Abstract
Whole strawberries were osmodehydrated at low temperature (5 °C) in a sucrose syrup (500 g/kg of solution) for different processing times (24, 48, 72, 96, and 120 h); after the treatment, water loss, solid gain, and weight reduction were recorded. Next, the osmodehydrated samples were frozen, and after a 5-month storage at −18 °C they were analyzed for drip loss, titratable acidity, refractometric index, pH, and sensory acceptance. The phenolic profile of the samples was characterized by means of high performance liquid chromatography (HPLC). After thawing, osmodehydrated samples presented a high acceptability for consumers. In addition, they presented drip loss values that were inversely correlated with the processing time, with 120-h samples presenting negligible drip loss after thawing. The processing time influenced the final quality of strawberries, and samples treated for 24–48 h were very suitable for direct consumption after thawing, in substitution of fresh strawberries. The samples treated for 72–120 h also presented characteristics that may render them suitable for applications in the food industry.
Fresas enteras se osmo-deshidrataron a baja temperatura (5 °C) en un jarabe de sacarosa (solución a 500 g/kg) para diversos tiempos de procesamiento (24, 48, 72, 96, y 120 h); después del tratamiento, la pérdida de agua, ganancia sólida y reducción de peso se registraron. A continuación, muestras osmo-deshidratadas se congelaron, y después de ser almacenadas por 5 meses a −18 °C fueron analizadas en cuanto a pérdida de agua durante descongelamiento, acidez titulable, índice refractométrico, pH y aceptación sensorial. El perfil fenólico de las muestras fue caracterizado mediante HPLC. Después de descongelar, las muestras osmo-deshidratadas presentaron una alta aceptación para los consumidores. Adicionalmente, los valores de pérdida de agua por descongelación se correlacionaron inversamente con tiempo de procesamiento, siendo las muestras con 120 h las que presentaron menos pérdida de agua después de la descongelación. El tiempo de procesamiento tuvo influencia en la calidad final de las fresas, y las muestras tratadas por 24–48 h fueron muy aceptables para consumo directo después de descongelación, en substitución de fresas frescas. Las muestras tratadas por 72–120 h también presentaron características que pueden hacerlas aceptables para aplicaciones en la industria alimentaria.
Introduction
A good way to preserve strawberries (Fragaria × ananassa Duch.) from bruises and fungal attacks before processing is through the use of freezing technologies that combine low temperature and water activity (a w) reduction associated with cryoconcentration of the fruit liquid phase during ice crystal formation. Individually quick-frozen strawberries may have potential uses as ingredients in different high-quality processed foods, such as ice creams, yogurt, jams, and jellies (Duxbury, Citation1992). However, several chemical–physical and sensory deteriorations take place during thawing with subsequent loss of product quality (Blanda, Cerretani, Bendini, Cardinali, & Lercker, Citation2008a; Martínez-Navarrete, Moraga, Martínez-Monzó, Botella, Tirado, & Chiralt, Citation2001).
In recent years, several studies have highlighted the importance of dehydration pre-treatment before the freezing process (dehydrofreezing) to reduce the water content and limit ice crystal damage (Chiralt, Martínez-Navarrete, Martínez-Monzó, Talens, Moraga, Ayala, & Fito, 2001). Osmotic dehydration (OD) before freezing is used to produce several kinds of fruit ingredients that can be stored for long periods with good retention of texture, color, and flavor after thawing (Dalla Rosa & Spiess, Citation2000; Maestrelli, Lo Scalzo, Lupi, Bertolo, & Torreggiani, Citation2001; Sormani, Maffi, Bertolo, & Torreggiani, Citation1999). OD in hypertonic solutions cause a flow of water from the food matrix to the liquid and flow of solute from the liquid to the food matrix. A third mass transfer involves food solutes leaching into solution. Although leaching has been generally considered to be quantitatively negligible (Dixon & Jen, Citation1977), it may result in loss of the nutritional content of foods (Blanda, Cerretani, Bendini, Cardinali, Scarpellini, & Lercker, Citation2008b; Peiró-Mena, Dias, Camacho, & Martínez-Navarrete, Citation2006). Solute impregnation coupled with mild partial dehydration occurs at moderate temperatures (5–50 °C), and thus OD has a minimal impact on the overall structure and composition of foods (Torreggiani, Citation1993).
Solute incorporation from the osmotic medium may improve the nutritional and functional properties of the food product; however, excessive solid gain (SG) can be detrimental to the quality of the food product and should be avoided (Matuska, Lenart, & Lazarides, Citation2006).
