?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In agricultural production, intercropping is a widely used system with many benefits. Lupin (Lupinus angustifolius L.) is a legume that contains a large variety of plant secondary metabolites, which have multiple functions in the plant, e.g. signalling, nodulation and stress response. An untargeted metabolomics approach was applied to investigate how the metabolome of lupin was affected by intercropped barley (Hordeum vulgare L.). The only primary metabolite of lupin affected by intercropping was tryptophan. Several secondary metabolites were affected by intercropping in lupin, and five flavonoids were annotated hereof. The flavonoid levels were increased, and tryptophan levels decreased in lupin when intercropped. Two flavonoids are prenylated, and prenylated flavonoids are believed to play a role in the plant’s stress response. Furthermore, flavonoids are involved in plant defence and the nodulation process. Thus the present flavonoids may affect regulation of lupin N2-fixation activity.
Highlights
Intercropping caused a change in the secondary metabolites of Lupinus angustifolius L.
Prenylated flavonoids were generated in Lupinus angustifolius L. as a response to intercropping
Tryptophan was the only identified primary metabolite affected by intercropping
Introduction
Intercropping is a well-known technique in agricultural production, but is presently only implemented to a small extent in Europe. Mixing cereal crops with legumes, e.g. lupin or soybean, has several benefits, including reduced greenhouse gas emissions, improved pest and weed control and increased soil fertility (Stagnari et al. Citation2017). Leguminous plants can fix atmospheric nitrogen (N) in the soil via root nodules, and serve as natural fertilizers to benefit cropping systems (Stagnari et al. Citation2017; Jensen et al. Citation2004). Legumes are used both as a pre-crop in a rotation system, allowing the following crop, e.g. barley or wheat, to take up the nitrogen fixed in the soil, or they can be used as a co-crop where the non-leguminous crop will benefit from the fixed N (Fustec et al. Citation2010). Angus et al. (Citation2015), e.g. reported an increase in wheat yield with legumes as pre-crop (Angus et al. Citation2015), and positive effects, such as weed control, yield stability and grain quality are reported (Sarunaite et al. Citation2010; Bilalis et al. Citation2010). Furthermore, legumes add both carbon (C) and N to the soil via primary metabolites, which stimulates soil C stabilisation (Peixoto et al. Citation2022), and regulates plant microbial interactions via secondary metabolites (Czaban et al. Citation2018). Hence, we need deeper understanding of the regulation of primary and secondary metabolites in legumes with and without competing crops to optimise intercropping plant communities.
Lupin is a leguminous plant presently with limited use in European agriculture (Stagnari et al. Citation2017). It contains a large variety of secondary metabolites, including alkaloids, tannins and flavonoids (Hama et al. Citation2020; Khan et al. Citation2015). Flavonoids play a key role in the nodulation process (Liu et al. Citation2016; Weston et al. Citation2013). They are exuded into the soil from the legume roots, and induce specific nod genes in the rhizobia. This leads to the rhizobia’s infection of the legume roots and thereby, formation of root nodules in which atmospheric N is fixed (Jain et al. Citation2002). Although the nodulation mechanism has been investigated and reviewed to a large extent (Fustec et al. Citation2010; Ferguson et al. Citation2010; Wichern et al. Citation2008), little is known about which flavonoids play a role in the nodulation process. It was suggested that the plant species and soil microorganisms determine the specific flavonoids involved in the nodulation (Liu et al. Citation2016). Studies have been published on both flavonoid subclasses and specific flavonoids involved in nodulation. Zhang et al. (Citation2009) found that flavones, a flavonoid subclass, might be nod gene inducers in Medicago truncatula nodulated by Sinorhizobium meliloti (Zhang et al. Citation2009). Novak et al. (Citation2002) reported the nod inducing effect of the flavanone, naringenin, in Rhizobium leguminosarum bv. viciae in pea (Novák et al. Citation2002). Similar findings were reported by Abdel-Lateif et al. (Citation2013). Here, naringenin induced nodulation in a chalcone synthase knockout of Casuarina glauca by Agrobacterium rhizogenes strain ARqua1.
Despite several known benefits of growing legumes in intercropping systems (Jensen et al. Citation2004; Carton et al. Citation2020; Kirkegaard et al. Citation2008), knowledge of how intercropping affects the metabolome of lupin is scarce. Many factors can affect the plant metabolome, and large variations in plant secondary metabolites within a small field have been reported (Andersen et al. Citation2022). Previous studies have shown that co-crops affect the plant metabolome. Yang et al. (Citation2017) reported an increase in fatty acid content in intercropped soybean seeds compared to sole-cropped soybeans seeds. Duan et al. (Citation2021) showed that in tea plants intercropped with soybeans the biosynthesis of amino acids and flavonoids was affected in the tea plants. Similar effects on amino acid content in tea plants were reported by Wu et al. (Citation2021). Because the secondary metabolites of lupin play key roles in many biological processes, such as nodulation and plant defence (Falcone Ferreyra et al. Citation2012), it is imperative to investigate how a co-crop affects the lupin metabolome and thereby key biological processes.
Up to today, no studies have investigated the changes in the lupin metabolome caused by intercropping. A better understanding of intercropping and its effects on the crops will provide helpful knowledge for agricultural production to utilise the benefits of intercropping in the green transition. The present study aimed to investigate the effect of spring barley (Hordeum vulgare L.) on the metabolome of lupin (Lupinus angustifolius L.) when intercropped compared to lupin as a sole-crop. This was done in a pot experiment with lupin as the primary crop barley as the competing crop and with a liquid chromatography-mass spectrometry-based untargeted metabolomics approach.
