ABSTRACT
IgG4s are dynamic molecules that undergo a process called Fab-arm exchange. Disulfide bonds between heavy chains are transiently reduced, resulting in half antibodies that reform intact antibodies with other IgG4 half antibodies. In vivo, therapeutic IgG4s can recombine with endogenous IgG4s, resulting in a heterogeneous mixture of bispecific antibodies. A related issue that can occur for any therapeutic protein during manufacturing is interchain disulfide bond reduction. For IgG4s, this primarily results in high levels of half-mAb that persist through purification processes. The S228P mutation has been used to prevent half-mAb formation. However, we demonstrated that IgG4s with the S228P mutation are subject to half-mAb formation and Fab-arm exchange in reducing environments. We identified two novel mutations that stabilize the heavy–heavy chain interaction via incorporation of additional disulfide bonds in the hinge region. Individually, these mutations increase stability toward reduction and lessen Fab-arm exchange. Combination of all three mutations, Y219C, G220C, and S228P, has an additive benefit resulting in an IgG4 with ˃7-fold increase in stability toward reduction while preventing Fab-arm exchange. Importantly, the mutations do not affect antigen binding or Fc effector function. These mutations hold great promise for solving mAb reduction during manufacturing and preventing Fab-arm exchange in vivo.
Introduction
Antibody-based therapeutics are entering the clinic at record numbers, with over 570 molecules across the different clinical stages.Citation1 All therapeutic mAbs are based on IgG1, IgG2, or IgG4 isotypes. Each of the different isotypes has unique structural features that control immune effector functions.Citation2 The most commonly used format is IgG1, likely due to its strong effector functions, including antibody-dependent cell-mediated cytotoxicity (ADCC).Citation3 However, there are many instances where it is desirable to have reduced effector function for a therapeutic mAb. IgG4s naturally have low effector function and are commonly selected as the format for a therapeutic mAb when depletion of the antigen-expressing target cells would have adverse effects on the health of the patient.Citation2
IgG4 antibodies are dynamic molecules that are unique among immunoglobulins in their ability to undergo a process called Fab-arm exchange.Citation4 In this process, an IgG4 antibody will form two-half antibodies (heavy chain + light chain) that can then reform complete antibodies with other IgG4 half-mAbs. It has been demonstrated in vivo that a therapeutic IgG4 antibody can recombine with an endogenous IgG4.Citation5 The therapeutic antibody then becomes a heterogeneous mixture of bispecific antibodies, with one Fab binding to unknown antigens. In the case of a combination therapy with two IgG4s, the therapeutic mAbs can recombine with each other, creating bispecific mAbs that might have adverse effects. An additional consequence is that the newly formed bispecific mAbs are functionally monovalent for the intended target antigen. The combined effects of Fab-arm exchange can affect the pharmacokinetics and efficacy of the biotherapeutic.Citation6
All antibodies rely on interchain disulfide bonds to maintain the correct structure for function and activity. During manufacturing processes, all antibodies are susceptible to reduction of the interchain disulfide bonds that hold the heavy and light chains together.Citation7,Citation8 Antibody reduction has been observed industry-wide and has been attributed to components of the glutathione and thioredoxin systems that are released from lysed cells during manufacturing operations.Citation8-Citation10 Antibody reduction can result in loss of product, increased complexity of the manufacturing process, and reduced stability of the formulated drug product.Citation8,Citation11-Citation14 For an IgG4, antibody reduction typically manifests as high levels of half-mAb that persist through the purification process.Citation15
Since the first step of Fab-arm exchange is reduction of the heavy–heavy chain disulfide bonds to form a half-mAb and antibody reduction for IgG4 results in high levels of half-mAb, we hypothesized that strengthening the heavy–heavy chain interaction would both make the IgG4 more stable toward reduction during manufacturing and prevent Fab-arm exchange. To this end, we identified two novel mutations in the IgG4 hinge region, tyrosine to cysteine at position 219 (Y219C) and glycine to cysteine at position 220 (G220C). Previously, the mutation of serine to proline at position 228 (S228P) in the hinge region of IgG4 antibodies has been shown to reduce half-mAb formation and prevent Fab-arm exchange with endogenous IgG4 antibodies in vitro.Citation16,Citation17 We evaluated all three hinge mutations by incorporating them into the same IgG4 heavy chain sequence and comparing the relative stability toward reduction of interchain disulfide bonds, the amount of half-mAb formed, and the amount of Fab-arm exchanged for the mutants compared to the unmodified IgG4. It is important to note that the amount of half-mAb formed and the stability toward reduction are not necessarily correlated. A mAb could be easily reduced without forming any half-mAb via reduction of the heavy-light chain disulfide bonds or, conversely, be relatively stable toward reduction, yet form high levels of half-mAb when ultimately reduced. However, half-mAb formation is required for Fab-arm exchange; therefore, we hypothesized that IgG4s with decreased half-mAb formation would also be less susceptible to Fab-arm exchange.
We found that the Y219C mutation resulted in a minor increase in stability toward reduction and decreased both half-mAb formation and Fab-arm exchange to very low levels. The G220C mutation also resulted in increased stability toward reduction, decreased half-mAb formation, and undetectable levels of Fab-arm exchange. Interestingly, the S228P mutation increased the stability of the mAb toward reduction, but the mAb still formed similar levels of half-mAb as the unmodified IgG4 and moderate levels of Fab-arm exchange. After the initial assessment of each individual mutation, we evaluated all combinations of the mutations into the same IgG4 antibody. We found the effects of the mutations were additive in increasing the stability of the mAb toward reduction and decreasing the levels of both half-mAb formed and Fab-arm exchange.
