Abstract
The total phenolic content, flavonoid content and antioxidant activity of methanol, ethanol, water and ethyl acetate extracts of the leaves and berries of myrtle (Myrtus communis) were measured. Total phenolic content was assessed by the Folin-Ciocalteau assay, total flavonoid content was assessed by a colorimetric method using rutin as standard flavonoid, whereas antioxidant activity was assessed by measuring the ability of the extracts to scavenge the 2,2′-azinobis (3-ethylbenzothiazoline-6- sulphonic acid) diammonium salt (ABTS+) radical. The results pointed to the significant antioxidant activities of the methanol and water extracts, the overall strength being in the order of methanol > water > ethanol > ethyl acetate in both, leaf and berry extracts. In all cases, the extracts obtained from leaves showed higher antioxidant activity and higher total phenolic and flavonoid contents than the corresponding extracts obtained from berries. The phenolic content exhibited a strong association (r 2 = 0.9452) with antioxidant activity.
Se determinó el contenido de compuestos fenólicos totales y de flavonoides, así como la actividad antioxidante de diferentes extractos (metanol, etanol, acetil éter y agua) de hojas y frutos de myrtus (Myrtus communis). El contenido de compuestos fenólicos totales se determinó mediante el método de Folin-Ciocalteau, el contenido de flavonoides mediante un método colorimétrico usando como flavonoide estándar la rutina, mientras que la actividad antioxidante se determinó midiendo la capacidad de los extractos para captar el radical ABTS+. Los resultados mostraron una significante actividad antioxidante de los extractos metanólicos y acuosos, siendo la intensidad de dicha actividad antioxidante en el siguiente orden: metanol > agua > etanol > acetil éter, tanto en el caso de los extractos procedentes de hojas como de frutos. Para todos los extractos, los procedentes de las hojas de myrtus presentaron mayor actividad antioxidante y mayor contenido en polifenoles totales y en flavonoides que los correspondientes extractos de frutos. El contenido total de compuestos fenólicos totales presentó una alta correlación (r 2 = 0,9452) con la actividad antioxidante.
Palabras clave:
Introduction
Myrtus communis L. (Myrtaceae), myrtle, is an evergreen shrub widespread in Mediterranean woodlands, maquis, and garrigues. In Morocco, it is a popular ornamental plant and has traditionally been consumed for medicinal purposes to adjust metabolic disturbances, although there is no scientific evidence to support such medicinal properties. The plant is traditionally used in the treatment of urinary infections, digestive problems, vaginal discharge, bronchial congestion, sinusitis, and dry coughs (Bown, Citation1995; Genders, Citation1994). The leaves are aromatic, balsamic, hemostatic and tonic (Chiej, Citation1984) and are used in cooked savory dishes as flavoring (Bown, Citation1995). The fruit is carminative and is used in the treatment of dysentery, diarrhea, hemorrhoids, internal ulceration, and rheumatism (Chopra, Nayar, & Chopra, Citation1986), and also to flavor sauces, syrups, etc. (Facciola, Citation1990). Carlo, Andrea, Alberto, Erika, & Filippo, (Citation2005) studied the chemical composition of the essential oils from leaves and berries of myrtle and found the major compounds to be: α-pinene (30.0 and 28.5%), 1,8-cineole (28.8 and 15.3%), and limonene (17.5 and 24.1%). However, considerable variability in the composition of oils from different locations has been reported (Olga, Stavros, & Ioanna, Citation2007). Although many plants from the Myrtaceae family are reported to have antibacterial or antifungal activities (Mansouri, Foroumadi, Ghaneie, & Gholamhosseinian, Citation2001; Shahidi, Citation2004), very little has been reported on the antioxidant activity of this plant (Hayder et al., Citation2004; Romani et al., Citation2004).
It is well known that many medicinal plants are also excellent sources of phytochemicals such as phenolic and polyphenolic compounds (e.g. phenolic acids, tannins, flavonoids, etc.), many of which have potent antioxidant activities which are often exploited in food products and in various medicinal treatments (Li, Hao, Wang, Huang, & Li, Citation2009; Liu, Qiu, Ding, & Yao, Citation2008; Tawaha, Alali, Gharaibeh, Mohammad, & El-Elimat, Citation2007). The antioxidant activity of phenolic compounds is mainly because of their redox properties, which allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers. In addition, they show metal-chelating potential (Rice-Evans, Miller J, Bolwell, & Bramley, 1995; Tung, Wu, & Chang, Citation2007). Moreover, phenolic compounds have different biological activities as antibacterial, anticarcinogenic, anti-inflammatory, anti-viral, anti-allergic, estrogenic, and immune-stimulating agents (Larson, Citation1988; Viuda-Martos, Ruiz-Navajas, Fernández-López, & Pérez-Álvarez, Citation2007, Citation2008).