Material and methods
Pot experiment
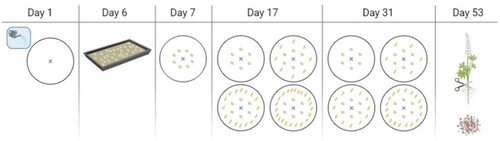
Eighteen 10-L pots were filled with a sandy loam soil (8% clay (<2 µm), 30% silt (2–63 µm), 59% sand (63–2000µm)) from the Aarhus University (AU) experimental station Foulumgaard (56°30´N, 9°34´E). The pots were placed in a semi-field facility at AU Flakkebjerg with controlled watering daily (34 mL/min for 10 min), and plants were grown in July – September 2021. All pots were irrigated six days before planting the lupin seeds, and weeds were removed continuously. The primary crop, lupin, and the competing crop, barley, were planted in triplicate (Berg et al. Citation2013; Fernie et al. Citation2011; Salem et al. Citation2020) in the following setup: lupin pure, lupin:barley (1:1), lupin:barley (1:2) and lupin:barley (1:3), in the following referred to as LP, LB(1:1), LB(1:2) and LB(1:3), respectively. Prior to planting, the lupin seeds were inoculated with HiStick® Lupin inoculum mixture (Bradyrhizobium spp.) (BASF A/S, Copenhagen, Denmark). Inoculum (∼5 g) and 10 mL 0.1 g/mL sucrose solution were mixed in a 50-mL Falcon tube. The inoculum mixture was transferred to the lupin seeds, mixed and left for drying in the fume hood overnight. The inoculated seeds were planted in surplus, eight seeds, and after 17 days reduced to six plants in all pots. Hereafter, barley seeds were planted in surplus, 8 seeds in LB(1:1) pots, 16 seeds in LB(1:2) pots and 22 seeds in LB(1:3) pots around the germinated lupin seeds. The number of barley plants were reduced to 6, 12 and 18 for LB(1:1), LB(1:2) and LB(1:3), respectively, after 14 days (Hazrati et al. Citation2022). After an additional 22 days, plants were harvested. An illustrated timeline is shown in . The lupin plants were carefully removed from the pots, the soil attached to the roots were collected, and the roots were washed with tap water. Lupin shoots and roots were separated and weighed (Table S1), and the individual plant parts of each pot were combined and immediately immersed in liquid nitrogen. All samples were stored at −20 °C prior to freeze drying. The lupin plants had developed nodules and seed pods, and the seed pods were gently separated with forceps from the lupin shoot samples after freeze-drying. The samples were freeze-dried for 48 hr, ground and stored at room temperature.
Figure 1. Timeline of the pot experiment. The watering system is marked with a blue X in the centre of each pot. Round seeds are lupin and long oval seeds are barley. Four pots are shown: LP, LB(1:1), LB(1:2) and LB(1:3). Day 1: Daily irrigation of all pots is initiated. Day 6: Inoculation of lupin seeds. Day 7: Sowing of 8 lupin seeds. Day 17: Reduction to 6 lupin seeds and sowing of 8, 16 and 22 barley seeds, respectively. Day 31: Reduction to 6, 12 and 18 barley seeds, respectively. Day 53: Harvest of both lupin and barley plants.
Extraction of metabolites
Extraction of root and shoot samples
Approx. 20 mg of ground lupin roots or shoots was transferred to a 2-mL Eppendorf tube, and 1.0 mL 70% MeOH was added to the sample (Salem et al. Citation2020). After sonication for 1 hr, the sample was centrifuged (4,500 G for 10 min). The supernatant was removed. 1.0 mL 70% MeOH was added to the sample, and the sonication and centrifugation was repeated. The supernatants were combined, filtered (KX Syringe filter, PTFE 13 mm, 0.22 μm), and 500 μL was transferred to a 1.5-mL Eppendorf tube and evaporated to dryness. The dry samples were stored at −20 °C until analysis.
Extraction of soil samples
Approx. 0.5 g of lupin soil was transferred to a 50-mL Falcon tube, and 25 mL 70% MeOH was added to the sample. After sonication for 1 hr, the sample was centrifuged (4,500 G for 10 min). The supernatant was removed, 25 mL 70% MeOH was added to the sample, and the sonication and centrifugation was repeated. The supernatants were combined, filtered (KX Syringe filter, PTFE 13 mm, 0.22 μm), and 500 μL was transferred to a 1.5-mL Eppendorf tube and evaporated to dryness. The dry samples were stored at −20 °C until analysis.
Sample preparation
Dry lupin soil, root and shoot extracts were dissolved in 60% EtOH in a volume corresponding to 50% of the evaporated volume, vortexed, heated at 60 °C for 1 hr, and 100 μL of each samples was transferred to a 96-well plate with seal and stored at 5°C until analysis.
Untargeted metabolic analysis using ultra high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF MS)
The untargeted analysis in this study consists of an LC-MS analysis of both primary and secondary metabolites. The term ‘feature’ will refer to an ion with a specific retention time and mass to charge ratio (m/z) throughout this article.
UHPLC-QTOF MS analysis
The samples were analysed on a UHPLC system with a VanGuard HSS T3 C18 column (2.1 × 100 mm2, 1.8 μm) coupled with a Premier QTOF MS system (Waters Corporation, Manchester, United Kingdom). The injection volume was set to 5 µL. The mobile phases (MPs) consisted of 0.1% FA in Milli-Q water (MP A), 10% 1 M ammonium acetate in MeOH (MP B), MeOH (MP C) and isopropanol (MP D). The gradient is described in Table S2. All samples were analysed in both positive and negative electrospray ionisation mode with a capillary voltage of 3.2 and 2.8 kV, respectively. The cone voltage was set to 20 kV. The temperature of the ion source and desolvation gas (nitrogen) was set to 120 and 400 °C, respectively (Bejder et al. Citation2021). For every 14 experimental samples, an additional sample (metabolome standards mixture (Barri et al. Citation2012) for quality control and analytical stability of the system was injected, and MP A was used as blank.
