ABSTRACT
Viscum combreticola Engl., an evergreen semi-parasitic mistletoe plant, is utilized in herbal medicines. Herein, a UHPLC-q-TOF-MS profiling and molecular networking approach were used to investigate the chemical interactions between V. combreticola and two of its host plants, Combretum erythrophyllum and Pseudolachnostylis maprouneifolia Pax. Moreover, in vitro grown V. combreticola seedlings were used to explore the host independence of the phytochemistry of these plants. The phytochemistry of V. combreticola was found to be independent and distinct from that of the host plants. Moreover, both mature V. combreticola and in vitro seedlings displayed diverse polyphenolic compounds but exhibited distinct metabolic profiles. Notably, the esterification of phenolic acids (hydroxycinnamic and benzoic acids) to quinic acid was a unique chemistry that was observed in both mature and in vitro grown V. combreticola. Beyond providing metabolic fingerprints of the studied samples, the UHPLC-q-TOF-MS and molecular networking effectively visualized plant-plant chemical relationships at a metabolite level.
1. Introduction
A leafless mistletoe, Viscum combreticola Engl. (Santalacaeae), is a semi-parasitic evergreen plant widely distributed in tropical and southern Africa (Okubamichael et al. Citation2016; Schmitt et al. Citation2018). This Viscum species parasitizes various hosts plants such as Acacia karroo, Combretum erythrophyllum, Dombeya rotundifolia and Sclerocarya birrea (Okubamichael et al. Citation2016; Moyo et al. Citation2022) through the haustorium which serves as a vasculature (Kokla and Melnyk Citation2018). Parasitic plants have been reported to disrupt the host’s growth and reproductive systems, with the degree of parasitism effects being dependent on the severity of the mistletoe infection (Mathiasen et al. Citation2008; Mutlu et al. Citation2016; Pietrzak and Nowak Citation2021). Severe mistletoe infections can weaken the defence system of the host plant and make it susceptible to pathogens and insects and eventually lead to its death (Adesina et al. Citation2013; Lázaro-González et al. Citation2019; Lázaro-González et al. Citation2021). Regardless of the adverse effects that parasitic plants pose to their hosts, mistletoe plants play a significant ecological role such as providing nesting sites to birds due to their dense clumps of semi-succulent foliage. In addition, they are a food source to wildlife (Mathiasen et al. Citation2008; Hartley et al. Citation2015) and provide nutrients to the surrounding plant communities including the host plant, through their nutrient-dense litterfall (Mellado and Zamora Citation2017; Maponga et al. Citation2021). More importantly, mistletoes are widely used in ethnomedicine in several parts of the world for treating various ailments (Singh et al. Citation2016; Song et al. Citation2022). Due to the rich chemical composition of most Viscum species, various potential health benefits of these plants, such as anticancer (Ma et al. Citation2015; Ostermann et al. Citation2020), immunomodulating effects (Urech and Baumgartner Citation2015; Singh et al. Citation2016), curing knee-waist malaise and hepatitis (Nag et al. Citation2020), have been reported.
A few previous investigations on the chemical interactions of different Viscum species and their host plants reported that the metabolite composition of the parasites is dependent on that of the host plant (Jäger et al. Citation2021; Pietrzak and Nowak Citation2021; Zhang et al. Citation2022). However, these studies focused on selected metabolites to determine the host specificity/dependency of the Viscum species (Pietrzak and Nowak Citation2021). Therefore, chemical interactions between host and parasitic plants are still not fully understood given that they may affect the medicinal properties of mistletoe plants. The emergence of various computational tools in the past years for deconvoluting large and complex tandem mass spectrometry (MS/MS) data sets simultaneously, has improved data analysis and, accordingly biochemical insights in plant metabolomics studies. Molecular networking, an unsupervised computational tool that groups a set of MS/MS spectra of related compounds into molecular families (Nothias et al. Citation2018; Farag et al. Citation2020), allows the comparison between different samples with ease. Despite the wide use of this tool in various studies, its application in investigating host-parasitic plant chemical interactions is still limited and most studies are still employing statistical tools such as principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), for analyzing data (Jäger et al. Citation2021; Zhang et al. Citation2022). To the best of our knowledge, no research study has comprehensively compared the metabolomes of the host and semi-parasitic plants. Song et al. (Citation2022) attempted to compare the metabolome of Viscum coloratum and its host; however, researchers in this study used a supervised method, PLS-DA to highlight differences in the chemical profiles of the two plants. In a study done by Lázaro-González et al. (Citation2019), the effect of parasitism of Viscum album L. on pine trees was investigated by comparing the chemical differences of these two plants. However, colorimetric methods were used to compare the total content of phenolics and tannins and liquid chromatographic (LC) analysis was only focused on terpenoids. In view of this, the aim of the current study was to investigate the chemical relationship between V. combreticola and two of its different hosts; namely, Combretum erythrophyllum and Pseudolachnostylis maprouneifolia Pax, through the liquid chromatography-tandem mass spectrometry (UHPLC-q-TOF-MS) profiling and classical molecular networking approach. Moreover, the secondary metabolite composition of seedlings grown in vitro from the seeds of mature V. combreticola was compared to that of the naturally growing plant on its host plants, to further explore the dependency of the chemistry of these plants on their hosts.
