Abstract
Mao-tofu was fermented by Mucor spp. for 3–9 days. Soluble protein fractions were then extracted by three solvents with ethanol/water ratios of 2:8, 4:6 or 6:4 (v/v), respectively, under fixed weight–volume ratio of 3:10 (w/v). Analysis results showed that the longer fermentation time of Mao-tofu, the higher extraction yield or degree of hydrolysis (DH) of the protein fractions. The solvent with higher ethanol content trended to extract less protein fractions but lead them higher DH. When Mao-tofu was fermented for five days and extracted by the solvent with ethanol/water ratio of 6:4 (v/v), the extract exhibited the highest ACE-inhibitory activity in vitro. Amino acid composition analysis showed that the extract with higher activity contained more total hydrophobic amino acids or proline. Further hydrolysis of the extract by five proteases in vitro might damage or enhance its activity, depending on the extract hydrolyzed and the protease applied.
Se fermentó Tofu Mao por Mucor spp entre tres y nueve días. Fracciones de proteína soluble fueron extraídas mediante tres soluciones con proporción etanol/agua de 2:8, 4:6 o 6:4 (v/v), respectivamente, bajo una proporción fija peso-volumen de 3:10 (w/v). Los resultados de los análisis mostraron que cuanto más largo era el tiempo de fermentación, más alto el rendimiento de extracción o grado de hidrólisis (DH) de las fracciones de proteína. El solvente con mayor contenido de etanol tendió a extraer menos fracciones de proteína, pero conllevó un mayor DH. Cuando el tofu Mao fermentó durante cinco días y fue extraído por solución con proporción de etanol/agua de 6:4 (v/v), el extracto mostró la mayor actividad inhibidora de ACE in vitro. El análisis de composición de aminoácidos mostró que el extracto con la mayor actividad contenía más aminoácidos hidrofóbicos totales o prolina. Una mayor hidrólisis de extracto por cinco proteasas in vitro pudo dañar o mejorar su actividad, dependiendo del extracto hidrolizado y la proteasa aplicada.
Palabras claves:
Introduction
Angiotensin-I-converting enzyme (ACE, EC 3.4.15.1) plays an important physiological role in regulating blood pressure (Skeggs, Kahn, Kahn, & Shumway, Citation1957). Since the discovery of ACE inhibitors in snake venom, many studies were directed toward the attempt to synthesize ACE inhibitors such as captopril, enalapril, alacepril, and lisinopril (Ondetti, Rubin, & Cushman, Citation1977; Patchett et al., Citation1980). As some synthetic drugs were found to have side effects such as cough, taste disturbances or skin rashes (Atkinson & Robertson, Citation1979), development of ACE inhibitors from food resources becomes an interesting topic. After a first report about the ACE-inhibitory peptides from food proteins by digestive proteases (Oshima, Shimabukuro, & Nagasawa, Citation1979), many ACE-inhibitory peptides from protein hydrolysates were studied, including those peptides from soybean proteins (Shin et al., Citation2001; Wu & Ding, Citation2001), casein (Kim & Chung, Citation1999; Tauzin, Miclo, & Gaillard, Citation2002), chicken muscle or egg (Fujita, Yokoyama, & Yoshikawa, Citation2000; Yoshii et al., Citation2001), whey proteins (Mullally, Meisel, & FitzGerald, Citation1997; Pihlanto-Leppälä, Citation2001), and fish proteins (Curis, Dennes, Waddell, Macgillivray, & Ewart, Citation2002; Wako, Ishikawa, & Muramoto, Citation1996).
Fermentation as one of the oldest food processing techniques can induce protein breakdown and ACE-inhibitory peptide formation in fermented foods, such as in Korean traditional rice wine (Kim, Lee, Choi, Park, & Lee, Citation2006) and fermented milk or soymilk (Apostolidis, Kwon, Ghaedian, & Shetty, Citation2007). Some traditional fermented soybean products, e.g. soybean sauce (Kinoshita, Yamakoshi, & Kikuchi, Citation1993), Korean soybean paste (Shin et al., Citation2001), tempeh (Gibbs, Zougman, Masse, & Mulligan, Citation2004), natto (Okamoto,Hanagata, Kawamura, & Yanagida, Citation1995; Okamoto, Hanagata, Matsumoto, Kawamura, & Yanagida, Citation1995), and douchi (Fan et al., Citation2009), were reported to have ACE-inhibitory peptides resulted from enzymatic digestion of soybean proteins. Sufu, a mould-fermented tofu (soybean protein curd) in China, was also found to have ACE-inhibitory activity (Wang, Saito, Tatsum, & Li, Citation2003). Mao-tofu is another type of mould-fermented tofu in China primarily by Mucor spp. capable of degrading soybean proteins (Zhao & Zheng, Citation2009) or milk proteins (Zhang & Zhao, Citation2010). Unfortunately, no information is available in the scientific articles to report the physiological functions including ACE inhibition of Mao-tofu.
