Abstract
1,2-Dehydro-pyrrolizidine alkaloids (PA), their corresponding N-oxides (PANO) and tropane alkaloids (TA), are toxic plant metabolites. If plant material, containing these toxins, is present in the feed of dairy cows these toxins can be transferred into milk. Here, milk was sampled directly from dairy farms in the German federal states of Bavaria and Schleswig-Holstein in 2020–2022 in order to investigate a possible contamination of milk at the production stage. In total, 228 milk samples were analysed for 54 PA/PANO and two TA by a sensitive LC–ESI-MS/MS method. In addition, a subset of milk samples (n = 85) was independently analysed for TA by a cooperating laboratory for verification. PA/PANO were found in 26 samples (11%) with a low median sum content of the contaminated samples of 0.024 µg/L. The highest level of contamination was 5.6 µg/L. Senecionine-, lycopsamine- and heliotrine-type PA/PANO were detected. In four samples (1.8%), atropine was determined up to 0.066 µg/L. The toxin levels in the milk samples hardly contributed to the total daily exposure. These data are first-time results on contamination rates and levels occurring in milk from individual dairy farms, based on a large sample number.
Keywords:
Introduction
1,2-Dehydro-pyrrolizidine alkaloids (PA) and tropane alkaloids (TA) are toxic secondary plant metabolites synthesised by various plant species. PA and their corresponding N-oxides (PANO) are mainly produced by the plant families Asteraceae, Boraginaceae and Leguminosae (Smith and Culvenor Citation1981). PA/PANO are protoxins which induce hepatotoxicity, genotoxicity and carcinogenicity in mammals after metabolic activation (Fu et al. Citation2004; Ruan et al. Citation2014). Acute poisoning with PA/PANO is pathognomonic of hepatic sinusoidal obstruction syndrome (HSOS). Long-term exposure even to low PA/PANO doses is associated with liver cirrhosis, cancer, pulmonary arterial hypertension (PAH) and adverse effects for other organs (Edgar et al. Citation2015).
The plant families Solanaceae, Brassicaceae, Erythroxylaceae, Proteaceae, Euphorbiaceae, Rhizophoraceae and Convolvulaceae are known to synthesise TA (Griffin and Lin Citation2000). The best-studied and most important TA occurring in food and feed are (−)-hyoscyamine (or L-hyoscyamine, pharmaceutical potent enantiomer of the racemic mixture atropine) and (−)-scopolamine (or L-scopolamine) produced by plants of the Solanaceae family (EFSA Citation2018; González-Gómez et al. Citation2022). (−)-Hyoscyamine and (−)-scopolamine are cholinergic antagonists which bind non-specifically to muscarinic acetylcholine receptors of the central and autonomous nervous system (Brown and Laiken Citation2011). Intoxication with (−)-hyoscyamine or (−)-scopolamine results in clinical symptoms such as tachycardia, ataxia, pupillary dilation, decreased production of secretions and disorientation (Perharič Citation2005; Perharič, Juvan et al. Citation2013).
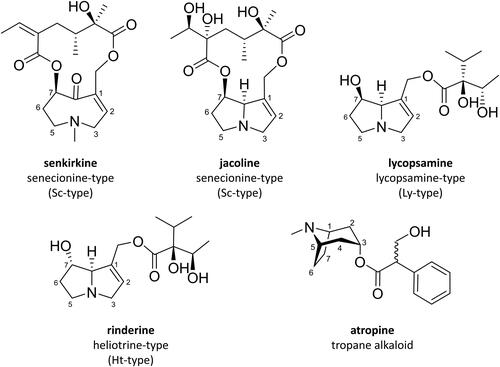
Plants containing PA/PANO and TA can grow on meadows, pastures and farmland used for feed production. Therefore, feedstuffs can be contaminated with these phytotoxins (Mulder et al. Citation2009; EFSA Citation2013; Gottschalk et al. Citation2015). Members of the genera Senecio (S., Asteraceae) or Jacobaea (J., Asteraceae), for example, J. vulgaris Gaertn. (tansy ragwort, syn. S. jacobaea L.) or J. aquatica (Hill) G. Gaertn., B. Mey. & Scherb (marsh ragwort, syn. S. aquaticus L.), are substantial sources for PA/PANO contamination of feed originating from Central Europe (Suter and Lüscher Citation2008; Mulder et al. Citation2009; EFSA Citation2011; Gottschalk et al. Citation2015). These plants produce PA/PANO of the senecionine-type (Sc-type) (). Other plants from the families Asteraceae and Boraginaceae may also contribute to PA/PANO contamination of feed (EFSA Citation2011). Boraginaceae are known to produce lycopsamine-type (Ly-type) and heliotrine-type (Ht-type) PA (). In particular, imported feed, such as ingredients used in compound feed, may contain PA/PANO compounds originating from plants not common in Europe, for instance PA/PANO of the Ht-type (EFSA Citation2011). For Europe, it is concluded that on arable land relevant weeds containing high levels of (−)-hyoscyamine are Datura (D.) stramonium L. (jimson weed), Hyoscyamus spp. (henbane) and Atropa (A.)belladonna L. (deadly nightshade) (de Nijs et al. Citation2023).
Figure 1. Exemplary chemical structures of different types of pyrrolizidine alkaloids and tropane alkaloids prevalent in milk samples.
For ruminants, the transfer rates of PA/PANO from plant material to milk were reported to be between 0.01% and 0.1%, depending on the study design and the plant material (Dickinson et al. Citation1976; Hoogenboom et al. Citation2011; Mulder et al. Citation2020). Individual PA/PANO varied in their transfer rate. Data on the transfer of atropine and scopolamine from feed into the milk are scarce. After oral administration of atropine and scopolamine to dairy cows, their transfer rates were estimated to be 0.037% and 0.007%, respectively (Lamp et al. Citation2021).
Regarding the occurrence of these alkaloids in milk, previous studies revealed small amounts of PA/PANO up to 0.17 µg/L in 6% and up to 0.061 µg/kg in 13% of milk samples from retail in Europe (Huybrechts and Callebaut Citation2015; Mulder et al. Citation2015). The low concentrations of PA/PANO in retail milk were attributed to a high dilution of milk during processing (Mulder et al. Citation2015). In industrial processing, milks of individual dairy farms are mixed to a large extent which might effectively dilute higher contaminant concentrations potentially present in milk from a few individual dairy farms. Raw milk sold by individual dairy farms via vending machines does not undergo this dilution. Hence, higher concentrations of PA/PANO or TA than commonly found in retail milk samples are to be expected in milk samples from individual dairy farms. So far, only a small study investigated the occurrence of PA/PANO and TA in 15 milks from vending machines and regional marketers in Bavaria (Germany) (Klein et al. Citation2022). In three samples, PA/PANO sum contents up to 0.035 µg/L were found and were therefore in a similar concentration as in retail milk. TA were not detected.
This study aimed to systematically investigate whether PA/PANO levels in milk from individual farms are potentially higher than in retail milk from large dairies and whether TA occur in milk intended for human consumption. Furthermore, we aimed to provide more insight into whether single, highly contaminated milk from individual farms account for the occasional occurrence of low contamination levels in retail milk, or if these are attributed to more frequent occurrence of low contamination levels in milk from several dairy farms. For this, PA/PANO and TA levels were examined in milk sampled directly from milk collecting tanks or vending machines of individual dairy farms over a two-year period. The German federal states of Bavaria and Schleswig-Holstein, with a known high incidence of ragwort (J. aquatica or J. vulgaris), were chosen as model regions (Neumann and Huckauf Citation2016; Suttner et al. Citation2016). The samples were analysed for 54 PA/PANO and two TA (atropine and scopolamine) by liquid chromatography–tandem mass spectrometry (LC–MS/MS). In parallel, a second independent LC–MS/MS method for the analysis of atropine and scopolamine was applied to a subset of milk samples to further verify the results of the multi-analyte LC–MS/MS method, as TA have not yet been detected in milk intended for human consumption.
The determined toxin levels were evaluated regarding the possible botanical origins of the detected toxins and discussed regarding their contribution to the dietary exposure and potential health risks for special consumer groups like children of farmer families or customers of milk from vending machines who consume milk from only a singular origin during longer periods of time.