Phenolic compounds may play an important role in protecting body cells from injury by hydrogen peroxide and from the damage caused by unsaturated fatty acids and lipid peroxides, absorbing and neutralizing free radicals (Sroka & Cisowski, Citation2003).
Lipid peroxidation is one of the major causes of deterioration in foods because it results in the formation of potentially toxic compounds (Karpinska, Borowski, & Danowska-Oziewiez, Citation2001). Synthetic antioxidants, such as tertiary butyl hydroquinone, butylated hydroxyanisole, and butylated hydroxytoluene, are the food additives widely used to protect against deterioration, although their use is increasingly restricted because of their toxicity and potential risk to health (Gordon, Citation1996). Moreover, there is a growing awareness among consumers regarding food additive safety (Moure et al., Citation2001).
There has been an upsurge in interest in phytochemicals as potential sources of natural antioxidants, the goal being to use them in foods and pharmaceutical preparations to replace synthetic antioxidants.
The main objective of this study was to evaluate the antioxidant activity of different extracts (methanolic, ethanolic, water, and acetate ethyl) from leaves and berries of myrtle (Myrtus communis) as potential sources of natural antioxidants. The relationship between phenolic content and antioxidant activity was also statistically investigated.
Materials and methods
Chemicals
2,2′-azinobis (3-ethylbenzothiazoline-6- sulphonic acid) diammonium salt (ABTS), 2,4,6-tripyridyl- S-triazine (TPTZ), potassium persulphate, Folin-Ciocalteu reagent, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), gallic acid, and rutin were purchased from Sigma-Aldrich. All chemicals and reagents used were of analytical grade.
Plant materials
Myrtus communis L. (Myrtaceae) leaves and berries were collected from the Chefchaouen region (NW Morocco) during vegetation period. The taxonomic identification of plant material was confirmed by A. Ennabili (from The National Institute of Medicinal and Aromatic Plants, Sidi Mohamed Ben Abdellah University). Herbarium specimens were deposited in the National Institute of Medicinal and Aromatic Plants, Sidi Mohamed Ben Abdellah University (eccicata no. INP67).
The collected plant materials were dried in an oven (Selecta, Barcelona, Spain) at 35 °C for 3 days for leaves and 5 days for berries and then the leaves and berries of plants were separated and ground in a grinder (Moulinex, France).
Preparation of extracts
Dried powders of leaves and berries from M. communis were extracted with different solvents (methanol, ethanol, ethyl acetate, and water). For methanolic, ethanolic and ethyl acetic extraction, a 25 g aliquot of each dried sample was extracted using 100 ml of methanol, ethanol or ethyl acetate, respectively, at room temperature for 7 days. For aqueous extraction, a 25 g aliquot of each dried sample was extracted using 100 ml of boiling water for 15 min using a water bath. Two extraction replicates of each solvent were prepared for each plant sample. The extracts were filtered through a filter paper Watman No. 1 and the solvents were eliminated using a rotary evaporator (Buchi Heating Bath B-490, Buchi Rotvapor R-200) to obtain a dry extract. The extracts were stored at −20 °C until use.
Determination of total phenolic content
The concentration of total phenols in extracts was measured by UV spectrophotometry (UV Spectrometer UNICAM, Cambridge, UK), based on a colorimetric oxidation/reduction reaction. The oxidizing agent used was Folin-Ciocalteu reagent (AOCS, Citation1990). To 0.5 ml of diluted extract (100 μg dry extract/ ml solvent), 2.5 ml of Folin-Ciocalteu reagent (diluted 10 times with water) was added and, after that (within a time interval from 0.5 to 8 min), 2 ml of Na2CO3 (75 g/l) were added. The sample was incubated for 5 min at 50 °C and then cooled. For a control sample, 0.5 ml of distilled water was used. The absorbance of the resulting blue-colored solutions was measured at 760 nm. Quantitative measurements were performed, based on a standard calibration curve of gallic acid in methanol. The mean (±SD) results of triplicate analyses were expressed as gallic acid equivalents (GAE) in milligrams per gram of dry-material.