In the present study, tofu was fermented by a strain of Mucor spp. isolated from Mao-tofu, and three ethanol/water-based solvents were used to extract soluble protein fractions from the Mao-tofu fermented for different times. The extracts were evaluated for extraction yield and degree of hydrolysis (DH) of the protein fractions, and ACE-inhibitory activity in vitro. Amino acid content and resistance to enzymatic digestion in vitro of some prepared extracts were measured. The goal was to reveal the impacts of fermentation time and extraction solvent system on ACE-inhibitory activity of Mao-tofu extract.
Materials and methods
Materials
Tofu used for Mao-tofu preparation was purchased from a local market. Soybean protein isolates (SPI) with protein content of 92.0% (on dry basis) were prepared as per the method of Petruccelli and Añón (Citation1994). N-(3-[2-furyl]acryloyl)-l-phenylalanylglycylglycine (FAPGG) and rabbit lung acetone powder were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Neutrase (24 kU/mL) and alcalase (100 kU/g) were purchased from Novozymes (China) Biotechnology Co., Ltd (Tianjin, China). Papain (18 kU/g), trypsin (40 kU/g), and pepsin (3000 kU/g) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China), Sigma-Aldrich Co., and Hui Shi Biochem. Reagent Co. (Shanghai, China), respectively. All chemicals used were of analytical grade. The water used was redistilled water.
Preparation of Mao-tofu
The spore suspension of the Mucor (identified as Mucor micheli ex fries) was prepared as per the method of Sparringa and Owens (Citation1999), and stored at 5°C ready for Mao-tofu preparation. Fermentation of the tofu was carried out as per the previous work (Zhao & Zheng, Citation2009). Fresh tofu was cut into cubes (18 cm × 14 cm × 10 cm) by a knife. After being cooked in boiling water for 20 min to inactivate the micro-organisms or enzymes contaminated, the cooled cubes were cut into dice (3 cm × 3 cm × 3 cm) aseptically and inoculated with the Mucor inoculum (105 spores/mL) over the surface. All dice were put in sterilized bamboo trays separately to facilitate air circulation and mycelia development, and fermented at 20 ± 1°C with a relative humidity about 73–76% for nine days. Some dice were selected randomly at 3, 5, 7, and 9 days as analysis samples and subjected to later extraction or analysis.
Analysis of Mao-tofu
The prepared Mao-tofu was analyzed for its moisture content by the AOAC Methods 926.08, total protein or water soluble protein content by the Kjeldahl method 920.123 (AOAC, Citation2000) on a Kjeltec 2300 Analyzer (Foss Tecator AB, Höganäs, Sweden), expressed as the weight in 100-gram sample (g/100g). For total protein content analysis, the sample was homogenized, and then 1 g homogenate was weighed exactly for analysis. For total water soluble protein content analysis, the water soluble proteins of the Map-tofu were prepared as a reported method (Moatsou, Moschopoulou, & Anifantakis, Citation2004) with some modifications. The Mao-tofu of 10 g was homogenized in 50 mL water by a high speed homogenizer (Type DS-1; Shanghai Jingke Ltd., Shanghai, China) for 5 min, centrifuged at 4000 g for 20 min and filtered through filter paper (Whatman 40) to obtain total water soluble protein extract. The obtained extract was diluted into 50 mL with water, and 10 mL of the diluted extract was weighed for nitrogen content analysis. A conversion factor of 5.71 was used to calculate the total protein or water soluble protein content of the Mao-tofu.
Preparation of Mao-tofu extract
The prepared Mao-tofu was extracted with three ethanol/water-based extraction solvents, namely the solvent I, II, and III with ethanol/water ratios of 2:8, 4:6, and 6:4 (v/v), respectively. The extraction was carried out as follow: the Mao-tofu of 75 g was mixed with the solvent of 250 mL at room temperature in a plastic beaker to give a weight (kg)–volume (L) ratio of 3:10 (w/v), homogenized by the homogenizer (Type DS-1; Shanghai Jingke Ltd.) at 10,000 rev/min for 2 min, centrifuged at 4000 g for 20 min, and then filtered through filter paper (Whatman 40) to obtain the soluble extract. The extract was evaporated under vacuum at 50°C to remove ethanol and reconstituted to 250 mL with water. Nitrogen content in the prepared extract or the Mao-tofu was measured by the Kjeldahl method (AOAC, Citation2000) and used to calculate the extraction yield of the soluble protein fractions as below.