Material and methods
Chemicals
For the PA/PANO and TA analysis reference standards were obtained from PhytoLab (Vestenbergsgreuth, Germany) and CFM Oskar Tropitzsch (Marktredwitz, Germany) as described by Klein et al. (Citation2022). A mixture of all analytical standards in methanol (10 µg/mL) was prepared. Analytical grade formic acid, analytical grade n-hexane, analytical grade 25% ammonia solution, LC–MS-grade methanol and LC–MS-grade acetonitrile were acquired from Th. Geyer (Renningen, Germany). HPLC-grade ammonium carbonate and LC–MS-grade 25% ammonia solution were purchased from Fisher Scientific (Schwerte, Germany) and from Merck (Darmstadt, Germany), respectively, as additives for LC solvents. Ultrapure water was prepared by water purification using an UltraClear™ TP UV UF TM device from Evoqua Water Technologies (Barsbüttel, Germany).
For the independent verification analysis for tropane alkaloids, standard reference substances were purchased from Biopure (Tulln, Austria) and Sigma Aldrich (Taufkirchen, Germany) as described by Lamp et al. (Citation2021). Analytical grade methanol used as extraction solvent and LC–MS-grade methanol for chromatography were provided by Honeywell/Riedel-de Haën (Seelze, Germany). Formic acid (98%) was obtained from Sigma Aldrich (Taufkirchen, Germany). Ultrapure water was delivered by a Milli-Q® Advantage A10® system from Merck (Darmstadt, Germany).
Samples
Milk was sampled over a two-year period to account for possible seasonal differences. Raw milk samples from Bavaria were acquired from the same 30 dairy farms in three or four consecutive seasons (named A–D) starting from March 2020 (A) or July 2020 (B), respectively (). In Schleswig-Holstein, samples of raw milk were taken from 35 dairy farms in two consecutive seasons starting from September 2020 (named B–C) and from 28 different dairy farms in two consecutive seasons starting from August 2021 (named D–E). For six dairy farms located in Schleswig-Holstein, sampling was performed only in the first respective sampling period (B or D, Supplementary Table S1).
Table 1. Overview of milk samples collected from farms in Bavaria and Schleswig-Holstein throughout consecutive seasons named A–E.
The milk was either directly sampled from the milk collecting tank of the farms or obtained from milk vending machines, cooled during transportation and frozen at −20 °C afterwards until analysis. All 30 dairy farms located in Bavaria and 28 out of 63 dairy farms located in Schleswig-Holstein volunteered for this study (active participation Supplementary Table S1). The remaining 35 dairy farms located in Schleswig-Holstein were selected randomly and sampling was conducted at their vending machines (passive participation). Overall, 108 and 120 milk samples were collected in Bavaria and Schleswig-Holstein, respectively. Information associated with the milk samples (i.e. farming conditions, number of milkings in the milk collection tank, direct marketing of milk) were documented if available (Supplementary Table S1).
Sample preparation and analysis of milk
Milk samples were analysed in duplicates for the occurrence of 54 PA/PANO and two TA according to Klein et al. (Citation2022). In brief, each milk sample was thawed and homogenised by shaking. An aliquot of 3.0 mL was extracted with 30 mL of 2.0% aqueous formic acid and 15 mL n-hexane. After mixing and centrifugation, the aqueous phase of the sample extract was cleaned by solid-phase extraction. The cartridge (Bond Elut Plexa PCX 200 mg Agilent, Waldbronn, Germany) was activated with methanol and conditioned with 2% aqueous formic acid; 15 mL of the aqueous phase of the sample extract were applied, then the cartridge was washed with water and methanol. The analytes were eluted with ammoniacal methanol (5.0%) and the eluate was dried under nitrogen (50 °C). The residue was reconstituted in 500 µL of methanol/water (10/90, v/v) and filtered (0.2 µm, PVDF, Wicom, Heppenheim, Germany). The chromatographic separation was performed on a Shimadzu high performance liquid chromatography apparatus (LC, Duisburg, Germany) using a Kinetex™ EVO C18 column (2.6 µm, 100 × 2.1 mm, 100 Å, Phenomenex, Aschaffenburg, Germany) and aqueous 10 mM ammonium carbonate buffer at pH 9 (solvent A) and acetonitrile (solvent B) as the mobile phases. The gradient conditions at a flow rate of 0.3 mL/min were slightly modified to: 0.0 min 0.0% B, 0.2 min 5.0% B, 6.0 min 10.0% B, 19.0 min 28.6% B, 22.8 min 34.0% B, 23.3 min 90.0%, 24.8 min 90.0% and 25.8 min 0.0% B. The column was equilibrated to starting condition for 7 min prior to each run. The oven temperature was set to 30 °C and the injection volume was 10 µL. An API 4000 triple quadrupole mass spectrometer (Sciex, Darmstadt, Germany) was used in positive electrospray ionisation mode for detection of the analytes in multiple reaction monitoring (MRM) mode (more information on the LC–MS/MS parameters is available in Supplementary Material S2). For quantification, external matrix-matched calibration (0–2.5 ng/mL in blank milk extract) was used. Where the content of individual PA/PANO/TA exceeded the range of the calibration curve, sample solutions were diluted (1 + 2 with methanol/water (10/90, v/v)) and analysed with calibration standards in equally diluted blank milk matrix. All results were not corrected for recovery. A compound was considered present if the ion ratio (±20%) and the retention time (±0.10 min) of the peaks present in both sample replicates matched the quantifier and qualifier mass transitions obtained from the calibration standards. LC–MS/MS system stability was assessed by injecting the calibration standards before and after a sequence of sample runs. To calculate PA/PANO and TA sum contents, individual analytes detected at a calculated concentration between the limit of detection (LOD) and the limit of quantification (LOQ) were set as 0.5 LOQ; values below the LOD were calculated with 0.0 µg/L (medium bound approach). Individual LOD and LOQ of the multi-analyte method, assessed using the calibration approach according to German DIN 32645, ranged from 0.005 to 0.054 µg/L and from 0.009 to 0.123 µg/L, respectively. The LC–MS/MS method’s linearity was R2 > 0.993 for all analytes (Klein et al. Citation2022). For 51 PA/PANO and both TA, recovery rates were between 64% and 127% with repeatability (RSDr) values below 15%. Merepoxine N-oxide, merenskine N-oxide and riddelliine N-oxide were assessed only semiquantitatively (recovery rates 12.5–172%, RSDr ≤ 27.3%) (Klein et al. Citation2022).
Independent verification analysis for tropane alkaloids
A subset of samples (samples from seasons C–E (winter/spring 2021, summer/autumn 2021, winter/spring 2022) from the region Schleswig-Holstein) was also independently analysed for their TA content according to Lamp et al. (Citation2021) for quality assurance and validation purposes of the methods and results. In brief, the milk samples were thawed and homogenised by shaking. An aliquot of 2 mL was extracted with 4 mL methanol/formic acid (99/1, v/v). After mixing and centrifugation, an aliquot of the extract was frozen at −20 °C for at least 12 h. After additional ultracentrifugation, the supernatant was analysed by LC–MS/MS. Measurements were carried out on a Shimadzu LC 20 (Shimadzu, Duisburg, Germany) coupled to a mass spectrometer 4000 QTrap (Sciex, Darmstadt, Germany) using electrospray ionisation in positive ionisation mode (more information on the LC–MS/MS parameters is available in Supplementary Material S2). The chromatographic separation was performed using a Luna Phenyl-Hexyl-column (5 µm, ID 2 mm × 150 mm, Phenomenex, Aschaffenburg, Germany) combined with gradient elution with water containing 0.1% formic acid (solvent A) and methanol (solvent B) as mobile phases. The gradient conditions at a flow rate of 0.5 mL/min were: 0.0 min 10% B, 2.0 min 10% B, 12.0 min 80% B, 14.0 min 80% B, 15.0 min 10% B. The column was equilibrated to starting conditions for 2 min prior to each run. The oven temperature was set to 40 °C and the injection volume was 10 µL. The samples were screened for the presence of atropine and scopolamine. For quantification, positive samples were analysed in duplicates. The atropine and scopolamine contents were determined using external standard calibration (0.05–5.0 ng/mL) in methanol/water (60/40, v/v) with 0.4% formic acid. LODs of the independent verification analysis were 0.025 ng/ml for both analytes based on a signal to noise ratio of 3. The LC–MS/MS method’s linearity was R2 ≥ 0.9998 for both compounds. Recovery rates ranged from 95-99% and RSDr values were ≤3.0% (Lamp et al. Citation2021). The reporting limit for the analysis within this study was set to 0.15 µg/kg sample to equal the concentration of the lowest calibration standard.
