Abstract
Proteins were produced by fed-batch fermentation of Saccharomyces cerevisiaecultivated on date solution. Protein content was 52.5% (dry basis). Protein solubility was studied as a function of pH, NaCl concentration, and solvent. The minimum protein solubility was at pH 4 and the maximum was at pH 12. As a function of NaCl concentration, protein solubility was 647.6 g/kg for 1 M. The best solubility was 916.3 g/kg under pH 12 and 1 M NaCl. Solubility was very low, 2.1 g/kg, when ethanol was used as a solvent. Biomass was dried by air drying, vacuum drying, and freeze drying. The best solubility of 937.5 g/kg was obtained using freeze and vacuum drying methods.
Se produjeron proteínas por fermentación semicontínua de Saccharomyces cerevisiaecultivada en solución de dátil. El contenido de proteína fue 52,5% (base seca). Se estudió la solubilidad de proteína como función de pH, concentración de NaCl y solvente. La solubilidad mínima de proteína fue a pH 4 y la máxima fue a pH 12. Como función de concentración NaCl, la solubilidad de proteína fue 647,6 (g/kg) para 1M. La mayor solubilidad fue 916,3 (g/kg) a pH 12 y 1M de NaCl. La solubilidad resultó muy baja, 2,1 (g/kg), cuando el solvente usado fue etanol. La biomasa se secó por aire, a vacío y por congelado. La mayor solubilidad de 937,5 (g/kg) se obtuvo usando los métodos de secado por congelado y a vacío.
Introduction
Because of the growing world population, nonconventional sources of proteins for human nutrition require more attention in order to meet the future demand. Protein production by fermentation of Saccharomyces cerevisiaecultivated on musts of dates has been carried out successfully. Dates were chosen due to their availability and their high sugar content. Proteins produced by fermentation of S. cerevisiaeyeast have similar quality as animal proteins (Acourene, Khalid, Bacha, Tama, & Taleb, Citation2007; Bessah & Touzi, Citation2001).
Protein solubility is an important functional property in the food industry as it defines other functional properties (emulsion, water retention, viscosity, etc.). Functional properties are also used to define the quality of food products such as texture, taste, and structure (Wong & Kitts, Citation2003). There are two groups of proteins: proteins soluble in solvents such as water, alkaline, acid, salt, and organic solvent, and insoluble proteins with very limited uses in food (Bernard & Carlier, Citation1992). Many researchers (Aluko & Yada, Citation1993; Khalid, Babiker, & EL Tinay, Citation2003; Lee, Morr, & Ha, Citation1992; Yu, Ahmedna, & Goktepe, Citation2007) investigated the solubilityofanimal and plant proteins as a function of pH, saltconcentration, organic solvents, and temperature. However,few research works have been carried out on microbial food proteins generated by S.cerevisiae(Alais & Linden, Citation1994).
The effect of drying on the functional and nutritional properties of proteins has been investigated by different researchers. Studies of Cepeda, Villaran, and Aranguiz (Citation1998), Wong and Kitts (Citation2003), Jovanovic et al. (Citation2006), Yu, Johnston, and Williams (Citation2006), and Yu et al. (Citation2007) on plant proteins (soya, sesame, and peas) or animal proteins (milk and eggs) showed the influence of processing on the functional properties such as the solubility of proteins and on digestibility. To date, little research has been conducted on the effect of drying methods on the functional properties (including solubility) of S. cerevisiaeproteins. Moderate heating allows partial denaturation of proteins, which often improves the digestibility of essential amino acids. However, extensive denaturation of proteins often results in insolublization, which can impair functional properties that are dependent on solubility (Fennema, Citation1996).
The main objectives of this study were to determine the effects of pH, NaCl concentration, and ethanol on the solubility of proteins of fresh S. cerevisiaebiomass, to include the best solubility parameters of fresh biomass into the protocol of studying dried biomass and to compare the effects of three drying methods on the solubility of proteins of dried S.cerevisiaebiomass.
