ABSTRACT
Introduction
Frankincense (Boswellia sp.) gum resins have been employed as an incense in cultural and religious ceremonies for many years. Frankincense resin has over the years been employed to treat depression, inflammation, and cancer in traditional medicines.
Areas covered
This inclusive review focuses on the significance of frankincense diterpenoids, and in particular, incensole derivatives for establishment future treatments of depression, neurological disorders, and cancer. The authors survey the available literature and furnish an overview of future perspectives of these intriguing molecules.
Expert opinion
Numerous diterpenoids including cembrane, prenylaromadendrane, and the verticillane-type have been isolated from various Boswellia resins. Cembrane-type diterpenoids occupy a crucial position in pharmaceutical chemistry and related industries because of their intriguing biological and encouraging pharmacological potentials. Several cembranes have been reported to possess anti-Alzheimer, anti-inflammatory, hepatoprotective, and antimalarial effects along with a good possibility to treat anxiety and depression. Although some slight drawbacks of these compounds have been noted, including the selectivity of these diterpenoids, there is a great need to address these in future research endeavors. Moreover, it is vitally important for medicinal chemists to prepare libraries of incensole-heterocyclic analogs as well as hybrid compounds between incensole or its acetate and anti-depressant or anti-inflammatory drugs.
1. Introduction
Frankincense has been grown in those areas having a distinct monsoon climate in the Arabian Peninsula, Ethiopia, Somalia, and India since ancient times. In addition, frankincense has been traded between Europe and China for the past 5000 years [Citation1]. Another important aspect to be noted about frankincense is that it is expensive and thus considered to be a sign of wealth. In ancient times, frankincense was one of the most important prosperity indicators in the Arabian Peninsula and considered to be a most precious resource in Europe. Frankincense resin has been employed for religious rituals in both Orthodox and Catholic churches and in funeral ceremonies. Frankincense has been used in numerous traditional medicines to treat cancer, stomach issues, flatulence, diabetes, Alzheimer's disease, central nervous system diseases, constipation, and inflammatory diseases [Citation1–4].
Historically, frankincense has been employed since 2800 BCE and this plant is mentioned numerous times in ancient Egyptian medical records. It was employed in perfumes and as a burn incense along with being a component for the preparation of balm and unguents for mummification. This is supported by writings about frankincense and myrrh being renowned at the time the Bible was being written because both these compounds were extensively mentioned and observed to be the most often used together with important aromatic resins [Citation5,Citation6]. Frankincense was also employed in Islamic traditional medicine in Arabian countries because this plant is stated in Avicenna’s (Ibn Sina’s) Canon of Medicine. It was employed to treat tuberculosis, amnesia, infections, bruises, diarrhea, burns, stomach issues, and eye sores. Moreover, frankincense is employed in Ayurvedic medicine to treat various other diseases as well [Citation2,Citation7]. Notably, frankincense was approved at the beginning of the 20th century to treat various inflammation diseases and is mentioned in the 7th supplement of the European Pharmacopoeia [Citation2]. On the other hand, frankincense is also employed in Chinese traditional medicine because frankincense-based pills named ‘xihuang’ have been employed to treat bronchial, nasopharyngeal, or pancreatic carcinomas [Citation2,Citation8].
Frankincense is essentially a resin derived from the tree of the genus Boswellia and mainly from five species, i.e. B. carterii, B. serrata, B. papyrifera, B. sacra, and B. frerana. The Boswellia genus, incorporating over 30 species out of which 16 grow in tropical Africa and Asia [Citation2]. Chemical investigation of frankincense resin has revealed that it comprises over 200 different natural products, including penta- and tetracyclic triterpenoids, diterpenoids, polyphenols, essential oils, and tannins [Citation2,Citation9–15]. Terpenes are considered to be one of the most structurally diverse groups among the spectrum of natural products. Furthermore, over 55,000 terpenes have been reported as isolated from various natural sources featured intriguing chemical diversity along with interesting biological properties. Among the terpenes, diterpenes are one of the largest groups of secondary metabolites with over 18,000 molecules derived from GGPP (E,E,E-geranylgeranyl diphosphate). Moreover, these compounds can be classified according to their biogenesis and over 126 different carbon skeletons have been reported to date [Citation16]. Quite recently, Al-Harrasi et al. [Citation17] published a review about the cembrane diterpenoids from the Boswellia species but their focus was more on the chemistry rather on their biology. In this review, we provide a comprehensive overview of detailed biological investigations of frankincense diterpenoids (cembrane and prenylaromadendrane-type diterpenes).
2. Clinical investigation of frankincense
In this part, mainly randomized clinical trials (RCT) are presented () excluding anticancer and anti-inflammatory activity because these two trials have already been covered in detail [Citation2]. Mehrzadi et al. investigated the antidiabetic effects of B. Serrata in 56 diabetic patients and found that there was a marginal reduction in glycosylated hemoglobin, blood sugar, and triglyceride in the B. serrata gum resin group [Citation18]. In another randomized, placebo-controlled trial, Azedmehr et al. [Citation19] investigated the B. serrata gum resin effects on the 71 type 2 diabetes patients with frankincense resin of 400 mg, which was taken orally for the duration of 12 weeks. Additionally, the patients were also being treated with metformin. Notably, a significant reduction was observed in serum insulin, HbA1c, and fasting blood glucose along with some decrease in triglycerides, serum cholesterol, and LDL in comparison with the placebo group.
Table 1. Clinical studies of frankincense
In another placebo and randomized controlled trial, Mehrzadi et al. [Citation20], investigated B. serrata effects on 56 type 2 diabetic patients and results showed that B. serrata gum resin did not affect the patients with a dose of 250 mg for 8 weeks. Moreover, this discrepancy between the Azedmehr et al. [Citation19] study and this one [Citation20] could be due to the 400 mg twice daily dose (higher dose of the resin) along with 12 weeks treatment rather than the 8 weeks treatment and 250 mg twice daily dose. Ahangarpour et al. [Citation21] studied antidiabetic effects of B. serrata gum resin on patients with a 900 mg daily dose for 6 weeks. In this case, the authors noticed a remarkable enhancement in blood HDL along with a significant reduction in triglyceride, cholesterol, SGPT, LDL, fructosamine, and SGOT levels. Schrott et al. [Citation22] in 2014 published a case report about the antidiabetic effects on one patient treated for 8.5 weeks with a B. serrata extract and the tyrosine phosphatase A2 antibody (IA2–A) marker was reduced from 25 K/U/1 to 10 K/U/1. In another report, Silybum marianum seeds, Urtica dioica leaves, and B. serrata resin capsules reduced the plasma glucose, glycosylated hemoglobin (HbA1c), serum triglyceride, and cholesterol [Citation23].
Craft et al. [Citation24] in a double-blind, randomized, placebo-controlled clinical trial demonstrated that B. serrata along with Melissa officinalis extract tablets improved total memory score in 70 elderly patients. Asadi et al. [Citation25] showed that frankincense resin had a remarkable effect on the retention and acquisition of explicit motor memory, while Aghajani et al. [Citation26] reported that 70 elderly patients they were treating experienced an enhanced memory after taking frankincense extract for 30 days. Givad et al. [Citation27] showed that 60 patients taking capsules containing 500 mg of powdered frankincense experienced improved muscle strength in their left limbs but interestingly, did not experience the same effect in their right limbs. Moreover, B. carterii essential oil inhalation aromatherapy was found to have a positive effect on the intensity of labor pain among women [Citation28] and menstrual bleeding duration was reduced in the B. serrata and ginger group [Citation29].
3. Frankincense diterpenes
3.1. Tetrahydrofurano cembranoids
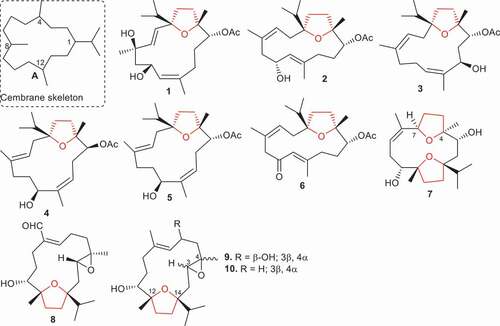
The cembrane skeleton comprises a 14-membered carbocyclic core system ‘A’ () featuring an isopropyl group at C-1 along with three methyl groups at C-4, C-8, and C-12. Moreover, this skeleton is also diversified with other core skeletons having 12 or 13-membered carbon skeletons along with lactone, cyclic ether, or furan groups around the macrocyclic system [Citation30].