Importantly, we determined that all seven of the IgG4s with hinge region mutations retained similar effector function and antigen binding compared to the unmodified IgG4. In summary, we identified two novel IgG4 hinge region mutations that increase stability toward disulfide bond reduction during manufacturing and have the potential to prevent Fab-arm exchange in-vivo.
Results
Comparison of reduction kinetics and pathways across IgG isotypes
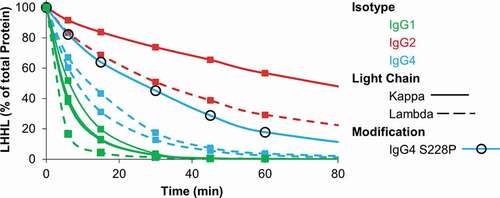
The glutathione and thioredoxin enzymatic systems both provide a reducing environment in vivo and have been shown to similarly reduce mAbs during manufacturing operations.Citation8,Citation10,Citation18 We selected the thioredoxin enzyme system because its role in mAb reduction during manufacturing is better characterized than the glutathione system. We initially compared the relative stability toward reduction of a variety of mAbs by incubating each with thioredoxin, thioredoxin reductase, and nicotinamide adenine dinucleotide phosphate hydrogen (NADPH). Stability toward reduction represents the loss of intact mAb (contains two heavy and two light chains) to any fragment that does not contain the expected two heavy and two light chains of a canonical mAb. The library of mAbs that we evaluated included IgG1s, IgG2s, and IgG4s. Four of the mAbs had lambda light chains, five had kappa light chains, and one of the IgG4s had the commonly used S228P mutation. In line with previous results, we found that IgG2s are more stable toward reduction than IgG1s and that mAbs with kappa light chains are more stable than mAbs with lambda light chains ().Citation19-Citation21 Interestingly, despite the propensity of IgG4s to form half-mAbs, we found IgG4 mAbs to be more stable toward reduction than IgG1s. The S228P mutation has been incorporated into several therapeutic IgG4s to prevent the formation of half-mAbs.Citation1 Expectedly, the IgG4 with the S228P mutation was more stable toward reduction than IgG4s with the wild-type hinge sequence.
Figure 1. Relative stability of IgGs toward reduction by the thioredoxin system. IgG1, IgG2, and IgG4 mAbs were incubated with the components of the thioredoxin system. The figure contains data for 4 distinct IgG1s, 3 distinct IgG4s, and 2 distinct IgG2s. Samples were taken at the indicated timepoints and intact mAb was quantified using capillary electrophoresis. Experiment was repeated twice with single replicates; 1 replicate is shown.
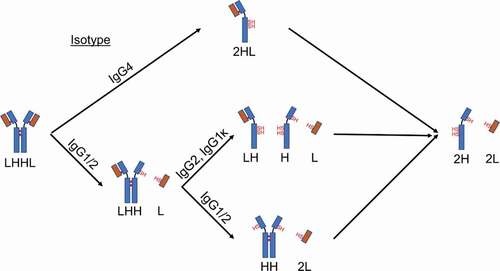
Next, we examined the intermediate species formed for each of the mAbs as they were reduced from intact mAbs to free heavy and light chains by the thioredoxin system. A representative set, containing IgG1s, IgG2s, IgG4s, each with kappa or lambda light chains, is compared in and Fig. S1. The results demonstrate that similar intermediate species are formed for IgG1s and IgG2s and these species are dependent on the light chain type. The dominant intermediate species formed for IgG1 and IgG2 mAbs with lambda light chains are heavy chain dimers (HH), while IgG1 and IgG2 mAbs with kappa light chains form similar or greater amounts of half-mAb containing a single heavy and light chain (HL) compared to HH. These results demonstrate that the disulfide bond between a lambda light chain and the heavy chain is more easily reduced than the disulfide bond between a kappa light chain and the heavy chain.
Figure 2. Intermediate species formed during reduction by the thioredoxin system. IgG1, IgG2, and IgG4 mAbs were incubated with the components of the thioredoxin system. Samples were taken at the indicated timepoints and mAb fragments were quantified using capillary electrophoresis. For all figures intact mAb (LHHL) is on the left axis and fragment species (HHL, HH, and HL) are on the right axis. Free heavy and light chain were not shown to aid in visualization, they are however, included in Fig S1. Experiment was repeated twice with single replicates; 1 replicate is shown.