2. Materials and methods
2.1. Chemicals and reagents
Acetonitrile, methanol and water of LC-MS grade were supplied by Romil SpS (Cambridge, UK). Sigma-Aldrich (Johannesburg, South Africa) supplied analytical grade formic acid.
2.2. Sample preparation and extraction of metabolites from mature V. combreticola plants
The whole V. combreticola plant (stems and berries) alongside samples from host plants that it parasitized; C. erythrophyllum (stem and leaves) and P. maprouneifolia (stem and leaves) were collected at Shakadza village (22°61’7’’S; 30°55’’6’E) around Nwanedi region, Limpopo province, South Africa. All the collected samples were stored separately to avoid contamination and air dried at room temperature according to previous similar studies (Pietrzak and Nowak Citation2021; Zhang et al. Citation2022). Moreover, the haustorium was not collected to avoid possible contamination from the host plants. Unparasitized host plants were used as control samples. All the samples were harvested in triplicates (three different plants of the same species for each). Samples were prepared according to a previous study conducted by Moyo et al. (Citation2022). Briefly, 2 g of the ground samples were weighed and, extracted overnight in 20 mL of 80% methanol (1:10 m/v) by shaking on a digital rotisserie tube rotator (Dlab Scientific, Beijing, China) at 70 rpm at room temperature. A benchtop fixed angle centrifuge (Thermofischer, Johannesburg, South Africa) was employed for the centrifugation of the crude extracts at 2739 g for 20 min. Prior to chromatographic analysis with mass spectrometric detection on the UHPLC-q-TOF-MS, the samples were filtered through 0.22 µm nylon filters into HPLC vials.
2.3. In vitro propagation of V. combreticola seedlings.
Berries from one of the mature V. combreticola plants collected were utilized for in vitro propagation of the seedlings after being washed with sterile distilled water. Green seeds were squeezed out of the red pericarp, removed with a forceps and rinsed with sterile distilled water. Surface sterilization was done in a sterile environment by means of 70% ethanol for 20 s, followed by 1% sodium hypochlorite for 20 min. The seeds were then repeatedly rinsed with sterile distilled water and placed on a solidified agar medium under sterile conditions. The composition of the medium was 8% plant tissue culture agar containing Murashige and Skoog (MS) macro- and micro-elements with the inclusion of 30% sucrose and MS vitamins (Duchefa, Haarlem, Netherlands). Seeds were allowed to germinate in an incubator with cool white fluorescent lights at 22°C with a 12 h /12 h light/dark cycle. In the first week the seed extended green hypocotyls. After 5–6 weeks, germinated seeds with developed ‘holdfasts’ (Fig. S1) were combined and weighed in sterile 50 mL Falcon tubes. Analytical grade methanol (80%) was added in a 1:10 m/v ratio and the tissue homogenized using an Ultraturrax homogenizer (Cat, Berlin, Germany) for 2 min. Homogenates were centrifuged at 5500 g in a swinging bucket centrifuge (Beckman, Allegra) at 5°C to pellet cellular debris. The supernatants were carefully removed with a pipette, concentrated under vacuum in a rotary evaporator at 40°C to 1 mL, transferred into 2 mL Eppendorf tubes and dried overnight in a fume hood in a heating block at 40°C. The dried samples were reconstituted in 1 mL of Romil MS-grade methanol (SpS, Cambridge, UK), filtered and analyzed by UHPLC-q-TOF-MS.
2.4. Chromatographic separation and mass spectrometric detection
A slightly modified chromatographic method by Moyo et al. (Citation2022) was used in this study. Briefly, an injection volume of 5 µL of sample extracts was analyzed using a liquid chromatography-quadrupole time of flight tandem mass spectrometer (LC/MS-9030 q-TOF, Shimadzu Corporation, Kyoto, Japan). A Shim-pack Velox C18 column (100 × 2.1 mm, 2.7 µm) (Shimadzu Corporation, Kyoto, Japan) maintained at 55°C was employed for the chromatographic separation of secondary metabolites. A binary mobile phase gradient elution at a flowrate of 0.3 mL min−1 was used to separate the compounds in the plant extracts. Mobile phases A and B contained 0.1% (v/v) formic acid in ultra-high purity water and 0.1% formic acid in methanol, respectively. From 0 to 3 min, the mobile phase composition was 10% mobile B, which was maintained until 40 min. The composition of mobile phase B was increased to 30% between 40 and 43 min, and to 90% between 45 and 49 min. Around 50 min, the gradient was returned to 10% mobile phase B and it was held at this composition until 53 min. A q-TOF high resolution MS with an electrospray ionization interface (ESI) operating in negative ionization mode was used for mass spectral analysis. The following parameters were set: 4.0 kV interface voltage, 300°C interface temperature, 3 L min−1 nebulization and dry gas flow (nitrogen), 400°C heat block temperature, 280°C DL temperature, 1.8 kV detector voltage, and 42°C flight tube temperature. For monitoring high mass accuracy, sodium iodide was used as a calibration solution. In data-dependent acquisition (DDA) mode, MS1 and MS2 were acquired simultaneously for all the ions with an intensity threshold above 5000 at a m/z range from 100 to 1000 Da. For the MS2 experiments, argon was employed as a collision gas with a collision energy of 30 eV with no spread. Representative UHPLC-MS chromatograms of the analyzed sample extracts are presented as supplementary data, Fig. S2 and S3.