Data pre-processing and cleaning of UHPLC-QTOF MS data
All raw data files (.raw) were converted into.mzml files using the freely available converter ProteoWizard (https://proteowizard.sourceforge.io/index.htm) (Chambers et al. Citation2012). A significant shift in retention time was observed for sample ‘LB(1:1) roots_1’, thus this sample was excluded and replaced with the mean of the remaining two sample replicates ‘LB(1:1) roots_2’ and ‘’LB(1:1) roots_3’ to allow for the statistical analysis. MZmine 2.53 software (http://mzmine.github.io/) (Pluskal et al. Citation2010) was used for mass detection, chromatogram builder, chromatogram deconvolution (local minimum search), isotopic peaks grouper, peak alignment (join aligner), duplicate peak filer, peak list row filter, and gap filling (peak finder). The settings for each step are available in the supplementary material (see Table S3). The MZmine-pre-processed data were further cleaned using MATLAB (R2017b) by removing features present in the initial blank sample, features eluting early (Rt < 0.3 min and Rt > 9.49 min (positive mode) or > 8.75 min (negative mode)) and potential isotopes and duplicates. In MetaboAnalyst 5.0 (Xia et al. Citation2009), the cleaned data were log-transformed and Pareto-scaled, and PCA and ANOVA with post hoc Tukey’s test were performed.
Data-dependent acquisition and metabolite annotation
Features presenting a clear separation between treatments (LP and LB(1:3)) and with a peak intensity 300 cps (listed in Table S4) were further investigated by obtaining collision-induced dissociation mass spectra with the application of 15, 25 and 40 eV, on a Vion QTOF MS (Waters) using the UHPLC method described above. The DDA involved a single full scan followed by 5 MS/MS scans with a dynamic exclusion of 3 s. The spectral information was compared to data available in several databases, FooDB (https://foodb.ca/), mzCloud (https://www.mzcloud.org/), MoNA (https://mona.fiehnlab.ucdavis.edu/), PubChem (https://pubchem.ncbi.nlm.nih.gov/) and an in-house, authentic standard-based library (Xi et al. Citation2021), to facilitate the annotation of the metabolites. Tryptophan was identified at level 1, whereas the flavonoid structures and compound annotations in this study are at level 3 (Sumner et al. Citation2007), meaning that the compound groups have been identified clearly by comparison with spectral databases but with some residual uncertainty about the exact structures. A level 2 identification requires that the obtained spectra are compared with published spectra, however no exact spectral matches could be observed. A level 1 identification requires comparison with an authentic standard, which was impossible in this study due to the unavailability of commercially available flavonoid standards.
Results
The effect of intercropping on lupin metabolites
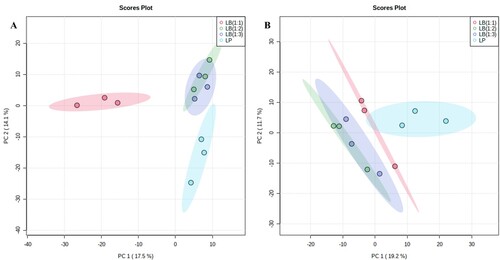
In total, 12,826 and 4,682 features were obtained after pre-processing and data cleaning in positive and negative ionisation mode, respectively, in lupin root, shoot and soil samples. By PCA and ANOVA, 27 (+) and 33 (-) features in roots were selected, discriminating the treatments. For the shoots, 29 (+) and 32 (-) features were selected, discriminating the treatments. Lastly, 32 features discriminated by treatment were selected for the soil in positive ionisation mode, but no features were discriminant in negative ionisation mode. The PCA score plots showed a separation of LP and the lupin intercropped with barley (LB(1:1), LB(1:2) and LB(1:3)). The PCA score plots for the root samples are shown in , and PCA score plots for shoot and soil samples are shown in Figures S1 and S2, respectively. For both root, shoot and soil samples, a distinct separation of intercropped lupins (LB(1:1), LB(1:2) and LB(1:3)) and LP was observed.
Structure elucidation
Because no distinct separation was observed between the intercropped lupins (LB(1:1), LB(1:2) and LB(1:3)), the following is focused on the comparison of LP and LB(1:3).
The majority of the selected features were present at very low concentrations and therefore excluded from MS/MS analysis and further compound annotation. Six metabolites in either lupin roots or shoots, of which the concentration was affected by the presence of barley, were annotated by comparing the MS spectra to spectral databases. The annotated metabolites are summarised in with m/z values of precursor and fragment ions with 4 and 3 decimals, respectively. In the Results and Discussion section only two decimals are used for easier reading. The spectra for each of the six metabolites are given in Figures S3–S8.
Table 1. Overview of annotated metabolites in lupin roots and shoots affected by intercropping with barley (LP compared to LB(1:3)).
In positive ionisation mode, five flavonoid-based metabolites were annotated. The MS spectra suggest that metabolite 1, 3 and 5 are branched flavonoids, and metabolite 2 and 6 are glycosylated. An example of the structure elucidation following MS/MS fragmentation is shown in . However, using this method it is not possible to determine the position of sugars, branches and hydroxyl groups, which may be placed in ortho, meta or para position, nor can the structure of any glycoside be identified. Metabolite 4 is identified as the primary metabolite tryptophan.
Figure 3. Example of the structural elucidation following MS/MS fragmentation of metabolite 1 with spectral peaks in MS1 (top) and MS2 (bottom) (A) representing the three structural fragments, (m/z 165.018, 365.102 and 347.092) and the precursor ion (m/z 421.165) (B).
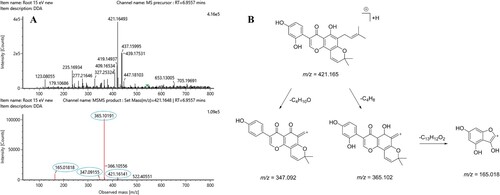
The database searches for metabolite 1 gave a few potential metabolite matches, which were further reduced based on the chemical structure and the likelihood of being a plant metabolite. Metabolite 1 is categorised as a branched flavonoid with m/z 421.16 ([M + H]+) as the precursor ion and m/z 365.10 as the main fragment. Minor fragment ions observed are m/z 165.02 and m/z 347.09. The loss of m/z 56.06 (precursor ion minus the main fragment ion) was reported in the literature as a characteristic mass loss in prenylated flavonoids (Simons et al. Citation2009), which is in accordance with the suggested compound structure for metabolite 1. The minor fragment, m/z 165.02, is found in the fragmentation pattern of kaempferol (March et al. Citation2004; Cuyckens et al. Citation2004), suggesting the flavonoid back bone of metabolite 1 to be kaempferol or a constitutional isomer as shown in .1.