The ratio of water loss (WL) to SG is a useful parameter to control the final product quality in fruit osmodehydration. A higher WL/SG ratio is associated with higher processing temperatures, and this is especially true if short processing times are used (2–3 h). A high WL/SG ratio, is presumably obtained by combining long processing times and low temperatures, however this aspect needs further investigation. To the best of our knowledge, low temperature osmotic processes have not been widely studied as they are not economically favorable as the treatment solutions must be cooled and the process is time-consuming. On the other hand, we have shown that (Blanda, Cerretani, Cardinali, Barbieri, Bendini, & Lercker, Citation2009) these conditions lead to high quality products with a higher WL/SG ratio, higher sensory quality, and retention of healthy compounds. Thus, better understanding of the low temperature phenomena associated with these processes could be useful to obtain high quality frozen products characterized by a longer shelf life with important advantages in the formulation of processed foods.
To provide insight into low temperature OD, whole strawberries were submitted to OD at 5 °C for a processing time ranging from 1 to 5 days in an industrial scale pilot plant. Changes in chemical composition and sensory properties were determined. The aim of the article is to investigate the mass transfer occurring at low temperature for long processing time, to produce osmodehydrated strawberries for direct consumption for the consumer or for use as food ingredients in food industry.
Materials and methods
Instruments
HPLC analyses on phenolic extracts were performed using a HP 1100 instrument (Agilent Technologies, Palo Alto, CA), equipped with a binary pump delivery system, a degasser, an autosampler, a diode array UV-VIS detector (DAD), and a mass spectrometer detector (MSD). The HPLC column used was a C18 Luna column, 5 μm, 15 cm × 3.0 mm (Phenomenex, Torrance, CA), with a C18 pre-column (Phenomenex) filter.
Reagents, stock solutions, and reference compounds
p-coumaric acid, pelargonidin chloride, ellagic acid, and kaempferol were acquired from Sigma-Aldrich (Sigma, St Louis, MO). Stock solutions containing these analytes were prepared in methanol at 2.0 mg mL−1 for p-coumaric, pelargonidin, and ellagic acid and 2.5 mg mL−1 for kaempferol. These standard solutions were used to prepare calibration curves in a range of 1–500 μg mL−1. Methanol and HPLC-grade water were from Merck (Darmstadt, Germany). Distilled water was deionized by using a Milli-Q system (Millipore, Bedford, MA).
Experimental design
Strawberries (cv. Camarosa) were bought at a local market. A subsample of 90 kg strawberries without damage nor fungal attacks and ranging from 23 to 27 g each was obtained from 120 kg of strawberries. After stalk removal, strawberries were accurately mixed, and divided into six aliquots of 15 kg.
Control sample
The strawberries were immediately frozen in a freezing chamber in direct contact with dry ice pellets (2 mm diameter). After 30 min, strawberries reached a temperature of −30 °C at the core, and were then put in a conventional freezer and stored at constant temperature of −18 °C for 5 months.
The 24, 48, 72, 96, and 120 h samples
For each sample, strawberries were put in a large stainless steel tank containing sucrose syrup, prepared with 750 kg of sucrose of commercial grade (Chimab, Milan, Italy) in 750 kg of water, and were kept submerged using a grid. The syrup was kept at a constant temperature of 5 °C during the entire processing period by using a syrup chiller equipped with a pump with a flow rate of 250 L h−1. At the end of each processing time, the grid was removed and the strawberries were withdrawn from the tank. Strawberries were drained, rapidly washed with tap water and dried with air, frozen in a dry ice cabinet as described above and stored at −18 °C for 5 months.
The below-described analyses were carried out in triplicate on aliquots of 19 strawberries (about 475 g). The representativeness of the aliquot dimension was studied in a previous work (Blanda et al., Citation2009) where it was found that an aliquot size of 19 strawberries was a good compromise between subsample representativeness and laboratory constraints.
Mass transfer determination
SG, WL, and weight reduction (WR) were calculated as described (Giangiacomo, Torreggiani, Abbo, Citation1987) using the equations reported below. Changes in weight and dry matter (DM) were determined in four replicates for each processing time: for each replicate, 19 strawberries were put in a plastic net and processed in the same soaking tank with the global sample.
Analyses of dry matter, pH, soluble solids, titratable acidity
DM, pH, soluble solids (SS), and titratable acidity (TA) of fresh and frozen slices were determined according to AOAC method 932 (AOAC, 2000).
Drip loss determination
Nineteen frozen strawberries for each replicate were put upon a metallic grid in plastic boxes and hermetically sealed with a lid. The plastic boxes were kept at a controlled temperature of 22 °C. After 8 h, the weight of juice lost by the strawberries was determined and expressed as a percentage of fruit initial weight.