Yu et al. [Citation31] isolated six tetrahydrofuran cembranoids named boscartins AP (1), AR (2), AS (3), AT (4), AU (5), and AW (6) from B. carterii. Moreover, compounds 1–6 all featured a tetrahydrofuran ring along with an isopropyl group. Biological investigations of compounds 1–6 showed that compound 1, bearing two hydroxyl groups (C-4 and C-6) along with one acetoxy group, illustrated significant anti-inflammatory effects toward LPS-induced RAW 264.7 cells with IC50: 13.1 μM () while the remaining compounds were not active (IC50: >50 μM). In addition, compounds 1–6 were tested for their activity toward hepatoprotective effects induced by APAP toward HepG2 cells. Notably, compound 1 proved to be the most potent and illustrated hepatoprotective activity with percentage inhibition: 30.7% at 10 μM and its effect was higher than the standard bicyclol (percentage inhibition: 27.2%). In addition, the hepatoprotective activity of compounds 4 and 6 showed significant inhibitions with percentage inhibition: 26.7% and 25.9%, respectively, which are slightly less than bicyclol ().
Table 2. Biological effects of diterpenes 1–44
Ren et al. [Citation32] reported four new cembranoids, viz. boscartins A (7), and E-G (8–10) from the gum resin of B. carterii and tested them for their anti-ulcerative colitis effects. Compound 7 featured two tetrahydrofuran rings between C-4⁄C-7 and C-12⁄C-14 while the remaining compounds have only one tetrahydrofuran ring (C-12⁄C-14) and one epoxide ring (C-3⁄C-4). Notably, compounds 7–10 activated XBP 1 transcription a concentration of 10 μM. Moreover, compound 8 bearing one tetrahydrofuran epoxide ring along with an aldehydic group, illustrated more potent effects than the 8-oxodihyrocoptisine (positive control). On the other hand, compounds 7, 9, and 10 lacking the aldehyde group showed lower biological activity compared to compound 8. In addition, compounds 7–10 demonstrated a promising dose−effect relationship observed for these tested metabolites with EC50: 0.34, 1.14, 0.88, and 0.42 μM, respectively.
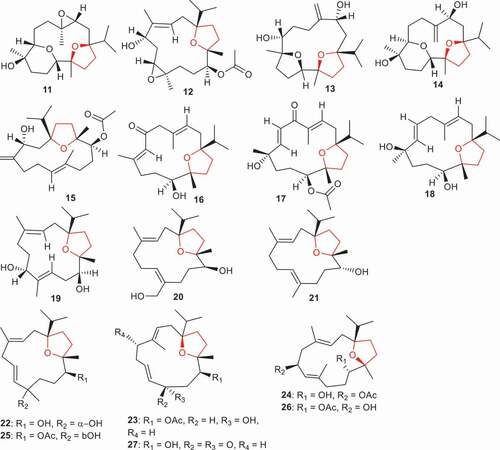
Boscartins P (11), U-Y (12–16), along with boscartins AA (17), AB (18), AE (19), AF (20) and 1,4-epoxy-8,13-cembrandien-5,12-diol (21) (), are produced by B. carterii and featured a tetrahydrofuran ring between C-1 and C-12. All the compounds were tested for hepatoprotective effects toward D-galactosamine-induced HL-7702 cell damage [Citation33]. Of note, compound 16 bearing a keto group at C-6 and a hydroxyl group at C-11, was the most potent and illustrated hepatoprotective activity with a percentage inhibition of 53.7% and this effect was 1.5 times higher than the standard bicyclol (percentage inhibition: 38.3%). In addition, hepatoprotective activity of compound 18, having two hydroxyl groups at C-8 and C-11, was quite significant and its activity was similar to the standard bicyclol (38.3%). Of further note was that the hepatoprotective effects of diterpene 21, which featured two double bonds and one hydroxyl group, were slightly higher (39.9%) than the standard bicyclol. It was found that compounds 15, 19, and 20 that do not feature and share common functional groups or similar structures, possessed almost the same good hepatoprotective activity with all having the same percentage inhibition (31%) [Citation33].
Boscartins AN (22), AP (23), AR (24), AC (25), AG (26), and compound 27 were isolated from B. sacra and tested for their hepatoprotective and neuroprotective effects. Diterpenes 22, 23, 25 and 27 illustrated hepatoprotective effects and cell viability rates ranged from 72.5% to 80.5%. Notably, these compounds manifested better hepatoprotective effects than the standard bicyclol (70.1%) [Citation34]. Interestingly, compound 22 was the most active with a cell viability rate of 80.5%. On the other hand, compound 25, which features the same structure as compound 22 except for having a β-hydroxyl and acetate group, possesses slightly less activity than 22 and demonstrated hepatoprotective effects with a cell viability rate of 76.6%. Notably, diterpene 23 also showed good hepatoprotective effects with a cell viability rate of 77.3%. On the other hand, it would appear that a keto group reduces the activity because diterpene 27, having a similar core structure to 23 except for an additional keto group, illustrated hepatoprotective effects with a cell viability rate of 72.5%. In addition, diterpenes 24 and 26 illustrated equal or better neuroprotective effects than the standard PHPB: 68.9% toward glutamate-induced toxicity with cell viability rates of 70.2% and 73.8%, respectively. Moreover, results of neuroprotective effects toward oxygen–glucose deprivation toxicity in SK-N-SH cells illustrated that diterpenes 23 and 26 possess significant protection effects with cell viability rates of 74.6% and 71.6%, respectively, which effects were higher than the standard PHPB: 70.0%) [Citation34].
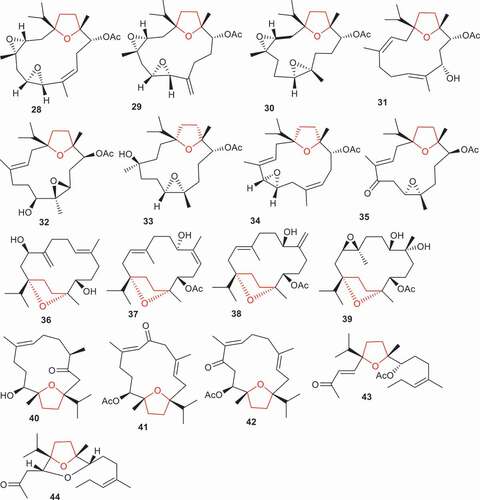
Boscartins AH–AO (28–35) () is produced by B. carterii and only compound 35, which features an α,β-unsaturated keto group along with an epoxide and acetyl group, illustrated significant anti-inflammatory effects via inhibition of NO production with an IC50: 14.8 µM. On the other hand, the same compound was the least active in terms of its hepatoprotective effects with cell viability rates of 44.0%. Moreover, compounds 28 and 29 displayed potent hepatoprotective effects with cell viability rates of 75.5% and 68.8% respectively [Citation35]. Notably, these compounds have better hepatoprotective effects than the standard bicyclol (67.7%). SAR studies showed that compounds 28 and 29 possessed better effects and it should be noted that they possess one furan ring and two epoxide rings. Additionally, both compounds have the same core structure except for the position of their double bonds viz. (28: C-8/C-9 double bond vs 29: C-8/C-19 double bond). Although compound 30, which is less active than compounds 28 and 29, also has one furan ring and two epoxide rings. However, this former compound (30) has the second epoxide ring at a different position compared to compounds 28 and 29 [Citation35].
(1S, 3 R, 11S, 12 R, 7E)-1,12-Epoxy-4-methylenecembr-7-ene-3,11-diol (36) along with boscartins L–M (37–39) were isolated from B. sacra. It was found that these compounds illustrated moderate hepatoprotective effects with cell viability rates ranging from 18.6% to 36.6% and these activities were less than the standard bicyclo (53.0%) () [Citation36]. Moreover, the position of the double bonds and stereochemistry of the hydroxyl group impact the biological effects because diterpene 38 showed better effects (32.8%) when compared to isomer 37 (21.9%). On the other hand, sacraoxides C (40), E (41), and F (42) were reported to also be isolated from B. sacra and were evaluated for their anti-inflammatory effects. Secondary metabolites 41 and 42 having keto, double bond and acetate groups demonstrated the most significant inhibitory effects toward LPS-induced NO production with IC50: 36.4 and 24.9 μM, respectively, while compound 40 which featured two double bonds, one hydroxyl, and one acetate group proved to be only moderately active with IC50: 72.1 μM [Citation37].
Literature revealed that Boswellia species, interestingly, demonstrated wound healing properties. For example, B. serrata oleo-gum resin was able to effectively induce wound contraction [Citation38]. In addition, B. serrata resin possesses peptic ulcer and colon ulcer healing properties [Citation39]. Boswellians B (43) and C (44), two 4,5-seco cembrane-type diterterpenes, were isolated from B. papyifera and displayed significant wound healing properties at a 20 μ mol/L concentration. Both molecules effectively stimulate proliferation, tuber formation, and mobility of HUVECs (vein endothelial cells) [Citation40].