Figure 4. Proposed chemical structure of the six metabolites altered by the presence of barley in a lupin cropping system. Metabolite 1: a branched flavonoid – here kaempferol as backbone (1), metabolite 2: a flavonoid with a hexose – here luteolin as backbone with glucoside (2), metabolite 3: a branched flavonoid – here apigenin as backbone (3), metabolite 4: tryptophan (4), metabolite 5: a branched flavonoid – here kaempferol as backbone (5) and metabolite 6: a flavonoid with two hexoses and a malonyl – here apigenin as backbone with a diglucoside, one of which with a malonic acid ester (6).
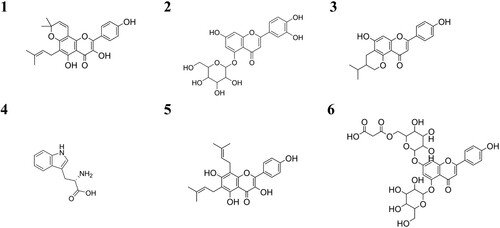
For metabolite 2, the database searches gave more than 50 potential structures, the majority being flavonoid glycosides. The main precursor ion and main fragment ion of metabolite 2 is m/z 449.11 ([M + H]+) and m/z 287.05, respectively. A loss of m/z 162.05 corresponds to a hexose moiety, e.g. glucose losing oxygen or rhamnose. Sugar-substituted flavonoids in plants have been reported extensively (Hazrati et al. Citation2021; Wojakowska et al. Citation2013). The main fragment m/z 287.05 suggests a flavonoid structure with four hydroxyl groups, such as luteolin and kaempferol. The fragment, m/z 153.08, is a common flavonoid fragment (Zhang et al. Citation2015), and together with the presence of several minor fragments, e.g. m/z 161.02 and m/z 259.06, and the absence of m/z 165, the fragmentation supports the annotation of luteolin or a constitutional isomer (Cuyckens et al. Citation2004) with a hexose moiety as shown in .2.
The database searches for metabolite 3 gave several potential metabolite matches, which were further reduced based on the chemical structure and the likelihood of being a plant metabolite. Like metabolite 2, the common flavonoid fragment m/z 153.02 is present in metabolite 3. The precursor ion m/z 353.10 ([M + H]+) and several characteristic fragments (m/z 271.06, m/z 283.06, m/z 299.05 and m/z 311.04) correspond to a branched flavonoid. In particular, m/z 271.06 is the [M + H]+ precursor ion for multiple flavonoids, e.g. apigenin and genistein (Wojakowska et al. Citation2013). A suggested structure of metabolite 3 is shown in .3.
Close to 100 suggested structures were obtained in the database search for metabolite 4, the majority being nitrogen containing structures. The best matches were tryptophan and different indoles. The ion m/z 205.10 ([M + H]+) is the precursor of metabolite 4 with five distinct fragments; m/z 91.05, m/z 118.06, m/z 146.06, m/z 170.06 and m/z 188.07. The fragment, m/z 188.07, corresponds to the loss of the primary amine, m/z 118.06, corresponds to the indole, and the smallest fragment (m/z 91.05) corresponds to a partial indole structure. The remaining two fragments represent different fragmentations of the formed acrylic acid. Tryptophan was confirmed by spectral comparison with an authentic standard, and therefore, .4 is the identified structure of metabolite 4, tryptophan.
Less than 20 structures were suggested for metabolite 5 in the database search. The best matches were branched flavonoids, and other structures were excluded based on their likelihood of being plant metabolites. In the spectra of metabolite 5, m/z 423.18 is the precursor ion and two major fragments are observed, m/z 367.12 and m/z 311.06. The two fragments correspond to a loss of 1× and 2× m/z 56.02, respectively, and is caused by the loss of prenyl group(s) in analogy to metabolite 1. Precursor ion m/z 423.18 ([M + H]+) and the fragment ions suggest that metabolite 5 is a branched flavonoid. The fragment m/z 165.06 suggests that the flavonoid back bone may be kaempferol or a similar structure, as shown in .5.
For metabolite 6, the database searches gave approx. 40 potential structures, the majority being flavonoids and flavonoid glycosides. The precursor ion of metabolite 6 is m/z 681.17 ([M + H]+), with the major fragment being m/z 271.06. Minor fragments are m/z 519.11, m/z 231.05, m/z 153.02 and m/z 109.03. Metabolite 6 is suggested to be a flavonoid with two hexoses and a malonyl group attached. The fragment m/z 271.06 is likely to be a flavonoid, e.g. apigenin or genistein, as stated above for metabolite 3. This chemical structure is further supported by two fragments, m/z 519.11 and m/z 595.17, where m/z 519.11 corresponds to the flavonoid with a hexose and a malonyl group attached. In MS1, m/z 595.17 is detected which is a loss of m/z 86.00, representing the loss of the malonyl group (Cuyckens et al. Citation2004). In addition, these two fragments indicate that the structure of a flavonoid with a linear branch of hexose-hexose-malonyl is not possible, as both the malonyl group and one of the hexoses can be lost from the molecule independently. A possible molecular structure may be the two hexoses substituted on two different hydroxyl groups in the flavonoid backbone, and the malonyl may either be linked to one of the hexoses or yet another flavonoid hydroxyl group. A suggested structure in shown in .6.