Where, P E and P S are total proteins in the prepared extract and Mao-tofu of 75 g, respectively.
Evaluation of degree of hydrolysis
An o-pthaldialdehyde (OPA) method (Spellman, McEvoy, O'Cuinn, & Fitz, Citation2003) was used to measure the amount of free amino groups of the soluble protein fractions in the prepared extract. The OPA reagent was prepared by combining the following reagents to a volume of 100 mL with water: 0.2 mol/L sodium borate buffer (pH 9.5) of 75 mL, 400 g/L SDS solution of 5 mL, 80mg OPA (in 1.0 mL methanol), and 0.4 mL β -mercaptoenthanol. The reagent was prepared daily and stored in the dark. The assay was carried out by adding 3.0 mL analysis sample to 3.0 mL OPA reagent. The absorbance of the mixture was measured at 340 nm in an UV spectrophotometer (UV-2401PC; Shimadzu Corporation, Kyoto, Japan) and taken after 5 min. l-Leucine solution (12–36 μg/mL) was used as standard. Nitrogen content of the extract was determined by the Kjeldahl method (AOAC, Citation2000). DH of the soluble protein fractions was calculated as the reported method of Adler-Nissen (Citation1979).
Assaying of ACE-inhibitory activity in vitro
ACE-inhibitory activity in vitro was measured as a reference method (Murray, Walsh, & FitzGerald, Citation2004) with FAPGG as substrate and the extract of rabbit lung acetone powder as ACE source. The reaction mixture contained 0.1 mL of the diluted Mao-tofu extract (or SPI) fixed at 0.5 mg proteins/mL, 0.5 mL of 1.6 mmol/L FAPGG in 0.1 mol/L sodium borate buffer (pH 8.3) with 0.3 mol/L NaCl. Then, 0.3 mL 10x diluted ACE extract in 0.1 mol/L sodium borate buffer (pH 8.3) containing 5% (v/v) glycerol was added into the mixture to start the reaction. The reaction was carried out at 37°C for 30 min and terminated by addition of 0.1 mol/L EDTA 0.1 mL. The EDTA was added immediately into the mixture before the ACE extract in zero-time control assays. The whole mixture was diluted with 3 mL water. The absorbance decrease (ΔA I) was determined in the spectrophotometer at 340 nm and taken as a measure of ACE activity. A control sample (0.1 mL of deionized water) was also assayed as above. The ACE inhibition of the extract (or SPI) was calculated as below.
Where, ΔA I and ΔA C are the absorbance decrease at 340 nm of the inhibitor (the extract or SPI) and control, respectively, which is equal to the absorbance difference between zero-time control assay and 30-min reaction assay.
Enzymatic hydrolysis of Mao-tofu extract
The Mao-tofu with the highest ACE-inhibitory activity was extracted with the three solvents. Each extract prepared was freeze-dried to obtain powdered extract. Part of the powdered extract was reconstituted in water to prepare a solution of 50 mg proteins/mL. Aliquot of the solution was then hydrolyzed by papain (pH 6.5, 45°C), neutrase (pH 7.0, 50°C), alcalase (pH 8.0, 55°C), trypsin (pH 8.0, 37°C), and pepsin (pH 2, 37°C) for 0.5 h, with enzyme addition of 10, 0.5, 0.5, 4, and 30 kU/g proteins, respectively. The hydrolyzed solutions were heated at 100°C for 10 min, cooled to room temperature, adjusted to pH 8.3, diluted to 0.5 mg proteins/mL, and assayed for their ACE-inhibitory activities.
Analysis of amino acid composition
The prepared powdered extracts of 0.2 g were hydrolyzed under reduced pressure in 6 mol/L HCl at 110°C for 24 h. Seventeen amino acids except for Trp were analyzed by using amino acids analyzer 835-50 (Hitachi, Tokyo, Japan) with the procedure provided by the manufacturer. Total hydrophobic amino acids (THAA) were calculated as the sum of eight amino acids including Ala, Val, Leu, Ile, Phe, Pro, Tyr, and Met.
Statistical analysis
All experiments were carried out in triplicate. All data were expressed as means ± standard deviation. Differences between the mean values of multiple groups were analyzed by one‐way analysis of variance (ANOVA) with Tukey's test. Microsoft Excel version 2003 software (Microsoft Corporation, Redmond, WA, USA) and SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA) were used to analyze or report the data.