Estimation of the corresponding PA/PANO intake of dairy cows via feed
Putative PA/PANO intake of dairy cows and potentially associated amounts of ragwort in the feed resulting in the measured PA/PANO levels were estimated. A milk yield of 25–35 L/d and an overall PA/PANO transfer rate of 0.05–0.1% for ragwort were used for calculations (Hoogenboom et al. Citation2011; Mulder et al. Citation2020). Intake of J. vulgaris plant material was estimated based on a PA/PANO content of 3.1–6.6 g/kg dry weight, a fresh biomass of 200–255 g per plant, and a dry weight of 28–35% for fully grown and flowering plants (Hama and Strobel Citation2021).
Evaluation of possible health concerns
Health risks for adults and children associated with PA/PANO () and TA () exposure via the consumption of the tested milk were estimated in order to give a better understanding of the relevance of the detected toxin levels. A mean case scenario was calculated using the mean toxin concentration for PA/PANO (). Exemplary single case calculations ( and ) were performed for estimations of the acute risk of exposure to singular high values. For adults, 131 g/d mean and 577 g/d 95% percentile milk intake (consumption of ‘milk and milk mixed beverages’ assessed via dietary history interviews) was used for exposure calculations (MRI Citation2008). For young children (2 to <5 years), 230.4 g/d (mean consumption) and 712.6 g/d (high consumption, 97.5% percentile) were used (Banasiak et al. Citation2005). For all calculations, 1 kg of milk was regarded as equal to 1 L of milk. Body weights (b.w.) of 16.15 and 70 kg were applied for children and adults, respectively (Banasiak et al. Citation2005; EFSA Citation2012).
Table 6. Exemplary scenarios (mean case and exemplary single case estimations) of consumer exposure to pyrrolizidine alkaloids (PA) and pyrrolizidine alkaloid N-oxides (PANO) via milk. The percentage of health-based guidance value (HBGV, 0.1 µg/kg b.w./d, BfR (Citation2016b)) (%), representing the risk of acute health impairment, and the margin of exposure (MOE, EFSA. (Citation2017)), representing an elevated risk for long-term adverse effects through PA/PANO, are shown for mean and high consumers. For calculation, 1 kg of milk was regarded as equal to 1 L.
Table 7. Exemplary single case estimations of consumer exposure to tropane alkaloids (TA) using two positive samples of milk (calculation of a mean case scenario not possible due to mean concentration < limit of detection). The percentage of the acute reference dose (ARfD, EFSA. (2013)) for the group of (−)-hyoscyamine and (−)-scopolamine, representing the risk of acute health impairment through TA intake, is shown for mean and high consumers. For calculation, the analytically determined atropine content was regarded as 100% (−)-hyoscyamine and 1 kg of milk was regarded as equal to 1 L.
For exposure calculations the mean PA/PANO sum content of all 228 milk samples was used. Exemplary calculations were also performed for two higher contaminated samples in order to assess a worst-case scenario. The risk associated with a long-term (chronic) PA/PANO intake was evaluated using the margin of exposure (MOE) approach, based on a benchmark dose lower confidence limit for a 10% increased cancer risk (BMDL10) of 237 µg/kg b.w./d for the sum of PA/PANO intake (EFSA Citation2017). The risk of an acute non-carcinogenic health impairment through short-term PA/PANO intake was assessed using a health-based guidance value (HBGV) of 0.1 µg/kg b.w./d for the sum of PA/PANO, based on a no observed adverse effect level (NOAEL) of 10 µg/kg b.w./d for the occurrence of non-neoplastic liver damages in rats and by applying an extrapolation factor of 100 (BfR Citation2016b).
Exposure to TA via the intake of contaminated milk was exemplarily calculated for the lowest and highest determined TA level. The risk of an acute health concern was assessed using the group acute reference dose (ARfD) of 0.016 µg/kg b.w. for the sum of (−)-hyoscyamine and (−)-scopolamine (EFSA Citation2013). Assuming a natural source, the analytically determined atropine content was regarded as 100% (−)-hyoscyamine (EFSA Citation2013).
Results
PA/PANO occurrence and pattern in milk
In total, 228 milk samples were analysed for the occurrence of 54 PA/PANO. PA/PANO were detected in milk samples of every season and from both regions. In 26 samples (11%), at least one PA/PANO was detected ( and ); 18 out of 108 samples (17%) from Bavaria and eight out of 120 samples (6.7%) from Schleswig-Holstein contained PA/PANO. Sum contents in contaminated samples ranged from 0.007 to 5.6 µg/L with a median of 0.024 µg/L and a mean of 0.30 µg/L ( and ). The mean PA/PANO sum content of all 228 milks was 0.034 µg/L. Only four milk samples (samples 1, 20, 134, 207) showed higher PA/PANO sum contents of 0.59 µg/L (milk collection tank, organic production), 0.13 µg/L (milk collection tank, organic production), 0.73 µg/L (vending machine, organic production) and 5.6 µg/L (vending machine, conventional production) ().
Figure 2. Pyrrolizidine alkaloid (PA) and pyrrolizidine alkaloid N-oxide (PANO) sum content (µg/L) of PA/PANO-positive milk (n = 26) sampled between winter/spring 2020 and winter/spring 2022 in the regions Bavaria (sample numbers 1–108) and Schleswig-Holstein (sample numbers 109–228). Sorted by region and sum content. Dark grey: organic production, light grey: conventional production.
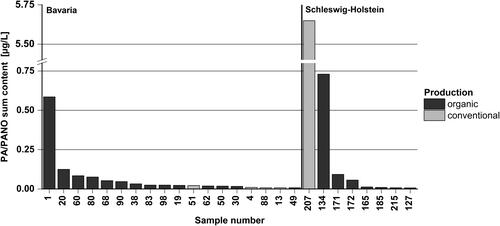
Table 2. Pyrrolizidine alkaloid (PA) and pyrrolizidine alkaloid N-oxide (PANO) content (µg/L) of PA/PANO-contaminated milk sampled in the region Bavaria. The samples originated from several farms with either organic or conventional production (for additional information see Supplementary Table S1). Non-listed samples had no detectable PA/PANO contents (<limit of detection). Only analytes detected in at least one sample from either Bavaria or Schleswig-Holstein are listed. PA/PANO contents were rounded to two significant figures where applicable.
Table 3. Pyrrolizidine alkaloid (PA) and pyrrolizidine alkaloid N-oxide (PANO) content (µg/L) of PA/PANO-contaminated milk sampled in the region Schleswig-Holstein. The samples originated from several farms with either organic or conventional production (for additional information see Supplementary Table S1). Non-listed samples had no detectable PA/PANO contents (<limit of detection). Only analytes detected in at least one sample from either Bavaria or Schleswig-Holstein are listed. PA/PANO contents were rounded to two significant figures where applicable.
57% of the milk samples from Bavaria and 43% of the milk samples from Schleswig-Holstein originated from organic production. However, the majority of PA/PANO-positive samples were organic milk samples (Bavaria: 78%; Schleswig-Holstein: 88%). Overall, 81% of PA/PANO-contaminated milk originated from organic production. In contrast, the milk sample with the highest PA/PANO sum content was from conventional farming.
Overall, 14 different Ly-type, Sc-type or Ht-type PA/PANO were detected ( and ). In 19 out of 26 PA/PANO-contaminated samples only one PA was detected.
Lycopsamine (Ly-type) and senkirkine (Sc-type) were the major occurring PA/PANO in milk from Bavaria. Co-occurrence of lycopsamine, senkirkine or erucifoline (Sc-type) was observed in four samples. Four farms from Bavaria (farms 1, 2, 12, 20) provided milk containing PA/PANO in three of four consecutive seasons. Thereby, PA/PANO patterns in the milk samples from each farm are similar but not identical throughout the seasons, except for sample 1 from farm 1 (). While in sample 19 and 49 from farm 1, only lycopsamine was detected in traces, in sample 1 from the same farm several Ht-type PA (rinderine, europine, heliosupine and heliotrine) were detected. Herein, rinderine was the predominant PA.
