Abstract
Rabbit hind legs were packaged under different modified atmospheres (Vacuum packaging (VP), 100% CO2 and a commercial gas blend) to determine an applicable packaging method for improving quality and durability of rabbit meat. The best atmosphere for preserving microbiological quality and extending shelf-life was 100% CO2 (5 weeks) followed by VP (between 3 and 4 weeks) and a commercial gas blend consisting of 35% CO2/35% O2/30% N2 (between 18 and 21 days). Reduction in acceptability was associated to exudate losses (100% CO2), “unspecific bad odours” (VP) and rancid off-odours (gas blend). The spoilage microflora in 100% CO2 and VP samples was dominated (up to 54%) by lactic acid bacteria (LAB). Under the gas blend atmosphere, Pseudomonas, LAB and Brochothrix thermosphacta accounted for 8.7%, 4.0%, and 2.4%, respectively. Among spoilage indicators, only extract-release volume significantly correlated (p < 0.05) with microbial changes. The 100% CO2 atmosphere was the best to increase the shelf-life and maintain the microbiological quality, with no significant changes in sensory properties.
Muslos traseros de conejo se envasaron en diferentes atmósferas modificadas (vacío (VP), 100% CO2 y una mezcla gaseosa comercial) con el objetivo de establecer un método aplicable de envasado que mejore la calidad y durabilidad de la carne de conejo. La mejor atmósfera para conservar la calidad microbiológica y aumentar la vida útil fue la consistente en 100% CO2 (cinco semanas), seguida por la de vacío (de tres a cuatro semanas) y por una mezcla comercial de gases constituida por 35% CO2/35% O2/30% N2 (de 18 a 21 días). La reducción en la aceptabilidad se asoció a pérdidas de exudado (100% CO2), “malos olores no específicos” (VP) y olores anormales a rancio (mezcla gaseosa). La microbiota alterante en las muestras bajo 100% CO2 y VP estuvo representada (hasta el 54%) por bacterias ácido lácticas (LAB). En la mezcla gaseosa, Pseudomonas, LAB y Brochothrix thermosphacta supusieron el 8,7%, 4,0% y 2,4%, respectivamente. Entre los indicadores de alteración, solamente el volumen de extracto liberado correlacionó significativamente (p < 0,05) con los cambios microbianos. La atmósfera que contenía solo CO2 fue la mejor para incrementar la vida útil y mantener la calidad microbiológica, sin modificar significativamente las propiedades sensoriales.
Introduction
The domestic rabbit (Oryctolagus cuniculus) is a significant provider of meat in some parts of the world. Global rabbit meat production in 2006 was estimated to be more than 1500,000 tons. Of these, 500,000 were produced in the European Union (EU) and this production was concentrated in three Mediterranean countries (Italy, Spain, and France), which together account for 77.3% of the total EU production (FAOSTAT 2008, http://apps.fao.org).
Major changes have occurred in the way fresh rabbit meat is marketed. In Spain, most rabbit meat is still sold as whole carcasses although in recent years the proportion of jointed and further processed products has increased as previously observed in the poultry sector. Early studies have shown that chilled rabbit meat in air is a highly perishable product for which storage life extension is economically desirable (Rodríguez-Calleja, García-López, Santos, & Otero, Citation2005b; Rodríguez-Calleja, Santos, Otero, & García-López, Citation2004). Vacuum packaging (VP) and modified atmosphere packaging (MAP) are effective means of prolonging the storage life of perishable foods. Many studies have provided detailed insight into the microbiology and spoilage of red meat and poultry during storage under VP and MAP (McMillin, Citation2008), but similar information on rabbit meat is very scarce (Berruga, Vergara, & Linares, Citation2005; Gariepy et al., Citation1986; Vergara, Berruga, & Linares, Citation2005).
The objective of this study was to examine the effects of VP, 100% CO2, and a commercial gas blend on microbiological, physicochemical and sensory properties of rabbit meat to determine the most convenient packaging procedure for extending its shelf life and keeping its quality.