Discussion
Identification and the role of tryptophan in lupin roots in intercropping
In MS1 of metabolite 4, m/z 205.10 is observed. This m/z corresponds to the pseudomolecular ion of tryptophan ([M + H]+), exact mass = 204.09 Da (https://pubchem.ncbi.nlm.nih.gov/), and the four observed fragments in the spectra are consistent with tryptophan as well. The structure of tryptophan was confirmed by using a reference standard. Tryptophan is a precursor for auxins, e.g. indole-3-acetic acid (Duca et al. Citation2020), plant hormones playing a role in several plant growth and developmental aspects (Chandler Citation2016), including nodulation in legumes (Wang et al. Citation2019). Liu et al. (Citation2017) reported an increase in nodules numbers and dry weight per nodule in faba bean when intercropped with wheat compared to monocropped faba bean. However, they could not unveil the underlying mechanism, as nodulation is a complex process (Liu et al. Citation2017). Similar findings of intercropping on nodulation have been reported (Bargaz et al. Citation2016; Hu et al. Citation2017). Thus, it may be speculated that intercropping increases the biosynthesis of auxins from tryptophan, reducing the amount of tryptophan in the roots and increasing the nodulation in lupin roots to increase the N-fixation as a response to the barley plants present in the intercropped system.
Structure elucidation of secondary metabolites affected by competing plants in lupin
Several thousands of flavonoids are known and have been reported in the literature (Falcone Ferreyra et al. Citation2012). The structure of flavonoids is based on a common backbone, and the large variety is to a great extent caused by different side chains and hydroxylation and glycosylation positions (Shi et al. Citation2021; Xiao et al. Citation2016). This can make it challenging and cumbersome to identify unknown flavonoids and obtain authentic standards for all possible isomers (Geng et al. Citation2016). Therefore, the annotated compounds in the present study are suggestions based on the data available in databases as well as information reported in the literature. Still, there are several different structural possibilities, especially for the exact position of hydroxyl groups, and thereby alternative candidates (Kind et al. Citation2006).
The suggested structures of metabolite 1, 2 and 6 are based on flavonoids, as fragment ions corresponding to the mass of common flavonoids are observed (m/z 271 and m/z 287) (Cuyckens et al. Citation2004; Wojakowska et al. Citation2013). In the spectra of metabolite 1 and 5 no fragment ion of a commonly known flavonoid was observed. Metabolite 1 and 5 are suggested to contain one and two prenyl groups, respectively. The fragmentation of a prenyl group on a flavonoid does not result in a flavonoid aglycone but in a flavonoid with a double bound carbon (Simons et al. Citation2009; Ye et al. Citation2019). This may explain why no flavonoid aglycone ion is observed for these two metabolites. Looking at the suggested structures, metabolite 1 and 5 are very similar with a mass difference of 2 Da and a difference of 0.1 min in retention time. Cyclisation between the prenyl group and hydroxyl group can take place chemically (Popłoński et al. Citation2018) and is believed to be occur in nature (Tanaka et al. Citation1997; Zhou et al. Citation2021). Cyclisation between a prenyl group and hydroxyl group in the A ring of metabolite 5 will lead to the formation of metabolite 1 with the loss of two hydrogen atoms, hence metabolite 5 may be the precursor for metabolite 1.
Changes in lupin secondary metabolites due to intercropping
Intercropping of lupin and barley caused a 3-fold decrease of metabolite 4 compared to pure lupin. The remaining five metabolites were not detected in pure lupin, but they were detected in lupin when the competing crop, barley, was present. Flavonoids have several functions in the plant related to growth and defence (Abdel-Lateif et al. Citation2013; Taylor et al. Citation1992), as well as the nodulation process in legumes (Dong et al. Citation2020). In addition, the presence of lupin in the intercropping setup caused a decrease in barley root and shoot mass compared to barley grown without lupin (see Table S1), likely due to lupin competition for resources (water, nutrients, light). Lupin root and shoot mass were not affected by the presence of barley as the competing plant, which may be explained by the time gap between sowing of lupin and barley seeds, allowing for the lupin seeds to germinate and grow.
Metabolites 1 and 5 are prenylated flavonoids, a compound subclass detected in different plant families, such as Euphorbiaceae (Yang et al. Citation2015) and Fabaceae (Araya-Cloutier et al. Citation2017). Previous work showed the presence of another prenylated flavonoid, luteone, in L. angustifolius roots in concentrations 126–354 µg/g DW (Andersen et al. Citation2022). In the literature, reports on the effect of prenylated flavonoids in plants are limited. However, the antimicrobial activity in both food and bacterial cultures of human pathogens were reported (Araya-Cloutier et al. Citation2017; Araya-Cloutier et al. Citation2018), which may be a significant effect of the compounds in the plants and their environment upon a microbial infection. Liu et al. (Citation2018) reported the upregulation of a prenyl transferase gene, LjG6DT, in L. japonicas by different hormones, suggesting prenylated flavonoids are involved in the stress response of the plant (Liu et al. Citation2018). This supports the hypothesis of prenylated flavonoids playing a role in plant defence. Intercropping may, like microorganisms and herbivores, apply stress to plants because they have to compete for the resources essential for plant survival and activation of the plant defence.
Glycosylation of flavonoids plays several roles in plants, such as increasing water solubility, antioxidant effects and reduction of toxicity (Plaza et al. Citation2014; Slámová et al. Citation2018). Both metabolite 2 and 6 are suggested to be glycosylated flavonoids; luteolin and apigenin, respectively. We found that metabolites 2 and 6 were increased in lupin in LB(1:3) samples compared to LP. Increase of glycosylated flavonoids in root exudates due to intercropping was reported in the literature to a limited extent. In 2022, Dong et al. (Citation2022) found that the concentration of glycosylated flavonoids were increased in the shared soil when peanut and maize were intercropped (Dong et al. (Citation2022). An increase in compound concentration in soil suggests that the concentration of the compounds is increased in the roots as well, or an increased exudation has taken place. However, Hazrati et al. (Citation2021) reported a decrease in the glycosylated flavonoid, kaempferol-rha-xyl-gal, in V. villosa roots and shoot when intercropped with S. cereale as the competing crop (Hazrati et al. Citation2021). This indicates that the intercropping response depends on several factors, such as crop species and flavonoid identity. Therefore, it is difficult to compare the results of the limited number of studies exploring this research area.