Results and discussion
Fermentation and extraction of Mao-tofu
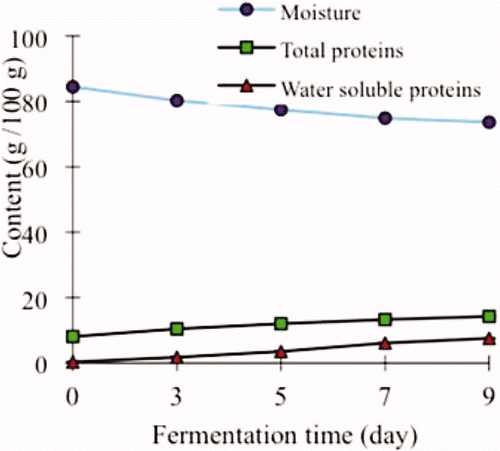
As fermentation time progressed, the main composition of the Mao-tofu exhibited changes as in . Moisture content of the Mao-tofu decreased from 84.46 to 73.64 g/100g, indicating water loss during fermentation. Meanwhile, the total protein or water-soluble protein content of the Mao-tofu increased from 8.11 or 0.33 to 14.26 or 7.56 g/100g, respectively, partly as the results of water loss or/and protein degradation during fermentation.
Figure 1. Moisture, total protein, and soluble protein content of Mao-tofu fermented by Mucor spp. during a fermentation period of nine days. Each evaluation was carried out triplicate.
Figura 1. Humedad, contenido total de proteína y de proteína soluble de tofu Mao fermentado por Mucor spp. durante un período de fermentación de nueve días. Cada evaluación se realizó por triplicados.
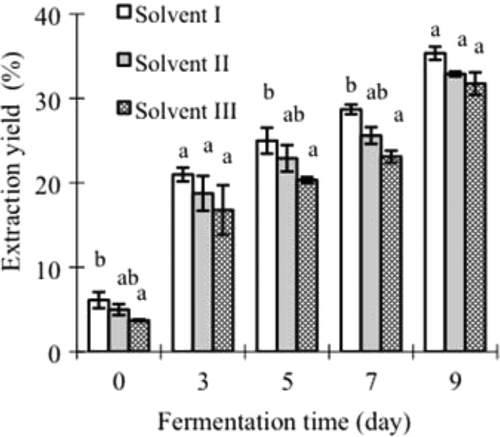
When ethanol/water solvents were used to extract protein fractions from the Mao-tofu, both fermentation time and solvent composition had some impacts on the extraction yield of soluble protein fractions (). Extraction yield of the protein fractions was enhanced as fermentation time progressed. At the late stage of fermentation (nine days), the extraction yield was the highest (31.8–35.3%), viz. nearly a third of total proteins in the Mao-tofu was extractable. Logically, when ethanol is mixed into water, the whole solvent has a lower polarity than water; the protein fractions should be less soluble in the solvent than in water. As more ethanol was mixed into water (e.g. the solvent III), less protein fractions would be extractable (viz. the solvent III should extract less protein fractions) as the trends shown in .
Figure 2. Extraction yield of soluble protein fractions from the Mao-tofu by ethanol/water solvent I, II, and III with volume ratios of 2:8, 4:6, and 6:4 (v/v), respectively. The different lowercase letters above the columns within same fermentation time indicate the data are significantly different (p < 0.05).
Figura 2. Rendimeineto de extracción de fracciones de proteína soluble de tofu Mao por solución acuosa de etanol I, II y III con proporciones de volumen 2:8, 4:6 y 6:4 (v/v), respectivamente. Las letras diferentes sobre las columnas en el mismo tiempo de fermentación indican que los resultados son significativamente diferentes (p < 0,05).
The DH of the protein fractions in the extract also showed protein degradation during Mao-tofu fermentation. Tofu was analyzed and served as a control. Fermentation time exhibited greater impact on the DH of the extracts. The DH of the protein fractions in the extract was enhanced significantly from 17.4 to 40.9% (p < 0.05) as fermentation time was increased (). This indicates the occurrence of protein degradation and the protein fractions to be small peptides or free amino acids (for the DH of the extract was very higher). In theory, the solvent III or I had the lower or higher polarity among the applied solvents, and might extract less or more protein fractions from the Mao-tofu; consequentially, the prepared extract would have a higher or lower DH. The data () revealed that solvent polarity indeed had some impacts on the DH of the prepared extract totally. The solvent III or I usual showed a trend to extract the protein fractions with a higher or lower DH. It was likely that the solvent polarity only showed less impact on the DH of the extract than fermentation time.
Table 1. Degree of hydrolysis (DH, %) of the protein fractions in Mao-tofu extract prepared by three extraction solvents.
Tabla 1. Grado de hidrólisis (DH, %) de fracciones de proteína en extracto de tofu Mao peparado por tres solventes de extracción.
