Abstract
The presence of furan in commercial fruit- and vegetable-based jarred baby food was studied. Furan values ranged from 7.7 to 32.1 μg/kg in fruit-based baby food and from 10.9 to 143.0 μg/kg in vegetable-based baby food. The higher furan content in the latter case may be related to either greater ascorbic acid degradation or furfural content in these samples. This could be due to pH and higher temperatures used in sample preparation. Furan intake by Spanish infants ranges from 0.9 to 16.8 μg/day, resulting in exposures of 0.1 to 2.1 μg/kg bw/day. The maximum furan exposure recorded in this study is much lower than the “no observable adverse effect level” of 0.08 mg/kg bw/day determined in experimental animals studies, and is close to the reported acceptable daily intake of 2 μg/kg bw/day.
Se estudió la presencia de furano en potitos comerciales de frutas y verduras. El contenido osciló entre 7,7–32,1 μg/kg en los potitos de frutas y 10,9–143,0 μg/kg en los de verduras. El mayor contenido de furano en los potitos de verduras podría estar relacionado con una mayor degradación de ácido ascórbico y con el contenido de furfural en estas muestras como consecuencia del pH y de las temperaturas más altas empleadas en su elaboración. La ingesta de furano en bebés españoles oscila entre 0,9–16,8 μg/día, resultando en exposiciones de 0,1–2,1 μg/kg peso/día. La máxima exposición de furano estimada en este estudio resulta muy inferior a 0,08 mg/kg peso/día, valor considerado como “nivel sin efectos adversos observados” determinado en estudios con animales de experimentación, y próxima al valor de 2 μg/kg peso/día, considerado como “ingesta diaria aceptable” de furano.
Palabras claves:
Introduction
Furan is a lipophilic, volatile compound formed during the thermal treatment of many foods and drinks, and is associated with sensory characteristics such as flavor. It can be generated from the thermal degradation of carbohydrates in the presence or absence of amino acids, from the thermal degradation of certain amino acids and from the thermal oxidation of ascorbic acid, polyunsaturated fatty acids, or carotenoids (Yaylayan, Citation2006).
In recent years, the study of this compound in foods has received special attention by various international organizations such as the US Food and Drugs Administration (FDA) and the European Food Safety Authority (EFSA), because of its carcinogenic and cytotoxic properties in animals and its association with harmful effects on human health (Byrns et al., Citation2006; Glatt, Schneider, & Liu, Citation2005). Furan has been classified as a possible carcinogenic to humans (Group 2B) by the International Agency for Research on Cancer (IARC, 1995) and by the Department of Health and Human Services (US Department of Health and Human Services, 2011). In the latest risk assessment on furan carried out by the joint FAO/WHO Expert Committee on Food Additives (FAO/WHO, 2010), it was concluded that furan exposure indicates a human health concern as this compound might act via a DNA-reactive genotoxic metabolite.
Recently, the European Commission requested Member States to collect data on furan concentrations in heat-treated commercial food products to obtain a better estimate of dietary exposure. Analytical results for furan content in foods have been reported by 18 countries (Spain not included), with coffee, baby foods and soups being the categories containing highest concentrations of furan (EFSA, 2010). The large amount of furan in canned and jarred foods is probably due to volatiles trapped in the food container. Lachenmeier, Reusch, and Kuballa (Citation2009) reported high levels of furan in commercially jarred baby foods; in contrast, it was not detected in the same types of foods when they were home-prepared. The long-term effects of furan on children's health is unknown, but its presence in baby foods is a matter of concern because of infants’ high sensitivity to carcinogens, as well as the large amounts (relative to body weight) of certain foods that are consumed. Moreover, commercial baby foods may comprise an important part of the diet for many infants. Baby foods are often fortified with vitamin C prior to thermal processing, which would increase furan formation (Fan, Huang, & Sokorai, Citation2008). Furthermore, food intended for consumption by babies may have been heated at high temperatures to ensure that the product is microbiologically safe, which again increases furan formation. Consequently, various studies have identified high furan content in baby foods (Altaki, Santos, & Galceran, Citation2007; Jestoi, Järvinen, Järvenpää, & Tapanainen, Citation2009; Lachenmeier et al., Citation2009); however, little is known about the relationship between furan content and its precursors in this kind of sample.