Materials and methods
Sample collection
Twelve lots of rabbit carcasses were studied. Each lot consisted of 10–14 chilled (0 °C) 24-h post-mortem carcasses from a single consignment of New Zealand white rabbits from the same flock and owner, which were processed at the same time in a medium-size abattoir.
Packaging, storage, and sampling
For VP trials, the two hind legs of each animal (56 carcasses) were placed on Cryovac U-BRT trays wrapped with Cryovac BB4L bags (Cryovac, Barcelona, Spain), which were immediately evacuated and heat sealed using a tabletop Multivac A300 packaging machine (Multivac Verpackungsmaschinen, Wolfertschwenden, Germany). For MAP trials, the air in the bags was replaced by 100% CO2 (56 carcasses) or by a commercial gas blend (40 carcasses) (35% CO2/35% O2/30% N2), with gas:meat volume ratios of about 1.5:1. The BB4L bags had an oxygen transmission rate of 30 cm3 m−2 24 h−1 at 23 °C and 0% relative humidity, and a CO2 transmission rate of 150 cm3 m−2 24 h−1 at 23 °C and 0% relative humidity. All packages were kept at 3 ± 1 °C and the air temperature was monitored using a Testo175-T2 (Instrumentos Testo S.A., Cabrils, Barcelona, Spain) data logger.
Two trays of each lot and atmosphere were examined immediately after packaging and at weekly intervals. Each sampling day, concentrations of CO2 and O2 were determined using an Oxy-Check 8003 gas analyser (Temac Instruments, Copenhagen, Denmark).
Sensory evaluation
Five expert panellists evaluated raw samples 4–5 min after the packages were opened. Sensory analyzed parameters were: (i) odour acceptability using a three-point scale; (ii) off odour if present (rancid, sweet, sour or acid, putrid or sulphide, and “others”); (iii) overall appearance (meat and/or drip color, fat appearance, slime and exudate formation, and flesh texture) using a structured hedonic scale with numerical scores from seven (excellent) to one (extremely undesirable). Texture was evaluated by panellists after performing a slight knife incision on the meat.
Shelf life was arbitrarily defined as the time in days that odour and appearance mean scores declined to 1.5 and/or 3.5, respectively.
Physicochemical analysis
Extract-release volume (ERV) was measured as previously described (Rodríguez-Calleja et al., Citation2004). The pH measurement was conducted in meat homogenates (10 g muscle per 10 ml distilled water) with a micropH 2001 pH meter (Crison).
The configuration and amount of lactic acid in hind legs were determined enzymatically using d-lactate and l-lactate dehydrogenase (R-Biopharm AG, Darmstadt, Germany). Standard solutions of d- and l- lactic acid provided by the manufacturer's kit were used as controls in each round of determinations. The recovery of lactic acid from samples was equal or higher than 99.5%.
Microbiological counts
One hind leg from each tray was placed in sterile Stomacher bags, rinsed with peptone water (1:5 dilution) and the rinse 10-fold diluted. Numbers of mesophilic and psychrotrophic bacteria (aerobic plate counts (APC)), Pseudomonas, lactic acid bacteria (LAB), Brochothrix thermosphacta, Enterobacteriaceae, psychrotrophic Clostridium spp. and fungi were determined and confirmed as described elsewhere (Rodríguez-Calleja et al., Citation2005b; Rodríguez-Calleja et al., Citation2004).
