Abstract
Antifungal properties of some plant extracts (pomegranate, rosemary, lemon and balsamic lemon) added in the elaboration of jam obtained from osmotically dehydrated strawberry were studied. Lemon extract exhibited the highest antifungal activity against the microbiota present. Subsequently, the effect of the addition of a combination of lemon extract, as antifungal, and pomegranate, as antimicrobial, on the microbiological and physico-chemical properties of this jam was evaluated through storage. The use of plant extracts reduces the microbial charge (aerobic mesophylls, lactic acid bacteria, molds, and yeasts) and is most effective when pomegranate and lemon extracts are used, as these do not cause significant changes in the physicochemical characteristics. Furthermore, adding pomegranate and/or lemon extract improved the consistency of jams during storage. In general, the addition of extracts involved an increase in stability of strawberry jam during storage at room temperature.
Se han estudiado las propiedades antifúngicas de algunos extractos de plantas (granada, romero, limón y limón balsámico) para su aplicación en la elaboración de mermelada obtenida con fresa osmóticamente deshidratada. El extracto de limón presentó la mayor actividad antifúngica frente a la microbiota presente. Posteriormente, se han evaluado los efectos sobre las propiedades microbiológicas y físico-químicas de esta mermelada, durante el almacenamiento, de la adición de una combinación de extractos de limón como antifúngico y granada como antimicrobiano. La utilización de extractos vegetales reduce la carga microbiana (mesófilos aerobios, bacterias ácido lácticas y mohos y levaduras) y es más efectiva cuando se utilizan los extractos de granada y limón, ya que no provocan cambios significativos en las características físico-químicas. Por otra parte, la adición de granada y/o extracto de limón mejora la consistencia de las mermeladas durante el almacenamiento. En general, la adición de extractos implica un aumento en la estabilidad de la mermelada de fresa durante el almacenamiento a temperatura ambiente.
Introduction
The jam industry needs to improve its competitiveness due to the changes that have taken place in consumption habits and the availability of alternative breakfast products in the market (Grigelmo-Miguel & Martín-Belloso, Citation1999). An attractive red color is one of the most important characteristics of strawberry jam, as consumers are attracted to the typical aroma and the bright red color (Goessinger et al., Citation2009). The color stability of red fruit products is affected by temperature, pH, oxygen, sugar content, ascorbic acid, and metals, which will in turn affect the pigments (Withy, Nguyen, Wrolstad, & Heatherbell, Citation1993). Anthocyanins are the main pigments accounting for the characteristic red color (Viguera, Zafrilla, & Barberan, Citation1997). Other important parameters are the typical sweet–sour strawberry flavor and the appropriate consistency. The selection of cultivar and its degree or ripeness are the major factors in determining the taste and color of strawberry jams (Redalen & Haffner, Citation2002; Sundfør, 2001). Pigment degradation results in discoloration of the product. During processing the pigments can be hydrolysed and degraded to anthocyanidin and sugar. Anthocyanidins are unstable when exposed to light and are more easily oxidized than anthocyanins, and consequently more susceptible to turn brown (Herrmann, Citation1972). Browning and discoloration during the storage of strawberry jams are common problems (García-Viguera, Zafrilla, & Tomás-Barberán, Citation1999).