In a previous paper, we determined ascorbic acid, dehydroascorbic acid and furfural as potential precursors of furan formation in different commercial presentations of fruit- and vegetable-based jarred baby foods (Mesías-García, Guerra-Hernández, & García-Villanova, Citation2010). The aim of the present study was to determine the furan content in these samples and to examine the relationship between this content and the compounds analyzed previously as potential precursors. In addition, we estimated the exposure of Spanish infants to furan, on the basis of the levels detected and the mean intake from bibliographic data.
Materials and methods
Chemicals
Furan (Fluka, Madrid, Spain) and furan-d4 (Isotec, Ohio, USA) were obtained at a minimum purity of 98%. HPLC grade methanol was purchased from Panreac (Barcelona, Spain). Demineralized water was obtained by filtering distilled water through a Milli-Q Ultrapure Water System (Millipore, Bedford, MA, USA).
Samples
A total of 15 different fruit-based and 9 vegetable-based jarred baby foods were purchased in a Spanish supermarket and used for the analysis. The ingredients of the different baby foods are shown in and (data provided by the manufacturer). The samples were stored unopened at + 4°C before the analysis.
Table 1. Ingredients and furan content of analyzed fruit-based jarred baby food.
Tabla 1. Ingredientes y contenido de furano de los potitos de frutas analizados.
Table 2. Ingredients and furan content of analyzed vegetable-based jarred baby food.
Tabla 2. Ingredientes y contenido de furano de los potitos de verduras analizados.
Standards and sample preparation
Analyses were carried out using the US FDA method (US Food and Drug Administration, 2004) with slight modification. Individual stock standard solutions (about 2.5 mg/ml) of furan and furan-d4 were prepared by adding 50 μl of each pure analyte via a micro-syringe through the septum of weighed 20 ml headspace vials containing 20 ml of methanol. The mixtures were then reweighed to determine the exact concentrations of furan and furan-d4. Solutions were stored at + 4°C and prepared weekly. Working standard solutions were individually prepared daily by dilution of the stock solutions using the same procedure. In these cases, 40 μl of each standard solution was added to 20 ml headspace vials containing 20 ml of water (reaching 5 μg/ml).
The content of furan in the samples was determined by the standard addition method. Various factors were tested in a preliminary study of the method, including water or saturated sodium chloride solution as dilutors of the samples, agitation times (15, 30, 45, and 60 min) and vial filling. The optimum values of furan concentration were obtained using water as the dilutor of baby foods, an agitation time of 15 min and a sample amount of 2.5 g of baby food + 2.5 ml of water; therefore, these parameter values were selected for our assay. A 2.5 g portion of each jarred baby food was weighed accurately into a 20 ml headspace vial and mixed with 2.35–2.5 ml of water, depending on the volume of furan solution added. Volumes of 0–50 μl of furan working standard solution were added to the vials containing fruit samples, whereas for vegetable samples the volume added was 0–150 μl in order to improve the slope and, consequently, the sensibility of the method. Six different standards were used in the construction of the calibration curve. A constant volume (40 μl) of internal standard working solution (furan-d4) was added to each vial. All standards and samples were prepared at + 4°C in order to avoid furan losses. Each baby food was analyzed in duplicate.
Validation of the method
The method was validated by determining quality parameters. Linearity was studied by spiking appropriate amounts of furan and furan-d4 into water samples (in the range 8–150 ng/ml). The precision of the instrumental technique was evaluated by analyzing different samples of baby foods on the same day (repeatability) and on different days (reproducibility). For the repeatability assay five series of baby food were analyzed. For the reproducibility assay, a selected baby food sample was weighed, mixed with water, and sealed in different headspace vials following the sample preparation procedure and stored at + 4°C for the following days until the moment of analysis. The determination was carried out in five different days. Moreover, baby foods from the same batch were likewise compared.