Kaempferol, luteolin and apigenin are suggested as backbone structures of the five flavonoids annotated in the present study. The three compounds were shown to play a role in the nodulation process by acting as a nod regulator or inducer (Hartwig et al. Citation1991; Begum et al. Citation2001; Ng et al. Citation2015); therefore, it may be speculated that the five generated metabolites here also play a role in the nodulation of lupin roots and regulation of N2 fixation activity. Czaban et al. (Citation2018) found that kaempferol changed the bacterial community in clover rhizosphere soil and affected the cycling of amino acids and nitrogen (Czaban et al. Citation2018). This indicates that kaempferol, and potentially, structurally similar compounds, can affect the bacterial community in the rhizosphere and thereby the nodulation process, although the function of each flavonoid is believed to be very specific and each compound should be tested to conclude on their function. Prenylated flavonoids have also shown to play a role in the nodulation process (Gagnon et al. Citation1998) and to have an increased concentration in stressed plants (Gagnon et al. Citation1997), which is in agreement with the increase of the prenylated metabolites 1 and 5. Hence, this study shows the need to improve the understanding of the role of secondary metabolites in the regulation of legume N2-fixation activity as a source of sustainable N in agroecosystems.
Conclusion
An untargeted metabolomics approach unveiled the effect of intercropping on the metabolome of lupin. The primary metabolite, tryptophan, was identified at level 1, and the concentration of tryptophan decreased when lupin was intercropped with barley. Several secondary metabolites were affected. Five flavonoids were identified at level 3 by clearly identifying functional groups by comparison with spectral databases, but with some residual uncertainty about the exact structures. Metabolites 1 and 5 were annotated as prenylated flavonoids, metabolites 2 and 6 were annotated as glycosylated flavonoids, and metabolite 3 was annotated as a branched flavonoids. The five flavonoids were not detected in pure lupin, but in lupin when intercropped with barley. Flavonoids are known to play a role in plant defence, and prenylated flavonoids have been reported to play a role in plants’ stress response. This suggests that intercropping causes stress on the primary plant which leads to the synthesis of these specific stress-related compounds. Furthermore, flavonoids are involved in the nodulation process in the roots of legumes and thereby the fixation of nitrogen in the soil, which may be relevant to consider when intercropping systems are designed. However, this study did not focus specifically on the effect of intercropping in the nodulation process, which would provide further knowledge on this particular subject and is relevant for future studies. Full identification of flavonoid metabolites might be possible by future synthesis of the suggested chemical structures to allow for spectral comparison and further study of their effects on nodulation in lupin plants. The present study investigates the effect of barley on lupin in an intercropping system, and in a future study it would be relevant to investigate the effect of lupin on the metabolome of barley.
Declaration of interest statement
The authors report there are no competing interests to declare.
Supplemental Material
Download MS Word (469.7 KB)Acknowledgements
We would like to thank Sarah F. Ben Soltane, Cătălina S. Cuparencu and Giorgia La Barbera, University of Copenhagen, and Bente B. Laursen, Aarhus University, for their much appreciated support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Ida K. L. Andersen
Ida K. L. Andersen PhD student in Natural Product Chemistry and Environmental Chemistry Group at Aarhus University with research focus on intercropping’s effect on the plant metabolome of narrow-leafed lupin and spring barley.
Lars O. Dragsted
Lars O. Dragsted Section leader of Preventive and clinical nutrition at University of Copenhagen with research focus on Bioactive compounds in food with an ability to prevent or alter the risk of life style disease using LC-MS based metabolomics.
Jim Rasmussen
Jim Rasmussen Senior researcher in Climate and Water Group at Aarhus University with research focus on nitrogen and carbon cycling in agricultural systems.
Inge S. Fomsgaard
Inge S. Fomsgaard Team leader of Natural Product Chemistry and Environmental Chemistry Group at Aarhus University with research focus on chemical compounds present in agricultural crops or in the agricultural environment using mass spectrometry.
References
- Abdel-Lateif K, et al. 2013. Silencing of the chalcone synthase gene in Casuarina glauca highlights the important role of flavonoids during nodulation. New Phytol. 199(4):1012–1021. doi:10.1111/nph.12326.
- Andersen IKL, et al. 2022. Optimised extraction and LC-MS/MS analysis of flavonoids reveal large field variation in exudation into Lupinus Angustifolius L. rhizosphere soil. Rhizosphere. 22:100516. doi:10.1016/j.rhisph.2022.100516.
- Angus JF, et al. 2015. Break crops and rotations for wheat. Crop Pasture Sci. 66(6):523–552. doi:10.1071/CP14252.
- Araya-Cloutier C, et al. 2017. The position of prenylation of isoflavonoids and stilbenoids from legumes (Fabaceae) modulates the antimicrobial activity against Gram positive pathogens. Food Chem. 226:193–201. doi:10.1016/j.foodchem.2017.01.026.
- Araya-Cloutier C, et al. 2018. Rapid membrane permeabilization of Listeria monocytogenes and Escherichia coli induced by antibacterial prenylated phenolic compounds from legumes. Food Chem. 240:147–155. doi:10.1016/j.foodchem.2017.07.074.
- Bargaz A, et al. 2016. Nodulation and root growth increase in lower soil layers of water-limited faba bean intercropped with wheat. J Plant Nutr Soil Sci. 179(4):537–546. doi:10.1002/jpln.201500533.
- Barri T, et al. 2012. Metabolic fingerprinting of high-fat plasma samples processed by centrifugation- and filtration-based protein precipitation delineates significant differences in metabolite information coverage. Anal Chim Acta. 718:47–57. doi:10.1016/j.aca.2011.12.065.