2.5. Molecular networking and metabolite annotation
Molecular networks were generated using the online workflow (https://ccms-ucsd.github.io/ GNPS Documentation/) on the Global Natural Product Social Molecular Networking (GNPS) website (http://gnps.ucsd.edu) (accessed on 4 December 2022) according to Aron et al. (Citation2020). The raw data from the Shimadzu LC-MS-9030 q-TOF was converted to an open-source format (.mzML) before uploading to the online workflow. All the MS2 fragment ions within +/−17 Da of the precursor ion m/z were filtered and removed from the data. Throughout the spectrum, MS2 spectra were window filtered by selecting the top 3 fragment ions in the +/−50 Da window. The mass tolerances for the precursor and MS2 fragment ions were set to 0.02 Da. The values for TopK and the maximum number of nodes that could be connected in a molecular family were set to 50 and 100, respectively. The Cytoscape software (https://cytoscape.org/, accessed on 4 December 2022) was used for the molecular network data integration, analysis and visualization (Aron et al. Citation2020). The putative annotation of matched and some unmatched nodes was confirmed by the empirical formulas generated from the accurate mass obtained from MS/MS data of the compounds. These annotations were also compared to some common databases for dereplication of natural products such as KNApSAck (http://www.knapsackfamily.com/knapsack_core/top.php, accessed on 23 Jan 2023). Furthermore, all the annotations / tentative identifications in this study were compared to previous research findings to ascertain accuracy in the identifications.
3. Results
3.1. Chemical composition of Viscum combreticola vs its host plants
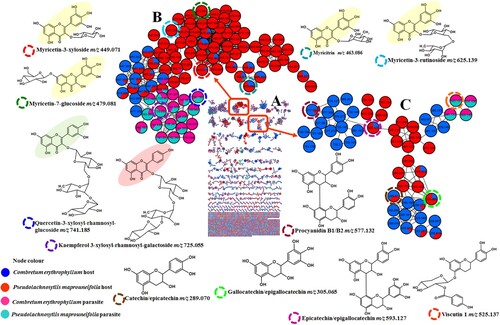
To investigate chemical interactions between a semi-parasitic plant, V. combreticola. and two of its host plants (C. erythrophyllum and P. maprouneifolia) at a secondary metabolite level, their metabolomes were compared using classical molecular networking which is currently a powerful tool in metabolomics studies for infographical comparison of samples. All the GNPS jobs are available on the website and the links are supplied in the supplementary information. In general, the visual inspection of (A) and Fig. S4 indicates that V. combreticola samples collected from the two different hosts exhibited similar secondary metabolite compositions. This similarity is evident from the presence of common compounds in both samples, although there are a few molecules that are specific to V. combreticola samples harvested from different host plants. On the other hand, the metabolomes of the host plants were distinct from that of the associated V. combreticola plants, suggesting that the chemical composition of this semi-parasitic plant might not be influenced by that of its host plants. Furthermore, the visual inspection of the chromatograms of V. combreticola and its host plants depicted different metabolite patterns (Fig. S2). Unparasitized host plants (C. erythrophyllum and P. maprouneifolia) showed a similar secondary metabolite composition to the parasitized host plants (Fig. S5 and Fig. S6), therefore further revealing that parasitism had no notable effect on the metabolite composition of the hosts in this study. Similar results were reported by Lázaro-González et al. (Citation2021), where the metabolome of mistletoe plants differed from that of its hosts. Moreover, both unparasitized and parasitized hosts showed similar metabolomes. This could be due to the fact that semi-parasitic plants can photosynthesize independently from their hosts, thereby producing a different set of secondary metabolites (Zhao et al. Citation2011). These findings align with those of Zhao et al. (Citation2011) who reported that host species does not affect the quality of V. coloratum that was harvested from different host plants. This could also be attributed to the degree and duration of parasitism on the host plants. Lázaro-González et al. (Citation2019) reported that parasitized plants showed unique chemical responses based on the parasitic load. V. combreticola and the host plants analyzed in the current study were found to be rich in flavonoids; however, they produced different subclasses of these