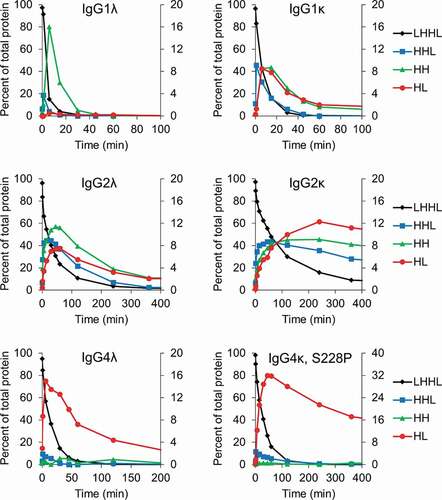
The intermediates formed for the IgG4 mAbs did not depend on the light chain isotype or even the presence of the S228P hinge mutation. As shown in , both IgG4s formed almost exclusively HL as they were reduced by the thioredoxin system. The IgG4 with the S228P mutation was more stable toward reduction by the thioredoxin system, but our results demonstrate that, upon reduction, the mAb still formed exclusively HL. This is somewhat surprising considering the S228P mutation has been engineered into multiple therapeutic mAbs to prevent the formation of half-mAb and Fab-arm exchange. However, this result is consistent with the observation that hinge-stabilized IgG4 mAbs containing the S228P mutation can undergo Fab-arm exchange, albeit under more reducing conditions than those required for unmodified IgG4s.Citation5,Citation22 Additionally, these results demonstrate that half-mAb formation and stability toward reduction are not correlated in all instances. The IgG4 mAbs were more stable toward reduction than IgG1s, yet the IgG1s formed comparatively low levels of half-mAb. The results in are summarized in , which illustrates the major intermediate species formed for different mAb types as they are reduced by the thioredoxin system.
Hinge mutations prevent half-mAb formation and increase stability toward reduction
For an IgG4 mAb, the first step in Fab-arm exchange and reduction during manufacturing is breaking the heavy–heavy chain disulfide bonds. IgG1 and IgG2 mAbs are not subject to Fab-arm exchange, and the first step in reduction during manufacturing for these molecules is removal of a light chain.Citation4 Based on these observations, we hypothesized that if the heavy–heavy chain interaction in an IgG4 were strengthened to be greater than the heavy-light chain interaction, we could both increase the stability of the IgG4 toward reduction and eliminate the possibility of half-mAb formation, and subsequently Fab-arm exchange. To that end, we compared the heavy chain hinge sequence for an IgG1, IgG2, and IgG4 (). IgG1 and IgG2 mAbs both contain a proline at position 228, while IgG4 mAbs contain a serine. The S228P mutation has been widely used across the industry to prevent Fab-arm exchange and was therefore included in our evaluation.Citation1 We identified two additional novel mutations based on the hinge sequence of an IgG2 mAb. IgG1, IgG2, and IgG4 mAbs contain disulfide bonds at positions 226 and 229, while IgG2s contain additional disulfide bonds at positions 219 and 220. IgG2 mAbs are the most stable toward reduction and we hypothesized that this is at least in part due to the four disulfide bonds in the hinge region compared to only two hinge disulfide bonds for IgG1 and IgG4 mAbs. Based on the sequence alignment (), we chose to evaluate the Y219C and G220C mutations in an IgG4λ; each of these mutations adds an additional disulfide bond between heavy chains in the hinge region. After synthesis and purification, intact mass spectrometry analysis was used to determine if the mutations had the desired effect of increasing the number of disulfide bonds. The results in Table S1 and Fig. S1 demonstrated that IgG4-Y291C and IgG4-G220C have an additional disulfide bond.
Table 1. Amino acid sequence comparison of the hinge region for IgG1, IgG2, and IgG4 antibodies using the EU index for antibody numbering. The positions of the mutations evaluated are indicated in red.
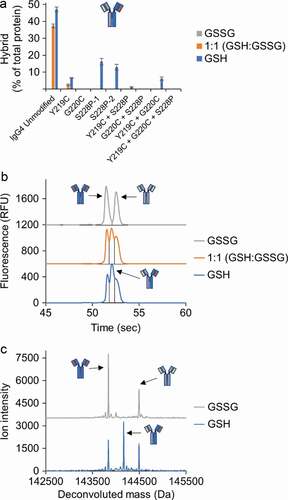
We first compared the relative stability of a set of IgG4λs (IgG4, IgG4-Y219C, IgG4-G220C, and IgG4-S228P) toward reduction by the thioredoxin system (). The results in were fit to a first-order exponential decay model to calculate the half-life of each mAb. The half-life of the mAb represents the stability of the mAb toward reduction by the thioredoxin system, and comparison of the half-life of the IgG4s with hinge mutations to the unmodified IgG4 provides the relative improvement in stability (). Our results show that the Y219C mutation provides only minimal improvement in stability while G220C and S228P mutations provide greater than twofold and threefold increases, respectively, in stability toward reduction. Next, we evaluated the intermediate species formed as these four mAbs were reduced to determine if there were differences in the amount of half-mAb formed (, Fig S.3). In line with the earlier results (), only the kinetics differed between the two mAbs; the unmodified IgG4 was quickly reduced to HL then to free heavy and light chain while the same process occurred more slowly for the IgG4-S228P. Interestingly, compared to the wild-type IgG4, the Y219C mutation nearly eliminated the formation of HL, while the G220C mutation reduced the amount of HL. Finally, we evaluated Fab-arm exchange for each mAb under three different redox conditions: oxidizing, mildly reducing, and reducing by utilizing buffers with (1) oxidized glutathione (GSSG), (2) a 1:1 ratio of GSSG to reduced glutathione (GSH), and (3) GSH, respectively. The IgG4s were all incubated with the same, unmodified IgG4, and Fab-arm exchange was determined by quantifying hybrid mAb formation using capillary electrophoresis (, Fig. S4) and confirmed using mass spectrometry (, Fig. S5, and Fig. S6). All IgG4s with hinge mutations had significantly reduced Fab-arm exchange compared to the unmodified IgG4. The IgG4-Y219C mAb had low levels of Fab-arm exchange in the buffer with the 1:1 GSH to GSSG that increased under the more reducing, GSH condition. The IgG4-G220C mAb had undetectable levels of Fab-arm exchange under all conditions. The IgG4-S228P mAb had undetectable levels of Fab-arm exchange with the 1:1 GSH to GSSG buffer and moderate levels under the more reducing, GSH condition. A second IgG4 with the S228P mutation was also included for comparison and yielded similar results.