Determination of total flavonoid content
Total flavonoid content was determined using a method described by Sakanaka, Tachibana, and Okada (Citation2005). Briefly, 0.25 ml of the extracts (0.625–5 mg/ml) or rutin standard solution (15–250 mg/ml) was mixed with 1.25 ml of distilled water in a test tube, followed by addition of 75 μl of a 5% (w/v) sodium nitrite solution. After 6 min, 150 μl of a 10% (w/v) aluminium chloride solution was added and the mixture was allowed to stand for a further 5 min before 0.5 ml of 1M NaOH was added. The mixture was made up to 2.5 ml with distilled water and mixed well. The absorbance was measured immediately at 510 nm. The mean (± SD) results of triplicate analyses were expressed as rutin equivalents (RE) in milligrams per gram of dry-material.
ABTS radical cation decolorization assay
The antioxidant capacity assay was carried out using the improved ABTS radical cation decolorization assay as described by Re et al. (Citation1999). ABTS+ radical cation was generated by oxidation of ABTS with potassium persulfate. ABTS was dissolved in deionized water to 7 mM concentration, and mixed with 2.45 mM potassium persulfate. The reaction mixture was left to stand at room temperature in the dark for 12–16 h before use. The ABTS+ solution was diluted with ethanol to an absorbance of 0.700±0.020 at 734 nm at 30 °C. Then, 1 ml of diluted ABTS solution was mixed with 10 μl aliquots of plant extracts, and the absorbance at 734 nm was measured at 30 °C exactly 6 min after mixing. Trolox standard solutions (concentrations from 0 to 2.5 mM) in 80% ethanol were prepared and assayed using the same conditions. Appropriate solvent blanks were run in each assay. The absorbance inhibition percentage at 734 nm was calculated and plotted as a function of trolox concentration. The absorbance of the resulting oxidized solution was compared to that of the calibrated trolox standard. Results were expressed in terms of trolox equivalent antioxidant capacity (TEAC, mM trolox equivalents per milligram dry extract). All determinations were carried out in triplicate.
Statistical analysis
The statistical analyses were performed by a multifactorial ANOVA and the Tukey's test using the general linear model of SPSS 14.0 for Windows (SPSS, Chicago, IL) software package. The results were expressed as means ± SD to show variations in the various experimental data. Differences are considered significant when P < 0.05. Relationships between ABTS and phenolic or flavonoid content were analyzed using the correlation and regression programmer in the EXCEL program (Microsoft Office 2007).
Results and discussions
Total phenolic content
As one of the most important antioxidant plant components, phenolic compounds have been widely investigated in many medicinal plants, fruits, and vegetables (Djeridane et al., Citation2006). This antioxidant activity is believed to be mainly because of their redox properties (Zheng & Wang, Citation2001), which play an important role in adsorbing and neutralizing free radicals (Laranjinha, Vieira, Madeira, & Almeida, Citation1995), quenching singlet and triplet oxygen (Hatano, Shoyama, & Nishioka, Citation1989) or decomposing peroxides (Das and Pereira, Citation1990; Rice-Evans et al., Citation1995).
The total phenolic content was estimated by the Folin-Ciocalteu colorimetric method, using gallic acid as a standard phenolic compound. The total phenolic content in methanol, ethanol, ethyl acetate, and water extracts of leaves and berries of Myrtus communis is shown in . The concentration of phenolics in the extracts was dependent on both the solvent and the part of the plant used in the extraction. Among the extracts investigated, total phenolics ranged from 7.81 to 35.56 mgGAE/g of extract. The extracts from leaves showed a higher (P <0.05) phenolic content than berry extracts, with all four solvents assayed. The amounts of phenolic compounds in the water extracts were highest, and lowest in the ethyl acetate extracts (in both leaves and berries) (P <0.05). The extraction yields of phenolic compounds increased with increasing solvent polarity as reported by other authors (Chua, Tung, & Chang, Citation2008; Goli, Barzegar, & Sahara, Citation2005).
Table 1. Total phenolic content (as mg gallic acid equivalents (GAE)/g of extract) in different extracts of M. communis.
Tabla 1. Contenido fenólico total (como mg equivalentes de ácido gálico (GAE)/g de extracto) en diferentes extractos de M. communis.