In samples collected in Schleswig-Holstein, jacoline (Sc-type) was the major PA in four out of eight PA/PANO-contaminated milk samples (). In samples 134 and 207 with a high jacoline content, jacobine and jaconine (Sc-type) co-occurred. In sample 207 (PA/PANO sum content 5.6 µg/L), jacoline-N-oxide and jacobine-N-oxide were detected besides jacoline, jacobine and jaconine. Jacoline always contributed >85% to the PA/PANO contents. In four other milk samples from Schleswig-Holstein heliosupine, intermedine/indicine (Ly-type), lycopsamine or rinderine were detected, but below the LOQ. All milk samples from Schleswig-Holstein contaminated with PA/PANO originated from different farms.
Assuming J. vulgaris as the potential origin of PA/PANO contamination in samples 134 and 207, this would account for less than one to less than three fully grown and flowering plants of J. vulgaris with a high PA/PANO content in the feed per dairy cow and per day ().
Table 4. Exemplary estimations on pyrrolizidine alkaloid/pyrrolizidine alkaloid N-oxide (PA/PANO) intake of a dairy cow per day and corresponding amounts of plant material potentially present in the diet of a dairy cow when milk is highly contaminated with PA/PANO.
TA occurrence in milk
In three milk samples from Bavaria (samples 1, 7, 64) and in one milk from Schleswig-Holstein (sample 217) atropine was detected in amounts from 0.028 to 0.066 µg/L (), corresponding to a very low rate of contamination of 1.8% of all milk samples. In contrast, scopolamine was not present in any of the samples. Milk samples collected in seasons C–E (winter/spring 2021, summer/autumn 2021, winter/spring 2022) from Schleswig-Holstein were analysed independently with a second LC–MS/MS method, targeting exclusively TA. The occurrence of atropine in sample 217 and the absence of both TA in all other analysed samples from Schleswig-Holstein was confirmed.
Table 5. Tropane alkaloid (TA) content (µg/L) of TA-contaminated milk sampled in the regions Bavaria and Schleswig-Holstein. The samples originated from several farms with either organic or conventional production. Data of the multi-analyte method and the independent verification analysis for tropane alkaloids are shown. Non-listed samples had no detectable TA contents (<limit of detection).
All atropine-contaminated samples from Bavaria originated from organic farming. In contrast, the atropine-contaminated sample from Schleswig-Holstein was obtained from a conventional farm. Samples containing atropine originated from different farms but were all sampled during winter/spring seasons (A, C, E). Sample 1 from Bavaria was simultaneously contaminated with PA and TA.
Evaluation of possible health concerns
Exposure to PA/PANO
The possible health risks associated with the selected exposure scenarios () were evaluated regarding an acute (non-carcinogenic effects, HBGV evaluation) and a chronic exposure (carcinogenic effects, MOE evaluation).
In a mean case scenario, neither acute nor chronic exposure scenarios accounted for a health risk for the consumers (). Even for the sensitive subpopulation of children with high milk intake (712.6 g/day, Banasiak et al. Citation2005) only a percentage of 1.5% of the HBGV and an MOE of 158,000 were calculated. In general, MOE values above 10,000 indicate a low concern regarding an elevated risk for long-term adverse effects from a public health point of view (EFSA Citation2017).
Also, the exemplary applied single case estimations based on calculations with highly contaminated milk from two individual farms (samples 134 and 207) revealed at most a very small to moderate contribution to the daily intake of PA/PANO in adults and children (, 1.4–32% of HBGV). Only the highest PA/PANO level (5.6 µg/L) would clearly contribute to the HBGV being reached or exceeded in mean or highly consuming children, respectively. An assessment of a chronic exposure using the MOE approach (EFSA Citation2017) was not applicable due to individual spot contaminations.
Overall, the risk of PA/PANO exposure through milk can be regarded as very low.
Exposure to TA
The risk associated with the intake of TA through the milk was exemplarily assessed for the lowest (0.028 µg/L) and the highest (0.066 µg/L) determined atropine concentration that was considered as (−)-hyoscyamine (, single case estimations). For adults with a mean or high milk intake, such atropine concentrations would only result in an exposure of less than 3.4% of the group ARfD of 0.016 µg/kg b.w./d set by EFSA (EFSA Citation2013). For children with a mean and high milk intake, such concentrations would correspond to a TA intake of 5.9% and 18% of the group ARfD, respectively.
Discussion
Sample acquisition
Schleswig-Holstein and Bavaria were selected as model regions as they are known for their spread of J. vulgaris and J. aquatica, respectively (Suttner et al. Citation2016; Gottschalk et al. Citation2018; Citation2020; Jung et al. Citation2020). In Bavaria, J. aquatica is particularly spread in the southern region ‘Allgäu’ (administrative district Swabia) (Suttner et al. Citation2016). Therefore, most of the actively participating dairy farms from Bavaria were in this region, due to higher awareness of the problem (Supplementary Table S1). Milk samples were completely (Bavaria) or partly (Schleswig-Holstein) acquired from volunteering farms (active participation). Due to the active participation, especially for Bavaria, the milk samples might not represent the overall situation of PA/PANO and TA contamination in raw milks of dairy farms. Farmers with less awareness of PA/PANO- or TA-containing plants on their grass or arable land might not have participated actively. The fact that the two highest contaminated milk samples were collected from milk vending machines where the sampling had been done via passive participation could be an indication for this.
PA/PANO occurrence in milk
PA/PANO were detected in 11% of all milk samples up to a contamination level of 5.6 µg/L, but generally at very low levels (median: 0.024 µg/L, mean: 0.30 µg/L) with low health concerns (). However, it was striking that the highest PA/PANO level quantified was about 30 times higher than levels determined in previous studies (Huybrechts and Callebaut Citation2015; Mulder et al. Citation2018; Klein et al. Citation2022). So far, the highest PA/PANO content reported for milk was 0.17 µg/L in a retail milk (n = 169 randomly taken cow milk samples from six European countries) (Mulder et al. Citation2018). Large scale industrial milk processing used to produce retail milk can cause effective dilution of potentially contaminated milk from individual dairy farms. This may result in an overall increased rate of low-level contaminated milk (Mulder et al. Citation2018). It was also hypothesised that some milk from singular origin might exhibit much higher levels as it has already been shown, for example, for honey (Gottschalk et al. Citation2020). Our data with a contamination rate of 6.7% and few higher PA/PANO sum contents in milk from Schleswig-Holstein support this hypothesis. In Bavaria, a contamination rate of 17% was observed, with 12 of 18 PA/PANO-contaminated milk samples originating from only four farms but with overall lower PA/PANO concentrations. The repeated occurrence of the same PA in samples collected in consecutive seasons indicated a persistent presence of the PA/PANO-containing plants on the farms’ grassland.
The contamination rates received here (6.7%, 17%) were similar to the contamination rates of 6% and 13% reported in studies conducted with retail milk (Huybrechts and Callebaut Citation2015; Mulder et al. Citation2018). The contamination rate of 17% of the Bavarian samples is similar to a former study with 15 milk samples obtained from vending machines and direct marketing from Bavaria (Klein et al. Citation2022).
A high percentage of PA/PANO-positive milk samples (81%) was from organic production. Mulder et al. (Citation2018) also observed that PA were found in milk from organic production more frequently. In general, only limited weed control methods are approved for use in organic farming. For example, there are limitations on herbicide use to conserve a higher level of biodiversity and restrictions on the application of mineral fertilisers. This may lead to a higher probability of PA/PANO contamination of milk. On the other hand, it is not necessarily linked to high PA/PANO contents in milk as the milk with the highest PA/PANO sum concentration detected in this study originated from conventional production. However, the overall number of positive samples was too low for statistically valid analysis of this point.
Regarding the milk sampling scheme, it is necessaryalso to take into account time and technical influences on the measured concentrations. Milk was sampled at different time intervals, potentially leading to different numbers of milkings in the milk collection tanks (Supplementary Table S1). Consequently, not all milk samples represent milk that was actually offered to customers or sold to dairy companies. Individual cows of one dairy farm might be exposed differently to PA/PANO as weeds may occur as spot contaminations in the animal feed or due to an individual weed intake while grazing. This is also the reason why the risk estimations for the higher contaminated samples are only of exemplary character for a better understanding of the measured concentrations ( and ). Hence dilution of contaminated milk may already take place on an intra-farm level due to varying exposure of cows. High contaminations of PA/PANO in milk from vending machines and milk collection tanks containing several milkings are probably associated with a more widespread contamination of PA/PANO-containing weeds in the provided feed.