Briefly, mesophilic and psychrotrophic APC were determined by the pour plate technique on Plate Count agar (PCA; Oxoid, Basingstoke, UK) incubated at 30 and 4.5 °C for 2 and 14 d, respectively. Pseudomonas numbers were determined after 2 days incubation at 25 °C on Pseudomonas agar base (Oxoid) to which CFC (cetrimide, fucidin, cephaloridine; Oxoid) supplement was added. The oxidase test (Oxidase Touch sticks, Oxoid) was performed on randomly selected colonies and only oxidase-positive colonies were counted as Pseudomonas. LAB were enumerated on overlaid plates of MRS (de Man, Rogosa and Sharpe; Oxoid) agar following 3 d incubation at 30 °C. B. thermosphacta was enumerated on streptomycin sulphate cicloheximide thallous acetate agar (STAA, Oxoid) after incubation for 2 days at 25 °C. B. thermosphacta suspect colonies were differentiated from Pseudomonas by performing an oxidase test. Overlaid plates of Violet Red Bile Glucose agar (Oxoid) were used for Enterobacteriaceae counts after 24 h incubation at 37 °C. Counts of presumptive psychrotrophic Clostridium spp. were obtained on plates of Shahidi Ferguson Perfringens agar (Oxoid) incubated for 14 days at 20 °C in an anaerobic jar with Anaerogen gas generating sachets (Oxoid). Fungi numbers were determined on Oxytetracicline Glucose Yeast Extract agar (OGYEA, Oxoid) plates incubated at 25 °C for 5 days.
Growth rates of the microbial groups
For each packaging trial, an estimation of the daily growth rate of each microbiological group was calculated from counts on solid media (log CFU g−1) using the following formula: daily growth rate between days a and b = (counts on day b − counts on day a) / number of days between a and b (Rodríguez-Calleja et al., Citation2005a).
Statistical analysis
Microbiological counts were transformed and expressed as log CFU g−1. Basic descriptive statistics of each parameter (mean and standard deviation) were calculated and linear regression analysis was used to determine the relationship between parameters. The potential influence of atmosphere and sampling day on the investigated parameters was analyzed with the factorial analysis of variance (ANOVA). Subsequently, post-hoc pairwise comparisons were performed through the Tukey Honest Significant Difference HSD test. Data analysis was carried out with the Statistica software (StatSoft, Chicago).
Results
Concentrations of CO2 in headspaces during storage under 100% CO2 ranged from 95 to 85% while those of O2 were always lower than 2%. Under the commercial atmosphere, there was a final increase in CO2 to reach 42% and a gradual decrease in O2 to reach ca. 25%.
Mean values for pH, ERV, and l-lactic acid content of rabbit meat during storage under the three different packaging atmospheres are given in . d-lactic acid content was always under the detection limit (0.06 g kg−1). Data in and show the quantitative changes occurring in the microflora of VP and MAP rabbit meat throughout storage. After 21 days, samples packaged under the commercial gas blend were considered spoiled and parameters were not determined. Psychrotrophic clostridia and moulds numbers were always below 1.40 log CFU g−1. The daily growth rates of the microbial associations on rabbit meat under the assayed conditions are presented in . shows the evolution of odour and overall appearance scores throughout chilled storage of VP and MAP rabbit meat.
Figure 1. Odour and overall appearance of vacuum packaged and modified atmosphere packaged rabbit meat during storage at 3 ± 1 °C (mean ± standard error of four lots for each atmosphere): (a) Vacuum packaged rabbit meat; (b) 100% CO2 packaged rabbit meat; (c) Commercial atmosphere (35% CO2/35% O2/30% N2) packaged rabbit meat. ▪ Overall appearance, in a seven-point scale (seven, excellent; one, extremely undesirable). □ Odour, in a three-point scale (three, acceptable; one, off-odour).
Figura 1. Olor y aspecto general de carne de conejo envasada al vacío y en atmósfera modificada durante el almacenamiento a 3 ± 1 °C (media ± desviación estándar de cuatro lotes por cada atmósfera): (a) Carne de conejo envasada al vacío; (b) carne de conejo envasada en 100% CO2; (c) carne de conejo envasada en una atmósfera gaseosa comercial (35% CO2/35% O2/30% N2). ▪ Aspecto general, en una escala de siete puntos (siete, excelente; uno, extremadamente indeseable). □ Olor, en una escala de tres puntos (tres, aceptable; uno, olor anormal).