Acceptance test
The acceptance test was carried out on a laboratory scale (Stone & Sidel, Citation1985) in the Laboratory of Sensory Analysis of the “Campus di Scienze degli Alimenti” at the University of Bologna using individual booths with white neon light. An untrained panel of 33 consumers was used (13 males and 20 females between the age of 25 and 40 years, office-workers). No information about the normal fruit consumption habits of the judges was available. Strawberries were thawed at controlled temperature until they reached 18 °C, and then served to judges. Firstly, visual and odour acceptance was evaluated, and then judges tasted strawberries and rated the taste acceptance level. A nine-point hedonic scale was used for each descriptor with scores ranging from 1 (extreme dislike) to 9 (extreme likeability) and 5 as the indifference point (neither like nor dislike). Each judge could freely express notes or comments on a scorecard.
Phenolic extraction and clean-up
Phenolic extracts were obtained by adapting the method reported by Blanda et al. (Citation2009). Briefly, 500 g of strawberries (about 19 strawberries) were ground in a blender with 500 g methanol for 1 min to prevent enzymatic degradations. Next, 10 g of this homogenate was centrifuged at 22,000 rpm (39,600g) for 10 min at 10 °C (Avanti J25, Beckman Coulter, Fullerton, CA). The supernatant was recovered and a second extraction was performed by homogenizing the sample residue with 10 mL of methanol/water (950 mL/L) in a centrifuge tube. An Ultra Turrax blender (IKA-Werke mod. T 25 basic, Staufen, Germany) was used at 15,000 rpm for 3 min. Then, the tube was centrifuged again at 22,000 rpm. The supernatant was recovered and the two extracts were combined and evaporated in a vacuum centrifuge to complete dryness (MIVAC DUO, Genevac, Ipswich, England). The concentrated sample was dissolved in 5 mL of acidified water (30 mL/L formic acid) and then passed through a Strata C18-E 55 μm 70 A cartridge (Phenomenex), previously activated with methanol followed by formic acid/water (30 mL/L). Anthocyanins and other phenolics were adsorbed onto the column while sugars, organic acids and other highly water-soluble components were eluted with 10 mL formic acid/water (30 mL/L). The anthocyanins and other phenolic compounds were then recovered with 2.0 mL of formic acid/methanol (25 mL/L).
HPLC-DAD/MSD analysis of phenols
Methanolic extracts were filtered through a 0.45 μm filter (Whatman, Clifton, NJ) and injected in HPLC 1100 Series (Agilent Technologies, Palo Alto, CA). A Luna C18 (Phenomenex, St. Torrance, CA) column (5 μm particle size, 250 mm, 3.00 mm ID) was used and 20 μL of phenolic extract were injected. Mobile phases were: A, formic acid/water (25 mL/L); and B, formic acid/methanol (25 mL/L). The elution gradient was linear: at 0 min 85% solvent A held for 5 min, from 5 to 20 min 65% A was reached and held constant until 25 min, from 25 to 35 min 50% held constant until 45 min, from 45 to 50 min 34% and finally at 59 min 85% solvent A was restored. A 10 min post run equilibration was performed. The detector wavelengths were set at 280, 320, 350, and 520 nm. Identification was also made using MSD, with an electrospray (ESI) interface operating in positive and negative mode using the following conditions: drying gas flow, 9.0 L/min; nebulizer pressure, 50 psi; gas drying temperature, 350 °C; capillary voltage, 3000 V; fragmentor voltage, 60 V. Phenolic compounds were tentatively identified based on their UV-VIS and mass spectra obtained by HPLC-DAD/MSD () and comparison with data from the literature (Lopes da Silva, De Pascual-Teresa, Rivas-Gonzalo, & Santos-Buelga, Citation2002; Määttä– Riihinen, Kamal–Eldin, & Törrönen, 2004).
For quantification in HPLC-DAD, four standard calibration curves were constructed using four commercial reference compounds, p-coumaric acid, pelargonidin chloride, ellagic acid, and kaempferol. Anthocyanins (compounds 1–8 in ) were quantified using the calibration curve of pelargonidin chloride at 520 nm (r 2 = 0.9952). Phenolic acids (compounds 9–13) were quantified on the basis of a p-coumaric standard calibration curve at 320 nm (r 2 = 0.9926); ellagic acid (compound 17) and an ellagic derivative (compound 15) were quantified using an ellagic acid curve at 350 nm (r 2 = 0.9998); finally, flavonols (compounds 14, 16, and 18–21) were quantified using a kaempferol calibration curve at 350 nm (r 2 = 0.9890).