The identification of the causative plant species is important to give advice regarding agricultural practices to prevent future contamination of feed and milk. It is known that PA/PANO patterns shift greatly from supplemented plant material to milk due to differing transfer rates of individual PA/PANO (Mulder et al. Citation2020). In the present work it was observed that in milks with high PA/PANO sum content, one PA dominated the PA/PANO pattern while co-occurring with other PA/PANO in lower amounts. Due to that, tracing back the potential botanical origin of the PA/PANO contamination in milk based on the analyte pattern obtained from plant material is very limited. Data on the actual transfer of PA/PANO from plant material to milk is only available for few plant species, mainly Jacobaea spp. (Dickinson et al. Citation1976; Hoogenboom et al. Citation2011; Mulder et al. Citation2020). Taenzer et al. (Citation2022) showed that, in the rumen environment, PANO were reduced to their corresponding PA and further metabolised to hydrogenated structures. Biotransformation to the hydrogenated metabolites was slower for some PA, in particular for jacoline. This offers an explanation for the higher transfer rates of some PA and the shifts of PA/PANO patterns from the feed into the milk.
The co-occurrence of jacoline, jacobine and jaconine match PA/PANO patterns in milk after dairy cows were exposed to ragwort (Mulder et al. Citation2020). In case of a low contamination level, the individual transfer rates of these PA/PANO would mean that only jacoline, with the highest transfer rate (1.4% and 4%, depending on the study), can be detected (Hoogenboom et al. Citation2011; Mulder et al. Citation2020). Thus, we assumed that the PA/PANO contamination of the four samples containing jacoline originated from feed contaminated with J. vulgaris (tansy ragwort, Asteraceae). This is in line with the documented wide spread of J. vulgaris in Northern Germany (Neumann and Huckauf Citation2016; Gottschalk et al. Citation2020; Jung et al. Citation2020). Jacoline was also detected in milk by Mulder et al. (Citation2018).
The four Ht-type PA in sample 1 from Bavaria potentially originate from plants of the Boraginaceae family, for example, Heliotropium europaeum (heliotrine, europine, heliosupine, rinderine) and Cynoglossum officinale (rinderine, heliosupine) (Mädge et al. Citation2020). Individual transfer rates of these PA from feed into the milk are not available.
The comparatively high frequency of senkirkine and lycopsamine occurrence in the milk samples from Bavaria suggests that certain plant species cause the PA/PANO contamination. Furthermore, PA/PANO were repeatedly detected in milk from four dairy farms in Bavaria. Even though not identical, PA/PANO patterns in milk samples from these farms were very similar over the seasons. This indicated a permanent presence of the PA/PANO-containing plant species in the feed supplied.
Lycopsamine is mainly produced by plants of the Boraginaceae family (Teuscher and Lindequist Citation2010). In Southern Germany, plants producing that compound are, for example, Symphytum spp. (e.g. comfrey, Symphytum officinale) and Eupatorium cannabinum L. (FloraWeb Citation2000Citation– ; Brown et al. Citation2016; Kast et al. Citation2018; Mädge et al. Citation2020). Senkirkine is synthesised by species of the Asteraceae family, for example, marsh ragwort (J. aquatica) and Petasites hybridus (L.) Gaertn., Mey. and Scherb., both growing in Southern Germany (FloraWeb Citation2000– ; Schenk et al. Citation2015; Gottschalk et al. Citation2018). Lycopsamine and senkirkine were also detected more frequently in other studies on retail milk (Huybrechts and Callebaut Citation2015; Mulder et al. Citation2015). The co-occurrence of senkirkine and lycopsamine in two milk samples suggests that at least two different PA/PANO-containing plants were present in the feed of the dairy cows.
The presence of low PANO-concentrations only in the highest contaminated milk sample is in line with previous studies (Mulder et al. Citation2020; Taenzer et al. Citation2022). During rumen metabolism, PANO undergo a rapid conversion to their corresponding tertiary amine PA predominantly followed by a biotransformation to 1,2-saturated metabolites (Taenzer et al. Citation2022).
Feeding patterns of ruminants differ seasonally in Germany: in winter only conserved feed (hay/silage/concentrate feed) is available. During summer, the diets vary between exclusively receiving preserved feed, solely grazing on pastures or a combination of both. Furthermore, the PA/PANO content and the size of PA/PANO producing plants also vary considerably during a growing season (Flade et al. Citation2019; Hama and Strobel Citation2021; Chizzola and Eller Citation2022). Distinct seasonal differences in PA/PANO contamination of the milk samples analysed in this study were not possible to evaluate due to the low number of PA/PANO-positive samples. Also, it remains unclear whether PA/PANO levels in the milk of the summer seasons (B, D) were caused by conserved feed or grazing. In general, it is assumed that cattle tend to avoid plants containing PA/PANO while grazing due to their bitter taste (Hoogenboom et al. Citation2011; Kalač and Kaltner Citation2021). The fact that the highest PA/PANO contamination in this study was observed in winter season C implicates that only conserved feed caused the contamination at least in this sample.
Estimations on the intake of J. vulgaris plant material by dairy cows were made based on parameters associated with high natural variances (e.g. PA/PANO content and weight of a plant) (Carvalho et al. Citation2014; Flade et al. Citation2019). For J. vulgaris plants during flowering time, PA/PANO contents of 0.8–6.6 g/kg dry weight were reported (Jung et al. Citation2020; Hama and Strobel Citation2021). It needs to be noted that these data correspond to fully grown and flowering J. vulgaris plants with a high PA/PANO content (Hama and Strobel Citation2021). In an animal feeding study conducted with dairy cows, 200 g of dried plant material of a ragwort mixture (J. vulgaris/S. inaequidens, 84/16, w/w, mean PA/PANO content of 3767 mg/kg dry matter) per day accounted for a mean PA/PANO concentration of 12.1 µg/L in the milk (Mulder et al. Citation2020). Applying the PA/PANO content of this specific ragwort mixture to the concentrations of 5.6 µg/L (sample 207) and 0.73 µg/L (sample 134) would account for 93 and 12 g dry plant material, respectively. These amounts are also within the range for the putative dried J. vulgaris plant material intake as calculated here (). Mulder et al. (Citation2020) estimated that the 200 g would account for 1% of the daily dry matter intake. The estimations on the plant numbers show that high PA/PANO levels found in milk within this study may be associated with a number of plants easily found on highly infested grassland (Wiggering et al. Citation2022). In particular, if infested grassland is used to prepare conserved feed stuff, dairy cows are barley able to avoid these plants. This highlights the importance of good grassland management.
TA occurrence in milk
The transfer of TA (atropine and scopolamine) into the milk was shown recently (Lamp et al. Citation2021). In our study, the milk samples showed a very low contamination rate and only traces of atropine. To the best of our knowledge, this is the first report of TA in authentic milk samples, that are not originating from animal feeding studies. During further processing in dairies, atropine contents would probably be diluted to levels below the LOD.
Parallel, an independent verification analysis for TA was conducted on a subset of milk samples from seasons C–E Schleswig-Holstein. This was meant to evaluate the TA determination of the analytical methods, as TA were not detected in authentic milk samples, not originating from animal feeding studies, before. For the TA contaminated milk sample within this subset, only a qualitative confirmation of atropine was possible as the concentration was below the independent verification method’s reporting limit. Neither of the analytical methods achieved separation of the racemic mixture atropine into (−)-hyoscyamine and (+)-hyoscyamine.
Another study investigated the occurrence of TA (−)-hyoscyamine and scopolamine in 10 milk samples from the Korean market (Zheng et al. Citation2019). Neither (−)-hyoscyamine nor scopolamine were found above their LOD of 0.8 and 1 µg/kg, respectively. The LOD of the used method were more than 10 times higher than the atropine concentrations determined within our study.