Table 2. The relative stability of the IgG4λs was assessed by fitting the results in to a first-order exponential decay to determine the half-life of each mAb. An IgG1λ and IgG2λ were included for comparison
Figure 4. Hinge mutations increase stability of IgG4 mAbs toward reduction. IgG4 mAbs with the indicated hinge mutations were incubated with the components of the thioredoxin system. Samples were taken at the indicated timepoints and intact mAb was quantified using capillary electrophoresis. Data represent the mean ± SD of triplicate experiments.
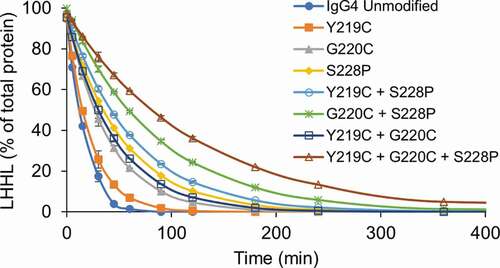
Figure 5. Hinge mutations decrease half-antibody formation. IgG4 mAbs with the indicated hinge mutation(s) were incubated with the components of the thioredoxin system. Samples were taken at the indicated timepoints and mAb fragments were quantified using capillary electrophoresis. Free heavy and light chain were not shown to aid in visualization, they are however, included in Fig S3. Data represent the mean ± SD of triplicate experiments.
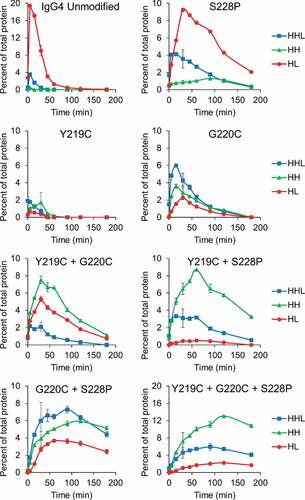
Figure 6. Hinge mutations decrease Fab-arm exchange. (a) Quantification of hybrid mAb formation for IgG4 mAbs with the indicated hinge mutation(s) after incubation with a second, unmodified IgG4. Hybrid mAb was quantified using capillary electrophoresis and quantification of each parent mAb is included in Fig. S4. Data represent the mean ± SD of triplicate experiments. (b) Example electropherogram from the capillary electrophoresis for the unmodified IgG4. (c) Example mass spectrometry data for the unmodified IgG4. Mass spectrometry for remaining samples are in Fig. S5 and S6.
In summary, the Y219C mutation provided only a minimal increase in stability toward reduction, but nearly eliminated half-mAb formation and significantly decreased Fab-arm exchange; the G220C mutation increased the stability of the mAb toward reduction, reduced half-mAb formation and eliminated Fab-arm exchange; and the S228P mutation increased the stability of the mAb toward reduction and decreased Fab-arm exchange, but did not affect the amount of half-mAb formed.
Effects of the hinge mutations are additive
Next, we evaluated combinations of the hinge mutations to determine if the beneficial effects of each individual mutation are additive when combined in the same molecule.
The first double mutant evaluated contained both the Y219C and S228P mutations. The IgG4-Y219C+S228P significantly improved stability toward reduction (, ), reduced half-mAb formation compared to the unmodified IgG4 (), and had only low levels of Fab-arm exchange under the most reducing condition (). Importantly, the IgG4-Y219C+S228P had slightly improved stability toward reduction compared to the IgG4-S228P mAb, comparable half-mAb formation compared to the IgG4-Y219C and lower Fab-arm exchange than either IgG-Y219C or IgG-S228P. These results show that the effects of the mutations are additive; addition of the Y219C mutation to the IgG4 containing the S228P mutation slightly increased the stability of the mAb toward reduction while also decreasing half-mAb formation and Fab-arm exchange.
The second double mutant evaluated contained both the G220C and S228P mutations. The IgG4-G220C+S228P had significantly improved stability toward reduction (, ), reduced half-mAb formation compared to the unmodified IgG4 (), and undetectable levels of Fab-arm exchange (). Importantly, the IgG4-G220C+S228P had greater stability toward reduction than either the G220C or S228P IgG4s individually. Additionally, the IgG4-G220C+S228P had half-mAb formation and Fab-arm exchange, in line with the IgG4-G220C. These results provided further evidence that the effects of the mutations are additive.
The last double mutant evaluated contained both the Y219C and G220C mutations. The IgG4-Y219C+G220C had significantly improved stability toward reduction (, ) and reduced half-mAb formation and Fab-arm exchange compared to the unmodified IgG4 ( and ). The IgG4-Y219C+G220C had improved stability toward reduction compared to the IgG4-G220C, but the amount of half-mAb formed was similar to IgG4-G220C and Fab-arm exchange was similar to IgG4-Y219C. In this case, the addition of the Y219C mutation to the G220C mAb did provide the minor improvement in stability, but the additional decrease in half-mAb formation and Fab-arm exchange was not observed. Intact mass spectrometry analysis of this molecule revealed only two disulfide bonds formed in the hinge region rather than the expected four (Table S1, Fig. S1). Presumably, if all four disulfide bonds had formed, we might have observed an additive effect in terms of stability, half-mAb formation, and Fab-arm exchange rather than just the minor improvement in stability we observed.