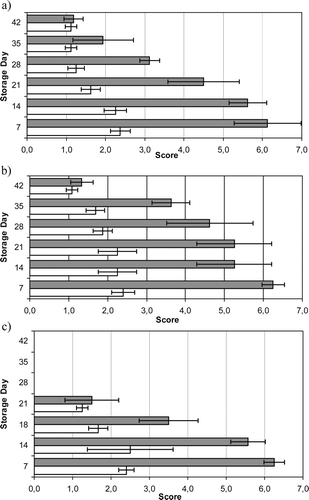
Table 1. Evolution of pH, extract-release volume (ERV), and l-lactic acid content during chilled storage (3 ± 1 °C) of rabbit meat packaged under vacuum, 100% CO2, and a commercial gas blend (35% CO2/35% O2/30% N2).
Tabla 1. Evolución del pH, volumen de extracto liberado (ERV), y contenido en ácido l-láctico durante el almacenamiento refrigerado (3 ± 1 °C) de carne de conejo envasada al vacío, en 100% CO2 y, en una mezcla gaseosa comercial (35% CO2/35% O2/30% N2).
Table 2. Evolution of microbial populations (mesophilic and psychrotrophic organisms and Pseudomonas) during chilled storage (3 ± 1 °C) of rabbit meat under vacuum, 100% CO2 and a commercial gas blend (35% CO2/35% O2/30% N2).
Tabla 2. Evolución de las poblaciones microbianas (microorganismos mesófilos y psicrotrofos, y Pseudomonas) durante el almacenamiento refrigerado (3 ± 1 °C) de carne de conejo envasada al vacío, en 100% CO2 y en una mezcla gaseosa comercial (35% CO2/35% O2/30% N2).
Table 3. Evolution of microbial populations (lactic acid bacteria, B. thermosphacta, Enterobacteriaceae and yeasts) during chilled storage (3 ± 1 °C) of rabbit meat under vacuum, 100% CO2 and a commercial gas blend (35% CO2/35% O2/30% N2).
Tabla 3. Evolución de las poblaciones microbianas (bacterias ácido lácticas, B. thermosphacta, Enterobacteriaceae y levaduras) durante el almacenamiento refrigerado (3 ± 1 °C) de carne de conejo envasada al vacío, en 100% CO2 y en una mezcla gaseosa comercial (35% CO2/35% O2/30% N2).
Table 4. Daily growth rates of several microbial groups throughout three weeks storage at 3 ± 1 °C of rabbit meat packaged under vacuum, 100% CO2 and a commercial gas blend (35% CO2/35% O2/30% N2).
Tabla 4. Tasa de crecimiento diario de varios grupos microbianos durante tres semanas de almacenamiento a 3 ± 1 °C de carne de conejo envasada al vacío, en 100% CO2 y en una mezcla gaseosa comercial (35% CO2/35% O2/30% N2).
Time of storage had a significant effect (p < 0.01) on counts of all microbial groups and ERV, but did not significantly affect (p > 0.05) pH values and l-lactic acid content. Packaging atmosphere significantly affected (p < 0.01) pH values and microflora changes. The combined effect of time and atmosphere did not significantly affect (p > 0.05) physicochemical parameters and numbers of LAB and yeasts, but it had an effect (p < 0.05) in the remaining microbial counts.
Discussion and conclusions
Shelf life extension
In a previous work, the mean storage life of overwrapped rabbit hind legs kept at 3 ± 1 °C was estimated at 6.8 days (Rodríguez-Calleja et al., Citation2005b). As expected, this study demonstrates that VP and MAP significantly extended the durability of rabbit meat. Thus, the storage life was estimated at 5 weeks, between 3 and 4 weeks and between 18 and 21 days when rabbit hind legs were packed under 100% CO2, vacuum and the commercial atmosphere, respectively. Overall, our results are comparable to those obtained by Gariepy et al. (Citation1986) who, at a temperature of 3 °C, also estimated 5 weeks of storage life for whole rabbit carcasses in 100% CO2 and a slightly shorter durability (between 15 and 20 d) for VP carcasses.