GC-MS conditions
The determination of furan by headspace extraction gas chromatography mass spectrometry (HS-GC-MS) was carried out using a Trace GC Ultra gas chromatograph coupled to a Polaris Q ion-trap mass spectrometer (ThermoScientific, Barcelona, Spain). The capillary column used was a 30 m × 0.32 mm × 20 μm HP Plot-Q (Agilent Technologies, Santa Clara, CA, USA). The headspace incubation oven temperature was 40°C and that of the syringe needle, 100°C. Samples were incubated for 15 min with simultaneous shaking. The injection volume was 1 ml and the injection speed 30 ml/min. The injector temperature was 200°C with injection in the split mode (split ratio 1:6). Helium was used as a carrier gas with a flow rate of 1.7 ml/min. The oven temperature was programmed from 50 to 225°C at 10°C/min and held for 12.5 min.
The mass spectrometer was operated in electron impact ionization mode using automatic gain control with 70 eV of electron energy and 250 μA of emission current. The ion source and transfer line temperatures were 230°C and 225°C, respectively. Xcalibur version 2.0.7 software was used for control, general operation and data acquisition of the results.
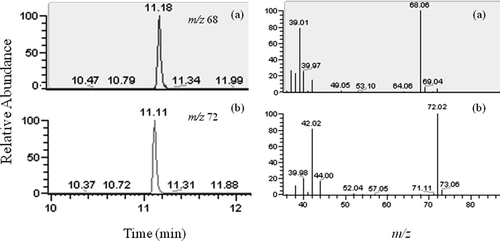
Furan was detected by selected ion monitoring of the major ion at m/z 68 and confirmed by monitoring of the ion at m/z 39. Furan-d4 was detected by monitoring the equivalent ions at m/z 72 and m/z 42. The ion ratio (m/z 68/39) of the samples was compared and used for further confirmation of a positive identification of furan. The retention time of both compounds was approximately 11.1–11.2 min. The extracted ion chromatograms and mass spectra for furan and furan-d4 are shown in .
Statistical analysis
SPSS for Windows, version 13.0 (SPSS Inc., 1999–2004, Chicago, IL, USA) was used to support the statistical analyses. Statistical evaluation of experimental results was performed using Student's t-test, after confirming normal distribution of the variables. Differences were considered to be significant at p < 0.05. The relationships between the different parameters analyzed were evaluated by computing the relevant correlation coefficient (Pearson linear correlation) at the p < 0.05 confidence level.
Results and discussion
Validation of the method
Regarding validation of the method, good linearity, with a correlation coefficient (r) higher than 0.999, was obtained. The relative standard deviations (RSD) for the repeatability and reproducibility assays were 12.6% and 13.2%, respectively; these variations probably were due to the matrix effect. The limit of detection (LOD) (three times the signal-to-noise ratio) was 1.2 μg/kg and the limit of quantification (LOQ) (10 times the signal-to-noise ratio) was 3.8 μg/kg; both values were lower than those of the samples analyzed. The LOD and LOQ values are comparable with those obtained by other authors (Nyman, Morehouse, McNeal, Perfetti, & Diachenko, Citation2006; Vranová, Bednáriková, & Ciesarová, Citation2007).
Furan in the baby food samples
The results obtained in the analysis of samples are shown in and . Furan was detected in all samples, at concentration levels ranging from 7.7 to 143.0 μg/kg, with a mean value of 33.9 μg/kg. These furan concentrations were in agreement with those reported by EFSA (0–224 μg/kg) (EFSA, 2010) and close to those reported by FDA, whose data ranged from 0 to 112 μg/kg (US Food and Drug Administration, 2004). Similar values in fruit and vegetable jarred baby foods have been reported by other authors (Jestoi et al., Citation2009; Lachenmeier et al., Citation2009).