4. Incensole derivatives
4.1. Anti-inflammatory effects
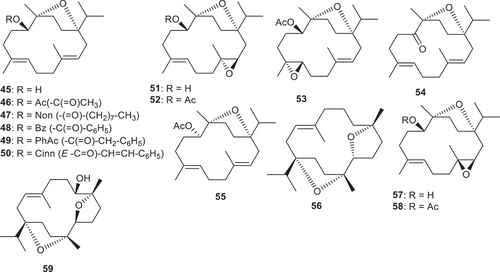
The frankincense resin containing incensole (45) and its acetate derivative, incensole acetate (IA, 46) () has been used globally to manage inflammatory disorders and is currently in use for its anti-inflammatory potential. It is used as an anti-inflammatory agent in Asia and Europe as Jerusalem Balsam [Citation41]. The extracts of Boswellia resin are also incorporated as supplements in foods for the management of arthritis in Europe and the USA. Moussaieff et al. [Citation42] reported that these readily available products need to be standardized for their active components, including incensole acetate (IA, 46) and incensole analogs along with boswellic acids. Moussaieff et al. [Citation41] in 2007 demonstrated that incensole (45) and incensole acetate (IA, 46) mediated the degradation of IκBα in HeLa cells stimulated with TNFα. IA (46) significantly inhibited IκBα degradation. It was demonstrated that incensole acetate (IA, 46) inhibited IKK phosphorylation and consequently, activation in vivo, but not in vitro, indicating that IA (46) exerted its effect upstream of IKK. Moreover, IA (46) prevented NF-κB activation () mediated through LPS and TNFα stimulation in Jurkat T cells that are co-stimulated. IA (46) reduced TAK/TAB mediated phosphorylation of the IKKα/β and subsequently, the activation pathway through interference with a step that couples TAK to the phosphorylation of IKK. However, this attenuation seems to be specific as IA (46) does not inhibit TNFα-mediated activation of p38 MAPK and JNK.
Table 3. Biological effects of diterpenes 45–59
IA (46) inhibits the phosphorylation of IκBα and its degradation, along with inhibition of phosphorylation of IKK at serine 536. The IKK plays a critical role both in p65 and IκBα phosphorylation [Citation43]. Furthermore, as IA (46) does not inhibit TRAF2-overexpressing cells and therefore supports a specific intervention of IA (46) at the TAK1-IKK activation stage. However, IA (46) effects on IKK are similar to that of tetracyclic kaurene diterpenes as reported earlier by Castrillo et al. [Citation44], since both are inhibitors of the signaling pathway. This fact illustrates that the action of IA (46) is more related to the NF-κB pathway as kaurenes also decrease the phosphorylation of ERK1, ERK2, p38, and MAPK. The mechanism through which terpenes reduce IKK activation is poorly understood [Citation44].
4.2. Neuroprotective effects
Moussaieff et al. [Citation45] reported that IA (46) produces neuroprotective effects in an animal model of traumatic brain injury (TBI). Their study demonstrated its prolonged effectiveness on memory and motor functions that suggested that the significant prolonged neuroprotection of IA (46) after CHI linked to decreased expression of IL-1β and TNF-α mRNAs and reduced activation of glial cells and thus neurodegeneration. The inhibition of inflammatory cytokines by IA (46) and the beneficial effect produced by its administration on cognitive and motor recovery, are in line with the view that reduced expression of proinflammatory cytokines following brain injury is helpful for recovery [Citation46]. Reduced immunoreactivity of GFAP is associated with the decreased labeling of microglial cells, indicating that IA (46) produces neuroprotection due to its anti-inflammatory effect. Since IA (46) produces an inhibitory effect on NF-kB, reported by the same author in 2007 [Citation41], and apoptosis in macrophages, it is possible that the anti-inflammatory potential of IA (46) following trauma is due to inhibition of NF-kB activation and occurs via apoptosis of the macrophage.
The potential of IA (46) to decrease neurodegeneration within the hippocampus and to enhance memory suggests that the enhanced memory ability is due to at least the decreased neuronal death within the hippocampus. Nevertheless, the involvement of other mechanisms responsible for IA (46) beneficial effects cannot be ruled out such as involvement of the modulation of the NMDA receptor in memory impairment and recovery [Citation47]. Further studies showed that IA (46) produces a mild hypothermia. Other studies have shown the involvement of crosstalk between anti-inflammatory action and hypothermia associated with injury in the central nervous system [Citation48,Citation49]. However, in studies conducted by Moussaieff et al. [Citation45], involving the prevention of this hypothermic effect of IA (46), they found that it did not reduce its neuroprotective action. This investigation illustrated that the IA (46)-mediated hypothermic effect plays no central role in the functional neuroprotective action produced by it. This finding is not unexpected due to the modest and brief nature of the observed reduction on temperature.
These results of Moussaieff et al. [Citation45] are in line with findings of Schuhmann et al. [Citation50], who demonstrated that B. carterii resin showed neuroprotective action following controlled cortical impact. However, the results of Moussaieff et al. [Citation45] indicate that the neuroprotection exhibited by B. carterii resin might be due to IA (46) and its derivatives. Nevertheless, in contrast to results by Schuhmann et al. [Citation50], the data of Moussaieff et al. [Citation45] indicated that IA (46) did not produce any substantial inhibitory action on post-traumatic edema formation within the cerebrum in animal model studies on mice. The inconsistency between these findings could be due to the difference in species (mice versus rats) and the models used (CHI versus controlled cortical impact). Therefore, it is also likely that Boswellia resin, as reported by Schuhmann et al. [Citation50], had additional compounds of a neuroprotective nature such as boswellic acids and their derivatives demonstrating their anti-inflammatory effects [Citation51].
Pollastro et al. [Citation52] evaluated incensole (45) and IA (46) along with the incensole semisynthetic derivatives 47–55 for their neuroprotective effects via TRPV3 activation. Incensole acetate (46) which features an acetate group at the C-5 hydroxyl group of incensole proved to be the most active compound and activated the TRPV3-mediated calcium influx with EC50: 1.4 μM. Notably, the EC50 value observed in rTRPV3 was less than that reported by Moussaieff et al. [Citation53] for mTRPV3 (EC50: 16 μM), indicating an excessive sensitivity to incensole acetate (46) for the rat orthologue. Moreover, cleavage of the acetate group leads to incensole (45) and this acetate cleavage reduces the activity (45: EC50: 2.1 μM). Oxidation of the C-5 secondary hydroxyl group in the later compound results in the formation of the corresponding keto comprising compound, incensone (54) and this keto group slightly decreases activity (EC50: 2.1 vs 2.4 μM) (). Conversely, inverting the stereochemistry of the C-5 hydroxyl group (epimerization) of incensole (45) followed by acetylation produced epi-incensol acetate (55) which exhibited a decrease of the TRPV3-activating effects (EC50: 7.3 μM). Additionally, epoxidation of incensole (45) to the 3-nonyl (52: EC50: 8.8 μM) and 7-epoxides (53: EC50: 8.6 μM) also displayed reduced efficacy [Citation52].
The decrease in activity was further evident after substitution of the acetate group with a cinnamate moiety (50: EC50: 10.5 μM). Conversely, the decrease in neuroprotective effects was evident for the benzoate derivative 48 (EC50: 4.4 μM) along with the nonanoate analog 47 (EC50: 5.6 μM). On the other hand, compounds bearing a phenylacetate group (compound 49: EC50: 1.2 μM) illustrated similar potency compared to incensole acetate (46). In addition, phenylacetate analog 49 demonstrated promising inhibitory effects on NF-κB with activations of IC50: 22.4 μM. Moreover, the incensol nonanoate (47) also inhibited STAT3 with IC50: 38.7 μM. However, the other incensole derivatives were inactive [Citation52].
4.3. Alzheimer’s disease and memory enhancement
Frankincense has demonstrated an ability to produce developmental alterations in the brains of laboratory animals such as an increase in neuronal volume and dendritic spines number and promoting memory formation [Citation54]. For instance, maternal administration of frankincense during gestation increased neuronal dendritic segments in the CA3 region of the hippocampus. Branching density of dendrites was also elevated in frankincense treated rats as compared to control animals [Citation55]. Moreover, it was shown that the volume of the cellular layer of CA3 and dentate gyrus as well as individual neurons in the hippocampus were significantly increased during lactation following maternal injection of frankincense [Citation56]. Furthermore, this study also demonstrated that maternal administration of frankincense upregulated the signaling mediator, reflecting an increased expression of calcium/calmodulin kinase II-α (CaMKII-α) mRNA in the hippocampus and an improvement in memory formation during gestation and lactation periods in juvenile rats [Citation57].
Recently, Beheshti et al. [Citation54] demonstrated that during gestation and lactation, maternal administration of frankincense significantly enhanced spatial memory formation in offspring male rats. Moreover, long-term administration of a B. serrata aqueous extract increased spatial learning formation in animals [Citation58,Citation59]. In Iran, frankincense was employed by the famous physician, Avicenna, to reduce amnesia and enhance memory function during the 10th century [Citation59].