Materials and methods
Strain
S. cerevisiaewas the strain used in this study. It was isolated from dates (variety Mech-degla) and preserved at 4°C on an inclined Sabouraud medium in the fermentation laboratory.
Vegetable material
Dates (var. Mech-degla) were used to prepare date solution, which was used as a carbon source in the current study. Date solution was prepared by washing, pitting, and then heating 1 kg of dry dates in 5 L of water at a temperature of 70°C for 1 h. Date solution was 15° Brix and pH fixed between 4.3 and 4.7. The juice was first filtered through two stainless sieves of diameter (200 and 125 μm; Fisher Scientific, Illkirch, France), filtered through a No. 1 Whatman filter paper using a Buchner funnel, then sterilized at 120°C for 20 min.
Fermentation
The strain was seeded in 100 mL of homogenized Carlsberg medium and incubated at 30°C for 24 h (Acourene et al., Citation2007). The prefermentation medium containing 250 mL of date solution at 15° Brix and 250 mL of the following mineral solution [((NH2)2CO212.70 g, (NH4)2H2PO44.80 g, MgSO40.44 g, and NH4SO 5.30 g) in 1 L of distilled water] was sterilized at 120 ± 1°C for 20 min. The strain with the prefermentation medium was incubated in a 2-L reactor (Hans W. Schmidt (HWS), Mainz, Germany, type) at 30°C, pH 4.5 for 15 h. The method of Tan, Zhang, and Gao (Citation2003) was used with a slight modification. The fed-batch fermentation was carried out in a 2-L reactor (HWS type) at 30 ± 1°C, pH 4–4.5, and air flow rate of 15 L/min. Substrate feeding of date solution 15° Brix and mineral solution were added each hour in order to maintain sugar concentration in the reactor around 50 g/L (Zidani & Fahloul, Citation2008).
Protein content and nitrogen solubility
Nitrogen content of the supernatant was determined by Kjeldahl method (920.53; AOAC, Citation1998). Protein content was calculated by multiplying the nitrogen content by a factor of 6.25, assuming that proteins contain 16% of nitrogen. Nitrogen solubility was expressed as the percentage of the nitrogen content of the sample.
Effect of pH on protein solubility
Methods of Yu et al. (Citation2007) and Khalid et al. (Citation2003) were used with slight modifications. Biomass solution prepared at 2% (w/v) was adjusted in a pH range of 1–12 using HCl or NaOH solutions, since some proteins are soluble only in acid (pH 2) and alkaline (pH 12) solutions (Damodaran, Citation1996). Solution was stirred at room temperature for 1 h, followed by centrifugation at 3000 × gfor 30 min.
Effect of NaCl concentration on protein solubility
The effect of NaCl on the solubility was studied following the method of Scopes (Citation1994). Briefly, biomass solution prepared at 2% (w/v) was adjusted with NaCl concentrations (0, 0.1, 0.25, 0.5, 1, 1.25, 1.5, 2, and 3 M) at pH 7. The solution was stirred at room temperature for 1 h, followed by centrifugation at 3000 × gfor 30 min.
Effect of NaCl concentration/pH on protein solubility
Testing the effects of both NaCl concentration and pH was proceeded as above, with a pH adjustment corresponding to the maximum value of soluble proteins (Abd El-aal, Hamza, & Rahma, Citation1986; Scopes, Citation1994).
Effect of ethanol on protein solubility
One gram of the biomass was dispersed in 50 mL of ethanol dissolved in distilled water at 70% (v/v) and then stirred at room temperature for 1 h. The solution was then centrifuged at 1650 × gfor 20 min (Abd El-aal et al., Citation1986).
Drying of biomass
Three drying methods were used to dry the biomass: air drying, vacuum drying, and freeze drying. For the three drying methods, samples of 5 g were put in stainless plates. The initial moisture content was 80.14 ± 0.37 kg water/kg product. Samples were weighed every 15 min until the final moisture content of dried biomass was around 0.08 kg water/kg product. All experiments were performed in triplicate.