- Begum AA, et al. 2001. Specific flavonoids induced nod gene expression and pre-activated nod genes of Rhizobium leguminosarum increased pea (Pisum sativum L.) and lentil (Lens culinaris L.) nodulation in controlled growth chamber environments. J Exp Bot. 52(360):1537–1543. doi:10.1093/jexbot/52.360.1537.
- Bejder J, et al. 2021. An untargeted urine metabolomics approach for autologous blood transfusion detection. Med Sci Sports Exercise. 53(1):236–243. doi:10.1249/MSS.0000000000002442.
- Berg M, et al. 2013. LC-MS metabolomics from study design to data-analysis – using a versatile pathogen as a test case. Comput Struct Biotechnol J. 4(5):e201301002. doi:10.5936/csbj.201301002.
- Bilalis D, et al. 2010. Weed-suppressive effects of maize–legume intercropping in organic farming. Int J Pest Manag. 56(2):173–181. doi:10.1080/09670870903304471.
- Carton N, et al. 2020. Intercropping winter lupin and triticale increases weed suppression and total yield. Agriculture. 10(8), doi:10.3390/agriculture10080316.
- Chambers MC, et al. 2012. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 30(10):918–920. doi:10.1038/nbt.2377.
- Chandler JW. 2016. Auxin response factors. Plant Cell Environ. 39(5):1014–1028. doi:10.1111/pce.12662.
- Cuyckens F, et al. 2004. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 39(1):1–15. doi:10.1002/jms.585.
- Czaban W, et al. 2018. Multiple effects of secondary metabolites on amino acid cycling in white clover rhizosphere. Soil Biol Biochem. 123:54–63. doi:10.1016/j.soilbio.2018.04.012.
- Dong Q, et al. 2022. Maize and peanut intercropping improves the nitrogen accumulation and yield per plant of maize by promoting the secretion of flavonoids and abundance of Bradyrhizobium in rhizosphere. Front Plant Sci. 13:957336. doi:10.3389/fpls.2022.957336.
- Dong W, et al. 2020. The significance of flavonoids in the process of biological nitrogen fixation. Int J Mol Sci. 21(16). doi:10.3390/ijms21165926.
- Duan Y, et al. 2021. The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis. BMC Plant Biol. 21(1):482. doi:10.1186/s12870-021-03258-1.
- Duca DR, et al. 2020. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl Microbiol Biotechnol. 104(20):8607–8619. doi:10.1007/s00253-020-10869-5.
- Falcone Ferreyra ML, et al. 2012. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 3(222), doi:10.3389/fpls.2012.00222.
- Ferguson BJ, et al. 2010. Molecular analysis of legume nodule development and autoregulation. J Integr Plant Biol. 52(1):61–76. doi:10.1111/j.1744-7909.2010.00899.x.
- Fernie AR, et al. 2011. Recommendations for reporting metabolite data. Plant Cell. 23(7):2477–2482. doi:10.1105/tpc.111.086272.
- Fustec J, et al. 2010. Nitrogen rhizodeposition of legumes. A review. Agron Sustainable Dev. 30(1):57–66. doi:10.1051/agro/2009003.
- Gagnon H, et al. 1997. Effects of various elicitors on the accumulation and secretion of isoflavonoids in white lupin. Phytochemistry. 44(8):1463–1467. doi:10.1016/S0031-9422(96)00735-2.
- Gagnon H, et al. 1998. Aldonic acids: a novel family of nod gene inducers of Mesorhizobium loti, Rhizobium lupini, and Sinorhizobium meliloti. Mol Plant-Microbe Interactions®. 11(10):988–998. doi:10.1094/MPMI.1998.11.10.988.
- Geng P, et al. 2016. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMS n and mass defect filtering. J Mass Spectrom. 51(10):914–930. doi:10.1002/jms.3803.
- Hama JR, et al. 2020. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ Sci Europe. 32(1):126. doi:10.1186/s12302-020-00405-7.
- Hartwig UA, et al. 1991. Release and modification of nod-gene-inducing flavonoids from alfalfa seeds. Plant Physiol. 95(3):804–807. doi:10.1104/pp.95.3.804.
- Hazrati H, et al. 2021. Targeted metabolomics unveil alteration in accumulation and root exudation of flavonoids as a response to interspecific competition. Journal of Plant Interactions. 16(1):53–63. doi:10.1080/17429145.2021.1881176.
- Hazrati H, et al. 2022. Integrated LC–MS and GC–MS-based metabolomics reveal the effects of plant competition on the Rye metabolome. J Agric Food Chem. 70(9):3056–3066. doi:10.1021/acs.jafc.1c06306.
- Hu F, et al. 2017. Improving N management through intercropping alleviates the inhibitory effect of mineral N on nodulation in pea. Plant Soil. 412(1):235–251. doi:10.1007/s11104-016-3063-2.
- Jain V, et al. 2002. Plant flavonoids: signals to legume nodulation and soil microorganisms. J Plant Biochem Biotechnol. 11(1):1–10. doi:10.1007/BF03263127.
- Jensen CR, et al. 2004. The effect of lupins as compared with peas and oats on the yield of the subsequent winter barley crop. Eur J Agron. 20(4):405–418. doi:10.1016/S1161-0301(03)00057-1.
- Khan MK, et al. 2015. Phytochemical composition and bioactivities of lupin: a review. Int J Food Sci Technol. 50(9):2004–2012. doi:10.1111/ijfs.12796.
- Kind T, et al. 2006. Metabolomic database annotations via query of elemental compositions: mass accuracy is insufficient even at less than 1 ppm. BMC Bioinformatics. 7:234. doi:10.1186/1471-2105-7-234.
- Kirkegaard J, et al. 2008. Break crop benefits in temperate wheat production. Field Crops Res. 107(3):185–195. doi:10.1016/j.fcr.2008.02.010.
- Liu C-W, et al. 2016. The role of flavonoids in nodulation host-range specificity: An update. Plants. 5(3), doi:10.3390/plants5030033.
- Liu J, et al. 2018. Genistein-Specific G6DT gene for the inducible production of wighteone in lotus japonicus. Plant Cell Physiol. 59(1):128–141. doi:10.1093/pcp/pcx167.