Fruits are the source of nutrients and antioxidant compounds. Fruits are most commonly consumed in the form of jams. However, the beneficial fruit properties are lost due to the high temperatures and long processing time involved in jam making. Osmotic dehydration could offer an alternative to the conventional jam-making process, since this technique uses lower temperatures (30–40°C) that are less aggressive against labile compounds in the fruit (García-Martínez, Ruiz-Diaz, Martínez-Monzó, Camacho, Martínez-Navarrete, & Chiralt, Citation2002; Igual, Contreras, & Martínez-Navarrete, Citation2010; Shi, Chiralt, Fito, Serra, Escoin, & Gasque, Citation1996). Nevertheless, this technique does not destroy some types of bacteria, molds, and yeasts, and there are osmophilic fungal species that can live in high-sugar concentrations (Pascual & Calderón, Citation2000). A possible solution to this problem could be the use of plant extracts such as pomegranate, lemon, balsamic lemon, and rosemary. Pomegranate (Punica granatum L.) is a very rich source of anthocyanins, ellagitannins, and other phenolic compounds with proven antioxidant activity (Madrigal-Carballo, Rodriguez, Krueger, Dreher, & Reed, Citation2009) and antimicrobial effects (Voravuthikunchai, Lortheeranuwat, & Jeeju, Citation2004). Pomegranate is effective against Aspergillus flavus (Krishnamurthy & Shashikala, Citation2006). Lemon (Citrus limon) has antimicrobial, antifungal (Viuda-Martos, Ruiz-Navajas, Fernandez-Lopez, & Perez-Alvarez, Citation2008), and antioxidant activities (Xu, Liu, Chen, Ye, Ma, & Shi, Citation2008). The antifungal activity is due to the presence of compounds such as d-limonene, linalool, or citral (Tepe et al., Citation2006; Veldhuizen, Tjeerdsma-Van Bokhoven, Zweijtzer, Burt, & Haagsman, Citation2006). Rosemary (Rosmarinus officinalis) slows down the growth of some bacteria and also acts as an antioxidant due to the presence of rosamarinic acid, carnosic acid, and its phenolic compounds such as carnosol, rosmanol, and rosmaridiphenol (Pérez-Fons, Garzon, & Micol, 2010; Yesil-Celiktas, Sevimli, Bedir, & Vardar-Sukan, Citation2010).
The objective of this work was to study the antifungal activity in osmotically dehydrated strawberry jam of plant extracts (pomegranate, rosemary, lemon, and balsamic lemon) and to evaluate the effect of selected plant extracts on microbiological and physicochemical properties of jam during storage.
Materials and methods
Raw materials
“Reina de los Valles” wild strawberry fruit (Fragaria vesca L.) was purchased from a local supermarket. The mean values (and standard deviation) of aw , °Brix, and pH of strawberry used were 0.980 (0.003), 12.6 (0.1), and 3.53 (0.01), respectively. An osmotic solution (OS) was prepared by mixing an amount of commercial food grade sucrose with distilled water until it was completely dissolved, thus forming a 65 °Brix syrup. Citrus peel pectin (60% degree of esterification, Fluka Biochemika, Switzerland) was added to the jam as gelling agent. Lemon, pomegranate, balsamic lemon, and rosemary extract (Nutracitrus, Spain) were used for the antifungal study.
Jam processing and storage conditions
Strawberries placed in the OS (ratio OS:fruit 5:1) were heated to 30°C (water bath P-Selecta Precisterm, Barcelona, Spain) with continuous stirring (200 rpm, Heidolph Instruments, RZR 2020, Schwabach, Germany) for 3 h, reaching ≈20 °Brix. Osmo-dehydrated samples were then ground together with part of the OS to obtain jam with 500 g fresh fruit/kg jam, with pectin (10 g/kg jam) as gelling agent, both with and without natural extracts. The jams thus obtained were placed in glass jars and stored at room temperature for 24 h until analysis and then stored at 25°C in darkness for 24 days.
Antifungal activity (in vitro) of extracts
Two types of microbiological analyses were carried out to obtain the minimal concentration of antifungal activity of the natural extracts: The first method was by agar diffusion in which the natural extract was applied to an agar plate: Chloramphenicol glucose agar (CGA) for molds and yeasts. 1 mL of inoculum (108 cfu/mL for molds and yeasts) was mixed with the agar on the plate. When the agar solidified, five wells were made and 100 μL of different concentrations of natural extracts were placed in each one. The plates were incubated at 25°C for 5 days, during which the extracts diffused through the agar, setting up a concentration gradient. The concentration was inversely proportional to the distance from the well. Inhibition, which is the measure of activity, is indicated by a no-growth zone around the well (Barry, Citation1986). The results of this test are qualitative, so that microorganisms are generally termed susceptible, intermediate or resistant, according to the diameter of the inhibitory zone (Davidson & Parish, Citation1989). The second analytical method was by broth dilution in which the compounds under study were serially diluted and distributed in a nutrient broth (Davidson & Parish, Citation1989). A broth containing a mixture of 200 g/kg glucose, 100 g/kg yeast extract and 200 g/kg tryptone (GYT) was used. Natural extracts were applied to these media in the form of an inoculum of altered jam in tubes (108 cfu/mL) (1:9 inoculum:nutrient broth ratio), which were incubated at 37°C for 24-48 h. 1 mL of the content of each tube was then placed on a CGA plate and incubated in the same way as described above.