Air drying
Biomass was subjected to convective drying in an oven dryer (Memmert, Schwabach, Germany), at temperatures ranging from 50 to 110°C and air velocity of 1 m/s.
Vacuum drying
Vacuum drying was carried out in a laboratory oven Ev100 (Jouan, Winchester, VA, USA) with parameters following the work of Kompany, Allaf, Bouvier, Guignon, and Maureaux (Citation1990). A set of three temperatures (25, 35, and 45°C) were combined with two pressures (30 and 60 cmHg) were used for the drying.
Freeze drying
Freeze drying was carried out in a laboratory freeze dryer Beta 1 (Martin Christ, Osterode/Harz, Germany) at a plate temperature which was adjusted to 10°C. Product was frozen at −30°C. Sublimation occurred at a pressure of 0.05 mbar for 8 h and product temperature increased until it reached 25°C.
Protein solubility of dried biomass
The best solubility parameters obtained for fresh biomass (pH 12 and 1 M NaCl concentration) were included into the protocol described by Abd El-aal et al. (Citation1986). One gram of biomass of S. cerevisiaewas extracted twice with 50 mL of distilled water for 1 h at room temperature using a stirrer. The extract was centrifuged at 1650 × gfor 20 min. The residue was consequently used for extraction with 1 M NaCl at pH 12 and 70% (v/v) ethanol. The supernatant of each extract was collected separately and used for protein determination using Kjeldahl method (962.10) and a conversion factor of 6.25 (AOAC, Citation1998). Soluble protein is calculated as the sum of soluble protein of each supernatant.
Statistical analysis
All experiments were carried out in triplicates. Solubility data of dried biomass were analyzed with analysis of variance using XLSTAT 2009.1.01 software. Means were separated at P = 0.05 using Fisher's least significant difference (LSD) test.
Results and discussion
Biomass production
Total protein content of the produced biomass was 52.5 ± 0.07% (dry basis). This value is similar to the result of 55% (dry basis) obtained by Alais and Linden (Citation1994), using S. cerevisiaeyeast cultivated on molasses medium. This high amount of proteins allows S. cerevisiaeyeast to be considered as a source of food protein, similar to other conventional sources such as meat (Botton et al., Citation1990), caseinate and whey protein (Lee et al., Citation1992), and soy protein isolate (Wong & Kitts, Citation2003).
Protein solubility of fresh biomass
Effect of pH
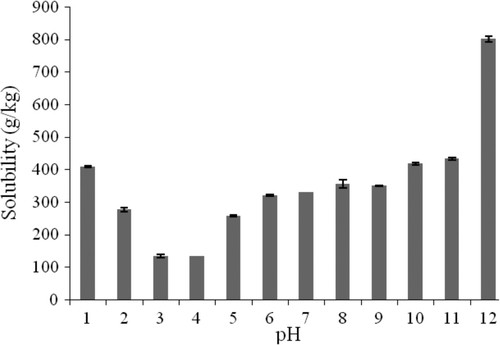
shows protein solubility over pH range from 1 to 12. The minimum solubility value was observed at a pH range of 3 and 4 with 132.5 g/kg and 134.6 g/kg of total proteins, respectively, with solubility increase at both ends of pH. The best solubility was observed in basic medium with a maximum value of 801.1 g/kg at pH 12. The isoelectric point of the protein of S. cerevisiaeyeast was estimated around pH 4. At this point, the electrostatic interactions between proteins are maximum and the net change close to zero. Therefore, the protein exhibited lowest solubility at this pH. In contrast, at pH 12, the electrostatic interactions are minimum; thus, protein solubility is the highest. The studied proteins showed solubility in both acid and alkaline regions, a criterion considered important in food formulation (Idouraine, Yensen, & Weber, Citation1991). Yu et al. (Citation2007) reported similar results in a study on peanut protein concentrate, with a minimum solubility at pH 3.5–4.5 and a maximum solubility at pH 10.