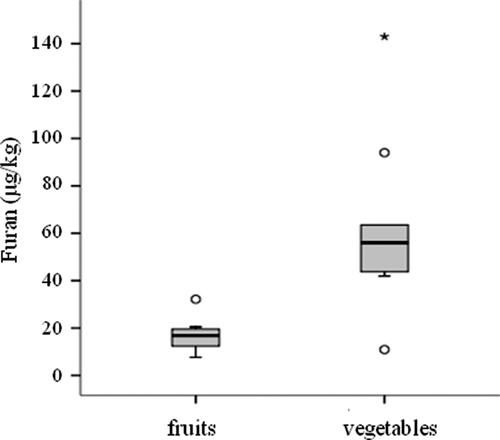
Statistical analysis shows that the fruit-based and vegetable-based jarred baby food groups have highly significant differences in furan content (p = 0.006), as shown in the distribution patterns in . The highest average content was found in the vegetable-based baby food group (63.0 ± 37.2 μg/kg) ranging from 10.9 to 143.0 μg/kg, whereas the fruit-based baby food group presented the lowest average content (16.6 ± 6.0 μg/kg) ranging from 7.7 to 32.1 μg/kg. These data agree with the findings of several authors (Jestoi et al., Citation2009; Lachenmeier et al., Citation2009; Ruiz, Santillana, Nieto, Cirugeda, & Sanchez, Citation2010; Zoller, Sager, & Reinhard, Citation2007), who have observed a higher furan content in vegetable-based baby food than in fruit-based samples. Moreover, the results are in accordance with the assumptions reported in a previous study by the present authors (Mesías-García et al., Citation2010), in which it was concluded that the higher furan content of vegetable-based baby food may be associated with greater ascorbic acid degradation in the presence of oxygen, which is favored by a higher pH in these samples (pH = 5.7) compared to fruit-based baby food (pH = 3.9). The different way of elaboration of vegetable- and fruit-based baby food may also influence the furan content in the samples. The chopped vegetables are cooked in boiling water, blended with water to adjust the consistency, bottled and sterilized, whereas the fruits are peeled, chopped, blended, bottled, and pasteurized. Wegener and López-Sánchez (Citation2010) found a higher furan content in sterilized than in pasteurized carrot juices. Moreover, the enhanced ascorbic acid decomposition in vegetables produces a higher level of furfural formation in this type of food, and both compounds, ascorbic acid and furfural, are known to be precursors of furan formation (Becalski & Seaman, Citation2005). In this sense, Lachenmeier et al. (Citation2009) noted that if ascorbic acid was added to potato-based baby foods, the furan content increased, although other factors besides ascorbic acid were also involved in the formation mechanism. In this respect, Limacher, Kerler, Conde-petit, and Blank (Citation2007) also suggested that the transformation of precursors into furan is accelerated by the presence of ascorbic acid.
Figure 2. Box plots for furan concentrations of the fruit and vegetable-based jarred baby foods analyzed. Asterisk means an outlier data.
Figura 2. Diagramas de cajas para las concentraciones de furano en los potitos de frutas y verduras analizados. El asterisco representa un valor atípico.
No correlation study was carried out between ascorbic acid or dehydroascorbic acid and furan content in the samples analyzed, previously published, because we did not find any trace of ascorbic acid in the vegetable-based baby food, perhaps because the samples in question were not enriched with this vitamin and its natural content could be destroyed during severe processing (Mesías-García et al., Citation2010). Neither was any correlation found between ascorbic acid or dehydroascorbic acid and furan content in the fruit-based baby foods. Owczarek-Fendor et al. (Citation2010) recently reported that a change in ascorbic acid concentration from 0.1 to 4.5 mg/g of sample (normally found in fruit-based baby food) (pH = 4) did not result in significantly different furan concentrations in a starch-based model system. On the contrary, furan concentrations were correlated with furfural content (r = 0.5904, p = 0.0024), which may be associated with greater ascorbic acid degradation.
The highest concentration of furan in vegetable baby food (143.0 μg/kg) was found in the “spinach cream” baby food. This result agrees with the observations of Van Lancker, Adams, Owczarek, De Meulenaer, and De Kimpe (Citation2009), who reported that furan can be formed from spinach in relatively high amounts, possibly because the vitamin C or carotenoids present in this food can be precursors of furan. In the same way, the presence of carotenoids from carrots is often referred to as an important source of furan, which may be another reason for the high furan content in vegetable-based baby foods.