Statistical analysis
The data were analysed using Statistica 7.0 (Statsoft, Tulsa, OK) statistical software. For all analyses, the significance of differences at 5% level between averages was determined by one-way ANOVA using Tukey's test. For sensory acceptance levels, the significance of differences at the 5% level between averages was determined by one-way ANOVA using the least significance different (LSD) test.
Results and discussion
Mass transfer
The WL and SG behavior agree with the kinetic models developed by other authors (Fickian diffusion laws) (Rastogi, Raghavarao, & Miranjan, Citation1997) reporting a higher WL and SG rate during the first days of treatment and a slow down in the latter days. The influence of processing time on mass transfers in osmodehydrated strawberries is reported in . The WL to SG ratio showed a remarkable increase during the first 2 days of the osmodehydrating treatment (from 4.9 to 6.2); after 3 days the WL/SG ratio reached a plateau until the 5th day (6.2 to 6.8).
Figure 1. Weight reduction (WR), water loss (WL), solid gain (SG), and WL/SG, at different processing times.
Figura 1. Reducción en peso (WR), pérdida de agua (WL), ganacia sólida (SG) y WL/SG, a diferentes tiempos de procesamiento.
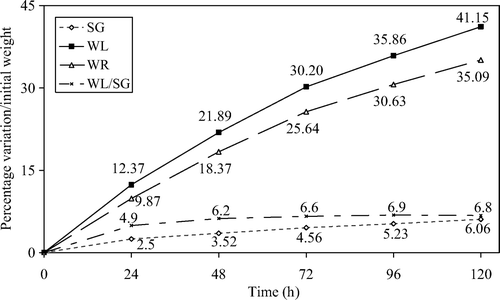
In a previous study (Blanda et al., Citation2009), we evaluated the nutritional and sensory quality of strawberries submitted to different combinations of OD processes and freezing. Moreover, the samples treated at lower temperature presented the highest WL/SG ratio, which is in apparent contrast with previous findings (Lazarides, Katsanidis, & Nickolaidis, Citation1995; Sereno, Moreira, & Martínez, Citation2001) that reported higher WL/SG values at higher processing temperatures. The reason behind these discrepancies may be related to the processing time used in our experiment, which was very long compared with other OD processes that have been reported. In fact, the rate of WL (and SG) depends on several factors such as the solution concentration, processing temperature, processing time, level of agitation, sample size and geometry, solution to solid volume ratio and operating pressure, and particularly the use of vacuum (Moreno, Chiralt, Escriche, & Serra, Citation2000; Rastogi et al., Citation1997; Shi & Fito, Citation1994). In fact, the WL/SG ratio calculated according to the kinetic model described by (Sereno et al., Citation2001) using a 24 h processing time and 5 °C processing temperature provides support for the supposition that the value is comparable with that obtained at higher temperature and shorter processing times.
Finally, a higher solute uptake is likely present in the first stages of the process, depending on the morphological structure of the fruit.
Dry matter, pH, soluble solids, titratable acidity
In the values of DM, pH, SS, and TA are reported. DM and SS increased with the processing time as expected. As these variables change significantly, it was interesting to note that pH values in fruit juice did not vary with the treatment time, in accordance with previous data (Blanda et al., Citation2009). On the other hand, TA increased significantly in the first 2 days of treatment, while it decreased in the following days. Because this behavior is difficult to understand, the observed variations could be explained by the concentration of organic acids during the process due to the decrease of water content, the higher mobility of H+ with respect to K+ and other cations during osmodehydration or the ex-novo formation of organic acids induced in the fruit maintained the pH constant at later times. In a previous work (Blanda et al., Citation2009), large amounts of volatile compounds (such as alcohols and acetaldehyde) deriving from fermentation were detected in osmodehydrated strawberries. Thus, fermentative metabolism could be activated in fruit, induced by the process itself causing changes in the organic acid profile. The decrease of TA in the subsequent days of treatment could be explained in the same way, by changes in the organic acids profile or by leaching of acids or protons into the treatment solution. These two factors could be active in strawberries at the same time during the process, with the latter being more important at 3–5 days of treatment. Better knowledge of metabolism pathways may be needed to better explain this behavior.
Table 1. Average values (n = 3) of pH.
Tabla 1. Valores promedio (n = 3) de pH.