Effect of NaCl concentration
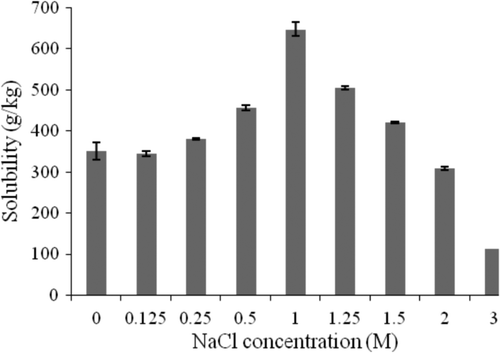
shows the total protein solubility as a function of NaCl concentration at pH 7. The solubility curve is divided into two phases. Increasing phase: where at low NaCl concentration, solubility increases with NaCl concentration from 0 to 1 M, corresponding, respectively, to 350.0 g/kg and 647.7 g/kg of total proteins. The maximum solubility value was obtained at 1 M which could be due to the salting-in phenomena, where electrostatic interactions between proteins and NaCl prevent protein cohesion and precipitation. Na+and Cl−ions surround proteins and allow their solubility (Scopes, Citation1994). Decreasing phase: where solubility decreases with NaCl concentration from 1 to 3 M, the minimum solubility reaches 113.1 g/kg due to the salting-out phenomenon (Scopes, Citation1994). In this situation, there are more NaCl ions and less solvent available to maintain protein solubility (Linden & Lorient, Citation1994). Therefore, solute–solute reactions are stronger than solute–solvent interactions (proteins–ions) leading to protein precipitation and a decrease in solubility.
Solubility profile as a function of NaCl concentration/pH
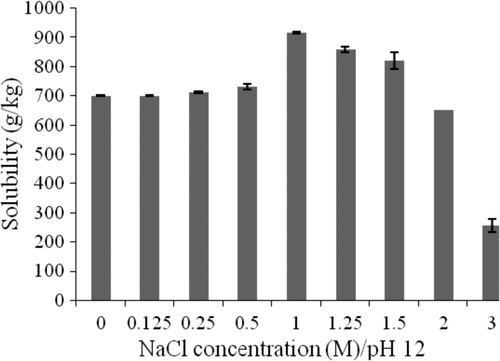
Total protein solubility as a function of NaCl concentration at pH 12 is depicted in . The combination of NaCl concentration and pH allowed the optimization of protein solubility with a value of 916.3 g/kg at 1 M NaCl concentration and pH 12. This is in agreement with the finding of Alais and Linden (Citation1994) on the solubility of whey protein concentrate and soya protein isolate.
Effect of ethanol on solubility
According to Damodaran (Citation1996), proteins are classified into four categories based on the solubility characteristics, and prolamines are those that are soluble in 70% ethanol (e.g., zein and gliadins). Protein solubility of the biomass in ethanol (70%, v/v) was 2.1 g/kg. This amount of solubilized protein is almost negligible. In this case, ethanol is rather used to precipitate than to dissolve proteins. Scopes (Citation1994) showed that proteins aggregate in ethanol. On the other hand, some proteins such as prolamines are almost soluble in ethanol (70%; Javillier et al., Citation1959). This is not the case in the current study of microbial proteins. Similar results were presented by Chin, Wheeler, and Klibanov (Citation1994), where the solubility of lysozyme of egg white was in the range of 0–3 g/kg in different organic solvents (octanol, tert-amyl alcohol, hexanol, acetonitrile, nitrobenzene, methylene chloride, and ethylene glycol).