As mentioned above, furan can be generated from thermal degradation of carbohydrates in the presence or otherwise of amino acids (no enzymatic browning or Maillard reaction) (Yaylayan, Citation2006). However, in our study, in disagreement with this, furan was negatively correlated with carbohydrate content (r = −0.6402, p = 0.0024), and moreover with hydroxymethylfurfural (HMF) concentrations in the baby foods analyzed (r = −0.4756, p = 0.0188). HMF is formed as an intermediate in the Maillard reaction, and also from the degradation of sugars, being recognized as an indicator of quality deterioration caused by excessive heating or inadequate storage (Delgado-Andrade, Rufián-Henares, & Morales, Citation2009; Guerra-Hernández, García-Villanova, & Montilla-Gómez, Citation1992). This compound was found in higher concentrations in the fruit-based baby food, probably favored by the greater content of sugars and by the lower pH of the fruits. This hypothesis was corroborated by the negative, significant correlation observed between HMF content and pH in the fruit-based baby foods (r =−0.5209, p = 0.032). This fact may explain the negative relation between HMF and furan concentration, since HMF is favored at acidic pH and, on the contrary, furan formation at higher pH values. Moreover, the fact that both furan formation and ascorbic acid degradation take place at higher pH values suggests that vitamin C may be a high precursor of furan in this kind of food, in comparison with carbohydrate thermal degradation, as has been suggested by Becalski and Seaman (Citation2005).
Regarding furan formation from the thermal oxidation of fatty acids, we were unable to test this hypothesis, due to a lack of data about the lipid content of the samples. We only know whether the vegetable-based baby foods contain olive oil or not, and no differences were found in relation to this fact. In this sense, Becalski and Seaman (Citation2005) showed that monounsaturated acid (oleic) does not form furan.
Lachenmeier et al. (Citation2009) reported that cereals have no influence on furan formation. This might be the reason why the lowest furan content among the vegetable-based baby food group was found in the sample containing mostly rice (no. 9 vegetables with rice, ), corroborating the findings of Zoller et al. (Citation2007).
Exposure estimates
Furan exposure in infants was estimated taking into account the range of concentration levels observed in our study (7.7–143.0 μg/kg). Six-months-old babies were selected for this estimation, since these infants generally consume baby foods containing fruits and vegetables and no other ingredients such as meat or fish. A typical consumption of 117.8 g/day of baby foods was calculated from the Spanish consumption database (Mercasa, 2010), this value being lower than those quoted for Germany (195 g/day) and Finland (172 g/day) for manufactured baby foods (Jestoi et al., Citation2009; Kersting, Alexy, Sichhert-Hellert, Manz, & Schöch, Citation1998). Assuming a mean body weight of 8.1 kg for a Spanish 6-months-old baby, according to Carrascosa-Lezcano et al. (2008), the furan intake in Spanish infants, in our study, ranged from 0.9 to 16.8 μg/day, resulting in exposures of 0.1 μg/kg bw/day and 2.1 μg/kg bw/day, respectively. These results agree with those reported by Jestoi et al. (Citation2009) for Finnish infants, being within the range from below 0.03 to 3.5 μg/kg bw/day estimated by EFSA in 2004 and including the mean value of 0.85 μg/kg bw/day recently associated with infant exposure to furan from jarred food at age 6 months (EFSA, 2004, 2009). The maximum furan exposure estimated in our study is considerably lower than the value of 0.08 mg/kg bw/day for no observable adverse effect level (NOAEL) established by the National Research Council for experimental animals (NRC, 2000), and close to the acceptable daily intake (ADI) estimated to be 2 μg/kg bw/day (Kuballa, Stier, & Strichow, Citation2005). It should be taken into account that the data considered for baby food consumption represent a mean value; therefore, higher consumption would be associated with higher risk. However, according to Morehouse, Nyman, McNeal, Dinovi, and Perfetti (Citation2008), the furan intake from the products analyzed in this survey may be overestimated, since the can/jar contents are sometimes warmed and stirred prior to use, which could lead to lower furan levels in the baby foods consumed, due to volatilization.
Therefore, following observations made by other authors, safety precautions such as heating in an open can and applying stirring are recommended in order to decrease furan levels in baby food (Bianchi, Careri, Mangia, & Musci, Citation2006; Zoller et al., Citation2007). On the other hand, Lachenmeir et al. (2009) and Limacher et al. (Citation2007) reported that it is not recommended to fortify canned and jarred food products with vitamin C before thermal treatment, which might be a suggestion to be taken into account in the food industry.