- Liu YC, et al. 2017. Intercropping influences component and content change of flavonoids in root exudates and nodulation of Faba bean. J Plant Interactions. 12(1):187–192. doi:10.1080/17429145.2017.1308569.
- March RE, et al. 2004. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int J Mass Spectrom. 231(2):157–167. doi:10.1016/j.ijms.2003.10.008.
- Ng JLP, et al. 2015. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre. Plant Cell. 27(8):2210–2226. doi:10.1105/tpc.15.00231.
- Novák K, et al. 2002. Effect of exogenous flavonoids on nodulation of pea (Pisum sativum L.). J Exp Bot. 53(375):1735–1745. doi:10.1093/jxb/erf016.
- Peixoto L, et al. 2022. Deep-rooted perennial crops differ in capacity to stabilize C inputs in deep soil layers. Sci Rep. 12(1):5952. doi:10.1038/s41598-022-09737-1.
- Plaza M, et al. 2014. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J Agric Food Chem. 62(15):3321–3333. doi:10.1021/jf405570u.
- Pluskal T, et al. 2010. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 11:395. doi:10.1186/1471-2105-11-395.
- Popłoński J, et al. 2018. Synthesis and antiproliferative activity of minor hops prenylflavonoids and new insights on prenyl group cyclization. Molecules. 23(4), doi:10.3390/molecules23040776.
- Salem MA, et al. 2020. Metabolomics in the context of plant natural products research: from sample preparation to metabolite analysis. Metabolites. 10(1), doi:10.3390/metabo10010037.
- Sarunaite L, et al. 2010. Intercropping spring wheat with grain legume for increased production in an organic crop rotation. Žemdirbystė-Agriculture. 97(3):51–58.
- Shi S, et al. 2021. A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids. Phytochemistry. 191:112895. doi:10.1016/j.phytochem.2021.112895.
- Simons R, et al. 2009. A rapid screening method for prenylated flavonoids with ultra-high-performance liquid chromatography/electrospray ionisation mass spectrometry in licorice root extracts. Rapid Commun Mass Spectrom. 23(19):3083–3093. doi:10.1002/rcm.4215.
- Slámová K, et al. 2018. “Sweet flavonoids”: glycosidase-catalyzed modifications. Int J Mol Sci. 19(7), doi:10.3390/ijms19072126.
- Stagnari F, et al. 2017. Multiple benefits of legumes for agriculture sustainability: an overview. Chem Biol Technol Agric. 4(1):2. doi:10.1186/s40538-016-0085-1.
- Sumner LW, et al. 2007. Proposed minimum reporting standards for chemical analysis. Metabolomics. 3(3):211–221. doi:10.1007/s11306-007-0082-2.
- Tanaka M, et al. 1997. Fad-dependent epoxidase as a key enzyme in fungal metabolism of prenylated flavonoids. Phytochemistry. 46(3):433–439. doi:10.1016/S0031-9422(97)00322-1.
- Taylor LP, et al. 1992. Conditional male fertility in chalcone synthase-deficient petunia. J Hered. 83(1):11–17. doi:10.1093/oxfordjournals.jhered.a111149.
- Wang Y, et al. 2019. GmYUC2a mediates auxin biosynthesis during root development and nodulation in soybean. J Exp Bot. 70(12):3165–3176. doi:10.1093/jxb/erz144.
- Weston LA, et al. 2013. Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol. 39(2):283–297. doi:10.1007/s10886-013-0248-5.
- Wichern F, et al. 2008. Nitrogen rhizodeposition in agricultural crops: Methods, estimates and future prospects. Soil Biol Biochem. 40(1):30–48. doi:10.1016/j.soilbio.2007.08.010.
- Wojakowska A, et al. 2013. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry. 92:71–86. doi:10.1016/j.phytochem.2013.04.006.
- Wu T, et al. 2021. Non-targeted and targeted metabolomics profiling of tea plants (Camellia sinensis) in response to its intercropping with Chinese chestnut. BMC Plant Biol. 21(1):55. doi:10.1186/s12870-021-02841-w.
- Xi M, et al. 2021. Discovery of urinary biomarkers of seaweed intake using untargeted LC–MS metabolomics in a three-Way cross-over human study. Metabolites. 11(11):1–15. doi:10.3390/metabo11010011.
- Xia J, et al. 2009. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 37:W652–W660. doi:10.1093/nar/gkp356.
- Xiao J, et al. 2016. Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr. 56(sup1):S29–S45. doi:10.1080/10408398.2015.1067595.
- Yang C-q, et al. 2017. Targeted metabolomics analysis of fatty acids in soybean seeds using GC-MS to reveal the metabolic manipulation of shading in the intercropping system. Anal Methods. 9(14):2144–2152. doi:10.1039/C7AY00011A.
- Yang D-S, et al. 2015. Three new prenylated flavonoids from Macaranga denticulata and their anticancer effects. Fitoterapia. 103:165–170. doi:10.1016/j.fitote.2015.04.001.
- Ye J-B, et al. 2019. Characterization and identification of prenylated flavonoids from artocarpus heterophyllus Lam. roots by quadrupole time-of-flight and linear trap quadrupole orbitrap mass spectrometry. Molecules. 24(24), doi:10.3390/molecules24244591.
- Zhang J, et al. 2009. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 57(1):171–183. doi:10.1111/j.1365-313X.2008.03676.x.
- Zhang J, et al. 2015. Rapid characterization and identification of flavonoids in radix astragali by ultra-high-pressure liquid chromatography coupled with linear Ion trap-orbitrap mass spectrometry. J Chromatogr Sci. 53(6):945–952. doi:10.1093/chromsci/bmu155.
- Zhou K, et al. 2021. Naturally occurring prenylated chalcones from plants: structural diversity, distribution, activities and biosynthesis. Nat Prod Rep. 38(12):2236–2260. doi:10.1039/D0NP00083C.