Protein solubility of dried biomass
Effect of air drying
shows a stable solubility in the temperature range between 60 and 80°C with the values between 751.2 and 763.7 g of soluble proteins/kg of total proteins. Mean solubility values between 60 and 80°C were nonsignificantly different (P > 0.05). This stability is due to the fact that the critical temperature where protein denaturation begins was not reached yet. The supplied energy was not enough to break the energy of structure bonds. At 50°C, a low solubility was observed (530.1 g/kg; very significant difference P < 0.0001). This is similar to the results presented by Linden and Lorient (Citation1994), where a moderate heat treatment (40–55°C) induces a low solubility due to increasing protein–protein interactions. Linden and Lorient (Citation1994) showed that the interactions between milk protein increase at moderate temperatures (50–55°C), causing a decrease in protein solubility.
Table 1. Effect of air drying on S. cerevisiaeprotein solubility.
Tabla 1. Efecto de secado por aire en la solubilidad de la proteína S. cerevisiae.
Increasing the temperature of drying causes a solubility decrease with a minimum value around 50% of total proteins at 110°C. At this stage, thermo-denaturation becomes severe, and the primary structure is affected by desulphurization, formation of new derivatives, etc. (Adamiec, Kaminski, Markowski, & Strumillo, Citation2006). The modification of lateral chains of amino acid residues responsible for protein repulsion–attraction phenomena induces a loss of surface activity and hence decreases solubility. In other words, appearance of hydrophobic groups at the surface decreases solubility. In addition, at a temperature range between 100 and 140°C, Maillard reactions occur (between proteins and carbohydrates). Linden and Lorient (Citation1994) showed that a temperature less than 100°C preserves solubility. However, a higher temperature causes a negative effect on the solubility of plant proteins. According to Fennema (Citation1996), hydrophobic reactions are endothermic, stable at high temperatures, and unstable at low temperatures. However, their stability cannot increase infinitely with increasing temperature. Hydrophobic interactions reach a maximum stability at around 60–70°C.
Effect of vacuum drying
According to Fennema (Citation1996), pressure and temperature are two thermodynamic variables affecting protein denaturation, and hence their functional properties (including solubility). shows that solubility increases slightly with temperature increase (25, 35, and 45°C) for pressure 60 cmHg. At this stage, temperature is more dominant than vacuum. However, at lower pressure (30 cmHg), solubility is higher than that at a higher pressure (60 cmHg) and decreases with temperature increase (Richardson, Citation2001). A maximum solubility of 937.5 g/kg was obtained for the combination (25°C/30 cmHg). Therefore, it is not advantageous to work at temperatures higher than 25°C since a solubility decrease was observed. Vacuum drying allows water vaporization at a low temperature and heat transfer occurs mainly by conduction and radiation. Thus, protein solubility is higher during vacuum drying than air drying. This confirms the positive effect of vacuum. Thus, vacuum drying better preserves the structure and functional properties (solubility) of S. cerevisiaeproteins.
Table 2. Effect of vacuum drying on S. cerevisiaeprotein solubility.
Tabla 2. Efecto del secado por vacío en la solubilidad de la proteína S. cerevisiae.
Effect of freeze drying
Soluble protein content of the freeze-dried biomass represents 937.5 ± 1.9 g/kg of total proteins. This high value is confirmed with the literature (Fennema, 1996). Liapi and Bruttini (Citation2006) showed that freeze drying minimizes reactions such as nonenzymatic browning, protein denaturation, and enzymatic reactions which occur during air drying. During freeze drying, 90% of water is removed as a vapor causing minimum salts and/or carbohydrates migration to the drying surface, hence minimizing interactions between components leaving solubility unaffected. Solubility values were greater than the values found by Cepeda et al. (Citation1998), where protein solubility of freeze-dried and spray-dried faba bean did not exceed 50%. Comparison between these results is not simple due to the fact that there are a wide variety of proteins with specific structure, function, and properties.
Comparison of the drying methods
shows that solubility of the dried biomass using the three drying methods is smaller than the solubility of proteins of fresh biomass. Difference is highly significant (for air drying P < 0.0001 for the seven temperatures) or nonsignificant [for vacuum drying (25°C/30 cmHg) and freeze drying P > 0.05]. According to Wong and Kitts (Citation2003), solubility decrease is due to the interactions between proteins–proteins and proteins with carbohydrates, lipids, and other food components (case of dried eggs and milk). Nevertheless, solubility for the three drying methods exceeds 50%, which is important compared to other studied proteins (animals or plants).