In addition to gaseous environment, which influence microorganisms, durability of meat in VP and MAP depends on intrinsic and extrinsic factors such as meat pH, initial microflora load, storage temperature, and packaging operations. Thus, the combined effects of high pH and high bacterial numbers will curtail the shelf life of the meat. Temperature is also a limiting factor and to achieve the best results the meat must be kept close to the freezing point (Gill, Citation1995; Hood & Mead, 1993). In this and other studies (Rodríguez-Calleja et al., Citation2005b; Rodríguez-Calleja et al., Citation2004), mean pH values of 24-h post-mortem rabbit hind legs were quite high (ca. 6.1) compared to pH values in other red meat species (ca. 5.6). The initial APC numbers at 30 °C (between 3.79 and 4.42 log CFU g−1) fall into the acceptable range according to EU microbiological criteria for red meat while Enterobacteriaceae numbers (between 0.85 and 1.14 log CFU g−1) were within the satisfactory range (Anonymous, Citation2005). Although carcass processing was properly controlled, the manner in which hung rabbit carcasses were washed could have contributed to their APC levels because water could spread hair and microorganisms from the hind feet furs that remain on carcasses after skinning. The optimum temperature for storage is considered −1.5 °C, but in current practice packaged rabbit meat portions experiences average temperatures between 2 and 4 °C.
Microflora and major spoilage defects
LAB are the main spoilage organisms associated with chilled VP fresh-meat products. Growth of other organisms, including B. thermosphacta, Enterobacteriaceae, and Pseudomonas may also occur (ICMSF, Citation2005; Nychas, Marshall, & Sofos, 2007). For VP rabbit meat, LAB, B. thermosphacta and Enterobacteriaceae were the fastest growing microorganisms examined with similar daily growth rates (). However, at the time of rejection, LAB accounted for 40–54% of the spoilage microflora while B. thermosphacta and Enterobacteriaceae were less than 3% and 1%, respectively, probably because of their lower initial numbers (). At this time and depending on the lot, Pseudomonas spp. represented between less than 1 and 33% of the psychrotrophic counts. Growth of presumptive Pseudomonas on VP meat have been attributed to residual oxygen, insufficiently impermeable packaging materials or the performance of Pseudomonas CFC-selective medium (Dainty & Mackey, Citation1992; Tryfinopoulou, Drosinos, & Nychas, Citation2001). Although some Pseudomonas strains grow quite well with as little as 200 Pa (0.2%) of oxygen (Hojberg, Schnider, Winteler, Sorensen, & Haas, Citation1999), in this study the overall increase of the aerobic Pseudomonas spp. counts () could be mainly related to localized growth in small pockets of atmosphere remaining in a number of packages in which irregular shaped cuts prevented perfect film adhesion.
For VP samples, off odour, described as “old dishcloth” or “unspecific bad odour” was the first evidence of spoilage when psychrotrophic counts exceeded 7 log CFU g−1 (). In day 28, both odour and appearance of rabbit meat were scored <1.5 and <3.5 points, respectively () and therefore considered as unacceptable. Progressive changes also included texture softening, greening of the drip, and sliminess. Under anaerobic conditions, softening might be related to autolytic activities, which during prolonged chilled storage tenderize the meat, and ultimately degrade the texture of muscle tissue (Jeremiah, Penney, & Gill, Citation1992). Greening of meat exudate stored anaerobically is caused by the formation of sulphmyoglobin by H2S binding with myoglobin and is usually associated with Enterobacteriaceae or LAB growing on high pH meats (Borch, Kant-Muermans, & Blixt, Citation1996). Slime formation may occur during the spoilage of VP meat associated to lactobacilli and other LAB (Holzapfel, Citation1998).