Drip loss
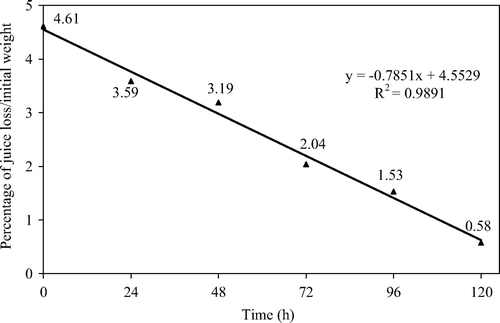
In , the results of drip loss determinations are reported. A linear relationship between processing time and the amount of drip loss can be observed, and a significant reduction in juice loss from fruit after thawing after 1 day of treatment was already evident. The reduction in drip loss was very high in samples treated for 3–4 days and accounted for only 0.58 g of juice in 100 g of strawberries after 5 days of treatment. Drip loss reduction in treated strawberries was supposedly due to less ice crystal formation during freezing of strawberries promoted by the reduction on free water after osmotic treatment and by the cryo-protective effect of sucrose impregnated in strawberries that increased the non-freezable water fraction.
Acceptance test
The results of the sensory acceptance test are presented in . An inverse correlation was found between visual acceptance scores and processing time. This could be substantially ascribed to the dry and rugged appearance of osmotically-treated fruit, particularly when processed for longer times. However, the high drip loss of control samples and of less treated samples did not appear to affect the acceptance level. With regards to the odour acceptance level, judgments were more variable and there were no statistically significant differences among treated samples; generally, untreated strawberries presented the lowest odour acceptance score. In any case, osmotic treatment seemed to improve the odour acceptance level of the fruits, as already reported by other authors (Blanda et al., Citation2009; Dalla Rosa & Spiess, Citation2000).
Table 2. Average values (n = 3) of the consumer's acceptance test of processed strawberries.
Tabla 2. Valores promedio (n = 3) de la prueba de aceptación del consumidor de fresas procesadas.
Analysis of taste acceptance levels provided interesting results. As it is already known, osmotic treatment allows for partial water removal from strawberries thus preventing damages caused by freezing (dehydrofreezing) and ice crystal formation that causes disruption of cell structures with dramatic changes in both texture and enzymatic activation. These phenomena cause off-flavor development, phenolic oxidation and overall declines in the sensory quality (Blanda et al., Citation2008a,Citationb, Citation2009; Chiralt et al., Citation2001; Dalla Rosa et al., Citation2000; Dixon et al., Citation1977; Maestrelli et al., Citation2001; Matuska et al., Citation2006; Peiró-Mena, Camacho, & Martínez-Navarrete, Citation2007; Peiró-Mena et al., Citation2006; Sormani et al., Citation1999; Torreggiani, Citation1993). This is quite evident in the differences between the taste acceptance level of untreated and treated samples. The very low score of untreated samples is likely due to the very low hardness level and atypical taste (as reported by the judges in their scorecards). Samples treated for 48 h were the most accepted, probably because the treatment had only minimal effects on the characteristics of the raw material, and limited the damages caused by freezing. In fact, samples treated for 48 h had an acceptability score higher than the indifference point. Samples treated for 24, 72, and 120 h were very similar (and not statistically different) to the 48 h sample, while the 96 h sample seemed to be the less accepted, although the score was still higher than untreated samples. The low score given to samples treated for 96 and 120 h are probably due to the low juiciness of strawberries and the gummy texture due to high level of dehydration, but were not statistically different from samples treated for 24 or 72 h; thus, there was not a trend towards lower acceptance levels that could be attributed to the low juiciness. Considering the chemical physical results and the comments given by panellists regarding taste, strawberries processed for 4–5 days could be used as an ingredient in food preparation (e.g. fruit cakes) as they showed low drip loss and very sweet taste.
HPLC-DAD/MS phenolic analysis
As reported in a previous study (Blanda et al., Citation2009) on osmotic processes, the natural components of strawberries can be affected by different processing variables, and their content may also change due to either biochemical or chemical transformations (enzymatic, hydrolytic, etc.) or leaching in the concentrated solution. In any case, the WL phenomenon causes the re-concentration of the constituents present in the fresh strawberries. In contrast, SG causes an increase in sample weight with an apparent decrease of the natural components of strawberries. Thus, at the end of the process, a final effect equal to WR = WL−SG takes place. Because of the higher value of WL with respect to SG, this effect results in concentration. Of course, solid loss (SL) may also occur, i.e. leaching of chemical compounds in the treatment solution.
shows different information (retention time, maximum of absorbance and the most abundant mass fragments in positive or negative modality) useful to identify the 21 considered phenolic compounds. The values of these phenolic compounds expressed in mg/kg of untreated and processed samples with (“cor”) and without (“uncor”) correction for the concentration effect using
EquationEquation (4) (see below) are reported in :
Table 3. HPLC-DAD/MSD of phenolic compounds.
