, Xing B. 2016. Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ Pollut. 212:605–614. doi:10.1016/j.envpol.2016.03.019.
- Tapan A, Biswas AK, Kundu S. 2010. Nano-fertiliser-a new dimension in agriculture. Indian J Fertilisers. 6(8):22–24.
- Tiwari DK, Dasgupta-Schubert N, Villaseñor Cendejas LM, Villegas J, Carreto Montoya L, Borjas García SE. 2014. Interfacing carbon nanotubes (CNT) with plants: enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl Nanosci. 4:577–591. doi:10.1007/s13204-013-0236-7.
- Vassell J, Racelis A, Mao Y. 2019. Effects of CuO nanoparticles on the growth of kale. ES Materials & Manufacturing. 5:19–23. doi:10.30919/esmm5f212.
- Velicogna JR, Schwertfeger DM, Beer C, Jesmer AH, Kuo J, Chen H, … Princz JI. 2020. Phytotoxicity of copper oxide nanoparticles in soil with and without biosolid amendment. NanoImpact. 17:100196. doi:10.1016/j.impact.2019.100196.
- Wang S, Liu H, Zhang Y, Xin H. 2015. The effect of CuO NPs on reactive oxygen species and cell cycle gene expression in roots of rice. Environ Toxicol Chem. 34(3):554–561. doi:10.1002/etc.2826.
- Wang W, Liu J, Ren Y, Zhang L, Xue Y, Zhang L, He J. 2020a. Phytotoxicity assessment of copper oxide nanoparticles on the germination, early seedling growth, and physiological responses in Oryza sativa L. Bull Environ Contam Toxicol. 104:770–777. doi:10.1007/s00128-020-02850-9.
- Wang W, Ren Y, He J, Zhang L, Wang X, Cui Z. 2020b. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L. Env Sci Pollut Res. 27:31505–31515. doi:10.1007/s11356-020-09338-3.
- Wang X, Xie H, Wang P, Yin H. 2023. Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Materials. 16(8):3097. doi:10.3390/ma16083097.
- Wang Y, Deng C, Cota-Ruiz K, Peralta-Videa JR, Sun Y, Rawat S, … Gardea-Torresdey JL. 2020c. Improvement of nutrient elements and allicin content in green onion (allium fistulosum) plants exposed to CuO nanoparticles. Sci Total Environ. 725:138387. doi:10.1016/j.scitotenv.2020.138387.
- Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B. 2012. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L). Environ Sci Technol. 46(8):4434–4441. doi:10.1021/es204212z.
- Xiong T, Dumat C, Dappe V, Vezin H, Schreck E, Shahid M, … Sobanska S. 2017. Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environ Sci Technol. 51(9):5242–5251. doi:10.1021/acs.est.6b05546.
- Xiong T, Zhang S, Kang Z, Zhang T, Li S. 2021a. Dose-dependent physiological and transcriptomic responses of lettuce (Lactuca sativa L.) to copper oxide nanoparticles—insights into the phytotoxicity mechanisms. Int J Mol Sci. 22(7):3688. doi:10.3390/ijms22073688.
- Xiong T, Zhang T, Xian Y, Kang Z, Zhang S, Dumat C, … Li S. 2021b. Foliar uptake, biotransformation, and impact of CuO nanoparticles in Lactuca sativa L. var. ramosa Hort. Environ Geochem Health. 43:423–439. doi:10.1007/s10653-020-00734-9.
- Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L. 2015. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int J Environ Res Public Health. 12(12):15100–15109. doi:10.3390/ijerph121214963.
- Yang Z, Xiao Y, Jiao T, Zhang Y, Chen J, Gao Y. 2020. Effects of copper oxide nanoparticles on the growth of rice (oryza sativa L.) seedlings and the relevant physiological responses. Int J Environ Res Public Health. 17(4):1260. doi:10.3390/ijerph17041260.
- Yasmeen F, Raja NI, Ilyas N, Komatsu S. 2018. Quantitative proteomic analysis of shoot in stress tolerant wheat varieties on copper nanoparticle exposure. Pl Mol Bio Rep. 36:326–340. doi:10.1007/s11105-018-1082-2.
- Yue L, Zhao J, Yu X, Lv K, Wang Z, Xing B. 2018. Interaction of CuO nanoparticles with duckweed (Lemna minor. L): uptake, distribution and ROS production sites. Environ Pollut. 243:543–552. doi:10.1016/j.envpol.2018.09.013.
- Zafar H, Ali A, Zia M. 2017. Cuo nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. Appl Biochem Biotechnol. 181:365–378. doi:10.1007/s12010-016-2217-2.
- Zhang D, Liu X, Ma J, Yang H, Zhang W, Li C. 2019. Genotypic differences and glutathione metabolism response in wheat exposed to copper. Environ Exp Bot. 157:250–259. doi:10.1016/j.envexpbot.2018.06.032.
- Zuverza-Mena N, Medina-Velo IA, Barrios AC, Tan W, Peralta-Videa JR, Gardea-Torresdey JL. 2015. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Env Sci Proc Impacts. 17(10):1783–1793. doi:10.1039/C5EM00329F.