The minimum inhibitory concentration (MIC) is defined as the minimum level of natural extract concentration that produces a 90% reduction in the growth (population) of microbial colonies (Ponce, Fritz, Valle, & Roura, 2003) or a complete inhibition of visible growth (Jia, Ji, Xing, Zhang, Zhu, & Wang, Citation2010). The minimum fungicidal concentration (MFC) is defined as the minimum level of natural extract concentration that produces at least a 99.9% reduction in the growth of fungal colonies (Ernst, Citation2005).
Microbiological and physicochemical properties of stored jams
The evolution of microbiota and physicochemical properties, color and consistency of jams with the selected extracts, were analyzed throughout the 24-day storage period at 25°C.
Previous studies on plant extracts (Gómez, Igual, Camacho, & Pagán, Citation2012), shown that pomegranate extract was very effective as natural antimicrobial and selected 0.001 g/mL as better dose. So, jams without extract, with antimicrobial extract and with antimicrobial and antifungal extract were characterized physicochemically and microbiologically and color and consistency were also observed throughout 24 days of storage at 25°C.
Microbiological characterization was based on the following parameters: aerobic mesophylls (AENOR, 2003), lactic acid bacteria (AENOR, 1998), coliforms (AENOR, 2006), molds and yeasts (AENOR, 2008). Moisture content (xw ), °Brix, pH, and water activity (aw ) were determined for all of the formulated jams. xw was determined by drying the sample to constant weight at 60°C in a vacuum oven (AOAC method 934.06, 2000). °Brix was measured in previously homogenized samples with a refractometer at 20°C (Zeiss, ATAGO model NAR-3T refractometer, Japan). A dew point hygrometer (FA-st Lab, GBX, France) was used to measure aw . pH was measured by means of a CRISON pH-meter (Crison Instruments, SA, Barcelona, Spain). Each analysis was carried out in triplicate.
Jam color was analyzed by a reflection spectrum (Minolta, CM 3600D, Tokyo, Japan) calibrated with a standard white reflective plate. CIE-L*a*b* uniform color space, 10° observer and D65 illuminant were selected to calculate color coordinates. Color data are provided as CIE-L*a*b* coordinates, which define color in a three-dimensional space. L* indicates lightness, a* indicates chromaticity on a green (−) to red (+ ) axis and b* chromaticity on a blue (−) to yellow (+ ) axis. The color coordinates were then used to calculate hue angle (), chrome (
) and color differences (
) with respect to the strawberry control jam sample.
The flow distance of the product was measured with a Bostwick consistometer. This instrument has two compartments, one measures 5 cm × 5 cm × 3.8 cm and is separated from the second by a spring-loaded gate. The second compartment measures 5 cm × 24 cm × 2.5 cm and its floor has a series of parallel lines drawn across it at 0.5 cm intervals from the gate to the far end. The controlled weight sample is placed in the first compartment and when the gate is opened the flowing distance after 30 s is measured (Bourne, Citation1982). So, the parameter used to characterize the consistency of the samples is the distance that the jam flows across the plate in 30 s in relation to the weight of the sample (mm/g).
Statistical analysis
Analysis of variance (ANOVA) with a confidence level of 95% (p < 0.05) was applied using Statgraphics Plus 5.1 Software (Statistical Graphics Corporation, USA) to evaluate the differences among samples and the different trend of samples during storage. Principal component analysis (PCA) was applied to the correlation matrix of the average values of color parameters, using the SPSS version 16.0 program.