compounds with various glycosylation patterns, including flavonols, flavan-3-ols and flavans. In the flavonols molecular family (B), myricetin-based glycosides at m/z 449.070 [M-H]- (myricetin-3-O-xyloside), 463.086 [M-H]- (myricitrin), 479.080 [M-H]- (myricetin-7-O-glucoside) and 625.138 [M-H]- (myricetin-3-O-rutinoside) were observed only in the host plants extracts. These compounds mainly showed a diagnostic product ion at m/z 316 which indicated the presence of an anionic radical of the myricetin aglycone ([M-H]•–) resulting from the homolytic cleavage of the O-glycosidic bond in these glycosides (Hvattum and Ekeberg Citation2003; Serni et al. Citation2022). The neutral losses of 132, 146, 162 and 308 Da were indicative of the xylosyl, rhamnosyl, glucosyl and rutinosyl moieties, respectively (Piccolella et al. Citation2019). On the contrary, V. combreticola samples showed the presence of kaempferol (m/z 725.191) [M-H]- and quercetin (m/z 741.186) [M-H]- containing flavonoids attached to trisaccharides. Quercetin-3-xylosyl-rhamnosyl-glucoside (m/z 741.186) and kaempferol-3-xylosyl-rhamnosyl-galactoside (m/z 725.191) exhibited fragment ions at m/z 301 (quercetin aglycone) and 285 (kaempferol aglycone) respectively, that were characteristic of the presence of flavonol aglycones and the loss of sugar moieties (Chen et al. Citation2016). Although a quercetin containing flavonoid (quercetin-3-O-arabinopyranoside, m/z 433.075 [M-H]-) was also observed in the host plants, it was a monoglycoside. Rutin (m/z 609.177 [M-H]-) was observed in both the host and mistletoe plants because it is one of the most ubiquitous flavonoids in nature. Meanwhile, host plants produced stereoisomers of catechin and their derivatives (flavan-3-ols), V. combreticola produced flavans as shown in (C). The diastereoisomers, catechin and epicatechin (m/z 289.070 [M-H]-) showed major fragment ions at m/z 137, 125 and 109, presumably resulting from the retro-Diels Alder (RDA) cleavage of ring C of the flavanol core structure, heterocyclic fission of ring A of the core structure and the presence of dihydrobenzene, respectively (Hamed et al. Citation2014; Yuzuak et al. Citation2018). Two isomers at m/z 305.064 [M-H]- showed a similar fragmentation pattern to catechin stereoisomers and these two compounds were suspected to be gallocatechin and epigallocatechin (Kelebek Citation2016). Host plants also showed dimeric type B proanthocyanidins where a compound at m/z 577.132 [M-H]- with two isomers exhibited an intense product ion at m/z 289, indicating the presence of epicatechin presumably resulting from quinone methide fragmentation (Jaiswal et al. Citation2012). Based on these results, the compounds were putatively identified as procyanidin B1 and B2. A compound with a precursor ion [M-H]- at m/z 593.127 showed 4 isomers which were suspected to be isomers of epicatechin-epigallocatechin. These isomers showed similar fragment ions at m/z 423 and 305, assumed to be resulting from the loss of a RDA fragment (152 Da) followed by dehydration and quinone methide fragmentation forming epigallocatechin, respectively (Jaiswal et al. Citation2012). The presence of a quinone methide product showed that epigallocatechin is a base unit (Ben Said et al. Citation2017), therefore, these compounds were annotated as isomers of epigallocatechin-epicatechin. On the other hand, viscutin 1 and a compound related to it (flavans) were observed in V. combreticola and they clustered with catechin and its derivatives in the same molecular family as depicted by (C). Viscutin 1 at m/z 525.137 was characterized by an intense fragment ion at m/z 137 which was suspected to be a product of the RDA cleavage of the C ring of the flavan scaffold. An unidentified compound at m/z 541.132 is related to viscutin 1, as it shared common fragment ions with viscutin 1 (Table S1).
Figure 1. Different flavonoid classes contained in Viscum combreticola samples and its host plants, Combretum erythrophyllum and Pseudolachnostylis maprouneifolia (A) full molecular network, (B) flavonols and (C) flavan-3-ols and, flavans. Each node in the molecular network represents a given compound and different samples are coded by different colors on the node as shown on the legend of the figure.