Han, Rombouts, and Nout (2004) found that total free amino acids had four-folds increases during sufu ripening. Zhu, Fan, Cheng, and Li (Citation2008) studied protein degradation in Chinese traditional fermented okara by SDS-PAGE. They found a gradually protein hydrolysis occurred during first 48 h, and after then observed the virtual disappearance of the proteins in the gels. Two reported results supported our present result that the Mucor induced protein degradation in the Mao-tofu during fermentation, which also reflected by the DH profiles of the soluble protein fractions in .
ACE-inhibitory activity in vitro of Mao-tofu extract
All Mao-tofu extracts exhibited higher ACE-inhibitory activity in vitro than the native soybean proteins (SPI). Both fermentation time and solvent composition exhibited impact on the activity of the extract as displayed. Totally, fermentation time exhibited different impacts on the activity of the extract. When the Mao-tofu was fermented for five days, the extract showed better activity (p < 0.05). Too long a fermentation time led the extract to lower activity. If the Mao-tofu was fermented for 3–7 days and extracted by a lower polarity solvent, the activity of the extract was also impacted by the solvent. For example, the extract prepared by solvent III usual exhibited higher activity than that prepared by solvent I. Extraction of the Mao-tofu fermented for five days by the solvent III would lead to the highest activity.
Table 2. In vitro ACE-inhibitory activity (%) of protein fractions in Mao-tofu extract prepared by three extraction solvents.
Tabla 2. Actividad inhibidora de ACE in vitro (%) de fracciones de proteína en extracto de tofu Mao preparado con tres .solventes de extracción.
Soybean sauce and paste had ACE-inhibitory activity (Kinoshita, Yamakoshi, & Kikuchi, Citation1993; Shin et al., 2001). The concentration of inhibitor needed to inhibit the ACE by 50% (IC50) of miso paste, tofuyu, and soybean sauce was measured as 1.27, 1.77, and 3.44 mg/mL (Kuba, Tanaka, Tawwata, Takeda, & Yasuda, Citation2003). Okamoto, Hanagata, and Matsumoto (1994) reported an IC50 of 5.35 mg/mL for soybean miso. The starter micro-organisms had impact on ACE-inhibitory activity of the fermented foods (Pihlanto-Leppälä, Rokka, & Korhonen, Citation1998; Vermeirssen, Smagghe, Beckers, & Camp, Citation2003), and the level of microbial enzymes in Mucor starters was higher than that in Aspergillus starters (Kang, Citation2001). More important, Fan et al. (2009) found that Mucor-type douchi had higher ACE-inhibitory activity than Aspergillus- or bacterial-type douchi, and accounted for the higher activity of Mucor-type douchi as the higher proteolytic activity and specificity of enzymes secreted by the starters. How the proteases secreted by the Mucor impacted the ACE-inhibitory activity of Mao-tofu remains unclear and needs to be studied in later work, but the findings in Fan et al. (Citation2009) provided support to the present result that the Mucor-fermented Mao-tofu had better ACE-inhibitory activity.
Influence of further hydrolysis on ACE-inhibitory activity of Mao-tofu extract
The stability of the Mao-tofu extracts against some proteases was examined in vitro. As the Mao-tofu fermented for other than five days showed a lower ACE-inhibitory activity, hence, three extracts from the Mao-tofu fermented for five days were selected to carry out further hydrolysis. After being hydrolyzed by one of five proteases, the hydrolyzed extracts had an increase in the amount of free amino groups about 40–180 μmol/g proteins (details not given here). ACE-inhibitory activity evaluation indicated that, compared to the original extract I (II or III), the hydrolyzed extract I (II or III) had similar or mostly different activity (), which was depended on what the extract was hydrolyzed and what the protease was applied. Totally, pepsin treatment showed no significant impact (p < 0.05) on the activity of the hydrolyzed extracts (viz. the extracts were resistant to pepsin) while neutrase treatment enhanced their activity. Papain or alcalase treatment improved the activity of two hydrolyzed extracts but trypsin treatment impaired the activity of two hydrolyzed extracts. The evaluation also showed that only the extract III remained its activity unimpaired (pepsin treatment) or enhanced by the further hydrolysis of other four proteases.
Table 3. Impacts of enzymatic hydrolysis of three Mao-tofu extracts on their ACE-inhibitory activity in vitro
a.
Tabla 3. Impacto de hidrólisis enzimática de tres extractos de tofu Mao en su actividad inhibidora de ACE in vitro.