Meat storage in 100% CO2 normally results in significant growth only of LAB and the longest shelf life (ICMSF, Citation2005; Nychas et al., Citation2007). In rabbit meat samples packaged under 100% CO2, LAB accounted for upto 52% of the spoilage flora while B. thermosphacta was less than 1% and Enterobacteriaceae less than 0.1%. For Pseudomonas spp., the bacteriostatic effect of CO2 resulted in the extension of the lag phase and a very low growth although at the spoilage time, numbers had increased by more than one logarithmic cycle. According to Dainty and Mackey (Citation1992), environmental conditions with residual oxygen levels as low as 1% are theoretically sufficient to support growth of these bacteria. More recently it was shown that when oxygen is limited or not available, alternative external electron acceptors can be utilized by several Pseudomonas species and also that the major regulator controlling the physiological switch between aerobic and anaerobic growth conditions in Pseudomonas is very similar to the anaerobic regulator of E. coli, a facultative anaerobe (Boes, Schreiber, Hartig, Jaensch, & Schobert, Citation2006; Filiatrault, Picardo, Ngai, Passador, & Iglewski, Citation2006; Hojberg et al., Citation1999).
Reduction in acceptability was associated to exudate losses and texture deterioration. At spoilage time, a viscous exudate imparted a shiny slime appearance similar to that observed for rabbit carcasses stored in pure CO2 by Gariepy et al. (Citation1986). Rejection was also due to tackiness and an unidentifiable persistent mild odour.
Packaging under the commercial atmosphere was the most effective method to maintain the rabbit meat color that remained comparable to that of fresh meat after 18 days (d) although the storage life was significantly lower than that attained in 100% CO2 or VP. Slime, discoloration, and rancid off odours were detected when psychrotrophic counts were ca. 8 log CFU g−1 (21 d; ). Oxidative rancidity occurs in rabbit carcasses stored in MAP + O2 due to the high proportion of unsaturated fatty acids in rabbit lipids (Berruga et al., Citation2005). Under this atmosphere, the fastest growing microorganisms studied were B. thermosphacta, Pseudomonas, and LAB (), which accounted for 2.4%, 8.7% and 4%, respectively of the total spoilage psychrotrophic microflora.
Spoilage indicators
Physicochemical changes and metabolites resulting from microbial growth have been proposed as meat spoilage indicators (Dainty, Citation1996; Nychas et al., Citation2007). Increases in d- and l-lactic acids associated to microbial growth have been detected during meat storage under anaerobic conditions (Nassos, King, & Stafford, Citation1985; Ordóñez, de Pablo, Pérez de Castro, Asensio, & Sanz, Citation1991) but some studies have reported a decrease in the concentrations of the l-isomer (Blixt & Borch, Citation2002; Kakouri & Nychas, Citation1994; Nychas & Tassou, Citation1997). In this study, d-lactic acid was always below 0.06 g kg−1 and overall but not significant (p > 0.05) decreases in l-lactic acid were observed at the end of storage (). d- lactic acid content can increase during storage due to the metabolic activity of Leuconostoc, Carnobacterium, Weisella, and lactobacilli (Nychas et al., Citation2007; Ordóñez et al., Citation1991). Nowadays, the identity of LAB involved in spoilage of VP/MAP rabbit meat is not known. Many workers have reported a decrease of l-lactate concentrations accompanied by an increase of acetate during anaerobic storage of fresh meat and poultry (Blixt & Borch, Citation2002; Borch & Agerhem, Citation1992; Kakouri & Nychas, Citation1994; Nychas & Tassou, Citation1997). This has been attributed to a shift from homo- to heterofermentative metabolism of LAB influenced by environmental factors such as substrate limitation and also to utilization by Pseudomonas (Nychas & Tassou, Citation1997).
Statistically significant negative (p < 0.05) correlations were found between ERV, which is a measure of the water holding capacity, and changes in microbial numbers, with the highest r values (between −0.53 and −0.78) corresponding to psychrotrophs, LAB, and B. thermosphacta. The major drawback with microbial spoilage indicators is that most of them are dependent on the storage condition, which provides unique environments for the growth of particular groups of bacteria (Tsigarida & Nychas, Citation2001).