Boswellia sp. containing IA (46) has been used folklorically for decades for neuroprotection against neurodegeneration within the hippocampus and to improve cognitive ability in patients with Alzheimer’s disease (AD) [Citation45,Citation60]. The hippocampus is that part of the limbic system known for memory and learning function. The neurodegeneration in the hippocampus causes memory loss due to increased oxidative stress, inflammation, apoptosis, and decreased brain-derived neurotrophic factor (BDNF) expression associated with an increased β-Amyloid peptide (Aβ) eventually resulting in Alzheimer’s disease. Apart from the hippocampus the neocortex is also involved in memory and learning functions [Citation61,Citation62].
It is known that oxidative stress is critically involved in the mediation of Alzheimer’s disease [Citation63,Citation64] involving neural stem cells (hOBNSCs) of the human olfactory bulb. IA (46) is known to produce neuroprotective, anti-oxidant, and anti-inflammatory properties. It also ameliorates neuronal cell death by preventing overproduction of ROS and improve the antioxidant enzymes [Citation65]. For example, it was reported that Aβ25–35 produces oxidative stress by increasing the level of ROS, while an IA (46) pretreatment ameliorates the ROS level. Additionally, IA (46) significantly decreased the expression of malondialdehyde (MDA) elevated with Aβ25–35. Furthermore, IA (46) increases the level of enzymes that possess antioxidant potential. These include catalase, superoxide dismutase, and glutathione peroxidase reduced by Aβ25–35 [Citation65]. Therefore, a strong association exists between AD and the increased oxidative stress, which might be due to an increased production of reactive oxygen species (ROS). The increased ROS formation causes apoptosis, cell death, and memory impairment [Citation66]. Further, heme oxygenase-1 (HO-1), an endogenous antioxidant [Citation64], not only prevents ROS production but also decreases the synthesis of the tau level involved in the progression of AD. IA (46) is known to produce its effect through a decrease in oxidative stress involving decreased ROS production, and improving the expression of antioxidant enzymes including SOD, HO-1, CAT, and GPX [Citation65].
B. carterii resin, due to its bioactive component IA (46), has been widely used to prevent memory impairment indicating its effectiveness in AD [Citation58,Citation67,Citation68] due to the reduced expression of inflammatory mediators such as TNFα, IL1β, TGFβ, Cox2, and NFkβ [Citation65]. As inflammation is the first response of the neuroimmune system to a disease, it plays a pivotal role in neurological disorders including stroke, Alzheimer’s disease, Parkinson’s disease, and other neuro-inflammatory conditions [Citation69]. Neuroinflammation during Alzheimer’s disease could be triggered through Aβ and/or astrocyte proliferation resulting in the accumulation of proinflammatory mediators, such as tumor necrosis factor-alpha (TNFα), interleukin 1β (IL-1β), interleukin-6 (IL-6), NFκB, and cyclooxygenase-2 (Cox2) activation [Citation70]. For example, lipopolysaccharide (LPS) is known to produce cognitive, learning, and memory impairment and hippocampal damage due to its inflammatory effects in animal models. The level of glial fibrillary acidic protein (GFAP) was, on the one hand, increased by IA (46) but, on the other hand, BDNF concentrations were decreased. The results also showed that LPS increased the NO level in the hippocampus compared to the control group [Citation71].
Further study showed that pretreatment with IA (46) also decreases memory impairment due to anti-inflammatory effects in the brain associated with reduced NFkB activation. Furthermore, a reduced expression of IL1β, TNFα, NFkB, and Cox2 against LPS-induced memory impairment was indicated by the passive avoidance and Morris water maze indicating IA (46) improved memory and spatial learning. It was also shown that IA (46) pretreatment improved avoidance memory as noted by an increased latency of the passive avoidance test [Citation71]. It was reported that IA (46) attenuates GFAP expression suggesting involvement of activated astrocytes [Citation65,Citation71] in memory deficits. IA (46) also reduced NO levels in the hippocampus due to NFkB inhibition [Citation34].
Since oxidative stress and inflammatory response are the main hallmarks of AD, IA (46) treatment might be considered the better option as it possesses both anti-inflammatory and antioxidant potential [Citation70]. Aβ deposition causes activation of pathways related to apoptosis that cause neuronal cell death in AD [Citation61,Citation72]. For example, cells treated with Aβ25–35 showed an increase in activity of caspase 3 and protein expression, inhibited by IA (46). This indicates the involvement of apoptotic pathways in the development of Aβ25–35-mediated neurotoxicity. Taken together, the above results suggest that IA (46) significantly decreased Aβ-mediated apoptosis through stimulation of antiapoptotic markers such as Bcl2, and inhibiting markers for pro-apoptosis, such as Bax, cyto c, caspase8, and caspase 3 in hOBNSCs [Citation65]. IA (46) pretreatment also significantly decreased mRNA and protein-level markers of apoptosis, such as Bax, caspase 8, cyto c and an increase in the mRNA and protein level of anti-apoptotic markers such as Bcl2 compared with controlled cells indicating its effectiveness against memory impairment [Citation65].
Studies have shown that BDNF plays a pivotal role in neurogenesis and is highly expressed in the hippocampus and neocortex [Citation73]. It was reported that IA (46) increased BDNF expression in the hippocampus that causes activation of tyrosine kinase B (TrkB) receptor to induce synaptogenesis and brain development [Citation74]. Furthermore, LPS decreases BDNF and TrkB expression during inflammation and memory deficit [Citation75]. It is, therefore, possible that IA (46) ameliorates memory impairment as indicated by the passive avoidance test and Morris water maze test through an increased expression of BDNF [Citation71] possibly involving the tyrosine kinase B (TrkB) receptor.
4.4. Anxiety and depression
Boswellia resin as an incense on burning remained a part of cultural and religious rituals in Eastern countries for centuries and is thought to lead to spiritual exaltation associated with such ceremonies [Citation53]. Moussaieff et al. [Citation53] showed that IA (46) exhibits a critical TRPV3-dependent anxiolytic and antidepressant effect. Due to slight differences that are usually expected among different strains, IA (46) produces substantial behavioral effects in both wild-type C57BL/6 and Sabra mice, indicating its effectiveness as a psychoactive agent. Furthermore, IA (46) also increases c-Fos expression in several areas of the mouse brain involved in depression and anxiety. c-Fos is a transcription factor, and changes in its expression indicate an alteration in neuronal activity. Therefore, any change in the level of c-Fos within the amygdala indicates its engagement in anxiety as shown by both anxiogenic and anxiolytic drugs [Citation76].
Moreover, the same authors reported that IA (46) causes calcium entry in TRPV3 channel expressing HEK293 cells. The effect of IA (46) on TRPV3 channels appears to be more selective, since it produces very modest or no effect on TRPV1, TRPV2, or TRPV4 expressing cells. As the IA (46) mediated charge response is delayed, it also results in a delayed calcium response. This may be due to intrinsic differences between an electrophysiology apparatus and calcium imaging system [Citation53].
In another research study, Moussaieff et al. [Citation77] indicated that IA (46) produces antidepressant action in laboratory animals. This antidepressant action was associated with alterations of both CRF and BNDF gene expression, along with serum corticosterone levels in animal studies. Antidepressant-like behavior was produced both after single and long-term administration of IA (46). As indicated in FST, a single injection of IA (46) (10 mg/kg) decreased the immobility time significantly in submissive animals. Likewise, the therapeutic benefits of antidepressants observed clinically after extended administration of IA (46) as shown by the DSR food competition test caused a time and dose-dependent reduction in animals’ submissive behavior. Earlier studies have shown that a decrease in submissive behavior with antidepressant drugs in the DSR test validated the efficacy of these drugs’ effectiveness on humans [Citation78]. Since IA (46) reduces the submissive behavior as indicated by the DSR test, it stands to reason that IA (46) has the potential to be an efficacious antidepressant agent.
Furthermore, IA (46) significantly decreased the serum corticosterone levels in laboratory animals. Corticosterone, a glucocorticoid, is released due to neuroendocrinal activation of the HPA axis from the adrenal gland in response to different stressors [Citation79]. Moussaieff et al. [Citation77] showed that stress due to physical restraint caused an increase in the level of corticosterone of vehicle-treated submissive mice compared to IA (46)-treated animals. In this study, the CRF mRNA contents in the hippocampus were decreased dose-dependently in submissive animals by long-term IA (46) administration. CRF plays a critical role in the regulation of glucocorticoids. The signaling and associated downstream transduction pathways, mediated through the CRF receptor, are well-studied potential drug targets for the management of anxiety and depression.
Moussaieff et al. [Citation77], in his studies, showed that reduction in the expression of CRF by IA (46) produces two-prolonged effects: i) decreased CRF that lowers the activity of HPA axis and in turn results in a reduced level of corticosterone, and ii) decreased expression of CRF within the hippocampus that may be observed due to behavioral effects. Furthermore, apart from the IA (46) effect on CRF levels within the hippocampus, IA (46) also affected the expression of BDNF in the hippocampus. In depression, BDNF involvement has received broad attention since both animal and clinical data associate an altered expression of BDNF with depressive-like behavior [Citation80]. The BDNF analyses indicated that increases in gene expression were significant among the IA (46) treatment groups. Thus, the result that IA (46) selectively affected BDNF and CRF contents may indicate an association between the antidepressant effect to decrease the HPA axis signaling on a molecular basis.