The last mAb evaluated contained all three mutations. The IgG4-Y219C+G220C+S228P had the greatest overall stability toward reduction (, ), formed very little half-mAb (), and had undetectable levels of Fab-arm exchange (). The effects of the mutations for this mAb were additive, while in the case of the double mutation, IgG4-Y219C+G220C, they were not. Intact mass spectrometry analysis of the triple mutation mAb demonstrated that all four hinge disulfide bonds were formed. Previous analysis of IgG4 mAbs with the S228P mutation has suggested that the increased stability is because of a conformation change in the hinge region due to the less flexible amino acid proline amino acid in place of serine.Citation2 It is likely that, in our case, the hinge conformational change caused by the S228P mutation was required for the proper alignment and formation of four hinge disulfide bonds in the IgG4-Y219C+G220C+S228P.
Hinge mutations do not affect antigen binding
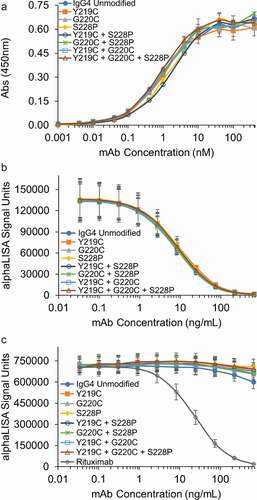
The three mutations we evaluated are all in the hinge region and are therefore not expected to affect antigen binding. However, it is possible that the additional disulfide bonds in the hinge region of an IgG4 could alter the conformation of the mAb, and lead to impaired antigen binding. Thus, antigen binding for all seven mAbs with hinge region mutations was compared to the unmodified IgG4 in an enzyme-linked immunosorbent assay (ELISA). As shown in , all seven mAbs demonstrated similar antigen binding to the unmodified IgG4. This result demonstrates that these mutations can be incorporated into an IgG4 without affecting antigen binding.
Figure 7. Hinge mutations do not affect antigen, FcRn or FcγRIIIa binding in vitro. (a) Antigen binding of the IgG4 mAbs with the indicated hinge mutations were compared to the unmodified IgG4 in an ELISA assay. An isotype control demonstrated no binding up to 5000 nM (not shown). Data represent the mean ± SD of duplicate experiments. (b) FcRn and (c) FcγRIIIa binding for the IgG4 s with hinge mutations were compared to the unmodified IgG4 in AlphaLISA assays. Rituximab was run as a known positive control in the FcγRIIIa AlphaLISA. Data represent the mean ± SD of duplicate independent experiments.
Hinge mutations do not affect FcRn binding
Binding to the neonatal Fc receptor (FcRn) provides IgGs long serum half-lives, enabling monthly or even less frequent dosing.Citation23 The FcRn binding site is on the heavy chains between the CH3 and CH2 domains, and was therefore not predicted to be impacted by the hinge mutations. However, due to the importance of FcRn binding to the efficacy of therapeutic mAbs, it was critical to demonstrate that the hinge mutations do not inhibit FcRn binding. As our results in demonstrate, all the IgG4s with hinge mutations have similar binding to FcRn compared to the unmodified IgG4. This result demonstrates that the hinge mutations do not reduce FcRn binding in vitro. Additionally, as the Fab remains unchanged, the hinge mutations are unlikely to adversely affect the pharmacokinetics compared to the unmodified IgG4.
Hinge mutations do not affect fc effector function
Fc effector function is critical for the efficacy of mAbs.Citation2 The FcγRIIIa receptor mediates ADCC, which can be an important part of the mechanism of action for IgG1-based therapeutics.Citation24 IgG4 mAbs have naturally low binding to this receptor and are often selected when depletion of the antigen-expressing target cells would have adverse effects on the health of the patient. Recently, it has been shown that afucosylation can increase ADCC activity for IgG4s to levels comparable to an IgG1 and that the increased ADCC activity is mediated via FcγRIIIa binding.Citation25 To confirm that the hinge modifications are unlikely to increase ADCC activity, we evaluated FcγRIIIa binding. Our results in demonstrate negligible FcγRIIIa binding for all IgG4s evaluated in this study. Combined, the results in demonstrate that the hinge mutations are unlikely to affect Fc effector function in vivo.
Discussion
Antibody reduction during manufacturing processes has been widely observed and occurs for all antibody isotypes. For an IgG4, this presents as high levels of half-mAb that persist through the purification process. Half-mAb is formed upon reduction of the heavy–heavy chain disulfide bonds. Similarly, the first step in Fab-arm exchange is the reduction of heavy–heavy chain disulfide bonds. Thus, mutations that increase the stability of the heavy–heavy chain interaction would both increase the stability of the IgG4 toward reduction during manufacturing and reduce the propensity for Fab-arm exchange. Further, if under reducing conditions, the IgG4 does not form half-mAbs, then Fab-arm exchange would not be possible.