3.2. Chemical composition of mature Viscum combreticola vs its in vitro propagated seedlings
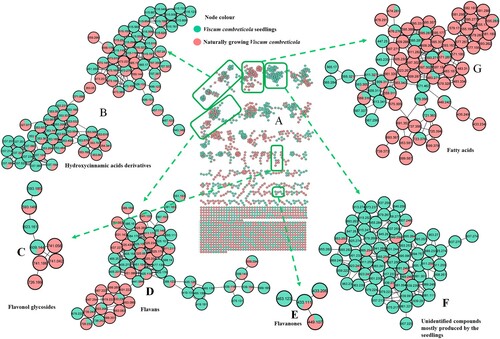
There is still a debate whether mistletoe plants acquire secondary metabolites from their host plants or they synthesize their own metabolites. As a result, a comparison between in vitro propagated seedlings and mature V. combreticola samples was conducted in this study. Fig. S1 shows the in vitro propagated seedlings at different growth stages. Interestingly, even though extraction methods for the mature V. combreticola and seedling samples were slightly different, seedlings contained most of the chlorogenic acids that were identified in a recent study done by Moyo et al. (Citation2022). These observations might suggest a common genetic/enzymatic machinery responsible for the production of these secondary metabolites and that the extraction method had minimal impact on the results. These compounds include different positional isomers of coumaroylquinic acid (m/z 337.092), caffeoylquinic acid (m/z 353.088), feruloylquinic acid (m/z 367.103), hydroxybenzoylcaffeoylquinic acid (m/z 473.109), coumaroylcaffeoylquinic acid (m/z 499.129), dicaffeoylquinic acid (m/z 515.119) and feruloylcaffeoylquinic acid (m/z 529.135) (B). This implies that in vitro grown seedlings of V. combreticola can serve as an alternative source for chlorogenic acids and these compounds exhibit multiple pharmacological activities (Farag et al. Citation2020). Interestingly, both mature and in vitro propagated samples of V. combreticola showed the presence of unique esters formed between quinic acid and benzoyl/hydroxybenzoyl at m/z 311.076 and 457.166 that were putatively annotated as hydroxybenzoylquinic acid and caffeoylbenzoylquinic acid, respectively due to the observed fragmentation patterns (Table S2). It is noteworthy that most of these hydroxycinnamic/benzoic acid derivatives were observed in mature V. combreticola samples only as shown on (A), hence, suggesting that these plants can synthesize their own secondary metabolites independent of the host plants. The seedlings also contained a triacylated chlorogenic acid, tricaffeoylquinic acid (m/z 677.171) [M-H]- that showed fragment ions at m/z at 515, 353 and 191 and this fragmentation pattern is consistent with previous reports (Ramabulana et al. Citation2020; Lee and Shaari Citation2022). A compound at m/z 451.058 [M-H]- was observed in the seedlings only (B) and it produced fragment ions at m/z 179 and 135, hence, indicating the presence of caffeoyl and decarboxylated caffeoyl moieties, respectively (Gutiérrez Ortiz et al. Citation2018). This compound was therefore tentatively annotated as a caffeoyl derivative. V. combreticola seedlings also showed the presence of some flavonoids that were observed in the naturally growing plant such as rutin (m/z 609.144) and kaempferol rutinoside (m/z 593.185), as shown on (C). (D) showed flavans including, viscutin 1 (m/z 525.137), viscutin 2 (m/z 567.148) and viscutin 3 (m/z 405.117), whereas (E) showed flavanones; eriodictyol-7-O-glucoside (m/z 449.107) and naringenin-7-O-glucoside (m/z 433.112). All these compounds were characterized by their unique fragmentation patterns as reported in Table S2. Most of the flavonoids in the study were annotated from spectral libraries on the GNPS platform and their identities were confirmed by MS/MS data. A glycerophospholipid, 1-hexadecanoyl-sn-glycero-3-phospho-(1'-myo-inositol) (m/z 571.287) was also tentatively identified in seedlings and mature V. combreticola samples as shown on (G) (lipids cluster). Nonetheless, most of the metabolites in this molecular family were observed in mature V. combreticola. Lipids provide structural barriers to the environment in plants since they are pivotal constituents of cellular membranes (Upchurch Citation2008). Although similarities exist between the in vitro propagated and the naturally growing V. combreticola samples, their metabolomes were generally different (A) and this might be attributed to a variety of factors such as the age and developmental differences in the two plants and the environment where they were growing. Metabolite levels change as a plant grows because plant metabolism is largely age dependant (Jäger et al. Citation2021). (E) shows a molecular family of unidentified compounds that were mostly produced by the seedlings, revealing that the chemistry of these samples is different from that of mature V. combreticola.
Figure 2. Different classes of secondary metabolites contained in mature Viscum combreticola and in vitro grown seedlings samples (A) full molecular network, (B) hydroxycinnamic acid derivatives, (C) flavanols, (D) flavans, (E) flavanones, (F) unidentified compounds mostly produced by seedlings and (G) lipids.
4. Discussion
Metabolomics approaches have gained popularity in understanding chemical relationships between host plants and their parasites in a comprehensive and unbiased manner (Pietrzak and Nowak Citation2021; Song et al. Citation2022; Zhang et al. Citation2022). Nonetheless, compound identification is still an enormous and challenging task. Even though different classes of secondary metabolites in plants can be visualized through molecular networking, the annotation of compounds is still limited to the commonly studied molecules. However, through molecular networking, unknown compounds can be grouped according to their spectral similarities into molecular families that can be further explored, and this may lead to the discovery of novel compounds. In this study, extracts from both V. combreticola and its host plants contained flavonoids of different subclasses which were specific to the two sets of samples as shown in . Moreover, there are classes of secondary metabolites such as hydroxycinnamic acids and many other compounds that were only observed in V. combreticola as shown on (A). These differences might be due to the fact that host plants modify their metabolome as a defense mechanism to parasitism (Lázaro-González et al. Citation2021) and hence, show a distinct metabolic profile. Lázaro-González et al. (Citation2021) reported that mistletoe plants may acquire primary metabolites such as amino acids from their host plants and synthesize their own secondary metabolites. Nonetheless, changes in the metabolite composition of host plants after parasitism is still a complex subject. Based on the findings of this study, it is suspected that V. combreticola plants have their own metabolic system that allows them to synthesize secondary metabolites according to their own specific needs since they can photosynthesize independently of their host (Hacham et al. Citation2016).