In a study analysing feed samples from Germany and the Netherlands, no (−)-hyoscyamine or (−)-scopolamine were detected above the LOD (4.5 and 2 µg/kg, respectively) in samples of forages and roughage, and products derived thereof (n = 301) as well as of legume seeds and products derived thereof (n = 13) (EFSA Citation2013). In the same study, 17 out of 29 samples of compound feed for ruminants were found to contain TA. As a result, the EFSA concluded that ruminants are more likely to consume TA-containing plant material through non-forage feed (e.g. compound feed) (EFSA Citation2013). In contrast, intoxications of dairy cows and bulls through maize silage contaminated with D. stramonium were reported (Bofill et al. Citation2007; Aboling et al. Citation2019). The source for the contamination in the milk samples of this study remains unknown but both possibilities should be considered. It is important to mention, that a transfer of TA into the milk can already take place when dairy cows are subclinically exposed to TA (Lamp et al. Citation2021).
Remarkably, atropine was only detected in samples from the winter/spring seasons when cows were fed with preserved feed stuff. However, the number of positive samples is too small to draw representative conclusions on a potential seasonal dependency. Information on the spread of weeds containing TA is limited (de Nijs et al. Citation2023). For Europe, D. stramonium (jimson weed), Hyoscyamus spp. (henbane) and A. belladonna (deadly nightshade) are considered to be relevant weeds with high levels of (−)-hyoscyamine.
Evaluation of possible health concerns
Exposure to PA/PANO
Milk is a highly consumed staple food. It has been known for decades that a transfer of PA/PANO from feed to milk is possible (Dickinson et al. Citation1976). In the framework of the joint research project PA-SAFE-FEED a selected scenario with milk from individual farms in model regions with known occurrence of ragwort should be studied under field conditions. Currently, significant exposure to PA/PANO via milk consumption is expected to be rare due to only trace amounts of PA detected in milk from supermarkets (up to 0.17 µg/L) (Mulder et al. Citation2018). In contrast to such retail milk, raw milk obtained from individual dairy farms is not mixed with raw milk from other origins. Therefore, potential toxin concentrations in the milk of individual dairy farms are supposed to be higher and may exhibit a higher risk for consumers, for example, if distributed via vending machines. Actually, our data revealed that most of the tested milk samples exhibited PA/PANO levels below the LOD (89% of the samples) and that their mean concentration was mostly irrelevant regarding consumers health (). Nevertheless, in individual cases, higher levels of PA/PANO in milk are possible, underlining that certain consumer groups like children of farmer families or customers of milk from vending stations who exclusively consume milk from one farm may be subject to a PA/PANO exposure reaching the HGBV. For example, the milk samples with 0.73 and 5.6 µg/L PA/PANO were obtained from vending machines and therefore intended for direct consumption (Supplementary Table S1). Such scenarios for highly exposed subpopulations have also been previously discussed for the occurrence of PA/PANO in regionally produced and marketed honey (Gottschalk et al. Citation2020). Undoubtedly, our results origin only from singular measurements of individual samples, hampering a chronic exposure assessment. Conducting repeated analysis of milk from a single source over a longer period would help to clarify the probability of high PA/PANO levels and to allow for a more comprehensive interpretation of the situation. However, the primary objective of the current study was to provide an overview of potential PA/PANO levels in milk from singular origins. In general, the exposure to PA/PANO should be kept as low as reasonably achievable (so-called ALARA principle, (BfR Citation2020)).
Within this study, mainly raw milks were tested but PA/PANO are considered stable during the heat treatments commonly applied in milk processing (de Nijs et al. Citation2017). Hence, a decay in PA/PANO is not expected after the recommended heating of raw milk for safe consumption regarding microbiological health risk (BfR Citation2016a).
In current European legislation and risk assessment, 1,2-dehydro-PA and their corresponding PANO are considered to have equal toxic potency (EFSA Citation2017; EC Citation2023). This is respected in the BMDL10 of 237 µg/kg b.w./d based on a toxicity study with riddelliine in rats that was also used for this evaluation (NTP Citation2003). Recently, there is data suggesting a more differentiated approach regarding the toxic potency of individual PA/PANO in the risk assessment instead of taking just the PA/PANO sum content into account (Kolrep et al. Citation2018; Geburek et al. Citation2020; Haas et al. Citation2023). This data indicated a reduced hepatic metabolisation for jacoline. This may suggest a less effective formation of the highly reactive intermediates due to a reduced metabolic activation of jacoline which may lead to a lower toxic potency compared to other 1,2-dehydro-PA/PANO (Geburek et al. Citation2020). In the case of the two highest contaminated milk samples from Schleswig-Holstein, jacoline contributed >85% to the PA/PANO sum content. Whether and to what extent the findings of Geburek et al. (Citation2020) can put this high jacoline contents into perspective is not assessable.
Exposure to TA
Cereal-based products are the main sources of TA for humans. For toddlers and other children it is reported that TA exposure via these products may lead to an exceedance of the ARfD for the group of (−)-hyoscyamine and (−)-scopolamine (EFSA Citation2018). Currently, it is considered unlikely that milk contributes significantly to the TA exposure (EFSA Citation2008). Our data confirmed that the probability of TA occurrence in general is very low. For adults, exemplary calculations showed that the low concentrations of atropine in milk are still negligible regarding health concerns. In children, atropine concentrations in milk may contribute to the overall exposure to TA due to their low body weight and higher milk intake (Banasiak et al. Citation2005). However, as the rate of contamination was so low even in milk from individual dairy farms which is not further mixed with milk from other dairy farms, such scenarios appear quite unlikely.
Many uncertainties still remain. For example, as the ratio of the atropine enantiomers remained unknown, the whole of its content in milk was set as (−)-hyoscyamine, as applied by the EFSA for atropine contents of plant-based products (EFSA Citation2013). There are no data available on the stability of TA in milk during storage, recommended heating processes like pasteurisation, or other processing. Here, mainly raw milk was investigated. For heating procedures of other foods, different decreases in atropine levels were reported depending on heating time, temperature and type of food (Friedman and Levin Citation1989; Perharič, Koželj et al. Citation2013; Marín-Sáez et al. Citation2019a, Citation2019b; Vera-Baquero et al. Citation2022). During tea preparation, partial degradation of TA with a more complex chemical structure, for example, atropine and scopolamine, to the core structures tropine and tropinone, and partly to other TA, for example, apoatropine and aposcopolamine, was reported (Marín-Sáez et al. Citation2019b). In contrast to atropine and scopolamine, information on the toxicity of other TA is scarce (Mulder et al. Citation2016). Overall, heating procedures may not automatically result in a safe product.
Conclusion
Overall, the PA/PANO contamination rates in milk sampled directly from dairy farms is similar to contamination rates determined for milk from retail. PA/PANO contamination levels are generally low, even in milk samples that originate from areas with a high occurrence of PA/PANO-containing plants in the grassland. In some samples PA/PANO levels up to 30 times higher than previously reported for retail milk were detected. This supports the hypothesis that in milk from individual dairy farms, PA/PANO levels are potentially higher than in milk from large dairies. Occasional high concentrations seem to be more likely to be responsible for contamination of retail dairy milk than frequently occurring low levels in many samples. While a wide range of Sc-type (Asteraceae), Ly-type (Boraginaceae) and Ht-type (Boraginaceae) PA/PANO was detected, jacoline, lycopsamine and senkirkine were the most abundant PA in the milk samples. In highly contaminated milk samples from Schleswig-Holstein, PA/PANO patterns are similar to those found in animal feeding studies with ragwort. As far as we know, this is the first report of TA occurrence in milk which was not obtained through animal feeding studies. Atropine showed very low frequency of occurrence and levels in trace amounts.
It was shown that directly marketed milk regularly has a very low contribution to the exposure of consumers to PA/PANO and TA. In order to prevent contamination of milk with PA/PANO and TA, identifying and eliminating possible sources of contamination as well as management aspects should be respected also in future. Estimations regarding the putative intake of toxic plant material by dairy cows show that raising the farmers’ awareness for relevant toxic plants in feed production is essential.