To this end, we identified two novel mutations, based on the IgG2 hinge sequence, that increase the stability of an IgG4 toward reduction, decrease half-mAb formation, and prevent Fab-arm exchange. The first mutation, Y219C, provided only a minimal increase in stability toward reduction, but nearly eliminated half-antibody formation and decreased Fab-arm exchange to very low levels. The second mutation, G220C, increased the stability of the IgG4 toward reduction, reduced half-mAb formation, and prevented Fab-arm exchange. It is important to note that the IgG4s with the S228P mutation still formed almost exclusively half-mAb and had moderate levels of Fab-arm exchange under reducing conditions. These results are consistent with previous reports that IgG4-S228P mAbs can still participate in Fab-arm exchange, but require a more reducing environment than unmodified IgG4 mAbs.Citation5,Citation22 These results also confirmed our hypothesis that decreasing the amount of half-mAb formed under reducing conditions also decreases Fab-arm exchange. However, it is important to note that Fab-arm exchange in vivo for an IgG4 with the S228P mutation has not been reported.
Additionally, we demonstrated additive effects by combining the hinge mutations. Both the Y219C and G220C mutations provided additional stability toward reduction, reduced half-antibody formation, and decreased Fab-arm exchange when combined with the S228P mutation. The IgG4 containing both the Y219C and G220C mutation did not have the same additive effects, but mass spectrometry analysis demonstrated only two disulfide bonds in the hinge region compared to the expected four. Finally, the IgG4 containing all three hinge mutations was the most stable toward reduction, had low levels of half-mAb formation, and undetectable levels of Fab-arm exchange. It is likely that the S228P mutation is required to allow proper hinge alignment for the formation of four hinge disulfide bonds, as the formation of four hinge disulfide bonds requires greater conformational constraints compared to the formation of three hinge disulfide bonds, which was the case for IgG4-Y219C and IgG4-G220C. The relative stability toward reduction, half-mAb formation, and Fab-arm exchange are summarized in . It should be noted that the disulfide bonds in the upper hinge region of IgG2 mAbs can shuffle, resulting in three different isoforms, representing an alternate location of the disulfide bond between the heavy-light chain on either or both sides of the IgG2 mAb.Citation26 In our study, the Fabs are IgG4s, and therefore may not be subject to disulfide bond shuffling. This possibility, however, would need to be explored before use as a therapeutic.
Table 3. Summary of the effect of each mutation on the relative stability toward reduction, half-mAb formation, and Fab-arm exchange
The mutations likely function by decreasing the flexibility of the hinge region. It has previously been shown that, in terms of hinge flexibility, IgG1> IgG4> IgG2,Citation2 which aligns with our results in where IgG1s were most easily reduced, followed by IgG4s, and then IgG2 mAbs. Additionally, the S228P mutation has been shown to stabilize the mAb toward reduction by inducing a more rigid, partial helical structure,Citation27 further supporting the hypothesis that reduced hinge flexibility decreases half-antibody formation. Incorporation of all three mutations likely further decreases the flexibility of the hinge region, thereby providing the additional stability toward reduction while also minimizing half-mAb formation.
Importantly, we demonstrated that these mutations do not affect the antibody’s ability to bind its antigen, or to FcRn or RcγRIIIa, which are key mediators of effector function. Taken together, the above-referenced mutations hold great promise for solving mAb reduction during manufacturing operations and for preventing Fab-arm exchange in vivo.
Materials and methods
mAb expression and purification
The hinge mutations were all incorporated into the same IgG4 heavy chain sequence using site-directed mutagenesis as previously described.Citation28,Citation29 All IgG4 used the same λ light chain. The cells were expressed via transient transfection in Chinese hamster ovary cells and purified using protein A chromatography followed by size exclusion purification to reduce product aggregates to <2%.
Stability toward reduction and protein size analysis
The relative stabilities of mAbs toward reduction by the thioredoxin system were assessed by spiking purified antibody at 1.2 mg/mL into solutions containing 2 µM thioredoxin (Abcam, Cambridge MA), 0.1 µM thioredoxin reductase (Cayman Chemical, Ann Arbor MI), and 0.24 mM NADPH (Millipore-Sigma, St. Louis) in phosphate buffer, pH 7.4, containing 10 mM EDTA. The amount of intact antibody and intermediates formed were quantified using a LabChip GXII Touch HT (Perkin Elmer). Samples were taken at the indicated time points, stored frozen at −80°C, and then analyzed using the Protein Express Assay kit under non-reducing conditions, following the standard protocol from Perkin Elmer. Fab-arm exchange was quantified using the ProteinEXact assay kit under non-reducing conditions, following the standard protocol from Perkin Elmer.
Mass spectrometry analysis
Intact mass analysis was performed with a Waters SYNAPT G2-Si High Definition Mass Spectrometer in conjunction with a UPLC system (Waters). Samples assessed for Fab-arm exchanged were pre-treated with PNGase F to remove Fc N-glycans. For non-reducing intact mass analysis, 1–2 µg of each sample was directly injected onto a reversed-phase column (Acquity UPLC BEH300, C4, 1.7 µm, 2.1 mm × 50 mm; Waters) and eluted on a linear gradient using a mobile phase A comprising 0.1% formic acid (FA) and 0.01% trifluoroacetic acid (TFA) in water and a mobile phase B comprising 0.1% FA and 0.01% TFA in acetonitrile. Mass spectra were collected at a range of 500–4,500 m/z. Molecular mass was determined by deconvolution of the m/z data, using the MaxEnt I in MassLynx software (Waters).