Table 3. Comparison of three drying methods on S. cerevisiaeprotein solubility.
Tabla 3. Comparación de tres métodos de secado en la solubilidad de la proteína S. cerevisiae.
The best drying methods are freeze drying and vacuum drying at (25°C/30 cmHg) with solubility of 937.5 g/kg for both methods. However, for air drying, solubility is lower with a percentage of 763.7 g/kg at 70°C (a decrease of 18%). In addition, based on the fact that freeze drying has a higher cost than vacuum drying. Therefore, vacuum drying method will be more advantageous than freeze drying.
Conclusion
Protein production by fermentation of S. cerevisiaecultivated on musts of dates produced a high amount of proteins, which allows S. cerevisiaeyeast to be considered as a source of food protein. The studied proteins showed solubility in both acid and alkaline regions, with the best solubility in alkaline region. The effect of NaCl concentration divided the solubility curve into two phases: increasing and decreasing phases with the maximum solubility value obtained at 1 M. The combination of NaCl concentration and pH allowed the optimization of protein solubility at 1 M NaCl concentration and pH 12. Protein solubility in ethanol (70%, v/v) was almost negligible. Ethanol was useful to precipitate rather than to solubilize proteins.
Solubility of the dried biomass using the three drying methods was smaller than the solubility of proteins of fresh biomass. Protein solubility is higher during vacuum drying than air drying. Soluble protein content of the freeze-dried biomass was similar to vacuum drying. Therefore, the best drying methods were freeze drying and vacuum drying.
References
- Abd El-aal , M.H. , Hamza , M.A. and Rahma , E.H. 1986 . In vitro digestibility, physico-chemical and functional properties of apricot kernel proteins . Food Chemistry , 19 : 197 – 211 .
- Acourene , S. , Khalid , A.Kh. , Bacha , A. , Tama , M. and Taleb , B. 2007 . Optimization of bakery yeast production cultivated on musts of dates . Journal of Applied Sciences Research , 3 ( 10 ) : 964 – 971 .
- Adamiec , J. , Kaminski , W. , Markowski , A.S. and Strumillo , C. 2006 . “ Drying of biotechnological products ” . In Handbook of industrial drying , Edited by: Mujumdar , A.S. 931 – 954 . New York , NY : Taylor & Francis .
- Alais , C. and Linden , G. 1994 . Biochimie alimentaire , Paris : Masson .
- Aluko , R.E. and Yada , R.Y. 1993 . Relationship of hydrophobicity and solubility with some functional properties of cowpea (Vigna unguiculata) protein isolate . Journal of the Science of Food and Agriculture , 62 : 331 – 335 .
- AOAC . 1998 . Official methods of analysis , Washington, DC , , USA : Association of Official Analytical Chemists .
- Bernard , A. and Carlier , H. 1992 . Aspects nutritionnels des constituants des aliments. Influence des technologies , 90 – 100 . Paris : Technique et Documentation .
- Bessah , R. and Touzi , A. 2001 . Production de protéines d'organismes unicellulaires (P.O.U) à partir de déchets des dattes . Revue des Energies Renouvelables , : 37 – 40 .
- Botton , B. , Breton , A. , Fevre , M. , Gauthier , S. , Guy , Ph. , Larpent , J.P. … and Veau , P. 1990 . Moisissures utiles et nuisibles. Importance industrielle , (2nd ed. , 135 – 320 . Paris : Masson .
- Cepeda , E. , Villaran , M.C. and Aranguiz , N. 1998 . Functional properties of faba bean (Vicia faba) protein flour dried by spray drying and freeze-drying . Journal of Food Engineering , 36 : 303 – 310 .
- Chin , J.T. , Wheeler , S.L. and Klibanov , A.M. 1994 . Protein solubility in organic solvents . Biotechnology and Bioengineering , 44 : 140 – 145 .