Results and discussion
Antifungal activity
Agar diffusion method showed that at the doses considered (1, 0.5 and 0.34 g/mL) only lemon extract had an antifungal action. In order to quantify this action with greater precision, the broth dilution technique was then used (Davison & Parish, 1989). The initial jam charge pattern at 24 h was 1.03 × 102 cfu/mL and MIC was 0.2 or 0.1 g/mL, as defined by Jia et al. (Citation2010) and Ponce et al. (Citation2003), respectively. Moreover, MFC was 0.1 g/mL. Therefore, the dose chosen for the lemon extract was 0.1 g/mL.
Effect of pomegranate and lemon extract addition on physicochemical and microbiological parameters
After dehydration, the fruit reached 20.7 ± 0.1 °Brix and 60 °Brix OS. In other studies (García-Martínez et al., Citation2002; Igual et al., Citation2010; Shi et al., Citation1996) using slices of osmotically dehydrated fruit, °Brix had similar values (26 for kiwi, 23 for orange, 20 for strawberry, and 30 for grapefruit).
shows the °Brix, aw and xw , pH values and the flow distance corrected for sample weight of the three jam batches: control with no extracts (OD), OD jam with 0.001 g/mL of pomegranate extract (OD+PG) and OD jam with 0.001 g/mL pomegranate extract and 0.1 g/mL lemon extract added by spraying (OD+PG+L).
Table 1. Mean values (and standard deviation) of °Brix, pH, water activity (a
w
), moisture content (x
w
) and the values of flow distance corrected for the sample weight of formulated jams.
Tabla 1. Valores medios (y desviación estándar) de °Brix, pH, actividad del agua (a
w
), contenido en humedad (x
w
) distancia del flujo por peso de muestra de las mermeladas formuladas.
There were no significant differences for xw and pH. The aw of OD+PG was significantly lower than OD and OD+PG+L jams, and consequently had a high °Brix value. The OD sample showed the lowest consistency (highest distance advanced). However, the sample containing both extracts had significantly (p < 0.05) higher consistency than OD. This agrees with similar studies by other authors (García-Martínez et al., Citation2002; Igual et al., Citation2010) who obtained similar consistency values for jams containing osmotically dehydrated fruit.
The color coordinates (L*, a*, and b*), h*ab and C*ab of jams shown in . The color differences (ΔE) of jam with extracts in relation with control jam are also indicated in this table. OD jam showed significantly (p < 0.05) higher values in L* than jams with extracts; however, the L* value of OD+PG+L was significantly lower (p < 0.05) than OD. The h*ab of OD+PG was significantly lower (p < 0.05) than the control. Although the values of ΔE with respect to control were small, OD+PG+L showed a color difference significantly greater than OD+PG. Other references (Gómez et al., Citation2012) show the results of jam obtained from var. Camarosa strawberries, in which the L* var. Camarosa jam values were lower than those obtained in this study. However the values of a*, b*, h*ab and C*ab were lower in the case of jams made from Fragaria vesca than var. Camarosa. It can therefore be said that the final color owes more to the variety of strawberry than to the extracts added.
Table 2. Mean values (and standard deviation) of colour parameters of formulated jams.
Tabla 2. Valores medios (y desviación estándar) de los parámetros de color de las mermeladas formuladas.
shows the counts of aerobic mesophylls, lactic acid bacteria, molds and yeasts and coliforms for the three batches immediately after processing. There was no coliform development in any of the jams. In general, the initial OD charge was significantly higher (p < 0.05) than the others.
Table 3. Mean values (and standard deviation) of microbial characterization of formulated jams.
Tabla 3. Valores medios (y desviación estándar) de los parámetros obtenidos de la caracterización de las mermeladas formuladas.