Fab-arm exchange
Three different solutions for each IgG4 mAb under evaluation were prepared in 50 mM tris buffer pH 7.4 containing 5 mM ethylenediaminetetraacetic acid (EDTA). All solutions contained the test IgG4 at 1.1 mg/mL, a second unmodified IgG4 at 1.1 mg/mL, and GSH/GSSG as indicated at a total concentration of 1.5 mM. The samples were incubated at 37°C for 90 minutes followed by buffer exchange using Amicon centrifugal spin filters with a 10 kDa cutoff membrane to remove glutathione. The samples were incubated for an additional 24 hours at 37°C prior to analysis as described above.
Antigen binding ELISA
The 96-well plates were coated with 4 µg/mL of the target antigen over night at 4°C. The plates were blocked with a 5% milk solution in phosphate-buffered saline (PBS) with 0.5% Tween 20. The primary mAb was detected with a secondary anti-human IgG4 horseradish peroxidase-conjugated antibody (1:1000 dilution, Invitrogen MH1742). Following the addition of tetramethylbenzidine, the reaction was stopped with 0.2 N sulfuric acid and absorbance was read at 450 nm. Between all steps, the wells were washed 4 times with PBS containing 0.05% Tween 20.
FcRn binding AlphaLISA
An AlphaLISA® FcRn competition binding assay kit (PerkinElmer, Catalog #AL3095) was used to measure relative FcRn binding. Increasing concentrations of antibody bound to FcRn competitively block the ability of the acceptor and donor beads to proximally interact, which in turn decreases the assay signal. IgG4 antibody samples were prepared to a starting concentration of 100 µg/mL in MES buffer, pH 6.0. A 10-point dilution series was prepared and incubated with biotinylated FcRn in a 96-well plate for 45 minutes at room temperature. Streptavidin-coated donor beads and human IgG conjugated acceptor beads were prepared to 20 µg/mL, added to the antibody-FcRn solution, and incubated for 60 minutes at room temperature in the dark. Chemiluminescence was quantified using an Envision. The resulting data were fit with SoftMax software using a 4-parameter fit.
FcγRIIIa binding AlphaLISA
An AlphaLISA® FcγRIIIa competition binding assay kit (PerkinElmer, Catalog #AL348) was used to measure relative FcγRIIIa-158 V binding. Biotinylated human FcγRIIIa-158 V is captured by streptavidin-coated donor beads. In the absence of antibody samples, human IgG Fc region conjugated to acceptor beads binds the FcγRIIIa-158 V and brings the donor and acceptor beads into proximity, generating a chemiluminescent emission. IgG4 antibody samples were prepared to a starting concentration of 20 µg/mL in HiBlock buffer and pre-incubated for 30 minutes at room temperature with biotinylated human FcγRIIIa-158 V to facilitate binding. Acceptor and donor beads were prepared to 20 µg/mL, then added to the antibody-receptor solution and incubated for 60 minutes at room temperature in the dark. Chemiluminescence was quantified using an Envision, and data fit with SoftMax software using a 4-parameter fit. Rituximab was run as a known positive control.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (5.9 MB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Kaplon H, Reichert JM. Antibodies to watch in 2019. mAbs. 2019;11:219–11. doi:https://doi.org/10.1080/19420862.2018.1556465.
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi:https://doi.org/10.3389/fimmu.2014.00520.
- Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9(1):63–73. doi:https://doi.org/10.1007/s13238-017-0473-8.
- van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–57. doi:https://doi.org/10.1126/science.1144603.
- Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, Killestein J, Polman CH, Aalberse RC, Schuurman J. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27(8):767–71. doi:https://doi.org/10.1038/nbt.1553.
- Correia IR. Stability of IgG isotypes in serum. mAbs. 2010;2(3):221–32. doi:https://doi.org/10.4161/mabs.2.3.11788.
- Trexler-Schmidt M, Sargis S, Chiu J, Sze-Khoo S, Mun M, Kao YH, Laird MW. Identification and prevention of antibody disulfide bond reduction during cell culture manufacturing. Biotechnol Bioeng. 2010;106:452–61. doi:https://doi.org/10.1002/bit.22699.
- Handlogten MW, Zhu M, Ahuja S. Glutathione and thioredoxin systems contribute to recombinant monoclonal antibody interchain disulfide bond reduction during bioprocessing. Biotechnol Bioeng. 2017;114:1469–77. doi:https://doi.org/10.1002/bit.26278.
- Kao YH, Hewitt DP, Trexler-Schmidt M, Laird MW. Mechanism of antibody reduction in cell culture production processes. Biotechnol Bioeng. 2010;107:622–32. doi:https://doi.org/10.1002/bit.22848.
- Koterba KL, Borgschulte T, Laird MW. Thioredoxin 1 is responsible for antibody disulfide reduction in CHO cell culture. J Biotechnol. 2012;157:261–67. doi:https://doi.org/10.1016/j.jbiotec.2011.11.009.
- Chung WK, Russell B, Yang Y, Handlogten M, Hudak S, Cao M, Wang J, Robbins D, Ahuja S, Zhu M, et al. Effects of antibody disulfide bond reduction on purification process performance and final drug substance stability. Biotechnol Bioeng. 2017;114(6):1264–74. doi:https://doi.org/10.1002/bit.26265.