Supplemental Material
Download Zip (542.4 KB)Acknowledgements
The authors wish to thank the dairy farms that volunteered for this study. Furthermore, the authors thank the Milchprüfring Bayern e.V., in particular, Dr. Christian Baumgartner, and the Milcherzeugervereinigung Schleswig-Holstein e.V., in particular, Nicolai Wree, for the support in the acquisition of volunteering dairy farms. The authors thank Julian Tänzer from the German Federal Institute for Risk Assessment for a comparison analysis of selected milk samples. The authors gratefully acknowledge the skilful technical assistance of Michaela Freitag, René Mamet, and Sebastian Schlef.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aboling S, Rieger H, Kölln M, Tenhündfeld J, Roerink G, Platje N, Kamphues J. 2019. Feed refusal in fattening bulls because of maize silage contamination by Jimson weed (Datura stramonium). Tierarztl Prax Ausg G Grosstiere Nutztiere. 47(2):125–130.
- Banasiak U, Heseker H, Sieke C, Sommerfeld C, Vohmann C. 2005. Estimation of the dietary intake of pesticide residues based on new consumption data for children. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 48(1):84–98. doi: 10.1007/s00103-004-0949-6.
- German Federal Institute for Risk Assessment (BfR). 2016a. Opinion No. 008/2016: raw milk: boiling protects against infection with Campylobacter. Berlin: BfR.
- German Federal Institute for Risk Assessment (BfR). 2016b. Opinion No. 030/2016: pyrrolizidine alkaloids: levels in foods should continue to be kept as low as possible. Berlin: BfR.
- German Federal Institute for Risk Assessment (BfR). 2020. Opinion No. 023/ 2020. Updated risk assessment on levels of 1,2-unsaturated pyrrolizidine alkaloids (PAs) in foods. Berlin: BfR.
- Bofill FX, Bofill J, Such G, Piqué E, Guitart R. 2007. Dos casos de intoxicación por contaminación de maíz con Datura stramonium en ganado vacuno. Rev Toxicol. 24(1):56–58.
- Brown JH, Laiken N. 2011. Muscarinic receptor agonists and antagonists. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s: the pharmacological basis of therapeutics. 12th ed. New York (NY): mcGraw-Hill Education; p. 219–237.
- Brown AW, Stegelmeier BL, Colegate SM, Gardner DR, Panter KE, Knoppel EL, Hall JO. 2016. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and intermedine in chicks (Gallus gallus domesticus). J Appl Toxicol. 36(5):716–725. doi: 10.1002/jat.3205.
- Carvalho S, Macel M, Mulder PPJ, Skidmore A, van der Putten WH. 2014. Chemical variation in Jacobaea vulgaris is influenced by the interaction of season and vegetation successional stage. Phytochemistry. 99:86–94. doi: 10.1016/j.phytochem.2013.12.004.
- Chizzola R, Eller A. 2022. Seasonal variability in pyrrolizidine alkaloids in Jacobaea alpina from the Trentino-Alto Adige region (Northern Italy). Chem Biodivers.e202200603. eng.
- de Nijs M, Crews C, Dorgelo F, MacDonald S, Mulder PPJ. 2023. Emerging issues on tropane alkaloid contamination of food in Europe. Toxins (Basel). 15(2):98. doi: 10.3390/toxins15020098.
- de Nijs M, Mulder PPJ, Klijnstra MD, Driehuis F, Hoogenboom RLAP. 2017. Fate of pyrrolizidine alkaloids during processing of milk of cows treated with ragwort. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 34(12):2212–2219. doi: 10.1080/19440049.2017.1364432.
- Dickinson JO, Cooke MP, King RR, Mohamed PA. 1976. Milk transfer of pyrrolizidine alkaloids in cattle. J Am Vet Med Assoc. 169(11):1192–1196.
- European Commission (EC). 2023. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 (Text with EEA relevance). Official Journal of the European Union. L119:103–157.
- Edgar JA, Molyneux RJ, Colegate SM. 2015. Pyrrolizidine alkaloids: potential role in the etiology of cancers, pulmonary hypertension, congenital anomalies, and liver disease. Chem Res Toxicol. 28(1):4–20. doi: 10.1021/tx500403t.
- European Food Safety Authority (EFSA). 2008. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission onTropane alkaloids (from Datura sp.) as undesirable substances in animal feed. EFSA J. 691:1–55.
- European Food Safety Authority (EFSA). 2011. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 9(11):2406.
- European Food Safety Authority (EFSA). 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 10(3):2579.
- European Food Safety Authority (EFSA). 2013. Scientific opinion on tropane alkaloids in food and feed. EFSA J. 11(10):3386.
- European Food Safety Authority (EFSA). 2017. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 15(7):4908.
- European Food Safety Authority (EFSA). 2018. Human acute exposure assessment to tropane alkaloids [Scientific Report]. EFSA J. 16(2):5160.
- Flade J, Beschow H, Wensch-Dorendorf M, Plescher A, Wätjen W. 2019. Occurrence of nine pyrrolizidine alkaloids in Senecio vulgaris L. Depending on developmental stage and season. Plants. 8(3):54. doi: 10.3390/plants8030054.
- FloraWeb. 2000– . Version 1.02. Bonn (Germany): German Federal Agency for Nature Conservation. [updated 2013 Dec 10; accessed 2024 Jan 23]. https://www.floraweb.de/pflanzenarten/pflanzenarten.html.
- Friedman M, Levin CE. 1989. Composition of jimson weed (Datura stramonium) seeds. J Agric Food Chem. 37(4):998–1005. doi: 10.1021/jf00088a040.
- Fu PP, Xia Q, Lin G, Chou MW. 2004. Pyrrolizidine alkaloids – genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 36(1):1–55. doi: 10.1081/dmr-120028426.
- Geburek I, Preiss-Weigert A, Lahrssen-Wiederholt M, Schrenk D, These A. 2020. In vitro metabolism of pyrrolizidine alkaloids – metabolic degradation and GSH conjugate formation of different structure types. Food Chem Toxicol. 135:110868. doi: 10.1016/j.fct.2019.110868.
- González-Gómez L, Morante-Zarcero S, Pérez-Quintanilla D, Sierra I. 2022. Occurrence and chemistry of tropane alkaloids in foods, with a focus on sample analysis methods: a review on recent trends and technological advances. Foods. 11(3):407. doi: 10.3390/foods11030407.
- Gottschalk C, Kaltner F, Zimmermann M, Korten R, Morris O, Schwaiger K, Gareis M. 2020. Spread of Jacobaea vulgaris and occurrence of pyrrolizidine alkaloids in regionally produced honeys from Northern Germany: inter- and intra-site variations and risk assessment for special consumer groups. Toxins (Basel). 12(7):441. doi: 10.3390/toxins12070441.
- Gottschalk C, Ostertag J, Meyer K, Gehring K, Thyssen S, Gareis M. 2018. Influence of grass pellet production on pyrrolizidine alkaloids occurring in Senecio aquaticus-infested grassland. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 35(4):750–759.
- Gottschalk C, Ronczka S, Preiß-Weigert A, Ostertag J, Klaffke H, Schafft H, Lahrssen-Wiederholt M. 2015. Pyrrolizidine alkaloids in natural and experimental grass silages and implications for feed safety. Anim Feed Sci Technol. 207:253–261. doi: 10.1016/j.anifeedsci.2015.06.014.
- Griffin WJ, Lin GD. 2000. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 53(6):623–637. doi: 10.1016/s0031-9422(99)00475-6.
- Haas M, Wirachowski K, Thibol L, Küpper J-H, Schrenk D, Fahrer J. 2023. Potency ranking of pyrrolizidine alkaloids in metabolically competent human liver cancer cells and primary human hepatocytes using a genotoxicity test battery. Arch Toxicol. 97(5):1413–1428. doi: 10.1007/s00204-023-03482-8.
- Hama JR, Strobel BW. 2021. Occurrence of pyrrolizidine alkaloids in ragwort plants, soils and surface waters at the field scale in grassland. Sci Total Environ. 755(Pt 1):142822. doi: 10.1016/j.scitotenv.2020.142822.
- Hoogenboom LAP, Mulder PPJ, Zeilmaker MJ, van den Top HJ, Remmelink GJ, Brandon EFA, Klijnstra M, Meijer GAL, Schothorst R, Van Egmond HP. 2011. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 28(3):359–372. doi: 10.1080/19440049.2010.547521.
- Huybrechts B, Callebaut A. 2015. Pyrrolizidine alkaloids in food and feed on the Belgian market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 32(11):1939–1951. doi: 10.1080/19440049.2015.1086821.
