Abstract
In the present study the effects of ultrasonic pretreatment and the types of enzyme on oil yield were investigated. The optimum ultrasonic pretreatment parameters were found to be 250 W of ultrasonic power, 30 min of ultrasonic time, and 50°C of ultrasonic temperature. Five types of enzyme, Cellulase, Viscozyme L, Alcalase 2.4L, Protex 6L, and Protex 7L, were evaluated for their effectiveness in releasing oil from ultrasonic pretreated perilla seeds. The highest oil yield of 81.74% was observed in cellulase treated perilla seed samples. The physicochemical properties of the control, hexane, and enzyme extracted perilla seed oils were compared. No significant (P > 0.05) differences were observed in iodine value, refractive index, unsaponifiable matter, saponification value, peroxide value, and acid value.
En el presente estudio se investigaron los efectos del pretratamiento ultrasónico y tipos de enzima en el rendimiento de aceite. Los parámetros óptimos de pretratamiento de ultrasonidos fueron 250 W de potencia ultrasónica, 30 min de tiempo de ultrasonidos, y 50°C de temperatura de ultrasonidos. Cinco tipos de enzima, celulasa, Viscozyme L, Alcalase 2.4L, Protex 6L, y 7L Protex, fueron evaluados por su eficacia en la liberación de aceite de semillas de perilla pretratadas con ultrasónicos. El mayor rendimiento de aceite de 81,74% se observó en las muestras de semillas de perilla tratadas con celulasa. Se compararon las propiedades fisicoquímicas del control y de aceites de semillas de perilla extraidas con hexano y enzimas. No se observaron diferencias significativas (P > 0,05) en el índice de yodo, índice de refracción, la materia insaponificable, valor de saponificación, el valor de peróxido y el valor ácido.
1. Introduction
Perilla (Perilla frutescens L.) is an annual grown edible herb belonging to the Lamiaceae family (Asif, Citation2011). Asia countries, such as China, Korea, Japan, Thailand, are the main producing area of perilla, and perilla seed oil has been deemed as edible and seasoning oil in those countries. The unique property of perilla seed oil is its naturally high content of omega-3 fatty acid (ranging from 53.6% to 64%) (Yoon & Noh, Citation2011). The high content of omega-3 confers perilla seed oil with a potential to lower risk for multiple chronic diseases, prevent abnormal clotting, reduce inflammation, relax blood vessels, and anticancer properties (Asif, Citation2011; Calder, Citation2006; Harris, Miller, Tighe, Davidson, & Schaefer, Citation2008).
Perilla seed oil is presently extracted by mechanical squeeze or organic solvent. The oil yield of mechanical squeeze extraction is quite low, and organic solvent extraction has healthy and environmental problems. For these reasons, it is desirable to find alternative extraction methods. Aqueous enzymatic extraction of oil as a healthy and green method has been widely applied to extract oil from a variety of oil-bearing seeds, such as corn germ (Moreau, Johnston, Powell, & Hicks, Citation2004), soybean (de Moura et al., Citation2008), sunflower (Latif & Anwar, Citation2009), and peanut (Jiang, Hua, Wang, & Xu, Citation2010).
Ultrasound can accelerate heat and mass transfer and has been successively applied to extraction field. Recent studies have shown that ultrasound enhances mass transfer mainly by cavitational effect (Chemat, Zill-e-Huma, & Khan, Citation2011). Ultrasonic treatment produces bubbles (filled with gas or vapour) when ultrasonic waves passes through a liquid. This phenomenon is widely known as cavitation. The explosion of cavitation bubbles yields localized high pressures and temperatures (up to 100 MPa and 5000 K, respectively). When these bubbles collapse close to the cell wall surface, the released high pressure and temperature generate liquid jets and shear forces directed toward the cell wall surface. These liquid jets and shear forces involved in this process cause physical damage of cell wall or cell membrane (Chemat et al., Citation2011; Knorr, Ade-Omowaye, & Heinz, Citation2002).
To the best of our knowledge, there has been no report on the ultrasound-assisted aqueous enzymatic extraction of oil from perilla seeds. The aim of the present study was to develop an efficient and environmental friendly method for the extraction of perilla seed oil. The effect of ultrasonic pretreatment and types of enzyme on oil yield was investigated. The quality of perilla seed oils extracted by different means were then evaluated by measuring fatty acid composition and physicochemical properties.