The downregulation in the expression of hippocampal CRF mediated by long-term IA (46) injection indicated that changes in the expression of BDNF, concomitant with reduced depressive behavior, are indeed in line with earlier studies that indicate involvement of BDNF in regulating the mood. Moussaieff et al. [Citation77] demonstrated robust antidepressant effects of IA (46) administration, which linked the stress with an altered hormonal and gene expression response. Future studies are required that might indicate the involvement of the signaling pathways through which IA (46) produces behavioral changes, possibly leading to some novel antidepressant drug developments.
In another study by Al-Harrasi et al. [Citation81], incensole (45), IA (46), incensone (54) and incensfuran (56) were examined for their potential antidepressant effects. These incensole derivatives showed antidepressant-like behavior. Compounds 45 and 46 significantly decreased immobility time at 46 and 55 mg/kg in mice, respectively, while compound 54 was reported to be highly potent, decreasing immobility time significantly at doses of 0.1–3 mg/kg. Moreover, compound 56 exhibited significant antidepressant-like effects at 3 mg/kg in the FST.
IA (46) is known to exhibit anti-depressive-like effects at a higher dose of 50 mg/kg following a single intraperitoneal (i.p) injection. Al-Harrasi et al. [Citation81] showed that compounds 45, 54 and 56 were efficacious even at lower dose (0.1–3 mg/kg) following a single i.p injection. In the same study, prazosin, SCH23390, ketanserin, and haloperidol pretreatment prior to 46 and 56 treatments, partly blocked anti-depressive-like activity of these agents, showing that antidepressant activity of 46 and 48 was produced involving the dopaminergic and alpha-adrenergic systems. However, pretreatment with bicuculline, pentylenetetrazole, and GABA antagonists did not block 46- and 56-mediated reductions in immobility time during FST, excluding the involvement of GABA.
Compounds 45 and 56 reduced immobility time as evidenced by the forced swim test that was partly blocked with prazosin, SCH23390, ketanserin, and haloperidol pretreatment indicating the involvement of other mechanisms responsible for this behavior. For example, involvement of TRPV3 and NF-κB in the mediation of anti-depressive-like actions of compounds 45 and 46 have been reported. Al-Harrasi et al. [Citation81] reported that compounds 45 and 46 reduced immobility time through NF-κB inhibition. Additionally, these compounds were identified to activate the central TRPV3, as evidenced by the lack of antidepressant potential in TRPV3-knockout mice [Citation53]. Furthermore, an elevated plus maze test indicated that compound 46 produced an anxiolytic effect reversed by bicuculline or pentylenetetrazole indicating involvement of GABAA receptors. These compounds were also shown to exhibit anticonvulsant effects in PTZ-induced seizures [Citation81].
4.5. Miscellaneous biological effects
Incensole (45), incensole epoxide (57), and the incensole epoxide acetate (58) () were isolated from B. carterii and these compounds were tested for their hepatoprotective effects toward D-galactosamine-induced HL-7702 cell damage [Citation33]. Of note, compounds 45 and 57 were the most potent and illustrated hepatoprotective activity with percentage inhibition: 58.8% and 60.4%, respectively, and these effects were over 1.5 times higher than the standard bicyclol (percentage inhibition: 38.3). In addition, the hepatoprotective activity of compound 58 also showed significant effects with a slightly higher percentage inhibition: 43.1% than the standard bicyclol [Citation33]. Diterpene isoincensole epoxide (59) and was isolated from B. serrata and possesses antimalarial effects against Plasmodium falciparum with IC50: 9.6 µM and with a selectivity index of 8.8. On the other hand, this compound was not effective in cytotoxic investigation toward L6 rat skeletal myoblasts (IC50: 84 µM) [Citation82].
4.6. Miscellaneous cembrane diterpenes with biological activities
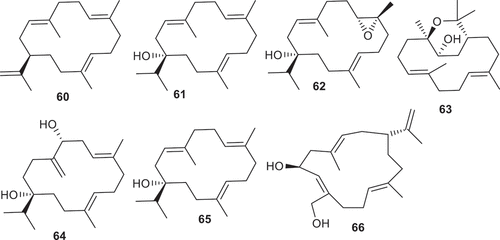
Diterpenes, cembrene A (60), serratol (61), 1S,3E,7 R,8 R,11E-7,8-epoxy-cembra-3,11-dien-1-ol (62), and isodecaryiol (63) () were reported to be isolated from B. serrata. Among these, compound 63 demonstrated better antimalarial effects against Plasmodium falciparum with IC50: 7.5 µM followed by compound 60 which possesses a similar but slightly lower activity with IC50: 9.9 µM (). On the other hand, only compound 62 demonstrated weak cytotoxic effects toward L6 rat skeletal myoblasts with IC50: 32 µM [Citation82]. Another diterpenoid, (rel)-(1S,5 R,7E,11E)-1-isopropyl-8,12-dimethyl-4-methylenecyclotetradeca-7,11-diene-1,5-diol (64) was isolated from B. carterii and illustrated anti-inflammatory effects via NO inhibition with IC50: 1.32 µM [Citation83].
Table 4. Biological effects of diterpenes 60–79.
Serratol (65) was isolated from B. serrata and tested for its activity against Plasmodium falciparum, Trypanosoma brucei rhodesiense, T. cruzi, and Leishmania donovani. Notably, this compound illustrated significant antimalarial effects toward P. falciparum (IC50: 2.5 μM) and T. brucei rhodesiense (IC50: 3.8 μM). In addition, this compound demonstrated weak to moderate effects toward the L6 rat skeletal myoblast cell (IC50: 39 μM), T. cruzi (IC50: 44 μM) and L. donovani (IC50: 12 μM) [Citation84]. In another study, serratol (65) illustrated potent neuroprotective effects and activated TRPV3 with IC50: 0.15 μM [Citation52]. Boscartin AU (66) (68.4%) isolated from B. sacra, illustrated equal neuroprotective effects as the standard PHPB (68.9%) toward glutamate-induced toxicity with cell viability rates [Citation34].
5. Prenylaromadendrane and verticillane-type diterpenes
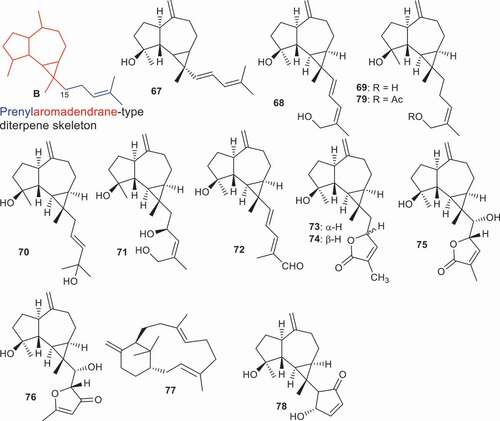
A prenylaromadendrane-type diterpene core skeleton ‘B’ is the combination of an aromadendrane sesquiterpene (; red color) with an attached prenyl group (; blue color) via C-15. A prenylaromadendrane-type diterpene named olibanumol D (67) was produce by B. carterii and illustrated inhibitory effects of 35.8% on NO production [Citation85]. In another report, B. carterii also produced another seven prenylaromadendrane-type diterpenes, boscartols A − C (68–70), E (71), F (72), H (73), and I (74). Of note, compound 72 bearing an aldehyde group at the C-20 position, was the most potent and illustrated hepatoprotective activity with a percentage inhibition: 69.6%, which effect was 1.6 times higher than the standard bicyclol (percentage inhibition: 42.5%). In addition, the hepatoprotective activity of compound 71 which has three additional hydroxyl groups (compared to compound 72), was less active than 72 but did demonstrate significant inhibition effects of 36.3% followed by compound 74 with 33.6%. However, these effects were lower than the standard bicyclol (). Notably, the position of the double bond at C-17/C-18 slightly decreased activity because diterpene 69 (33.6%) illustrated better effects than compound 68 (26.1%). Additionally, stereochemistry at C-16 plays a role in biological activity because compounds 73 (24.3%) and 74 (33.6%) are epimers [Citation86].
Yu et al. [Citation87] reported three new prenylaromadendrane-type diterpenes, boscartols K (75) and L (76) along with the known compounds, boscartols B (69), E (71), and F (72). Moreover, compounds 72, 75, and 76 possessed anti-inflammatory effects with IC50: 8.63, 2.22, and 1.10 µM, respectively. Notably, triterpene 76 illustrated greater effects than the standard dexamethasone (2.0 µM) while activity of compound 75 was slightly higher than the standard compound. All compounds have been tested for their cytotoxic effects toward breast cancer (MCF-7) and only diterpenes 69 and 71 illustrated activities with IC50: 9.5, and 6.3 µM, respectively.