Matsui et al. observed that when royal jelly hydrolysates were treated with pepsin followed by trypsin and chymotrypsin, its ACE-inhibitory activity had a four-fold increase, compared to with pepsin alone (Matsui et al., Citation2002). It was also reported that the inhibitory activity of the β-conglycinin hydrolysates markedly increased after successive digestion by pepsin and trypsin (Kuba, Tana, Tawata, & Yasuda, Citation2005). Two reports confirm our work that further hydrolysis of Mao-tofu extract by some protease might enhance its ACE inhibition. More detailed work is needed to reveal the possible mechanism behind.
Amino acid compositions of Mao-tofu extract
lists the composition of 17 amino acids except for Trp of three extracts obtained from the Mao-tofu fermented for five days. Among these extracts, the extract of the solvent III had the highest THAA, Pro and activity, while the extract of the solvent I had the lowest THAA, Pro and activity ( and ). It could be assumed that more THAA or Pro accounted for the highest activity of the extract.
Table 4. Amino acid composition (mol, %) of three Mao-tofu extracts prepared.
Tabla 4. Composición de aminoácidos (mol, %) de tres extractos de tofu Mao preparados.
Hydrophobic amino acids especially Pro are usually found in ACE-inhibitory peptides, such as Arg-Ala-Asp-His-Pro-Phe from albumin (He, Chen, Sun, & Zhang, Citation2004), Val-Pro-Pro from milk (Pan, Luo, & Tanokura, Citation2005), Asp-Leu-Pro and Leu-Ala-Ile-Pro-Val-Asn-Lys-Pro from soybean proteins (He et al., Citation2004; Kuba et al., Citation2005), Gly-Pro-Pro from buckwheat (Ma, Bae, Lee, & Yang, Citation2006), and Pro-Pro-Glu-Ile-Asn or Pro-Leu-Pro-Leu-Leu from yak milk casein (Mao,Ni, Sun, Hao, & Fan, Citation2007). Based on the above mentioned results, it is reasonable that the lowest polarity solvent III (ethanol/water ratio of 6:4) extracted more hydrophobic peptides from the Mao-tofu and lead the extract to the highest activity. More detailed work is needed to study the relationship among protein degradation, amino acid metabolism, and peptide activity during Mao-tofu fermentation.
Conclusions
Soluble extract of Mucor-fermented Mao-tofu extracted by ethanol/water solvent exhibited better in vitro ACE-inhibitory activity than SPI. Both fermentation time and solvent composition (ethanol/water ratio) showed different impacts on the extraction yield, DH and especially ACE-inhibitory activity in vitro of the extracts. Totally, fermentation time of Mao-tofu showed greater impact on DH and ACE-inhibitory activity in vitro of the extract than the applied solvent types. If Mao-tofu was fermented for five days and extracted by an ethanol/ water solvent at a ratio of 6:4 (v/v), the obtained extract had the highest activity, hydrophobic amino acids or proline. Further hydrolysis of the Mao-tofu extracts by five selected proteases in vitro might increase or decrease the ACE-inhibitory activities of the prepared extracts, which was dependant on what the extract prepared was hydrolyzed with, and what protease was applied.
Acknowledgements
The authors thank the anonymous reviewers and the editors for their constructive and valuable work on our article. The authors also thank Prof. Jing Wang, an analytical chemist in CAAS for her kind help in amino acid analysis.
References
- Adler-Nissen , J. 1979 . Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid . Journal of Agricultural and Food Chemistry , 27 : 1256 – 1261 .
- AOAC . 2000 . Official methods of analysis of AOAC international , (17th ed.) , Gaithersburg : Author .
- Apostolidis , E. , Kwon , Y.I. , Ghaedian , R. and Shetty , K. 2007 . Fermentation of milk and soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension . Food Biotechnology , 21 : 217 – 236 .
- Atkinson , A.B. and Robertson , J.I.S. 1979 . Captopril in the treatment of clinical hypertension and cardiac failure . Lancet , 2 : 836 – 839 .
- Curis , J.M. , Dennes , D. , Waddell , D.S. , Macgillivray , T. and Ewart , H.S. 2002 . Determination of angiotensin-converting enzymeinhibitory peptide Leu-Lys-Pro-Asn-Met (LKPNM) in bonito muscle hydrolysates by LC–MS/MS . Journal of Agricultural and Food Chemistry , 50 : 3919 – 3925 .
- Fan , J.F. , Hu , X.Z. , Tan , S. , Zhang , Y.Y. , Tatsumi , E. and Li , L.T. 2009 . Isolation and characterisation of a novel angiotensin I-converting enzyme-inhibitory peptide derived from douchi, a traditional Chinese fermented soybean food . Journal of the Science and Food Agriculture , 89 : 603 – 608 .