Mistletoe extracts are considered as potent traditional medicine in various parts of the world due to their rich and diverse phytochemistry. However, due to their semi-parasitic lifestyle, these plants cannot be easily propagated. Despite successful reports on the propagation of callus from V. album (Mavrikou et al. Citation2020; Tsekouras et al. Citation2020), this current study reported successful in vitro propagation of seedlings from V. combreticola seeds for the first time, but callus formation was not possible for reasons still unknown. Since variation in the secondary metabolite composition of mistletoes due to seasons and geographic location (Ninkovic et al. Citation2019) can affect the quality and quantity of natural product phytotherapeutics (that can be produced from these plants), biotechnology, through the in vitro propagation of these plants can be a viable solution to this. The results of the current study showed that the metabolic composition of the in vitro grown seedlings and the mature V. combreticola sampled from different host plants were distinct, although there were some similarities between these two samples. Interestingly, most of the chlorogenic acids that were produced by mature V. combreticola were also observed in the seedlings, thereby indicating that these compounds are part of this Viscum species chemistry. Moreover, the seedlings showed an additional hydroxycinnamic acid derivative that was not observed in the mature plants and this shows that seedlings have their own unique chemistry. More importantly, these results show that in vitro grown seedlings of V. combreticola could be an alternative source of pharmacological important metabolites. Moreover, this can serve as a conservation strategy of these plants as this may reduce harvesting from natural resources.
4. Conclusion
This study provided a holistic approach in uncovering the chemical relationship between V. combreticola and its host plants. The secondary metabolite composition of V. combreticola and two of its hosts was generally different at a metabolome level, suggesting that there was no transfer of pre-formed compounds from the host to the parasitic plant. It is however, possible that V. combreticola can tap into the host as a source of primary metabolites to supply needs for the synthesis of specialized secondary metabolites. Classical molecular networking proved to be a suitable and effective visualization tool for revealing differences in the chemical space of parasitic plants like V. combreticola and their hosts based on MS/MS data. Therefore, this study illustrated the potential of molecular networking in elucidating host-parasitic plant chemical relationships at a secondary metabolite level. The development stage of plants is crucial in the quality of secondary metabolites that they produce, as revealed by the difference in the metabolite composition of mature V. combreticola samples and their in vitro grown seedlings in this study.
Supplemental Material
Download MS Word (23.2 KB)Supplemental Material
Download MS Word (5.2 MB)Acknowledgments
The University of Venda is thanked for financial support. Mr Neshakadza is also thanked for helping with collection of plants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Babra Moyo
Babra Moyo is currently registered for a PhD in Biochemistry at the University of Venda and her research is on the investigation of chemical relationships between mistletoe plants and their host plants using the UHPLC-q-TOF-MS and molecular networking. She is also working as a Lab Technician in the Department of Food Science and Technology in the same institution. She obtained her MSc Chemistry from the University of Venda, BSc (Hons) Chemistry and, BSc Chemistry and Mathematics from the University of South Africa.
Nikita Tawanda Tavengwa
Nikita Tawanda Tavengwa is an Adjunct Senior Research Fellow in the Department of Chemistry at the University of Venda. His research interests lie in Analytical Chemistry in the pre-concentration of organic analytes (Including emerging pollutants such as poly-aromatic hydrocarbons, pesticides, nitro-aromatic compounds and antibiotics) to enhance their detection limits before their instrumental analysis. He is keen in investigating novel modern analytical extractive techniques and has authored more that 80 scientific articles in this field.
Ian Dubery
Ian A Dubery is a Research Professor in the Department of Biochemistry at the University of Johannesburg, South Africa. He is also the Director of the Research Centre for Plant Metabolomics at the same institution. His research interests include plant: microbe interactions, plant innate immunity and host defense responses, with a focus on secondary plant metabolites and metabolomics. He has published more than 200 research papers and has an h-index of 45.
Ntakadzeni Edwin Madala
Ntakadzeni Edwin Madala currently serves as an Associate professor specializing in biochemistry at the University of Venda. In his research pursuits, Edwin employs mass spectrometry as a tool to investigate the composition of plant metabolites and the underlying biochemical determinants contributing to the complexity of plant metabolites. He exhibits a strong passion for examining and unraveling the biological intricacies associated with derived isomers. He has published over 95 publications in peer reviewed journals.
References
- Adesina SK, Illoh HC, Johnny II, Jacobs IE. 2013. African mistletoes (Loranthaceae); ethnopharmacology, chemistry and medicinal values: an update. African Journal of Traditional, Complementary and Alternative Medicines. 10:161–170.
- Aron AT, Gentry EC, McPhail KL, Nothias LF, Nothias-Esposito M, Bouslimani A, Petras D, Gauglitz JM, Sikora N, Vargas F, et al. 2020. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat Protoc. 15:1954–1991. doi:10.1038/s41596-020-0317-5.
- Ben Said R, Arafa IH, Usam AM, Abdullah Sulaiman AA, Kowalczyk M, Moldoch J, Oleszek W, Stochmal A. 2017. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int J Mol Sci. 18:512. doi:10.3390/ijms18030512.