Tawaha et al. (Citation2007) estimated that a total phenolic content higher than 20 mgGAE/g dry weight could be considered as very high. On the basis of this, the methanol, ethanol, ethyl acetate, and water extracts of Myrtle leaves must be considered as good sources of phenolic compounds.
Total flavonoids
The total flavonoid content was estimated by a colorimetric method, using rutin as standard flavonoid. The total flavonoid content of methanol, ethanol, ethyl acetate, and water extracts of leaves and berries of Myrtus communis is shown in . As in the case of total phenolics, the concentration of flavonoids in the extracts was dependent on both the solvent and the part of the plant used in the extraction, the total flavonoid content ranging from 21.37 to 129.96 mgRE/g of extract. In this case too, the extracts from leaves showed a higher (P < 0.05) flavonoid content than the berry extracts except the ethyl acetate extracts, which showed similar levels (P > 0.05) in both parts of the plant. For both leaves and berries, the methanolic extracts showed the highest (P < 0.05) flavonoid content, followed by the aqueous extracts. As in the case of total phenolics, the ethyl acetate extracts (for both berries and leaves) yielded the lowest (P <0.05) flavonoid content. According to Liu et al. (Citation2008), all the extracts (including the ethyl acetate extracts) from leaves and berries of Myrtle could be considered as a plant material with a high total flavonoid content (>20 mgRE/g). The same authors reported a great variation (from 0.0 to 157.67 mg RE/g) in the total flavonoid content of the different Chinese herbals. Higher total flavonoid content (70.65–396.05 mgRE/g) has been reported by Li et al. (Citation2009) in different extracts from Lysimachia foenum-graecum Hance (Chinese herbal species).
Table 2. Total flavonoids (as mg rutin equivalents (RE)/g of extract) in different extracts of M. communis.
Tabla 2. Contenido total de flavonoides (como mg equivalentes de rutina (RE)/g de extracto) en diferentes extractos de M. communis.
Antioxidant activity
The improved ABTS method was used to determine the antioxidant capacity for the extracts examined in this work. ABTS radical cation decolorization assay measures the relative antioxidant ability to scavenge the radical ABTS·+ compared with trolox, and is considered an excellent tool for determining the antioxidant capacity of hydrogen donating antioxidants. ABTS, a protonated radical, has a characteristic absorbance maximum at 734 nm, which decreases with the scavenging of the proton radicals (Mathew & Abraham, Citation2006). The method is widely used to evaluate antioxidant activity in foods and biological systems (Meyer, Frankel, & Lester, Citation2001).
To express the antioxidant capacity of Myrtus communis extracts by the ABTS·+ scavenging assay, the TEAC values after 6 min of reaction time were calculated and the results are shown in . Although only TEAC values after 6 min are shown, it was noted that the reaction time with ABTS was fast and in almost all cases was completed within 1 min. High TEAC values demonstrate high antioxidant activity. The results showed that ABTS+ radical scavenging activity depends on both the solvent and the part of the plant used for the extraction process. The water and methanol extracts (in both leaves and berries) exhibited the highest (P <0.05) radical scavenging activities when reacted with the ABTS+ radicals. In contrast, the ethyl acetate extracts only showed low activity, approximately 4–6 times lower in berry extracts and 7–8 times lower in leaf extracts.
Table 3. Antioxidant activity (as mM trolox equivalent antioxidant capacity (TEAC)/g extract) of different extracts of M. communis.
Tabla 3. Actividad antioxidante (capacidad antioxidante de mM de equivalentes del Trolox/g de extracto) de diferentes extractos de M. communis.