Tabla 3. HPLC-DAD/MSD de los compuestos fenólicos.
The data relative to “cor” values will be used to discuss the absolute variations in strawberries after the treatment.
We have previously found that the phenolic compounds in osmodehydrated strawberries at 5 °C for 24 h showed an interesting behavior (Blanda et al., Citation2009). Under the conditions used, strawberries presented an absolute increase in the polyphenolic content, principally ascribed to an increase in anthocyanins. Such behavior was also found in the present study, and from examination of the data in and , after the first 2 days of treatment, the total phenolic (sum of HPLC detected compounds) content increased. Although the phenolic content in the treatment solution was not determined, it is obvious that a certain extent of phenolic leaching in the treating solution will occur (also demonstrated by the pink color of the solution within a few hours after fruit immersion). Thus, the absolute increase can be explained by the neo-formation of phenolic compounds derived by hydrolysis of polymeric compounds or by activation of anabolic pathways. As reviewed by Stintzing and Carle (Citation2004), anthocyanins play different roles in plant physiology, and appear to be important as monosaccharide transporters and osmotic adjusters during periods of drought and low temperatures. Strawberry cells submitted to osmotic stress induce anthocyanin synthesis (Suzuky, Citation1995), and thus it is not unexpected that strawberries osmodehydrated at low temperature had an increase in the anthocyanin content. This increase was evident in the first day of treatment, while the anthocyanin content undergoes a continuous decrease in the subsequent days. In fact, apart from the neo-synthesis effect, a leaching effect in the concentrated solution is present and probably becomes more important, prevailing in the latter stages of the process. This behavior was particularly evident for the most abundant compound, pelargonidin-3-glucoside, as shown in . It is evident that in addition to anthocyanins, other phenolic compounds also had a similar behavior, such as the p-coumaroyl-glucoside (compound 10). Compound 9 (a tentatively identified galloyl derivative) showed an absolute increase in the first day of treatment that continued until the second day. Not only anthocyanins, but also other phenolic compounds in strawberries, may possess functional properties as osmotic regulators. Other compounds appeared to be less affected by the process itself, and their content did not change significantly during the process. Other phenols decreased constantly as only leaching was present (compounds 11 and 16).
Figure 4. HPLC-DAD/MSD content of selected phenolic compounds of untreated and processed strawberries corrected for the concentration effect (see
EquationEquation (4)).
Figura 4. HPLC-DAD/MSD contenidos fenólicos totales de fresas no tratadas y procesadas, corregidas por el efecto de concentración (vea Ecuación (4)).
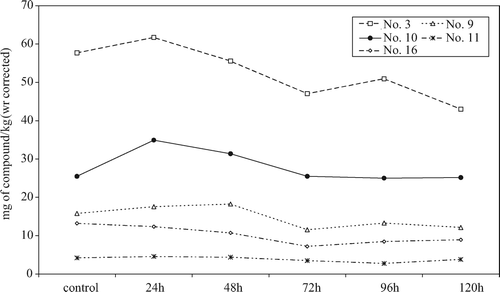
Table 4. HPLC-DAD/MSD phenolic contents of fresh and processed strawberries with (cor) and without (uncor) the correction for the concentration effect (see the
EquationEquation 4 ).
).
Tabla 4. HPLC-DAD/MSD contenidos fenólicos de fresas frescas y procesadas con (cor) y sin (uncor) la corrección del efecto de concentración (vea la Ecuación 4).
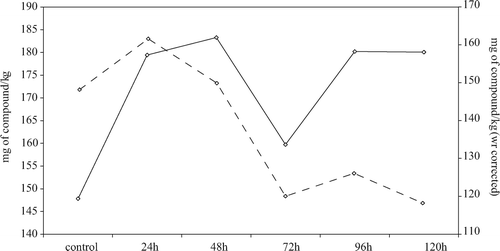
By examination of and , where data are expressed referring to the fresh weight of samples, it can be noticed that the combination of three effects (leaching in the treating solution, neo-formation and re-concentration caused by WL) caused a higher content of phenolic compounds in all samples with respect to the control. In particular, during the first 2 days of treatment, when the neo-synthesis effect was high and the concentration effect was important, the total phenolic content was significantly higher and remained constant in subsequent days. Considering the behavior of individual compounds ( and ), it can be seen that on the third day of treatment the leaching effect prevailed over the other two effects, while it was less important in the following days.
Figure 3. HPLC-DAD/MSD total phenolic contents of untreated and processed strawberries (continuous line) and of untreated and processed strawberries corrected for the concentration effect (dotted line) (see
EquationEquation (4)).