- Damodaran , S. 1996 . “ Amino acids, peptides and proteins ” . In Food chemistry , (3rd ed. , Edited by: Fennema , O.R. 370 – 375 . NewYork , NY : Marcel Dekker .
- Fennema , O.R. 1996 . Food chemistry , (3rd ed. , 321 – 425 . NewYork , NY : Marcel Dekker .
- Idouraine , A. , Yensen , S.B. and Weber , C.W. 1991 . Tepary bean flour, albumin and globulin fractions functional properties compared with soy protein isolates . Journal of Food Science , 56 : 1316 – 1318 .
- Javillier , M. , Polonovski , M. , Florkin , M. , Boulanger , P. , Lemoigne , M. , Roche , J. and Wurmser , R. 1959 . Traité de biochimie générale, Tome I , 708 – 1475 . Paris : Masson & Cie .
- Jovanovic , N. , Bouchard , A. , Hofland , G.W. , Witkamp , G.J. , Crommelin , D.J.A. and Jiskoot , W. 2006 . Distinct effects of sucrose and trehalose on protein stability during supercritical fluid drying and freeze-drying . European Journal of Pharmaceutical Sciences , 27 : 336 – 345 .
- Khalid , E.K. , Babiker , E.E. and EL Tinay , A.H. 2003 . Solubility and functional properties of sesame seed proteins as influenced by pH and/or salt concentration . Food Chemistry , 82 : 361 – 366 .
- Kompany , E. , Allaf , K. , Bouvier , J.M. , Guignon , P. and Maureaux , A. 1990 . Nouveau procédé de déshydratation des fruits et légumes à réhydratation rapide . Industries Alimentaires et Agricoles , 107 ( 12 ) : 1243 – 1248 .
- Lee , S.Y. , Morr , C.V. and Ha , E.Y.W. 1992 . Structural and functional properties of caseinate and whey protein isolate as affected by temperature and pH . Journal of Food Science , 57 ( 5 ) : 1210 – 1229 .
- Liapi , A.I. and Bruttini , R. 2006 . “ Freeze drying ” . In Handbook of industrial drying , Edited by: Mujumdar , A.S. 257 – 281 . New York , NY : Taylor & Francis .
- Linden , G. and Lorient , D. 1994 . Biochimie agro-industrielle valorisation alimentaire de la production agricole , 18 – 40 . Paris : Masson .
- Richardson , P. 2001 . Thermal technologies in food processing , Cambridge , England : Woodhead Publishing .
- Scopes , R.K. 1994 . Protein purification – Principles and practice , (3rded. , 71 – 78 . New York , NY : Springer-Verlag .
- Tan , T. , Zhang , M. and Gao , H. 2003 . Ergosterol production by fed-batch fermentation of . Saccharomyces cerevisiae. Enzyme and Microbial Technology , 33 : 366 – 370 .
- Wong , P.Y.Y. and Kitts , D.D. 2003 . A comparison of the buttermilk solids functional properties to nonfat dried milk, soy protein isolate, dried egg white and egg yolk powders . Journal of Dairy Science , 86 ( 3 ) : 746 – 754 .
- Yu , J. , Ahmedna , M. and Goktepe , I. 2007 . Peanut proteins concentrate: Production and functional properties as affected by processing . Food Chemistry , 103 : 121 – 129 .
- Yu , Z. , Johnston , K.P. and Williams , R.O. III . 2006 . Spray freezing into liquid versus spray-freeze drying: Influence of protein atomization on protein aggregation and biological activity . European Journal of Pharmaceutical Sciences , 27 : 9 – 18 .
- Zidani , S. and Fahloul , D. 2008, December . Etude de la solubilité des protéines de S. Cerevisiae produite dans un milieu à base de datte en fonction du pH, force ionique, et solvants organiques , Hammamet, Tunisia : 7èmes Journées Biotechnologiques .