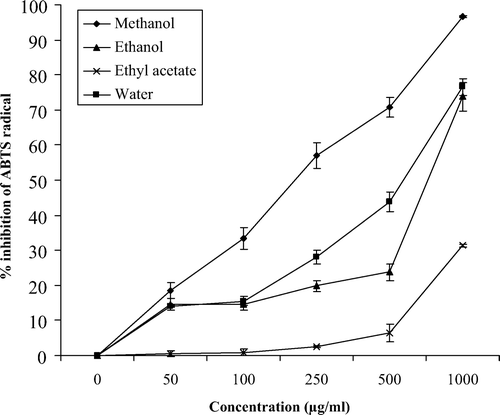
and showed the absorbance inhibition percentage of the ABTS+ radical in water, methanol, ethanol, and ethyl acetate extracts of M. communis at varying concentrations (0–1000 μg/ml) in leaves and berries, respectively. In general, the extracts demonstrated a dose-dependent inhibition of the ABTS+ radical, and all extracts obtained from M. communis leaves were more active than their counterparts from berries at the same concentrations. All the extracts obtained from M. communis leaves exhibited the ABTS+ radical scavenging capacity at all the concentrations assayed (). The same figure demonstrates the steady increase in the inhibition percentage of the ABTS+ radicals by all extracts. The methanol extracts of leaves of M. communis achieved maximum inhibition (100%) at 500 μg/ml and above of the dry extract. Water and ethanol extracts also reached this maximum inhibition but a higher concentrations (1000 μg/ml). In contrast, ethyl acetate extracts did not reach this maximum of inhibition at any the assayed concentrations, and only reached 80% inhibition at the highest concentration (1000 μg/ml). The water, methanol, and ethanol extracts obtained from M. communis berries exhibited ABTS+ radical scavenging capacity at all the concentrations assayed (). The methanol extracts had the highest (P <0.05) ABTS+ radical scavenging capacity at all concentrations assayed, showing a percentage inhibition near to 100% at the highest concentration. In contrast, ethyl acetate extracts needed concentrations higher than 100 μg/ml before such a capacity even started to slow, and it was necessary to use the highest concentration (1000 μg/ml) to reach a percentage of inhibition that exceeded 10%. When the ABTS+ radical scavenging capacity was compared with trolox (), similar results were obtained, suggesting that methanol and water extracts from leaves of M. communis exert their radical scavenging effects at much lower concentrations than the rest of the extracts. In addition, these results suggest that the antioxidants in the ethyl acetate extract are weak ABTS radical-scavengers and require extremely high concentration to have a significant effect.
Figure 1. ABTS+ radical scavenging capacity of leaf extracts of M. communis. The analyses were performed in triplicate and the results are expressed as % inhibition of the absorbance of ABTS radicals ± SD.
Figura 1. Capacidad de captación de radicales ABTS de extractos de hojas de M. communis. Los análisis se llevaron a cabo por triplicado y los resultados se expresan como % de inhibición de la absorbancia del radical ABTS ± SD.
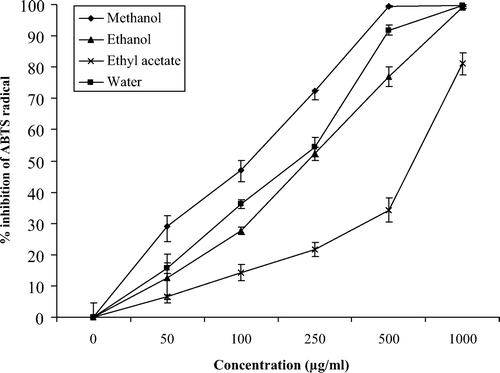
Figure 2. ABTS+ radical scavenging capacity of berry extracts of M. communis. The analyses were performed in triplicate and the results are expressed as % inhibition of the absorbance of ABTS radical ± SD.
Figura 2. Capacidad de captación de radicales ABTS de extractos de frutos de M. communis. Los análisis se llevaron a cabo por triplicado y los resultados se expresan como % de inhibición de la absorbancia del radical ABTS ± SD.
Relationship between antioxidant activity and total phenolic or total flavonoid content
The antioxidant activity, evaluated by the ABTS assay, showed a significant linear correlation (r 2 = 0.9452) with total phenolic contents of the M. communis extracts which suggests that the phenolic compounds of these extracts provide substantial antioxidant activity. These data are in accordance with previous research (Ao, Li, Elzaawely, Xuan, & Tawata, Citation2008; Liu et al. Citation2008; Zainol, Abd-Hamid, Yusof, & Muse, Citation2003), which has shown that a high total polyphenol content increases antioxidant activity and that a linear correlation exists between the phenolic content and antioxidant activity. Ao et al., (Citation2008) also reported that the correlation coefficient between antioxidant activity and polyphenols was higher when the method used for determining this antioxidant activity was the ABTS assay rather than other methods (1,1–diphenyl-2-picrylhydrazyil radical scavenging assay, phenazine methosulfate-nicotinamide adenine dinucleotide-reduced system superoxide-radical scavenging assay, or β-carotene/linoleic acid system antioxidant assay). The correlation coefficient between the total flavonoids and ABTS values was determined to be 0.5978, which was much smaller than that determined between total phenolics and ABTS values. These results indicate that, apart from flavonoids, there might be other phenolic compounds (phenolic acids, tannic acid, and others) contributing to the antioxidant activity of these extracts. These results also suggest that phenolic compounds in these extracts provide substantial antioxidant activity and so could be used as an important indicator of their antioxidant capacity, acting as a preliminary screen to choose extracts for use as natural sources of antioxidant, functional ingredients.