Conclusions
In summary, furan is found in jarred baby foods, with higher concentrations in vegetable-based baby foods compared with those in fruit-based baby foods, probably associated with greater ascorbic acid degradation and, moreover, with the furfural content in these samples. Due to the health risks associated with the consumption of this compound, the reduction of furan content by means of ingredient selection and modifications to processing methods should be investigated further in future studies.
Acknowledgements
This research was supported by a research project from Comisión Interministerial de Ciencia y Tecnología (AGL-2006-12656/ALI). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Altaki , M.S. , Santos , F.J. and Galceran , M.T. 2007 . Analysis of furan in foods by headspace solid-phase microextraction-gas chromatography-ion trap mass spectrometry . Journal of Chromatography A , 1146 : 103 – 109 .
- Becalski , A. and Seaman , S. 2005 . Furan precursors in food: A model study and development of a simple headspace method for determination of furan . Journal of AOAC International , 88 : 102 – 106 .
- Bianchi , F. , Careri , M. , Mangia , A. and Musci , M. 2006 . Development and validation of a solid phase micro-extraction-gas-chromatography-mass-spectrometry method for the determination of furan in baby-food . Journal of Chromatography A , 110 : 268 – 272 .
- Byrns , M.C. , Vu , C. , Neidigh , J.W. , Abad , J.L. , Jones , R.A. and Peterson , L.A. 2006 . Detection of DNA adducts derived from the reactive metabolite of furan, cis-2-butene-1,4-dial . Chemical Research in Toxicology , 19 : 414 – 420 .
- Carrascosa Lezcano , A. , Fernández García , J.M. , Fernández Ramos , C. , Ferrández Longás , A. , López-Siguero , J.P. , Sánchez González , E. and Yeste Fernández , D. 2008 . Estudio transversal español de crecimiento 2008. Parte II: valores de talla, peso e índice de masa corporal desde el nacimiento a la talla adulta . Anales de Pediatría , 68 : 552 – 569 .
- Delgado-Andrade , C. , Rufián-Henares , J.A. and Morales , F.J. 2009 . Hydroxymethylfurfural in commercial biscuits marketed in Spain . Journal of Food and Nutrition Research , 48 : 14 – 19 .
- EFSA (European Food Safety Authority) . 2004 . Report of the scientific panel on contaminants in the Food Chain on provisional findings on furan in food . EFSA Journal , 2 : 137
- EFSA (European Food Safety Authority) . 2009 . Scientific document: Results on the monitoring of furan levels in food . EFSA Journal , 7 : 304
- EFSA (European Food Safety Authority) . 2010 . Scientific Document: Update of results on the monitoring of furan levels in food . EFSA Journal , 8 : 1702
- Fan , X. , Huang , L. and Sokorai , K.J.B. 2008 . Factors affecting thermally induced furan formation . Journal of Agricultural and Food Chemistry , 56 : 9490 – 9494 .
- FAO/WHO (Food and Agricultural Organization of the United Nations/World Health Organization) . 2010 . Summary and conclusions report of the seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives , Rome : Joint FAO/WHO Expert Committee on Food Additives .
- Glatt , H. , Schneider , H. and Liu , Y. 2005 . V79-hCYP2E1-hSULT1A1, a cell line for the sensitive detection of genotoxic effects induced by carbohydrate pyrolysis products and other food-borne chemicals . Mutation Research , 580 : 41 – 52 .
- Guerra-Hernández , E. , García-Villanova , B. and Montilla-Gómez , J. 1992 . Determination of hydroxymethylfurfural in baby cereals by high performance liquid chromatography . Journal of Liquid Chromatography , 15 : 2551 – 2559 .
- IARC (Internacional Agency for Research on Cancer) . 1995 . Dry cleaning, some chlorinated solvents and other industrial chemicals, Monographs on the evaluation of carcinogenic risk to humans , 3194 – 3407 . Lyon , , France : World Health Organization .
- Jestoi , M. , Järvinen , T. , Järvenpää , E. and Tapanainen , H. 2009 . Furan in the baby-food samples purchased from the Finnish markets-Determination with SPME-GC-MS . Food Chemistry , 117 : 522 – 528 .
- Kersting , M. , Alexy , U. , Sichhert-Hellert , W. , Manz , F. and Schöch , G. 1998 . Measured consumption of commercial infant food products in German infants: results from the Donald study . Journal of Pediatric Gastroenterology and Nutrition , 27 : 547 – 551 .