Effect of storage on physicochemical and microbiological parameters
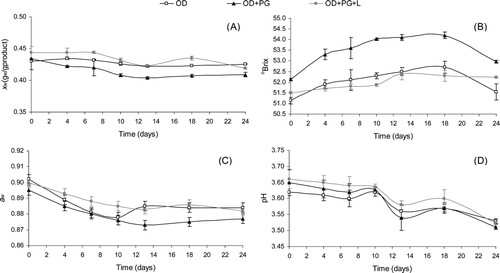
shows the evolution of xw , °Brix, aw and pH for the three jams stored at room temperature (25°C) for 24 days. The control of these parameters is very important since they are indicators of the stability of the product. Any physicochemical changes indicate alterations and/or deterioration during storage and affect their shelf-life. In general, the jams studied showed similar behavior during storage in terms of xw, aw , and pH. However, the °Brix of OD and OD+PG decreased significantly (p < 0.05) from day 18 of storage, while the °Brix of the OD+PG+L sample did not change significantly. The decrease of °Brix in the indicated samples could have been due to the action of microorganisms.
Figure 1. Evolution of (A) moisture content (xw ), (B) °Brix, (C) water activity (aw ), (D) pH of formulated jams throughout storage (24 days at 25°C).
Figura 1. Evolución de (A) contenido de humedad (xw), (B) °Brix, (C) actividad del agua (aw), (D) pH de las mermeladas estudiadas durante el almacenamiento (24 días a 25°C).
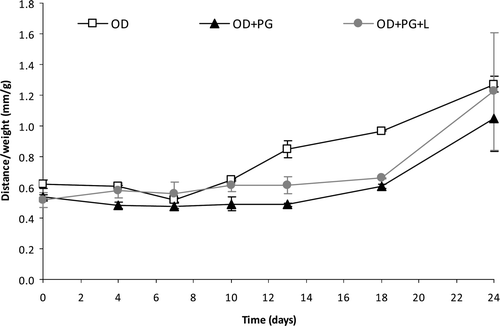
The consistency of the jams stored at room temperature () decreased at the end of the 24-day storage (increased advance distance). OD lost consistency from the 10th day, while OD+PG and OD+PG+L showed a significant decrease (p < 0.05) from day 18, probably due to attack by microorganisms.
Figure 2. Evolution of consistency (flow distance corrected for the sample weight) of formulated jams throughout storage (24 days at 25°C).
Figura 2. Evolución de la consistencia (distancia del flujo corregido por el peso de muestra) de las mermeladas formuladas durante el almacenamiento (24 días a 25°C).
On applying PCA () to the average values of the color coordinates L*, a* and b*, h*ab , and C*ab of the jams during storage, the first two components showed eigenvalues higher than 1. The consideration of both components accounted for 90.38% of the total variability. The first component (C1), explaining 66.78% of the variability, was associated with h*ab (r = 0.99), a* (r = 0.96), C*ab (r = 0.89), and b* (r = 0.78) values. The second component (C2) accounted for 23.60% of the variability and was mainly associated with the L* (r = 0.91) value. At the beginning of the storage period, strawberry jams were placed in the PCA plot on the right-hand side as a consequence of their lower b* and h*ab values and higher a* and C*ab values. In this case, C2 separated the OD+PG+L sample from the OD and DO + PG samples. During storage, a decrease of the C1 component was observed, which meant a decrease in a* and C*ab and an increase of b* and h*ab , that is jams become more brown (red color decrease and yellow increase) and color saturation decrease. This fact probably happens as a consequence of enzymatic reactions
Figure 3. Principal component analysis (PCA) of the values of color parameters of all the jams.
Figura 3. Análisis de componentes principales (PCA) de los valores de los parámetros de color de todas las mermeladas.
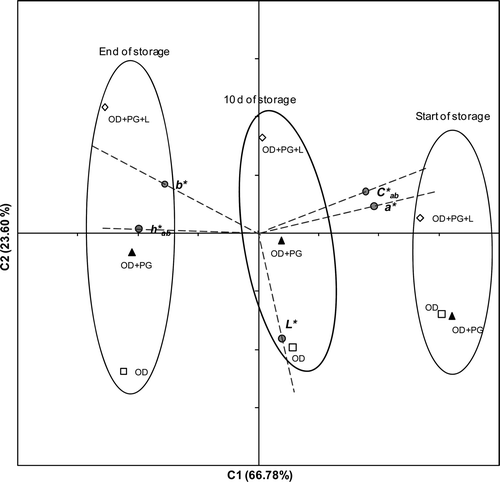
L* remained stable throughout the storage period. At the end of storage, the jams were placed on the left-hand side of the PCA plot. Applying a multifactor ANOVA to the C1 and C2 values of all the jam samples, there was a significant difference in C1 with storage time.