- Fujita , H. , Yokoyama , K. and Yoshikawa , M. 2000 . Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins . Journal of Food Science , 65 : 564 – 569 .
- Gibbs , B.F. , Zougman , A. , Masse , R. and Mulligan , C. 2004 . Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food . Food Research International , 37 : 123 – 131 .
- Han , B.Z. , Rombouts , F.M. and Nout , M.J.R. 2004 . Amino acid profiles of sufu, a Chinese fermented soybean food . Journal of Food Compositions and Analysis , 17 : 689 – 698 .
- He , H. , Chen , X. , Sun , C. and Zhang , Y. 2004 . Research progress in inhibitory peptides of angiotensin-converting enzyme . China Biotechnology , 24 : 7 – 11 . in Chinese
- Kang , M.G. 2001 . Handbook of processing methods of famous fermented foods in china and foreign countries , (2nd ed.) , [In Chinese] Beijing : Chemical Industry Inc .
- Kim , Y.K. and Chung , B.H. 1999 . A novel angiotensin I-converting enzyme inhibitory peptide from human as1-casein . Biotechnology Letters , 21 : 575 – 578 .
- Kim , J.H. , Lee , D.H. , Choi , S.Y. , Park , J.S. and Lee , J.S. 2006 . Effects of Lycii fructus and edible mushroom, Pholiota adiposa, on the quality and Angiotensin I-converting enzyme inhibitory activity of Korean traditional rice wine . Food Biotechnology , 20 : 183 – 191 .
- Kinoshita , E. , Yamakoshi , J. and Kikuchi , M. 1993 . Purification and identification of an angiotensin I-converting enzyme inhibitor from soy sauce . Bioscience, Biotechnology, and Biochemistry , 57 : 1107 – 1110 .
- Kuba , M. , Tana , C. , Tawata , S. and Yasuda , M. 2005 . Production of angiotensin-Iconverting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase . Processing Biochemistry , 40 : 2191 – 2196 .
- Kuba , M. , Tanaka , K. , Tawwata , S. , Takeda , Y. and Yasuda , M. 2003 . Angiotensin I- converting enzyme inhibitory peptides isolated from tofuyo fermented soybean food . Bioscience, Biotechnology, and Biochemistry , 67 : 1278 – 1283 .
- Ma , M.S. , Bae , I.Y. , Lee , H.G. and Yang , C.B. 2006 . Purification and identification of angiotensin-I-converting enzyme inhibitory peptide from buckwheat . Food Chemistry , 96 : 36 – 42 .
- Mao , X.Y. , Ni , J.R. , Sun , W.L. , Hao , P.P. and Fan , L. 2007 . Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides . Food Chemistry , 103 : 1282 – 1287 .
- Matsui , T. , Yukiyoshi , A. , Doi , S. , Sugimoto , H. , Yamada , H. and Matsumoto , K. 2002 . Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR . The Journal of Nutritional Biochemistry , 13 : 80 – 86 .
- Moatsou , G. , Moschopoulou , E. and Anifantakis , E. 2004 . Effect of different manufacturing parameters on the characteristics of graviera kritis cheese . International Journal of Dairy Technology , 57 : 215 – 220 .
- Mullally , M.M. , Meisel , H. and FitzGerald , R.J. 1997 . Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins . International Dairy Journal , 7 : 299 – 303 .
- Murray , B.A. , Walsh , D.J. and FitzGerald , R.J. 2004 . Modification of the furanacryloyl-L-phenylalanyl-glycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity . Journal of Biochemical and Biophysical Methods , 59 : 127 – 137 .
- Okamoto , A. , Hanagata , H. and Matsumoto , E. 1994 . “ Antihypertensive substances in viscous material of fermented soybean (natto) ” . In Food hydrocolloids: Structures, properties, and functions , Edited by: Nishinar , K. and Doi , E. New York , NY : Plenum Press .
- Okamoto , A. , Hanagata , H. , Kawamura , Y. and Yanagida , F. 1995 . Anti-hypertensive substances in fermented soybean, Natto . Plant Foods for Human Nutrition , 47 : 39 – 47 .
- Okamoto , A. , Hanagata , H. , Matsumoto , E. , Kawamura , Y. and Yanagida , F. 1995 . Angiotensin I-converting enzyme inhibitory activities of various fermented foods . Bioscience, Biotechnology, and Biochemistry , 59 : 1147 – 1149 .
- Ondetti , M.A. , Rubin , B. and Cushman , D.W. 1977 . Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents . Science , 196 : 441 – 444 .
- Oshima , G. , Shimabukuro , H. and Nagasawa , K. 1979 . Peptide inhibitors of angiotensin I-converting enzyme in digests of gelatin by bacterial collagenase . Biochimica et Biophysica Acta , 566 : 128 – 137 .