Conclusions
This study shows that of the four extracts (water, methanol, ethanol, and ethyl acetate) of Moroccan Myrtus communis, the first two possess significant antioxidant activities, their overall strength being in the order of methanol > water > ethanol > ethyl acetate. This order is observed in both leaf and berry extracts, and, in all cases, the extracts obtained from leaves show higher antioxidant activity, and total phenolic and flavonoid contents than their corresponding counterparts obtained from berries. Therefore, the best method for extracting phenolic compounds from Myrtus communis is to use the leaves and extraction with methanol or water. All the extracts obtained from leaves are high in phenolics (>20 mgGAE/g of extract) and their content exhibited strong association (r 2 = 0.9452) with antioxidant activity. The results suggest that phenolic compounds are the major contributors to the antioxidant activities of Myrtus communis, which could be used as an easily accessible source of natural antioxidants and as a possible food supplement or in the pharmaceutical industry. This study provides a basis for future studies in this area.
Acknowledgements
The authors thank the Spanish Agency for International Cooperation (AECI) for financing these research projects (A/9934/07 and A/16296/08) and the Centre Nacional pour la Recherche Scientifique et Technique (CNRST) from Morocco for support the doctoral grant of one of the authors.
References
- Ao , C. , Li , A. , Elzaawely , A. A. , Xuan , T. D. and Tawata , S. 2008 . Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. extracts . Food Control , 19 : 940 – 948 .
- AOCS . 1990 . Official methods and recommended practices of the American oil chemists' society , 4th ed. , Urbana, IL : American Oil Chemists' Society .
- Bown , D. 1995 . Encyclopaedia of herbs and their uses , London : Dorling Kindersley .
- Carlo , I. G.T. , Andrea , B. , Alberto , A. , Erika , S. and Filippo , M. P. 2005 . Chemical composition of volatiles in Sardinian myrtle (Myrtus communis L.) alcoholic extracts and essential oils . Journal of Agriculture and Food Chemistry , 54 : 1420 – 1426 .
- Chiej , R. 1984 . Encyclopaedia of medicinal plants , London : MacDonald Press .
- Chopra , R. N. , Nayar , S. L. and Chopra , I. C. 1986 . Glossary of Indian medicinal plants , New Delhi : Council of Scientific and Industrial Research .
- Chua , M. T. , Tung , Y. T. and Chang , S. T. 2008 . Antioxidant activities of ethanolic extracts from the twing of Cinnamomun osmophloeum . Bioresource Technology , 99 : 1918 – 1925 .
- Das , N. P. and Pereira , T. A. 1990 . Effects of flavonoids on termal autoxidation of palm oil: Structure–activity relationship . Journal of the American Oil Chemists' Society , 67 : 225 – 258 .
- Djeridane , A. , Yousfi , M. , Nadjemi , B. , Boutassouna , D. , Stocher , P. and Vidal , N. 2006 . Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds . Food Chemistry , 97 : 654 – 660 .
- Facciola , S. 1990 . Cornucopia – A source book of edible plants , California : Kampong Publications .
- Genders , R. 1994 . Scented flora of the world , London : Robert Hale .
- Goli , A. H. , Barzegar , M. and Sahara , M. A. 2005 . Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts . Food Chemistry , 92 : 521 – 525 .
- Gordon , M. H. 1996 . Dietary antioxidants in disease prevention . Natural Product Report , 14 : 265 – 273 .
- Hatano , K. , Shoyama , Y. and Nishioka , I. 1989 . Clonal propagation of Atractylodes japonica and A. ovata by tip tissue culture and the atractylon content of clonally propagated plants . Plant Medicinal , 56 : 131 – 132 .
- Hayder , N. , Abdelwahed , A. , Kilani , S. , Ammar , R. B. , Mahmoud , A. , Ghedira , K. and Chekir-Ghadira , L. 2004 . Anti-genotoxic and free-radical scavenging activities of extracts from (Tunisian) . Myrtus communis. Mutation Research , 564 : 89 – 95 .