The verticillane-type diterpene named (1S,3E,7E,11 R)-verticilla-3,7,12(18)-triene (77) was reported from B. serrata and demonstrated antimalarial effects against Plasmodium falciparum with IC50: 9.2 µM and a selectivity index of 8.8. On the other hand, this compound was not effective in a cytotoxic investigation toward L6 rat skeletal myoblasts [Citation82]. Prenylaromadendrane-type diterpenoids 78 and 79 along with boscartols B (69), C (70) and F (72) were isolated from B. sacra. Interestingly, all these diterpenoids displayed significant cytotoxic effects toward the U87-MG (malignant glioma) cell line with inhibitory rates ranging from 33.9 to 55.3 %. Notably, the activities of these molecules were higher than the standard 5-fluorouracil (30.4%) [Citation88].
6. Expert opinion
One major issue facing most of the developing world is provision of basic health care and the WHO demonstrated that countries having over half of the world‘s population cannot afford sufficient health-care services. This is mainly due to the fact that poor people are unable to afford the current health-care services. Medicinal plants offer alternative affordable medicine to such poor people leading to tremendous opportunities. Frankincense is one of the more important traditional plants and is thus an option for poor people for treatment of various types of diseases.
Frankincense diterpenoids, in particular incensole (45) and its acetate (46) are cembrane-type diterpenoids and possess interesting biological activities, such as anxiolytic, antidepressive, anticancer, and anti-inflammatory effects. There is an unfortunate paucity of simple chemical transformations of these scaffolds to prepare new and more potent anti-inflammatory agents. It is interesting to note that most of the drugs currently used are heterocyclic compounds. It seems to the authors that chemists should concentrate their talents on synthesizing heterocyclic analogs of active natural products. Thus, it is very important for medicinal chemists to establish new efficient protocols for the synthesis of different libraries of incensole-heterocyclic analogs for a comprehensive evaluation process. In addition, hybrid compounds between incensole or its acetate and anti-depressant or anti-inflammatory drugs should be synthesized in order to discover or identify new lead compounds based on the incensole scaffold.
There is a great concern regarding the sustainability of B. papyrifera which to date has been shown to contain the highest quantity of incensole (45) and its acetate (46). According to Groenendijk et al. [Citation89], over the next five decades, the population of B. papyrifera will decrease to about 10 % of its present population due to over-exploitation in harvesting of the plant. The obvious consequence is that this pharmacologically important material will become increasingly rare and expensive. Moreover, no drug is currently available in the market containing pure incensole (45) and its acetate (46). This may be due to their limited availability from nature as well as their poor solubility and bioavailability. Thus, better water-soluble analogs of compounds 45 and 46 need to be synthesized; this is a key feature for future drug development of these interesting natural products. In addition, it might be most useful to consider cultivating these plants on a commercial scale to ensure their survival and as sustainable sources of incensole (45) and its acetate (46). This must be supported by a more efficient synthetic methodology being devised for their industrial production since this will also open the doors for large libraries of analogs to be made and evaluated.
Incensole acetate (46) possesses very strong anti-inflammatory and antidepressant effects, and thus it is essential to study the molecular mechanisms by which it acts. In addition, future research should focus on gaining a fuller understanding on the particular molecular signaling pathways that incensole acetate (46) induces in order to maximize behavioral moderation. Previous studies have shown that neuroinflammation is one of the hallmarks in the pathophysiology of Parkinson’s disease involving activated microglia, astrocytes, and associated proinflammatory mediators [Citation90]. Therefore, incensole (45) and its acetate (46) should comprehensively be evaluated for their anti-Parkinsonism potential and their effect on proinflammatory mediators such as TNF-α, IL-1β, and IL-6. Additionally, high levels of proinflammatory cytokines have been found in the blood and cerebrospinal fluid of schizophrenic patients [Citation91]. As mentioned earlier, frankincense gum resins have been employed as incense in religious ceremonies. It is probable that incensole (45) and its acetate (46) may be effective against schizophrenia due their anti-inflammatory potential. Peripheral diabetic neuropathy is associated with rather painful symptoms involving activation of NF-κB and increased levels of TNF-α, IL-6, IL-1, and IL-18 [Citation92]. It is, therefore, possible that incensole (45) and its derivatives are potentially effective against diabetes-induced neuropathic pain. Moreover, since various Boswellia extracts have been marketed in the US and Europe as food supplements to treat arthritis, the authors suggest that these supplement products should be standardized for incensole acetate (46) and boswellic acids. Additionally, possible synergistic effects of these compounds need to be fully examined. Pharmacodynamics and pharmacokinetic data and in vivo studies of incensole (45) and its acetate (46) are thus required to give direction for future research. Furthermore, clinical studies are required to be carried after successful pharmacodynamics and pharmacokinetic studies.
Religious scholars have claimed for centuries that burning incense is good for the soul. It has been reported that frankincense smoke is employed for the treatment of postpartum depression, madness, restlessness, and sleepwalking. Moreover, Moussaieff et al. [Citation53] claimed that incensole acetate (46) possesses promising anxiolytic and antidepressive effects. The obvious question is whether it is the smoke derived from heating incensole acetate (46) in frankincense that is responsible for its antidepression action or some other ingredient. Basar [Citation93] investigated the pyrolysis products of B. carterii via heating with red-hot charcoal and surprisingly reported the presence of incensole (45; 22.8%) and incensole acetate (46; 15.5%) in the smoke that reflects quite a substantial amount. On the other hand, the essential oils of B. carterii contain only small quantities of incensole (45) (1.0% vs 22.8%) and incensole acetate (46) (2.3% vs 15.5%) as compared to its pyrolyzate given in brackets. We believe that an error may have been responsible for this apparent contradiction about the detection of incensole (45) and incensole acetate (46) in pyrolysis products of B. carterii. Second, Paul [Citation94] demonstrated that incensole acetate (46) completely decomposes at 60°C after 16 h, and it has also been observed that during investigations on frankincense smoke generation that boswellic acids were converted into 1,2,4a,9-tetramethyl-1,2,3,4,4a,5,6,14b-octahydropicene and 2,9-dimethylpicene that could be formed via demethylation, dehydration, decarboxylation, and dehydrogenation [Citation95]. The authors believe that the anxiolytic and antidepressive effects of frankincense smoke are more likely not only due to incensole acetate (46) but may be the result of synergistic effects and that presumably incensole acetate (46) is totally decomposed after heating the resin on hot charcoal after a certain time period. Based on the biological effects discussed in this review, the authors firmly believe that incensole-based molecules may emerge as a privileged scaffold to establish a host of lead compounds for the treatment of inflammation, Alzheimer’s disease, anxiety, and depression.
Herb–drug interaction (HDI) is a most crucial clinical consequence of herbal products practice. Frankincense has been employed over many years with Ayurvedic herbs without incident. Two reports have been published about the interaction between B. serrata and warfarin and the INR values were elevated in patients [Citation96]. In another report [Citation97] B. serrata showed an interaction with leukotriene inhibitors. Frankincense may create toxicity, or some other side effect via interacting with conventional medication. An advanced knowledge of interaction consequences of frankincense with western medicine will therefore equip people in their practice with vital knowledge during administration treatments. More clinical data is required in this area as the currently available information on frankincense HDI is insufficient and thus needs to be addressed.
Article highlights
Frankincense resin has over the years been employed in traditional medicines to treat depression, inflammation, and cancer
Various diterpenes have been isolated from frankincense and demonstrated intriguing biological activities
Among frankincense diterpenoids, incensole, and its acetate illustrated significant biological and pharmacological effects
Frankincense diterpenoids possess anti- inflammatory, neuroprotective, hepatoprotective, antimalarial, and cytotoxic effects
These diterpenoids have a great potential to be used in the treatment of Alzheimer’s disease, anxiety, and depression
This box summarizes key points contained in the article.
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Martinez D, Lohs K, Weihrauch JJM. Kulturgeschichte und wirtschaftliche Bedeutung. Botanik Chemie Medizin: Wissenschaftliche Verlagsgesellschaft, Stuttgart; 1989.
- Efferth T, and Franz O. Anti-inflammatory and anti-cancer activities of frankincense: targets, treatments and toxicities, Semin Cancer Biol, 2022;in print.
- Al-Harrasi A, Hussain H, and Csuk R, et al. Chemistry and bioactivity of boswellic acids and other terpenoids of the genus Boswellia.Amsterdam:Elsevier;2018.
- Hussain H, Al-Harrasi A, Green IR. Frankincense (Boswellia) Oils. In: Preedy VR, editor. Essential oils in food preservation, flavor and safety”. Elsevier UK: Academic Press; 2016. p. 431–440.
- Grbić ML, Unković N, Dimkić I, et al. Frankincense and myrrh essential oils and burn incense fume against micro-inhabitants of sacral ambients. Wisdom of the ancients? J Ethnopharmacol. 2018;12:1–14.