- Pan , D.D. , Luo , Y.K. and Tanokura , M. 2005 . Antihypertensive peptides from skimmed milk hydrolysate digested by cell-free extract of Lactobacillus helveticus JCM1004 . Food Chemistry , 91 : 123 – 129 .
- Patchett , A.A. , Harris , E. , Tristram , E.W. , Wyvratt , M.J. , Wu , M.T. , Taub , D. … & and Stone , C.A. 1980 . A new class of angiotensin-converting enzyme inhibitors . Nature , 298 : 280 – 283 .
- Petruccelli , S. and Añón , M.C. 1994 . Relationship between the method of orientation and the structural and functional properties of soy protein isolates. 1. Structural and hydration properties . Journal of Agricultural and Food Chemistry , 42 : 2161 – 2169 .
- Pihlanto-Leppälä , A. 2001 . Bioactive peptides derived from bovine proteins: Opioid and ACE-inhibitory peptides . Trends in Food Science and Technology , 11 : 347 – 356 .
- Pihlanto-Leppälä , A. , Rokka , T. and Korhonen , H. 1998 . Angiotensin I converting enzyme inhibitory peptides derived from bovine milk proteins . International Dairy Journal , 8 : 325 – 331 .
- Shin , Z.I. , Yu , R. , Park , S.A. , Chung , D.K. , Ahn , C.W. , Nam , H.S. … and Lee , H.J. 2001 . His-His-Leu, an angiotensin I converting enzyme inhibitory peptide derived from Korean soybean paste, exerts antihypertensive activity in vivo . Journal of Agricultural and Food Chemistry , 49 : 3004 – 3009 .
- Skeggs , L.T. , Kahn , J.E. , Kahn , J.E. and Shumway , N.P. 1957 . The preparation and function of the angiotensin-converting enzyme . The Journal of Experimental Medicine , 103 : 295 – 299 .
- Sparringa , R.A. and Owens , J.D. 1999 . Protein utilization during soybean tempe fermentation . Journal of Agricultural and Food Chemistry , 47 : 4375 – 4378 .
- Spellman , D. , McEvoy , E. , O'Cuinn , G. and Fitz , G.R.J. 2003 . Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis . International Dairy Journal , 13 : 447 – 453 .
- Tauzin , J. , Miclo , L. and Gaillard , J.L. 2002 . Angiotensin-I-converting enzyme inhibitory peptides from tryptic hydrolysate of bovine α S2 -casein . FEBS Letters , 531 : 369 – 374 .
- Vermeirssen , V. , Camp , J.V. , Decroos , K. , Wijmelbeke , L.V. and Verstraete , W. 2003 . The impact of fermentation and in vitro digestion on the formation of angiotensin-I-converting enzyme inhibitory activity from pea and whey protein . Journal of Dairy Science , 86 : 429 – 438 .
- Wako , Y. , Ishikawa , S. and Muramoto , K. 1996 . Angiotensin Iconverting enzyme inhibitors in autolysates of liver and mantle muscle . Bioscience, Biotechnology, and Biochemistry , 60 : 1353 – 1355 .
- Wang , L.J. , Saito , M. , Tatsum , E and Li , L.T. 2003 . Antioxidative and angiotensin I- converting enzyme inhibitory activities of Sufu (Fermented Tofu) extracts . JARQ-Japan Agricultural Research Quarterly , 37 : 129 – 132 .
- Wu , J. and Ding , X. 2001 . Hypertensive and physiological effect of angiotensin converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats . Journal of Agricultural and Food Chemistry , 49 : 501 – 506 .
- Yoshii , H. , Tachi , N. , Ohba , R. , Sakamura , O. , Takeyama , H. and Itani , T. 2001 . Antihypertensive effect of ACE inhibitory oligopeptides from chicken egg yolks . Comparative Biochemistry and Physiology-Part C , 128 : 27 – 33 .
- Zhang , N. and Zhao , X.H. 2010 . Study of Mucor spp. in semi-hard cheese ripening . Journal of Food Science and Technology , 47 : 613 – 619 .
- Zhao , X.H. and Zheng , X.T. 2009 . A primary study on texture modification and proteolysis of mao-tofu during fermentation . African Journal of Biotechnology , 8 : 2294 – 2300 .
- Zhu , Y.P. , Fan , J.F. , Cheng , Y.Q. and Li , L.T. 2008 . Improvement of the antioxidant activity of Chinese traditional fermented okara (Meitauza) using Bacillus subtilis B2 . Food Control , 19 : 654 – 661 .