- O’Mara B, Gao ZH, Kuruganti M, Mallett R, Nayar G, Smith L, Meyer JD, Therriault J, Miller C, Cisney J, et al. Impact of depth filtration on disulfide bond reduction during downstream processing of monoclonal antibodies from CHO cell cultures. Biotechnol Bioeng. 2019;116(7):1669–83. doi:https://doi.org/10.1002/bit.26964.
- Swope N, Chung WK, Cao M, Motabar D, Liu D, Ahuja S, Handlogten M. Impact of enzymatic reduction on bivalent bispecific antibody fragmentation and loss of product purity upon reoxidation. Biotechnol Bioeng. 2020;117:1063–71.
- Handlogten MW, Wang J, Ahuja S. n/a. Online control of cell culture redox potential prevents antibody interchain disulfide bond reduction.
- Derfus GE, Dizon-Maspat J, Broddrick JT, Velayo AC, Toschi JD, Santuray RT, Hsu SK, Winter CM, Krishnan R, Amanullah A, et al. Red colored IgG4 caused by vitamin B12 from cell culture media combined with disulfide reduction at harvest. mAbs. 2014;6(3):679–88. doi:https://doi.org/10.4161/mabs.28257.
- Silva JP, Vetterlein O, Jose J, Peters S, Kirby H. The S228P mutation prevents in vivo and in vitro IgG4 Fab-arm exchange as demonstrated using a combination of novel quantitative immunoassays and physiological matrix preparation. J Biol Chem. 2015;290:5462–69. doi:https://doi.org/10.1074/jbc.M114.600973.
- Angal S, King DJ, Bodmer MW, Turner A, Lawson AD, Roberts G, Pedley B, Adair JR. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30(1):105–08. doi:https://doi.org/10.1016/0161-5890(93)90432-B.
- Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med. 2015;80:148–57. doi:https://doi.org/10.1016/j.freeradbiomed.2014.11.013.
- Hutterer KM, Hong RW, Lull J, Zhao X, Wang T, Pei R, Le ME, Borisov O, Piper R, Liu YD, et al. Monoclonal antibody disulfide reduction during manufacturing: untangling process effects from product effects. mAbs. 2013;5(4):608–13. doi:https://doi.org/10.4161/mabs.24725.
- Liu H, Chumsae C, Gaza-Bulseco G, Hurkmans K, Radziejewski CH. Ranking the susceptibility of disulfide bonds in human IgG1 antibodies by reduction, differential alkylation, and LC-MS analysis. Anal Chem. 2010;82:5219–26. doi:https://doi.org/10.1021/ac100575n.
- Montano RF, Morrison SL. Influence of the isotype of the light chain on the properties of IgG. J Immunol. 2002;168:224–31. doi:https://doi.org/10.4049/jimmunol.168.1.224.
- Stubenrauch K, Wessels U, Regula JT, Kettenberger H, Schleypen J, Kohnert U. Impact of molecular processing in the hinge region of therapeutic IgG4 antibodies on disposition profiles in cynomolgus monkeys. Drug Metab Dispos. 2010;38:84–91. doi:https://doi.org/10.1124/dmd.109.029751.
- Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the Architect behind the immune and nonimmune functions of IgG and albumin. J Immunol. 2015;194:4595–603. doi:https://doi.org/10.4049/jimmunol.1403014.
- Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J. ImmunoTher. Cancer. 2014;2:29. doi:https://doi.org/10.1186/s40425-014-0029-x.
- Gong Q, Hazen M, Marshall B, Crowell SR, Ou Q, Wong AW, Phung W, Vernes J-M, Meng YG, Tejada M, et al. Increased in vivo effector function of human IgG4 isotype antibodies through afucosylation. mAbs. 2016;8(6):1098–106. doi:https://doi.org/10.1080/19420862.2016.1189049.
- Wypych J, Li M, Guo A, Zhang Z, Martinez T, Allen MJ, Fodor S, Kelner DN, Flynn GC, Liu YD, et al. Human IgG2 antibodies display disulfide-mediated structural isoforms. J Biol Chem. 2008;283(23):16194–205. doi:https://doi.org/10.1074/jbc.M709987200.
- Scapin G, Yang X, Prosise WW, McCoy M, Reichert P, Johnston JM, Kashi RS, Strickland C. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol. 2015;22(12):953–58. doi:https://doi.org/10.1038/nsmb.3129.
- Peng L, Oberst MD, Huang J, Brohawn P, Morehouse C, Lekstrom K, Baeuerle PA, Wu H, Yao Y, Coats SR, et al. The CEA/CD3-bispecific antibody MEDI-565 (MT111) binds a nonlinear epitope in the full-length but not a short splice variant of CEA. PLoS One. 2012;7(5):e36412. doi:https://doi.org/10.1371/journal.pone.0036412.
- Bezabeh B, Fleming R, Fazenbaker C, Zhong H, Coffman K, Yu X-Q, Leow CC, Gibson N, Wilson S, Stover CK, et al. Insertion of scFv into the hinge domain of full-length IgG1 monoclonal antibody results in tetravalent bispecific molecule with robust properties. mAbs. 2017;9(2):240–56. doi:https://doi.org/10.1080/19420862.2016.1270492.