- Chen G, Li X, Saleri F, Guo M. 2016. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules. 21:1275. doi:10.3390/molecules21101275.
- Farag MA, Hegazi NM, Donia MS. 2020. Molecular networking based LC-MS reveals novel biotransformation products of green coffee by ex vivo cultures of the human gut microbiome. Metabolomics. 16:1–5. doi:10.1007/s11306-019-1621-3.
- Gutiérrez Ortiz AL, Berti F, Navarini L, Crisafulli P, Colomban S, Forzato C. 2018. Aqueous extracts of walnut (Juglans regia L.) leaves: quantitative analyses of hydroxycinnamic and chlorogenic acids. J Chromatogr Sci. 56:753–760. doi:10.1093/chromsci/bmy041.
- Hacham Y, Hershenhorn J, Dor E, Amir R. 2016. Primary metabolic profiling of Egyptian broomrape (Phelipanche aegyptiaca) compared to its host tomato roots. J Plant Physiol. 205:11–19. doi:10.1016/j.jplph.2016.08.005.
- Hamed AI, Al-Ayed AS, Moldoch J, Piacente S, Oleszek W, Stochmal A. 2014. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun-an ancient Egyptian palm) by LC-ESI-MS/MS. J Mass Spectrom. 49:306–315. doi:10.1002/jms.3344.
- Hartley SE, Green JP, Massey FP, Press MC, Stewart AJ, John EA. 2015. Hemi-parasitic plant impacts animal and plant communities across four trophic levels. Ecology. 96:2408–2416. doi:10.1890/14-1244.1.
- Hvattum E, Ekeberg D. 2003. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J Mass Spectrom. 38:43–49. doi:10.1002/jms.398.
- Jäger T, Holandino C, Melo MN, Peñaloza EM, Oliveira AP, Garrett R, Glauser G, Grazi M, Ramm H, Urech K, Baumgartner S. 2021. Metabolomics by UHPLC-q-TOF reveals host tree-dependent phytochemical variation in Viscum album L. Plants. 10:1726. doi:10.3390/plants10081726.
- Jaiswal R, Jayasinghe L, Kuhnert N. 2012. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J Mass Spectrom. 47:502–515. doi:10.1002/jms.2954.
- Kelebek H. 2016. LC-DAD-ESI-MS/MS characterization of phenolic constituents in Turkish black tea: effect of infusion time and temperature. Food Chem. 204:227–238. doi:10.1016/j.foodchem.2016.02.132.
- Kokla A, Melnyk CW. 2018. Developing a thief: haustoria formation in parasitic plants. Dev Biol. 442:53–59. doi:10.1016/j.ydbio.2018.06.013.
- Lázaro-González A, Gargallo-Garriga A, Hódar JA, Sardans J, Oravec M, Urban O, Peñuelas J, Zamora R. 2021. Implications of mistletoe parasitism for the host metabolome: A new plant identity in the forest canopy. Plant Cell Environ. 44:3655–3666. doi:10.1111/pce.14179.
- Lázaro-González A, Hódar JA, Zamora R. 2019. Mistletoe versus host pine: does increased parasite load alter the host chemical profile?. J Chem Ecol. 45:95–105. doi:10.1007/s10886-018-1039-9.
- Lee SY, Shaari K. 2022. LC-MS metabolomics analysis of Stevia rebaudiana Bertoni leaves cultivated in Malaysia in relation to different developmental stages. Phytochem Anal. 33:249–261. doi:10.1002/pca.3084.
- Ma Y, Fan R, Duan M, Yu Z, Zhao Y. 2015. A study of pharmacokinetic interactions among co-existing ingredients in Viscum coloratum after intravenous administration of three different preparations to rats. Pharmacogn Mag. 11:455. doi:10.4103/0973-1296.160448.
- Maponga TS, Ndagurwa HG, Witkowski ET. 2021. Functional and species composition of understory plants varies with mistletoe-infection on Vachellia karroo trees in a semi-arid African savanna. Global Ecology and Conservation. 29:01897.
- Mathiasen RL, Nickrent DL, Shaw DC, Watson DM. 2008. Mistletoes: pathology, systematics, ecology, and management. Plant Dis. 92:988–1006. doi:10.1094/PDIS-92-7-0988.
- Mavrikou S, Tsekouras V, Karageorgou MA, Moschopoulou G, Kintzos S. 2020. Anticancer and biochemical effects of Viscum album L. protein extracts on HeLa cells. Plant Cell, Tissue and Organ Culture (PCTOC). 140:369–378. doi:10.1007/s11240-019-01733-0.
- Mellado A, Zamora R. 2017. Parasites structuring ecological communities: the mistletoe footprint in Mediterranean pine forests. Funct Ecol. 31:2167–2176. doi:10.1111/1365-2435.12907.
- Moyo B, Tavengwa NT, Madala NE. 2022. Diverse chemical modifications of the chlorogenic acid composition of Viscum combreticola Engl.: A premise for the state of readiness against excessive sunlight exposure. J Photochem Photobiol, B. 233:112501. doi:10.1016/j.jphotobiol.2022.112501.