- Karpinska , M. , Borowski , J. and Danowska-Oziewiez , M. 2001 . The use of natural antioxidant in ready to serve food . Food Chemistry , 72 : 5 – 9 .
- Laranjinha , J. , Vieira , O. , Madeira , V. and Almeida , L. 1995 . Two related phenolic antioxidants with opposite effects on vitamin E content in low density lipoproteins oxidized by ferrylmyoglobin: Consumption versus regeneration . Archives of Biochemistry and Biophysics , 323 : 373 – 381 .
- Larson , R. A. 1988 . The antioxidants of higher plants . Phytochemistry , 27 : 969 – 978 .
- Li , H. , Hao , Z. , Wang , X. , Huang , L. and Li , J. 2009 . Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance . Bioresource Technology , 100 : 970 – 974 .
- Liu , H. , Qiu , N. , Ding , H. and Yao , R. 2008 . Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses . Food Research International , 41 : 363 – 370 .
- Mansouri , S. , Foroumadi , A. , Ghaneie , T. and Gholamhosseinian , A. N. 2001 . Antibacterial activity of the crude extracts and fractionated constituents of Myrtus communis . Pharmaceutical Biology , 395 : 399 – 401 .
- Mathew , S. and Abraham , T. E. 2006 . Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models . Food Chemistry , 94 : 520 – 528 .
- Meyer , A. S. , Frankel , E. N. and Lester , P. 2001 . Antioxidant activity of hydroxycinnamic acids on human low-density lipoprotein oxidation . Methods Enzymo , 335 : 256 – 265 .
- Moure , A. , Cruz , J. M. , Franco , D. , Domínguez , J. M. , Sineiro , J. Dominguez , H. 2001 . Natural antioxidants from residual sources . Food Chemistry , 72 : 145 – 171 .
- Olga , G. , Stavros , L. and Ioanna , C. 2007 . Re-evaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes . European Food Research and Technology , 226 : 583 – 590 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Rice-Evans , C. A. , Miller , N. J. , Bolwell , P. G. and Bramley , P. M. 1995 . The relative antioxidant activities of plant-derived polyphenolic flavonoids . Free Radical Research , 22 : 375 – 383 .
- Romani , A. , Coinu , R. , Carta , S. , Pinelli , P. , Galardi , C. , Vincieri , F. F. and Franconi , F. 2004 . Evaluation of antioxidant effect of different extracts of Myrtus communis L . Free Radical Research , 38 : 97 – 103 .
- Shahidi , B. G.H. 2004 . Antibacterial screening of plants used in Iranian folkloric medicine . Fitoterapia , 75 : 231 – 235 .
- Sakanaka , S. , Tachibana , Y. and Okada , Y. 2005 . Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) . Food Chemistry , 89 : 569 – 575 .
- Sroka , Z. and Cisowski , W. 2003 . Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids . Food and Chemical Toxicology , 41 : 753 – 758 .
- Tawaha , K. , Alali , F. Q. , Gharaibeh , M. , Mohammad , M. and El-Elimat , T. 2007 . Antioxidant activity and total phenolic content of selected Jordanian plant species . Food Chemistry , 104 : 1372 – 1378 .
- Tung , Y. S. , Wu , J. H. and Chang , S. T. 2007 . Antioxidant activities of natural phenolic compounds from Acacia confuse bark . Bioresource Technology , 98 : 1120 – 1123 .
- Viuda-Martos , M. , Ruiz-Navajas , Y. , Fernández-López , J. and Pérez-Álvarez , J. A. 2007 . Antifungal activities of thyme, clove and oregano essential oils . Journal of Food Safety , 27 : 91 – 101 .
- Viuda-Martos , M. , Ruiz-Navajas , Y. , Fernández-López , J. and Pérez-Álvarez , J. A. 2008 . Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils . Food Control , 19 : 1130 – 1138 .
- Zainol , M. K. , Abd-Hamid , A. , Yusof , S. and Muse , R. 2003 . Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban . Food Chemistry , 81 : 575 – 581 .
- Zheng , W. and Wang , S. Y. 2001 . Antioxidant activity and phenolic compounds in selected herbs . Journal of Agricultural and Food Chemistry , 49 : 5165 – 5170 .