Figura 3. HPLC-DAD/MSD contenidos fenólicos totales de fresas no tratadas y procesadas (línea continua) y de fresas no tratadas y procesadas, corregidas por el efecto de concentración (línea punteada) (vea Ecuación (4)).
It is difficult to precisely understand the underlying reasons for the observed changes in polyphenolic content in osmodehydrated samples. The mechanism should involve the alteration of the cell walls and membranes causing an irreversible increase of permeability particularly after 2 days of treatment. The reaction of strawberries to OD is interesting and not only polyphenols, but also other compounds may be implicated in modifications during the process (e.g. organic acids or pectins). A better understanding of metabolic reactions and the chemical modifications would allow optimization of osmotic processes and production of high quality frozen fruits under the sensory and the nutritional point of view.
Conclusions
From a technological point of view, it is important to underline the high quality of samples treated for 96 and 120 h, and that they may potentially be employed as ingredients in foods (such as frozen desserts and cakes) due to the very low drip loss values and high acceptance level. At the same time, the phenolic content of treated samples was very high and comparable to fresh strawberries, and thus the product could be considered as healthy as fresh strawberries (with obvious marketing implications). The low temperature process probably induces a protective mechanism in strawberries that permits improvements in their freezing performance. This hypothesis needs to be confirmed by more in-depth studies on the textural, sensory, and chemical characteristics of fruits.
In the present study, we have provided an initial insight in the low temperature osmodehydro-freezing of whole strawberries. At the temperature used (5 °C), a few hours of treatment are not sufficient to obtain a substantial WL extent, thus making the technique relatively slow with respect to osmodehydration at higher temperatures. On the other hand, at low temperature the process has two major advantages: it permits a higher sensory acceptance level and a higher polyphenolic content with respect to osmodehydration at higher temperatures (as demonstrated in previous works) also with respect to the same fresh raw material. This last supposition is supported by the neo-synthesis of low molecular weight phenolic compounds, probably induced by the combination of osmotic stress and low temperatures. The increase in the phenolic content of strawberries occurs in the first day of treatment, and decreases slightly in subsequent days, when leaching in the osmotic solution prevailed. In any case, strawberries treated for 1–2 days at 5 °C presented a high acceptance level for consumers and a very high polyphenolic content (in particular anthocyanins), while strawberries treated for 3–5 days presented a reasonable acceptance level, very low drip loss and high phenolic content. Thus, the processing time influences the final quality of strawberries. The strawberries treated for 1–2 days are very suitable for direct consumption after thawing in substitution of fresh fruits, and samples treated for 2–5 days may have interesting applications as ingredients in the food industry.
References
- AOAC Official Methods of Analysis . 2000 . “ Numbers, 932,12; 934,01; 942,15 ” . In , 17th edn , Edited by: AOAC International . Urbana, IL, , USA : The American Oil Chemists' Society .
- Blanda , G. , Cerretani , L. , Bendini , A. , Cardinali , A. and Lercker , G. 2008a . Phenolic content and antioxidant capacity versus consumer acceptance of soaked and vacuum impregnated frozen nectarines . European Food Research and Technology , 227 : 191 – 197 .
- Blanda , G. , Cerretani , L. , Bendini , A. , Cardinali , A. , Scarpellini , A. and Lercker , G. 2008b . Effect of vacuum impregnation on the phenolic content of Granny Smith and Stark Delicious frozen apple cvv . European Food Research and Technology , 226 : 1229 – 1237 .
- Blanda , G. , Cerretani , L. , Cardinali , A. , Barbieri , S. , Bendini , A. and Lercker , G. 2009 . Osmotic dehydrofreezing of strawberries: polyphenolic content, volatile profile and consumer acceptance . Lebensmittel Wissenschaft und Technologie , 42 : 30 – 36 .
- Chiralt , A. , Martínez-Navarrete , N. , Martínez-Monzó , J. , Talens , P. , Moraga , G. Ayala , A. 2001 . Changes in mechanical properties throughout osmotic processes: Cryoprotectant effect . Journal of Food Engineering , 49 : 129 – 135 .
- Dalla Rosa , M. and Spiess , W. E.L. 2000 . “ Industrial application of osmotic dehydration/treatments of food ” . In Concerted action FAIR-CT96-1118 , Edited by: Dalla Rosa , M. and Spiess , W. E.L. Udine, , Italy : Forum .
- Dixon , G. M. and Jen , J. J. 1977 . Changes of sugars and acids of osmovac-dried apple slices . Journal of Food Science , 42 : 1126 – 1127 .