- Kuballa , T. , Stier , S. and Strichow , N. 2005 . Furan in kaffee und kaffeegetränken . Deutsche Lebensmittel-Rundschau , 101 : 229 – 235 .
- Lachenmeier , D.W. , Reusch , H. and Kuballa , T. 2009 . Risk assessment of furan in commercially jarred baby foods, including insights into its occurrence and formation in freshly home-cooked foods for infants and young children . Food Additives and Contaminants , 26 : 776 – 785 .
- Limacher , A. , Kerler , J. , Conde-petit , B. and Blank , I. 2007 . Formation of furan and methylfuran from ascorbic acid in model systems and food . Food Additives and Contaminants , 24 : S122 – S135 .
- Mercasa. Feeding in Spain 2010 (Alimentación en España 2010) . 2010 . Informe sobre Producción, Industria, Distribución y Consumo de alimentación en España Mercasa , , Spain
- Mesías-García , M. , Guerra-Hernández , E. and García-Villanova , B. 2010 . Determination of furan precursors and some thermal damage markers in baby foods: ascorbic acid, dehydroascorbic acid, hydroxymethylfurfural and furfural . Journal of Agricultural and Food Chemistry , 58 : 6027 – 6032 .
- Morehouse , K.M. , Nyman , P.J. , McNeal , T.P. , Dinovi , M.J. and Perfetti , G.A. 2008 . Survey of furan in heat processed foods by headspace gas chromatography/mass spectrometry and estimated adult exposure . Food Additives and Contaminants , 25 : 259 – 264 .
- NRC (National Research Council) . 2000 . Spacecraft maximum allowable concentrations for selected airborne contaminants , 307 – 329 . Washington , DC : National Academy Press .
- Nyman , P.J. , Morehouse , K.M. , McNeal , T.P. , Perfetti , G.A. and Diachenko , G.W. 2006 . Single-laboratory validation of a method for the determination of furan in food by using static headspace sampling and gas chromatography/mass spectrometry . Journal of AOAC International , 89 : 1417 – 1424 .
- Owczarek-Fendor , A. , De Meulenaer , B. , Scholl , G. , Adams , A. , Van Lancker , F. , Yogendrarajah , P. and De Kimpe , N. 2010 . Furan formation from vitamin C in a starch-based model system: Influence of the reaction conditions . Food Chemistry , 121 : 1163 – 1170 .
- Ruiz , E. , Santillana , M.I. , Nieto , M.T. , Cirugeda , M.E. and Sanchez , J.J. 2010 . Determination of furan in jarred baby food purchased from the Spanish market by headspace gas chromatography-mass spectrometry (HS-GC-MS) . Food Additives and Contaminants , 27 : 1208 – 1214 .
- US Department of Health and Human Services . 2011 . Report on carcinogens , (12th ed. , 499 NC : U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program . Research Triangle Park
- US Food and Drug Administration . 2004 . Determination of furan in foods , Department of Health and Human Services . Rockville, MD: FDA, US Food and Drug Administration
- Van Lancker , F. , Adams , A. , Owczarek , A. , De Meulenaer , B. and De Kimpe , N. 2009 . Impact of various food ingredients on the retention of furan in foods . Molecular Nutrition and Food Research , 53 : 1505 – 1511 .
- Vranová , J. , Bednáriková , A. and Ciesarová , Z. 2007 . In-house validation of a simple headspace gas chromatography-mass spectrometry method for determination of furan levels in food . Journal of Food and Nutrition Research , 46 : 123 – 127 .
- Wegener , J.W. and López-Sánchez , P. 2010 . Furan levels in fruit and vegetables juices, nutrition drinks and bakery products . Analytica Chimica Acta , 672 : 55 – 60 .
- Yaylayan , V.A. 2006 . Precursors, formation and determination of furan in food . Journal für Verbraucherschutz und Lebensmittelsicherheit , 1 : 5 – 9 .
- Zoller , O. , Sager , F. and Reinhard , H. 2007 . Furan in food: Headspace method and product survey . Food Additives and Contaminants , 24 : 91 – 107 .