In the microbiological study, the development of five kinds of microorganisms was evaluated: aerobic mesophyll, coliforms, lactic acid bacteria (LAB), molds, and yeasts.
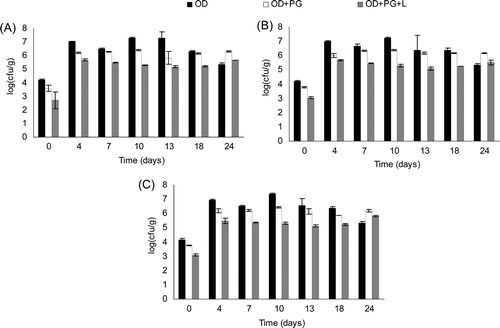
Coliforms were not present in any of the batches throughout storage. The results of aerobic mesophylls, lactic acid bacteria, molds, and yeast are shown in . OD showed higher counts of aerobic mesophylls, LAB, molds, and yeasts until 18 days of storage. The lowest microbial growth was found in OD+PG+L, which indicates the antimicrobial effect of the plant extracts studied. The use of pomegranate extract achieved a mean reduction of 0.71, 0.52, and 0.58 log units for aerobic mesophylls LAB, yeasts, and molds, respectively. In the case of OD+PG+L, the reductions were 1.52, 1.34, and 1.38 log units. On the other hand, there was a drop in the OD counts from days 10–13, which could be attributed to the exhaustion of nutrients in the product. However, the counts for the three groups of microorganisms tested were always higher for OD jam than OD+PG and OD+PG+L during the first 18 days.
Figure 4. Evolution of (A) aerobic mesophylls, (B) lactic acid bacteria (LAB) and (C) molds and yeasts of formulated jams throughout storage (24 days at 25°C).
Figura 4. Evolución of (A) mesófilos aerobicos, (B) bacterias ácido lácticas (LAB) and (C) hongos y levaduras de las mermeladas formuladas durante el almacenamiento (24 días a 25°C).
In order to analyze in great detail the evolution of the microbiota present in the three jams during storage, the evolution of aerobic mesophylls, LAB, molds, and yeasts, was modeled according to the Gompertz and Baranyi models (Baranyi & Roberts, Citation1994). shows the growth parameters obtained from these models. There were no high differences between the two proposed models. The maximum population density (N) was for aerobic mesophylls, around 6.7, 5.9, and 5 log units for OD, OD+PG and OD+PG+L, respectively. For LAB, the maximum levels were 6.5 (OD), 6 (OD+PG), and 5 (OD+PG+L) log units. Finally, in the case of molds and yeasts, N reached values of 6.8 (OD), 5.8 (OD+PG), and 5 (OD+PG+L).
Table 4. Maximum density population (N) for microbiota present in the three batches of strawberry jam.
Tabla 4. Máxima densidad de población (N) de la microbiota presente en los tres lotes de mermelada de fresa.
These results confirm the antimicrobial and antifungal capacity of pomegranate and lemon extracts mainly linking to anthocyanins, ellagitannins and other phenolic compounds found in pomegranate (Madrigal-Carballo et al., Citation2009) and to D-limonene, linalool or citral in lemon extract (Tepe et al., Citation2006; Veldhuizen et al., Citation2006).