- De Rapper S, Van Vuuren SF, Kamatou GPP, et al. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils – a combination from the pharaonic pharmacopoeia. Lett Appl Microbiol. 2012;54:352–358.
- Dennert G. the CAM-Cancer Consortium, Boswellia, concerted action for complementary and alternative medicine assessment in the cancer field (CAM-Cancer), (2020) www.cam-cancer.org.
- Shen T, Lou HX. Bioactive constituents of myrrh and frankincense, two simultaneously prescribed gum resins in Chinese traditional medicine. Chem Biodivers. 2008;5:540–541.
- Maupetit P. New constituents in olibanum resinoid and essential oil. Perfumer Flavorist. 1985;9:19–37.
- Morikawa T, Matsuda H, Yoshikawa M. A review of anti-inflammatory terpenoids from the incense gum resins Frankincense and Myrrh. J Oleo Sci. 2017;66:805–814.
- Al-Harrasi A, Ali L, Rehman N, et al. 11α-ethoxy-β-boswellic acid and Nizwanone: one new boswellic acid derivative and new triterpene, respectively, from Boswellia sacra. Chem Biodivers. 2013;10:1501–1506.
- Rehman N, Khan A, Al-Harrasi A, et al. New α-glucosidase inhibitors from the resins of boswellia species with structure–glucosidase activity and molecular docking studies. Bioorg Chem. 2018;79:27–33.
- Shamraiz U, Hussain H, Ur Rehman N, et al. Synthesis of new boswellic acid derivatives as potential antiproliferative agents. Nat Prod Res. 2020;34(13):1845–1852. DOI:https://doi.org/10.1080/14786419.2018.1564295.
- Bini Araba AB, Ur Rehman N, Al-Araimi A, et al. New derivatives of 11-Keto-β-Boswellic Acid (KBA) Induce Apoptosis in Breast and Prostate Cancers Cells. Nat Prod Res. 2021;35(5):707–716. DOI:https://doi.org/10.1080/14786419.2019.1593165.
- Hussain H, Ali I, Wang D. Boswellic acids: privileged structures to develop lead compounds for anticancer drug discovery. Expert Opin Drug Discov. 2021;16(8):851–867.
- Roncero AM, Tobal IE, Moro RF, et al. Halimane diterpenoids: sources, structures, nomenclature and biological activities. Nat Prod Rep. 2018;35:955–991.
- Al-Harrasi A, Avula SK, Csuk R, et al. Cembranoids from Boswellia species. Phytochemistry. 2021;191:112897.
- Mehrzadi S, Tavakolifar B, Huseini HF, et al. The effects of boswellia serrata gum resin on the blood glucose and lipid profile of diabetic patients: a double-blind randomized placebo-controlled clinical trial. J Evid-Based Integr Med. 2018;23:1–7.
- Azadmehr A, Ziaee A, Ghanei L, et al. A randomized clinical trial study: anti-oxidant, anti-hyperglycemic and anti-hyperlipidemic effects of olibanum gum in type 2 diabetic patients. Iran J Pharm Res. 2014;13:1003–1009.
- Mehrzadi S, Tavakolifar B, Husseini HF, et al. The efficacy of Boswellia serrata gum resin for control of lipid profile and blood glucose in diabetic patients. Iran J Med Sci. 2016;41:S66.
- Ahangarpour A, Heidari H, Fatemeh RA, et al. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type 2 diabetic patients. J Diabetes Met Disord. 2014;13:29.
- Schrott E, Laufer S, Lämmerhofer M, et al. Extract from gum resin of Boswellia serrata decreases IA 2 -antibody in a patient with “Late onset autoimmune diabetes of the adult“ (LADA). Phytomed. 2014;21:786.
- Khalili N, Fereydoonzadeh R, Mohtashami R, et al. A mixed herbal formulation in the treatment of Type II Diabetes: a Randomized, double-blind, placebo-controlled, clinical trial. J Evid Based Complementary Altern Med. 2017;22:603–608.
- Taghizadeha M, Maghaminejad F, Aghajanic M, et al. The effect of tablets containing Boswellia serrata and Melisa officinalis extract on older adults’ memory: arandomized controlled trial. Arch Gerontol Geriatr. 2018;75:146–150.
- Asadi E, Shahabikaseb M, Zeidabadi R, et al. Effect of 4 weeks of frankincense consumption on explicit motor memory and serum BDNF in elderly men. Turk J Med Sci. 2019;49:1033–1040.
- Aghajani M, Taghizadeh M, Maghaminejad F, et al. Effect of frankincense extract and lemon balm extract co-supplementation on memory of the elderly. Complement Med J. 2017;7:3.
- Givad N, Rafieian-Kopaei M, Rezaei-Kheirabadi F, et al. A study of the clinical efficacy of frankincense in the acute phase of ischemic stroke. J Adv Herb Med. 2015;1:4–10.
- Esmaelzadeh-Saeieh S, Rahimzadeh M, Khosravi-Dehaghi N, et al. The effects of inhalation aromatherapy with Boswellia carterii essential oil on the intensity of labor pain among nulliparous women. Nurs Midwifery Stud. 2018;7:45–49.
- Eshaghian R, Mazaheri M, Ghanadian, et al. The effect of frankincense (Boswellia serrata, oleoresin) and ginger (Zingiber officinale, rhizoma) on heavy menstrual bleeding: a randomized, placebo-controlled, clinical trial. Complement Ther Med. 2019;42:42–47.
- Rodrigues IG, Miguel MG, Mnif W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, sinularia, and lobophytum since 2016. Molecules. 2019;24:781.
- Yu J, Zhao L, Sun X, et al. Bioactive cembrane diterpenoids from the gum resin of Boswellia carterii. Fitoterapia. 2020;146:104699.
- Ren J, Wang YG, Wang AG, et al. Cembranoids from the gum resin of Boswellia carterii as potential antiulcerative colitis agents. J Nat Prod. 2015;78:2322–2331.
- Wang YG, Ren J, Ma J, et al. Bioactive cembrane-type diterpenoids from the gum-resin of Boswellia carterii. Fitoterapia. 2019;137:104263.
- Wang JJ, Sun HR, Suo XY, et al. Ten undescribed cembrane-type diterpenoids from the gum resin of Boswellia sacra and their biological activities. Phytochemistry. 2020;177:112425.
- Sun X, Geng Y, Wang X, et al. Cembrane-type diterpenoids from the gum resin of Boswellia carterii and their biological activities. RSC Adv. 2020;10:746–755.
- Wang J, Zhen B, Hu J, et al. Neuroactive and Anti-inflammatory frankincense cembranes: boscartins L–O: cembrane-type diterpenoids from the gum resin of Boswellia sacra Flueck. Phytochemistry. 2019;163:126–131.
- Zhang B, Liu D, Ji W, et al. Sacraoxides A–G, Bioactive Cembranoids from Gum Resin of Boswellia sacra. Front Chem. 2021;9:649287.
- Mallik A, Goupale D, Dhongade H, et al. Evaluation of Boswellia Serrata oleo-gum resin for wound healing activity. Der Pharmacia Lettre. 2010;2:457–463.
- Namjou A, Rouhi-Broujeni H. Antihyperglycemic, antihyperlipidemic and wound healing of Boswellia serrata on experimentally induced diabetic rats. Abanico Veterinario. 2020;10:1–17.
- Yu QH, Sura MB, Wang DW, et al. Isolation of Boswelliains A—E, Cembrane-Type Diterpenoids from Boswellia papyifera,and an Evaluation ofTheir Wound Healing Properties. Chin J Chem. 2021;39:2451–2459.
- Moussaieff A, Shohami E, and Kashman Y, et al. Incensole acetate, a novel anti-inflammatory compound isolated from boswellia resin, inhibits nuclear Factor-κB activation. Mol Pharmacol. 2007;72:1657–1664.
- Moussaieff A, Fride E, Amar Z, et al. The Jerusalem Balsam: from the Franciscan monastery in the old city of Jerusalem to Martindale 33. J Ethnopharmacol. 2005;101:16–26.
- Sizemore N, Lerner N, Dombrowski N, et al. Distinct roles of the IκB kinase α_and β subunits in liberating nuclear factor κ B (NF-κB) from IκB and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277:3863–3869.
- Castrillo A, de Las Heras B, Hortelano S, et al. Inhibition of the nuclear factor _B (NF-_B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-_B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J Biol Chem. 2001;276:15854–15860.
- Moussaieff A, Shein NA, and Tsenter J, et al. Incensole acetate: a novel neuroprotective agent isolated from Boswellia carterii. J Cereb Blood Flow Metab. 2008;28:1341–1352.
- Shohami E, Bass R, Wallach D, et al. Inhibition of tumor necrosis factor alpha (TNFa) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab. 1996;16:378–384.
- Yaka R, Biegon A, Grigoriadis N, et al. D-cycloserine improves functional recovery and reinstates longterm potentiation (LTP) in a mouse model of closed head injury. FASEB J. 2007;21:2033– 2041.
- Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on t he expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–134.
- Shein NA, Doron H, Horowitz M, et al. Altered cytokine expression and sustained hypothermia following traumatic brain injury in heat acclimated mice. Brain Res. 2007;1185:313–320.
- Schuhmann MU, Mokhtarzardeh M, Skardelly M, et al. Effect of Boswellia carterii on brain edema following contusion injury. J Cereb Blood Flow Metab. 2005;25:S261.
- Hussain H, Al-Harrasi A, and Csuk R, et al. Therapeutic potential of boswellic acids: a patent review (1990-2015). Expert Opin Ther Pat. 2017;27:81–90.
- Pollastro F, Golin S, Chianese G, et al. Neuroactive and Anti-inflammatory Frankincense Cembranes: a Structure−Activity Study. J Nat Prod. 2016;79:1762–1768.
- Moussaieff A, Rimmerman N, Bregman T, et al. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008;22:3024–3034.
- Beheshti S, Tohidloo S, Esmaeili A. Frankincense improves memory retrieval and downregulates the hippocampal synaptophysin mRNA during the development of the rat brain. Physiol Pharmacol. 2020;24:46–53.
- Hosseini-Sharifabad M, Esfandiary E. A morphometeric study on CA3 hippocampal field in young rats following maternal administration of boswellia serrata resin during gestation. Iran J Basic Med Sci. 2007;10:176–182.
- Hosseini-Sharifabad M, Esfandiari E. The effects of maternal administration of Boswellia gum resin (frankincense) during lactation on stereological parameters of rat hippocampus. J Isfahan Med Sch. 2012;29:2198–2207.
- Beheshti S, Skakakomi AG, and Ghaedi K, et al. Frankincense upregulates the hippocampal calcium/calmodulin kinase ii-alpha during development of the rat brain and improves memory performance. Int J Dev Neurosci. 2018;69:44–48.
- Mahmoudi A, Hosseini-Sharifabad A, Monsef-Esfahani HR, et al. Evaluation of systemic administration of boswellia papyrifera extracts on spatial memory retention in male rats. J Nat Med. 2011;65:519–525.
- Hosseini-Sharifabad M, Kamali-Ardakani R, Hosseini-Sharifabad A. Beneficial effect of Boswellia serrata gum resin on spatial learning and the dendritic tree of dentate gyrus granule cells in aged rats. Avicenna J Phytomed. 2016;6:189–197.
- Moussaief A, Yu J, and Zhu H, et al. Protective effects of incensole acetate on cerebral ischemic injury. Brain Res. 2012;1443:89–97.
- Butterfield DA, Drake J, Pocernich C, et al. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554.
- Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444.
- Chen DL, Zhang P, Shuai LO L, et al. Protective effect of Bajijiasu against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2013;33:837–850.
- Wang HQ, Sun XB, Xu YX, et al. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010;360:159–167.
- El-Magd MA, Khalifa SF, Alzahrani A, et al. Incensole acetate prevents beta-amyloid-induced neurotoxicity in human olfactory bulb neural stem cells. Biomed Pharmacother. 2018;105:813–823.
- Evelson P, Llesuy S, Filinger E, et al. Decreased oxidative stress in prehepatic portal hypertensive rat livers following the induction of diabetes. Clin Exp Pharmacol Physiol. 2004;31:169–173.
- Hosseini M, Hadjzadeh MA, Derakhshan M, et al. The benefcial effects of olibanum on memory defcit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010;33:463–468.
- Marshall S. Frankincense: festive pharmacognosy. Pharm J. 2003;271:862–864.
- Zipp F, Aktas O. The brain as a target of infammation: common pathways link infammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527.
- Ali SI, Zhang CR, Mohamed AA, et al. Major constituents of Boswellia carteri resin exhibit cyclooxygenase enzyme inhibition and antiproliferative activity. Nat Prod Commun. 2013;8:1365–1366.
- Narges M, Beheshti F, Vafaee F, et al. the efects of incensole acetate on Neuro-infammation, brain-derived neurotrophic factor and memory impairment induced by lipopolysaccharide in rats. Neurochem Res. 2021;46:2473–2484.
- Harada HJ, Sugimoto M. Activation of caspase-3 in beta-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res. 1999;842:311–323.
- Zhu L, Nang C, Luo F, et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinfammatory processes and depressive-like behavior in mice. Physiol Behav. 2016;163:184–192.
- Fulgenzi G, Tomassoni-Ardori F, Babini L, et al. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB T1 receptor activation. J Cell Biol. 2015;210:1003–1012.
- Gao J, Xiong B, Zhang B, et al. Sulforaphane alleviates lipopolysaccharide-induced spatial learning and memory dysfunction in mice: the role of BDNF-mTOR signaling pathway. Neuroscience. 2018;388:357–366.
- Thompson BL RJB. Immediate-early gene expression in the central nucleus of the amygdala is not specific for anxiolytic or anxiogenic drugs. Neuropharmacology. 2006;50:57–68.
- Moussaief A, Gross M, and Nesher E, et al. Incensole acetate reduces depressive-like behavior and modulates hippocampal BDNF and CRF expression of submissive animals. J Psychopharmacol. 2012;26:1584–1593.
- Malatynska E, Knapp RJ. Dominant-submissive behavior as models of mania and depression. Neurosci Biobehav Rev. 2005;29:715–737.
- de Kloet Er. About stress hormones and resilience to psychopathology. J Neuroendocrinol. 2008;20:885–892.
- Duman RS, Monteggia LM. A neurotrophic model for stressrelated mood disorders. Biol Psychiatry. 2006;59:1116–1127.
- Al-Harrasia A, Khan A, and Rehman NU, et al. Evidence for the involvement of a GABAergic mechanism in the effectiveness of natural and synthetically modified incensole derivatives in neuropharmacological disorders: a computational and pharmacological approach. Phytochemistry. 2019;16:358–374.
- Yu J, Zhao L, Sun X, et al. Terpenoids from the Oleo-Gum-Resin of Boswellia serrata and Their Antiplasmodial Effects In Vitro. Planta Med. 2017;83:1214–1226.
- Yu JQ, Geng YL, Wang DJ, et al. Terpenes from the gum resin of Boswellia carterii and their NO inhibitory activies. Phytochem Lett. 2018;28:59–63.
- Schmidt TJ, Kaiser M, Brun R. Complete Structural Assignment of Serratol, a Cembrane-Type Diterpene from Boswellia serrata, and Evaluation of Its Antiprotozoal Activity. Planta Med. 2011;77:849–850.
- Morikawa T, Oominami H, Matsuda H, et al. New terpenoids, olibanumols D–G, from traditional Egyptian medicine olibanum, the gum-resin of Boswellia carterii. J Nat Med. 2011;65:129–134.
- Wang YG, Ren J, Wang AG, et al. Hepatoprotective Prenylaromadendrane-Type Diterpenes from the Gum Resin of Boswellia carterii. J Nat Prod. 2013;76:2074–2079.
- Yu J, Geng Y, Zhao H, et al. Diterpenoids from the gum resin of Boswellia carterii and their biological activities. Tetrahedron. 2018;74:5858–5866.
- Wang JJ, Suo XY, Sun HY, et al. Prenylaromadendrane-type diterpenoids from the gum resin of Boswellia sacra flueck and their cytotoxic effects. Nat Prod Res. 2021. DOI:https://doi.org/10.1080/14786419.2021.1939331. in print.
- Groenendijk P, Eshete A, Sterck FJ, et al. Limitations to sustainable frankincense production: blocked regeneration, high adult mortality and declining populations. J Appl Ecol. 2012;49:164–173.
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19.
- Müller N, Weidinger E, Leitner B, et al. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372.
- Pop-Busui R, Ang L, Holmes C, et al. Inflammation as a therapeutic target for diabetic neuropathies. Curr Diab Rep. 2016;16:29.
- Basar S 2005. Phytochemical Investigations on Boswellia Species, Comparative Studies on the Essential Oils, Pyrolysates and Boswellic Acids. Dissertation, University of Hamburg, Germany.
- Paul M 2012. Ph. D thesis; Chemotaxonomic Investigations on Resins of the Frankincense Species Boswellia papyrifera, Boswellia serrata and Boswellia sacra, respectively, Boswellia carterii. Saarland University Saarbrücken, Saarland Germany.
- Al-Harrasi A, Hussain H, Hussain J, et al. Two pyrolysate products from Omani Frankincense smoke: first evidence of thermal aromatization of boswellic acids. J Anal Appl Pyrolysis. 2014;110:430–434.
- Milića N, Miloševića N, Kona SG, et al. Warfarin interactions with medicinal herbs. Nat Prod Commun. 2014;9:1211–1216.
- Basch E, Boon H, Davies-Heerema T, et al. Boswellia: an evidence-based systematic review by the natural standard research collaboration. J Herb Pharmacother. 2004;4:63–83.