In summary, storage under 100% CO2, VP and the commercial atmosphere extended the storage life of 24 h post-mortem rabbit meat between five to three times over that attainable in air. The best atmosphere for shelf life increase and for maintaining microbiological quality was that containing only CO2, but the commercial gas blend was the most effective in maintaining an attractive color. LAB were the major microbial group associated with the spoilage of rabbit meat stored in VP and 100% CO2 whereas a mixed flora of Pseudomonas, LAB and B. thermosphacta were found at the spoilage time under the commercial atmosphere.
Acknowledgements
J.M. Rodríguez-Calleja was supported by a grant from the Spanish MCYT (Project AGL2000-1159). This work was partially funded by project AGL2000-1159.
References
- Anonymous . 2005 . Regulation (EC) N° 2073/2005 of the commission of 15 November 2005 laying down microbiological criteria on food products . Official Journal of the European Union, L , 338 22.12.2005
- Berruga , M. I. , Vergara , H. and Linares , M. B. 2005 . Control of microbial growth and rancidity in rabbit carcasses by modified atmosphere packaging . Journal of the Science of Food and Agriculture , 85 ( 12 ) : 1987 – 1991 .
- Blixt , Y. and Borch , E. 2002 . Comparison of shelf life of vacuum-packed pork and beef . Meat Science , 60 ( 4 ) : 371 – 378 .
- Boes , N. , Schreiber , K. , Hartig , E. , Jaensch , L. and Schobert , M. 2006 . The Pseudomonas aeruginosa universal stress protein PA4352 is essential for surviving anaerobic energy stress . Journal of Bacteriology , 188 ( 18 ) : 6529 – 6538 .
- Borch , E. and Agerhem , H. 1992 . Chemical, microbial and sensory changes during the anaerobic cold storage of beef inoculated with a homofermentative Lactobacillus sp. or a Leuconostocsp . International Journal of Food Microbiology , 15 ( 1–2 ) : 99 – 108 .
- Borch , E. , Kant-Muermans , M. L. and Blixt , Y. 1996 . Bacterial spoilage of meat and cured meat products . International Journal of Food Microbiology , 33 ( 1 ) : 103 – 120 .
- Dainty , R. H. 1996 . Chemical/biochemical detection of spoilage . International Journal of Food Microbiology , 33 ( 1 ) : 19 – 33 .
- Dainty , R. H. and Mackey , B. M. 1992 . The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes . Journal of Applied Bacteriology, Symposium Supplement , 21 : 103S – 114S .
- Filiatrault , M. J. , Picardo , K. F. , Ngai , H. , Passador , L. and Iglewski , B. H. 2006 . Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth . Infection and Immunity , 74 ( 7 ) : 4237 – 4245 .
- Gariepy , C. , Amiot , J. , Simard , R. E. , Boudreau , A. and Raymond , D. P. 1986 . Effect of vacuum-packing and storage in nitrogen and carbon-dioxide atmospheres on the quality of fresh rabbit meat . Journal of Food Quality , 9 ( 5 ) : 289 – 309 .
- Gill , C. O. 1995 . “ MAP and CAP of fresh, red meats, poultry and offals ” . In Principles of modified atmosphere and sous vide product packaging , Edited by: Farber , J. M. and Dodds , K. L.E. 105 – 136 . Basel, , Switzerland : Technomic Publishing AG .
- Hojberg , O. , Schnider , U. , Winteler , H. V. , Sorensen , J. and Haas , D. 1999 . Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil . Applied and Environmental Microbiology , 65 ( 9 ) : 4085 – 4093 .
- Holzapfel , W. H. 1998 . “ The Gram-positive bacteria associated with meat and meat products ” . In The microbiology of meat and poultry , Edited by: Davies , A. and Board , R. 35 – 84 . London : Blackie Academic and Professional .