Conclusion
In vitro studies show that among plant extracts tested in this study (pomegranate, lemon, rosemary and lemon balsamic) lemon extract presented the highest antifungal activity against the microbiota present in strawberry jam, establishing MIC and MFC at 0.1 g/mL. Adding extracts increases the stability of this jam during storage at room temperature. The use of plant extracts reduces the microbial charge (aerobic mesophylls, lactic acid bacteria, molds, and yeasts) and is most evident in the case of pomegranate and lemon extracts, which do not cause significant changes in the physicochemical characteristics. Furthermore, adding pomegranate and/or lemon extract improved the consistency of jams during storage. Based on the physicochemical and microbiological parameters obtained in this study, the use of pomegranate and lemon extracts would therefore prolong the shelf-life of strawberry jam made with osmotically dehydrated fruit.
Acknowledgments
The authors wish to thank the Spanish Ministerio de Educación y Ciencia for the financial support given through Projects AGL 2005–05994. The proof-reading of this paper was funded by the Universidad Politécnica de Valencia, Spain.
References
- RSS Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of mesophilic lactic acid bacteria – Colony-count technique at 30 degrees C. ISO 15214:1998. AENOR. (1998).Madrid: AENOR
- AENOR. (2003). RSS Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of microorganisms – Colony-count technique at 30 degrees C. ISO 4833:2003. Madrid: AENOR
- AENOR. (2006). RSS Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coliforms – Colony-count technique. ISO 4832:2006. Madrid: AENOR
- AENOR. (2008). RSS Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of yeasts and moulds – Part 1: Colony count technique in products with water activity greater than 0,95. ISO 21527-1:2008. Madrid: AENOR
- AOAC . 2000 . Official methods of analysis of AOAC international , 17th ed , Gaithersburg , MD : USA .
- Baranyi , J. and Roberts , T.A. 1994 . A dynamic approach to predicting bacterial growth in food . International Journal of Food Microbiology , 23 : 277 – 294 .
- Barry , A.L. 1986 . “ Procedure for testing antimicrobial agents in agar media: Theoretical considerations ” . In Antibiotics in laboratory medicine , (2nd ed.) , Edited by: Lorain , V. 21 – 22 . Baltimore : Williams & Wilkins .
- Bourne , M. 1982 . Food texture and viscosity-concept and measurement , New York : Academic Press .
- Davidson , P.M. and Parish , M.E. 1989 . Methods for testing the efficacy of food antimicrobials . Food Technology , 1 : 148 – 155 .
- Ernst , E.J. 2005 . Antifungal agents. Methods and protocols , Totowa , , New Jersey : Humana Press Inc .
- García-Martínez , E. , Ruiz-Diaz , G. , Martínez-Monzó , J. , Camacho , M.M. , Martínez-Navarrete , N. and Chiralt , A. 2002 . Jam manufacture with osmodehydrated fruit . Food Research International , 35 : 301 – 306 .
- García-Viguera , C. , Zafrilla , P. and Tomás-Barberán , F.A. 1999 . Influence of processing and storage conditions in strawberry jam colour . Food Science and Technology International , 5 : 487 – 492 .
- Goessinger , M. , Mayer , F. , Radocha , N. , Hoefler , M. , Boner , A. , Groll , E. … and Berghofer , E. 2009 . Consumer's color acceptance of strawberry nectars from puree . Journal of Sensory Studies , 24 ( 1 ) : 78 – 92 .
- Gómez , F. , Igual , M. , Camacho , M.M. and Pagán , M.J. 2012 . Effect of the addition of plant extracts on the microbiota of minimally processed strawberry jam and its physicochemical and sensorial properties . CyTA – Journal of Food , doi: 10.1080/19476337.2012.712058
- Grigelmo-Miguel , N. and Martín-Belloso , O. 1999 . Influence of fruit dietary fibre addition on physical and sensorial properties of strawberry jams . Journal of Food Engineering , 41 : 13 – 21 .
- Herrmann , K. 1972 . Anthocyanins in food . Zeitschrift für Lebens-mittel-Untersuchung und Forschung , 148 : 290 – 302 .
- Igual , M. , Contreras , C. and Martínez-Navarrete , N. 2010 . Non-conventional techniques to obtain grapefruit jam . Innovative Food Science and Emerging Technologies , 1 : 335 – 345 .