- Duxbury , D. D. 1992 . Strawberries source of natural flavor and nutrition . Food Processing , 53 : 97
- Giangiacomo , R. , Torreggiani , D. and Abbo , E. 1987 . Osmotic dehydration of fruit: Part 1. Sugars exchange between fruit and extracting syrups . Journal of Food Processing and Preservation , 11 : 183 – 195 .
- Lazarides , H. N. , Katsanidis , E. and Nickolaidis , A. 1995 . Mass transfer kinetics during osmotic preconcentration aiming at minimal solid uptake . Journal of Food Engineering , 25 : 151 – 166 .
- Lopes da Silva , F. , de Pascual-Teresa , S. , Rivas-Gonzalo , J. and Santos-Buelga , C. 2002 . Identification of anthocyanin pigments in strawberry (cv. Camarosa) by LC using DAD and ESI-MS detection . European Food Research and Technology , 214 : 248 – 253 .
- Määttä–Riihinen , K. R. , Kamal–Eldin , A. and Törrönen , A. R. 2004 . Identification and quantification of phenolics compounds in berries of Fragaria and Rubus species (Family Rosaceae) . Journal of Agricultural and Food Chemistry , 52 : 6178 – 6187 .
- Maestrelli , A. , Lo Scalzo , R. , Lupi , D. , Bertolo , G. and Torreggiani , D. 2001 . Partial removal of water before freezing: Cultivar and pre-treatments as quality factors of frozen muskmelon (Cucumis melo, cv reticulatus Naud.) . Journal of Food Engineering , 49 : 255 – 260 .
- Martínez-Navarrete , N. , Moraga , G. , Martínez-Monzó , J. , Botella , F. , Tirado , N. and Chiralt , A. Proceedings of the Eighth International Congress on Engineering and Food. Mechanical and color changes associated to dehydrofreezing of strawberry . Edited by: Welti-Chanes , J. , Barbosa-Canvas , G. V. and Aguilera , J. M. pp. 793 – 797 . Lancaster, PA : Technomic Publishing Company .
- Matuska , M. , Lenart , A. and Lazarides , H. N. 2006 . On the use of edible coatings to monitor osmotic dehydration kinetics for minimal solids uptake . Journal of Food Engineering , 72 : 7285 – 7291 .
- Moreno , J. , Chiralt , A. , Escriche , I. and Serra , J. A. 2000 . Effect of blanching/osmotic dehydration combined methods on quality and stability of minimally processed strawberries . Food Research International , 33 : 609 – 616 .
- Peiró-Mena , R. , Camacho , M. M. and Martínez-Navarrete , N. 2007 . Compositional and physicochemical changes associated to successive osmodehydration cycles of pineapple (Ananas comosus) . Journal of Food Engineering , 79 : 842 – 849 .
- Peiró-Mena , R. , Dias , V. M.C. , Camacho , M. M. and Martínez-Navarrete , N. 2006 . Micronutrient flow to the osmotic solution during grapefruit osmotic dehydration . Journal of Food Engineering , 74 : 299 – 307 .
- Rastogi , N. K. , Raghavarao , K. S.M.S. and Miranjan , K. 1997 . Mass transfer during osmotic dehydration of banana: Fickian diffusion in cylindrical configuration . Journal of Food Engineering , 31 : 423 – 432 .
- Sereno , A. M. , Moreira , R. and Martínez , E. 2001 . Mass transfer coefficients during osmotic dehydration of apple in single and combined aqueous solutions of sugar and salt . Journal of Food Engineering , 47 : 43 – 49 .
- Shi , X. Q. and Fito , P. M. 1994 . Mass transfer in vacuum osmotic dehydration of fruits: A mathematical model approach . Lebensmittel Wissenschaft und Technologie , 27 : 67 – 72 .
- Sormani , A. , Maffi , D. , Bertolo , G. and Torreggiani , D. 1999 . Textural and structural changes of dehydrofreeze-thawed strawberry slices: Effects of different dehydration pre-treatments . Food Science and Technology International , 56 : 479 – 485 .
- Stintzing , F. C. and Carle , R. 2004 . Functional properties of anthocyanins and betalains in plants, food, and in human nutrition . Trends in Food Science and Technology , 15 : 19 – 38 .
- Stone H. Sidel J. L. Sensory evaluation practices Academic Press Orlando, , USA 1985
- Suzuky , M. 1995 . Enhancement of anthocyanin accumulation by high osmotic stress and low pH in grape cells (Vitis hybrids) . Journal of Plant Physiology , 147 : 152 – 155 .
- Torreggiani , D. 1993 . Osmotic dehydration in fruit and vegetable processing . Food Research International , 26 : 59 – 68 .