- Hood , D. E. and Mead , G. C. 1993 . “ Modified atmosphere storage of fresh meat and poultry ” . In Principles and applications of modified atmosphere packaging of foods , Edited by: Parry , R. T. 269 – 298 . London : Blackie Academic and Professional .
- ICMSF. 2005 . International Commission on Microbiological Specifications for Foods. Microorganisms in foods 6: Microbial ecology of food commodities , 2nd ed. , New York : Kluwer Academic and Plenum Publishers .
- Jeremiah , L. E. , Penney , N. and Gill , C. O. 1992 . The effects of prolonged storage under vacuum or CO2 on the flavour and texture profiles of chilled pork . Food Research International , 25 ( 1 ) : 9 – 19 .
- Kakouri , A. and Nychas , G. J.E. 1994 . Storage of poultry meat under modified atmospheres or vacuum packs. Possible role of microbial metabolites as indicator of spoilage . Journal of Applied Bacteriology , 76 ( 2 ) : 163 – 172 .
- McMillin , K. W. 2008 . Where is MAP going? A review and future potential of modified atmosphere packaging for meat . Meat Science , 80 ( 1 ) : 43 – 65 .
- Nassos , P. S. , King , A. D. and Stafford , A. E. 1985 . Lactic acid concentration and microbial spoilage in anaerobically and aerobically stored ground beef . Journal of Food Science , 50 ( 3 ) : 710 – 712 .
- Nychas , G.-J. E. , Marshall , D. L. and Sofos , J. N. 2007 . “ Meat, poultry, and seafood ” . In Food microbiology: Fundamentals and frontiers , Edited by: Doyle , M. P. and Beuchat , L. R. 105 – 140 . Washington, DC : American Society for Microbiology .
- Nychas , G. J.E. and Tassou , C. C. 1997 . Spoilage processes and proteolysis in chicken as detected by HPLC . Journal of the Science of Food and Agriculture , 74 ( 2 ) : 199 – 208 .
- Ordóñez , J. A. , de Pablo , B. , Pérez de Castro , B. , Asensio , M. A. and Sanz , B. 1991 . Selected chemical and microbiological changes in refrigerated pork stored in carbon dioxide and oxygen enriched atmospheres . Journal of Agricultural and Food Chemistry , 39 ( 4 ) : 668 – 672 .
- Rodríguez-Calleja , J. M. , García-López , M. L. , Santos , J. A. and Otero , A. 2005b . Development of the aerobic spoilage flora of chilled rabbit meat . Meat Science , 70 ( 2 ) : 389 – 394 .
- Rodríguez-Calleja , J. M. , Patterson , M. F. , García-López , I. , Santos , J. A. , Otero , A. and García-López , M. L. 2005a . Incidence, radioresistance, and behavior of Psychrobacter spp. in rabbit meat . Journal of Food Protection , 68 ( 3 ) : 538 – 543 .
- Rodríguez-Calleja , J. M. , Santos , J. A. , Otero , A. and García-López , M. L. 2004 . Microbiological quality of rabbit meat . Journal of Food Protection , 67 ( 5 ) : 966 – 971 .
- Tryfinopoulou , P. , Drosinos , E. H. and Nychas , G. J.E. 2001 . Performance of Pseudomonas CFC-selective medium in the fish storage ecosystems . Journal of Microbiological Methods , 47 ( 2 ) : 243 – 247 .
- Tsigarida , E. and Nychas , G. J.E. 2001 . Ecophysiological attributes of a Lactobacillus sp and a Pseudomonas sp on sterile beef fillets in relation to storage temperature and film permeability . Journal of Applied Microbiology , 90 ( 5 ) : 696 – 705 .
- Vergara , H. , Berruga , M. I. and Linares , M. B. 2005 . Effect of gas composition on rabbit meat quality in modified atmosphere packaging . Journal of the Science of Food and Agriculture , 85 ( 12 ) : 1981 – 1986 .