- Mutlu S, Osma E, Ilhan V, Turkoglu HI, Atici O. 2016. Mistletoe (Viscum album) reduces the growth of the Scots pine by accumulating essential nutrient elements in its structure as a trap. Trees. 30:815–824. doi:10.1007/s00468-015-1323-z.
- Nag M, Kar A, Chanda J, Mukherjee PK. 2020. RP-HPLC analysis of methanol extract of Viscum articulatum. J Ayurveda Integr Med. 11:277–280. doi:10.1016/j.jaim.2018.02.135.
- Ninkovic V, Rensing M, Dahlin I, Markovic D. 2019. Who is my neighbor? Volatile cues in plant interactions. Plant Signal Behav. 14:1634993. doi:10.1080/15592324.2019.1634993.
- Nothias LF, Nothias-Esposito M, Da Silva R, Wang M, Protsyuk I, Zhang Z, Sarvepalli A, Leyssen P, Touboul D, Costa J, Paolini J. 2018. Bioactivity-based molecular networking for the discovery of drug leads in natural product bioassay-guided fractionation. J Nat Prod. 81:758–767. doi:10.1021/acs.jnatprod.7b00737.
- Okubamichael DY, Griffiths ME, Ward D. 2016. Host specificity in parasitic plants-perspectives from mistletoes. AoB Plants. 8. doi:10.1093/aobpla/plw069.
- Ostermann T, Appelbaum S, Poier D, Boehm K, Raak C, Buessing A. 2020. A systematic review and meta-analysis on the survival of cancer patients treated with a fermented Viscum album L. extract (iscador): an update of findings. Complementary Medicine Research. 27:260–271. doi:10.1159/000505202.
- Piccolella S, Crescente G, Volpe MG, Paolucci M, Pacifico S. 2019. UHPLC-HR-MS/MS-guided recovery of bioactive flavonol compounds from Greco di Tufo vine leaves. Molecules. 24:3630. doi:10.3390/molecules24193630.
- Pietrzak W, Nowak R. 2021. Impact of harvest conditions and host tree species on chemical composition and antioxidant activity of extracts from Viscum album L. Molecules. 26:3741. doi:10.3390/molecules26123741.
- Ramabulana AT, Steenkamp P, Madala N, Dubery IA. 2020. Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites. 10:178. doi:10.3390/metabo10050178.
- Schmitt MH, Shuttleworth A, Ward D, Shrader AM. 2018. African elephants use plant odours to make foraging decisions across multiple spatial scales. Anim Behav. 141:17–27. doi:10.1016/j.anbehav.2018.04.016.
- Serni E, Tomada S, Haas F, Robatscher P. 2022. Characterization of phenolic profile in dried grape skin of Vitis vinifera L. cv. Pinot Blanc with UHPLC-MS/MS and its development during ripening. J Food Compos Anal. 114:104731. doi:10.1016/j.jfca.2022.104731.
- Singh BN, Saha C, Galun D, Upreti DK, Bayry J, Kaveri SV. 2016. European Viscum album: a potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC Adv. 6:23837–23857. doi:10.1039/C5RA27381A.
- Song C, Wang W, Xue Z, Peng H, Yang B. 2022. Characterization of the interaction between Viscum coloratum (mistletoe) and its host by ultra-high performance liquid chromatography-quadrupole-time-of-flight-mass spectrometry (UPLC-q-TOF-MS)-based metabolomics. Anal Lett. 12:1–6. doi:10.1080/22297928.2021.1980099.
- Tsekouras V, Mavrikou S, Vlachakis D, Makridakis M, Stroggilos R, Zoidakis J, Termentzi A, Moschopoulou G, Kintzios S. 2020. Proteome analysis of leaf, stem and callus in Viscum album and identification of lectins and viscotoxins with bioactive properties. Plant Cell, Tissue and Organ Culture (PCTOC). 141:167–178. doi:10.1007/s11240-020-01777-7.
- Upchurch RG. 2008. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 30:967–977. doi:10.1007/s10529-008-9639-z.
- Urech K, Baumgartner S. 2015. Chemical constituents of Viscum album L.: implications for the pharmaceutical preparation of mistletoe. Mistletoe: From Mythology to Evidence-Based Medicine. 4:11–23.
- Yuzuak S, Ballington J, Xie DY. 2018. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites. 8:57. doi:10.3390/metabo8040057.
- Zhang RZ, Zhao JT, Wang WQ, Fan RH, Rong R, Yu ZG, Zhao YL. 2022. Metabolomics-based comparative analysis of the effects of host and environment on Viscum coloratum metabolites and antioxidative activities. J Pharm Anal. 12:243–252. doi:10.1016/j.jpha.2021.04.003.
- Zhao Y, Yu Z, Fan R, Gao X, Yu M, Li H, Wei H, Bi K. 2011. Simultaneous determination of ten flavonoids from Viscum coloratum grown on different host species and different sources by LC-MS. Chem Pharm Bull. 59:1322–1328. doi:10.1248/cpb.59.1322.