- Jia , H.L. , Ji , Q.L. , Xing , S.L. , Zhang , P.H. , Zhu , G.L. and Wang , X.H. 2010 . Chemical composition and antioxidant, antimicrobial activities of the essential oils of Thymus marschallianus Will. and Thymus proximus Serg . Journal of Food Science , 75 ( 1 ) : E59 – E65 .
- Krishnamurthy , Y.L. and Shashikala , J. 2006 . Inhibition of aflatoxin B1 production of Aspergillus flavus, isolated from soybean seeds by certain natural plant products . Letters in Applied Microbiology , 43 : 469 – 474 .
- Madrigal-Carballo , S. , Rodriguez , G. , Krueger , C.G. , Dreher , M. and Reed , J.D. 2009). Pomegranate (Punica granatum) supplements: authenticity, antioxidant and polyphenol composition . Journal of Functional Foods , 1 : 324 – 329 .
- Pascual , M.R. and Calderón , V. 2000 . Microbiología Alimentaria. Metodología Analítica para Alimentos y Bebidas , 2a Edición Madrid , España
- Pérez-Fons, L., Garzón, M.T., & Micol, V. (2010). Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. Journal of Agriculture and Food Chemistry, 13, 161–171
- Ponce , A.G. , Fritz , R. , Valle , C. and Del , Roura, S.I. 2003 . Antimicrobial activity of essential oils on the native microflora of organic Swiss chard . Lebensmittel-Wissenschaft und –Technologie , 36 : 679 – 684 .
- Redalen , G. and Haffner , K. 2002 . Quality of raspberry jam individual cultivars after one year of storage . Acta Horticulturae , 585 : 525 – 530 .
- Shi , X.Q. , Chiralt , A. , Fito , P. , Serra , J. , Escoin , C. and Gasque , L. 1996 . Application of osmotic dehydration technology on jam processing . Drying Technology , 14 : 841 – 857 .
- Sundfør , W.M. 2001 . Evaluation of strawberry cultivars for jam with focus on colour characteristics , Agricultural University of Norway . (Master thesis)
- Tepe , B. , Akpulat , H.A. , Sokmen , M. , Daferera , D. , Yumrutas , O. and Aydin , E. 2006 . Screening of the antioxidative and antimicrobial properties of the essential oils of Pimpinella anisetum and Pimpinella flabellifolia from Turkey . Food Chemistry , 97 : 719 – 724 .
- Veldhuizen , E.J. , Tjeerdsma-Van Bokhoven , J.L. , Zweijtzer , C. , Burt , S.A. and Haagsman , H.P. 2006 . Structural requirements for the antimicrobial activity of carvacrol . Journal of Agricultural and Food Chemistry , 54 : 1874 – 1879 .
- Viguera , C.G. , Zafrilla , P. and Barberan , E.T. 1997 . Determination of authenticity of fruit jams by HPLC análisis of anthocyanins . Journal of Science and Food Agriculture , 73 : 207 – 213 .
- Viuda-martos , M. , Ruiz-Navajas , Y. , Fernandez-Lopez , J. and Perez-Alvarez , J. 2008 . Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils . Food Control , 19 : 1130 – 1138 .
- Voravuthikunchai , S. , Lortheeranuwat , A. and Jeeju , W. 2004 . Effective medicinal plants against enterohaemorrhagic Escherichia coli O157:H7 . Journal of Ethnopharmacology , 94 : 49 – 54 .
- Withy , L.M. , Nguyen , T.T. , Wrolstad , R.E. and Heatherbell , D.A. 1993 . Storage changes in anthocyanin content of red raspberry juice concentrate . Journal of Food Science , 58 : 190 – 192 .
- Xu , G. , Liu , D. , Chen , J. , Ye , X. , Ma , Y. and Shi , J. 2008 . Juice components and antioxidant capacity of citrus varieties cultivated in China . Food Chemistry , 106 : 545 – 551 .
- Yesil-Celiktas , O. , Sevimli , C. , Bedir , E. and Vardar-Sukan , F. 2010 . Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines . Plant Foods for Human Nutrition , 65 : 158 – 163 .