- Jung S, Lauter J, Hartung NM, These A, Hamscher G, Wissemann V. 2020. Genetic and chemical diversity of the toxic herb Jacobaea vulgaris Gaertn. (syn. Senecio jacobaea L.) in Northern Germany. Phytochemistry. 172:112235. doi: 10.1016/j.phytochem.2019.112235.
- Kalač P, Kaltner F. 2021. Pyrrolizidine alkaloids of European Senecio/Jacobaea species in forage and their carry-over to milk: a review. Anim Feed Sci Technol. 280:115062. doi: 10.1016/j.anifeedsci.2021.115062.
- Kast C, Kilchenmann V, Reinhard H, Droz B, Lucchetti MA, Dübecke A, Beckh G, Zoller O. 2018. Chemical fingerprinting identifies Echium vulgare, Eupatorium cannabinum and Senecio spp. as plant species mainly responsible for pyrrolizidine alkaloids in bee-collected pollen. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 35(2):316–327. doi: 10.1080/19440049.2017.1378443.
- Klein LM, Gabler AM, Rychlik M, Gottschalk C, Kaltner F. 2022. A sensitive LC–MS/MS method for isomer separation and quantitative determination of 51 pyrrolizidine alkaloids and two tropane alkaloids in cow’s milk. Anal Bioanal Chem. 414(28):8107–8124. doi: 10.1007/s00216-022-04344-5.
- Kolrep F, Numata J, Kneuer C, Preiss-Weigert A, Lahrssen-Wiederholt M, Schrenk D, These A. 2018. In vitro biotransformation of pyrrolizidine alkaloids in different species. Part I: microsomal degradation. Arch Toxicol. 92(3):1089–1097. doi: 10.1007/s00204-017-2114-7.
- Lamp J, Knappstein K, Walte HG, Krause T, Steinberg P, Schwake-Anduschus C. 2021. Transfer of tropane alkaloids (atropine and scopolamine) into the milk of subclinically exposed dairy cows. Food Control. 126:108056. doi: 10.1016/j.foodcont.2021.108056.
- Mädge I, Gehling M, Schöne C, Winterhalter P, These A. 2020. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 37(8):1339–1358. doi: 10.1080/19440049.2020.1757166.
- Marín-Sáez J, Romero-González R, Garrido Frenich A. 2019a. Degradation of tropane alkaloids in baked bread samples contaminated with Solanaceae seeds. Food Res Int. 122:585–592. doi: 10.1016/j.foodres.2019.01.027.
- Marín-Sáez J, Romero-González R, Garrido Frenich A. 2019b. Effect of tea making and boiling processes on the degradation of tropane alkaloids in tea and pasta samples contaminated with Solanaceae seeds and coca leaf. Food Chem. 287:265–272. doi: 10.1016/j.foodchem.2019.02.091.
- Max Rubner-Institut (MRI). 2008. Nationale Verzehrsstudie II. Ergebnisbericht, Teil 2: Die bundesweite Befragung zur Ernährung von Jugendlichen und Erwachsenen. MRI.
- Mulder PPJ, Beumer B, Oosterink E, de Jong J. 2009. Dutch survey pyrrolizidine alkaloids in animal forage. Wageningen: Institute of Food Safety.
- Mulder PPJ, De Nijs M, Castellari M, Hortos M, MacDonalds S, Crews C, Hajslova J, Stranska M. 2016. Occurrence of tropane alkaloids in food. EFSA supporting publication. EN-1140
- Mulder PPJ, Klijnstra MD, Goselink RMA, van Vuuren AM, Cone JW, Stoopen G, Hoogenboom RLAP. 2020. Transfer of pyrrolizidine alkaloids from ragwort, common groundsel and viper’s bugloss to milk from dairy cows. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 37(11):1906–1921. doi: 10.1080/19440049.2020.1798028.
- Mulder PPJ, López Sánchez P, These A, Preiss-Weigert A, Castellari M. 2015. Occurrence of pyrrolizidine alkaloids in food. EFSA supporting publication. EN-859.
- Mulder PPJ, López P, Castellari M, Bodi D, Ronczka S, Preiss-Weigert A, These A. 2018. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: results of a survey across Europe. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 35(1):118–133. doi: 10.1080/19440049.2017.1382726.
- Neumann H, Huckauf A. 2016. Tansy ragwort (Senecio jacobaea): a source of pyrrolizidine alkaloids in summer honey? J Verbr Lebensm. 11(2):105–115. doi: 10.1007/s00003-015-0986-0.
- National Toxicology Program (NTP). 2003. Toxicology and carcinogenesis studies of riddelliine in F344/N Rats and B6C3F1 mice (Gavage Studies). National Toxicology Program Technical Report. p. 508.
- Perharič L. 2005. Mass tropane alkaloid poisoning due to buckwheat flour contamination. Clin Toxicol. 43:413.
- Perharič L, Juvan KA, Stanovnik L. 2013. Acute effects of a low-dose atropine/scopolamine mixture as a food contaminant in human volunteers. J Appl Toxicol. 33(9):980–990. doi: 10.1002/jat.2797.
- Perharič L, Koželj G, Družina B, Stanovnik L. 2013. Risk assessment of buckwheat flour contaminated by thorn-apple (Datura stramonium L.) alkaloids: a case study from Slovenia. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 30(2):321–330. doi: 10.1080/19440049.2012.743189.
- Ruan J, Liao C, Ye Y, Lin G. 2014. Lack of metabolic activation and predominant formation of an excreted metabolite of nontoxic platynecine-type pyrrolizidine alkaloids. Chem Res Toxicol. 27(1):7–16. doi: 10.1021/tx4004159.
- Schenk A, Siewert B, Toff S, Drewe J. 2015. UPLC TOF MS for sensitive quantification of naturally occurring pyrrolizidine alkaloids in Petasites hybridus extract (Ze 339). J Chromatogr B Analyt Technol Biomed Life Sci. 997:23–29. doi: 10.1016/j.jchromb.2015.05.027.
- Smith LW, Culvenor CCJ. 1981. Plant sources of hepatotoxic pyrrolizidine alkaloids. J Nat Prod. 44(2):129–152. doi: 10.1021/np50014a001.
- Suter M, Lüscher A. 2008. Occurrence of Senecio aquaticus in relation to grassland management. Appl Veg Sci. 11(3):317–324. doi: 10.1111/j.1654-109X.2008.tb00448.x.
- Suttner G, Weisser WW, Kollmann J. 2016. Have the distribution and abundance of poisonous herb Senecio aquaticus increased in Bavarian grassland? Evaluation of extensive biotope mapping in the periods 1984–1995 and 1999–2013. Nat Landschaft. 91(12):544–552.
- Taenzer J, Gehling M, Klevenhusen F, Saltzmann J, Dänicke S, These A. 2022. Rumen metabolism of Senecio pyrrolizidine alkaloids may explain why cattle tolerate higher doses than monogastric species. J Agric Food Chem. 70(33):10111–10120. doi: 10.1021/acs.jafc.2c01332.
- Teuscher E, Lindequist U. 2010. Biogene Gifte – Biologie-Chemie-Pharmakologie-Toxikologie. 3rd ed. Stuttgart: Wissenschaftliche Verlagsgesellschaft.
- Vera-Baquero FL, Morante-Zarcero S, Sierra I. 2022. Evaluation of thermal degradation of tropane and opium alkaloids in gluten-free corn breadsticks samples contaminated with Stramonium seeds and baked with poppy seeds under different conditions. Foods. 11(15):2196. doi: 10.3390/foods11152196.
- Wiggering H, Diekötter T, Donath TW. 2022. Regulation of Jacobaea vulgaris by varied cutting and restoration measures. PLoS One. 17(10):e0248094. doi: 10.1371/journal.pone.0248094.
- Zheng W, Yoo K-H, Choi J-M, Park D-H, Kim S-K, Kang Y-S, Abd El-Aty AM, Hacımüftüoğlu A, Jeong JH, Bekhit AE-D, et al. 2019. A modified QuEChERS method coupled with liquid chromatography–tandem mass spectrometry for the simultaneous detection and quantification of scopolamine, L-hyoscyamine, and sparteine residues in animal-derived food products. J Adv Res. 15:95–102. doi: 10.1016/j.jare.2018.09.004.