2. Materials and methods
2.1 Perilla seeds and reagents
The perilla seed with an oil content of 39.96% (determined by AOAC 922.06) were purchased from the Xintai seedling field in China. The shell of perilla seed was carefully removed by hand. The cleaned perilla seed (perilla seed kernel) was kept in sealed polyethylene bags at 4°C until use. Alcalase 2.4L (from Bacillus licheniformis, 2.4 AU/g, AU: anson unit) and Viscozyme L (from Aspergillus aculeatus, multi-enzyme mixture containing arabanase, cellulase, β-glucanase, hemicellulase, and xylanase, 100 fungal β-glucanase units (FBG)/ml) were provided by Novo Nordisk A/S (Bagsvaerd, Denmark). Protex 6L (from a selected strain of Bacillus licheniformis, 580,000 DU/g, DU: dextrinizing unit) and Protex 7L (from Bacillus amyloliquefaciens, 1600 AU/g) were purchased from Genencor (Rochester, NY, USA). Cellulase (from Trichoderma longibrachiatum, 1.0 unit/mg solid, one unit will liberate 1.0 μmole of glucose from cellulose per hour at pH 5.0 at 37°C incubated for 2 hours) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Crushing of perilla seed kernels
Perilla seed kernels (about 50 g) were crushed into powder in a juice extractor (BE601AB, Media Group, China) with a particle size range of 0.8 to 1.2 mm. The crushed perilla seed kernel powder was collected and the remaining perilla seed kernel powder on the juice extractor’s chamber wall was washed off using deionized (DI) water. The collected and water-washed powders were filtered through a Whatman No. 1 filter paper. The filtered powder was waiting for ultrasonic pretreatment.
2.3. Ultrasonic pretreatment
Ultrasonic pretreatment was carried out on an ultrasonic machine (JY92-IIN, Ningbo Scientz Biotechnology Co. Ltd, Ningbo, China). Perilla seed kernel powder was mixed with DI water at a ratio of 6:1 liquid/solid in a beaker followed by immersing the ultrasonic horn into the beaker. The ultrasonic treatments were carried out under the conditions of ultrasonic power ranging from 100 to 350 W, ultrasonic time ranging from 5 to 50 min, and ultrasonic temperature ranging from 20 to 70°C. After sonication, the mixture was centrifuged at 8000 × g using a centrifuge (Model TGL-16G, Anting Scientific Instrument Factory, Shanghai, China) at room temperature to separate extracted oil. After centrifugation, extracted oil was located in the upper layer due to its less density than water, and can be easily separated using a micro-pipette. Oil yield was calculated as the weight of extracted oil divided by the total weight of oil present in perilla seed kernel sample (calculated based on wet basis), shown as Equation (1).
2.4. Aqueous enzymatic extraction
Aqueous enzymatic extraction was conducted on the ultrasonic pretreated perilla seed kernel powder. The ultrasonic power, time, and temperature were set to the optimum values obtained from the ultrasonic pretreatment part. Perilla seed kernel powder (approx. 50 g) was mixed with water to a ratio of 6:1 (water/sample), and then subjected to ultrasonic pretreatment. Prior to adding enzyme, the pH of the mixture was adjusted to the optimal value (recommended by the manufacturers) for each enzyme using 0.5 N NaOH and 0.5 N HCl. Enzymes (Alcalase 2.4L, Protex 6L, Protex 7L, Cellulase, and Viscozyme L) were then added according to their recommended dosage from the manufacturers. The mixture was incubated in a water bath at 50°C for 2 h with constant stirring. Afterwards, the mixture was subsequently centrifuged at 8000 × g for 20 min at room temperature. Three layers (oil, cream, and skim) were obtained after centrifugation. The supernatant oil layer was carefully removed by absorbing using a micro-pipette. The control group was treated identically, except for the addition of enzyme. The oil yield was calculated according to Equation (1).
2.5. Fatty acid composition analysis
The fatty acid composition analysis was performed according to our previous work (Li, Zhang, Wang, Jiang, & Sui, Citation2012). The fatty acid methyl esters (FAMEs) were prepared by the following two steps: (1) oils were saponified with 0.5 M KOH; (2) later methylated with 40% BF3 in methanol (Reena, Reddy, & Lokesh, Citation2009). Gas chromatography mass spectrometry (GC/MS) analysis was carried out with an Agilent 6890–5973 (Agilent Technologies, CA, USA) instrument. Separating procedure was achieved on an Agilent HP-88 capillary column (100 × 0.25 mm i.d., film thickness 0.2 μm). The operating conditions were as follows: carrier gas pressure, 100 kPa; carrier gas, helium; split ratio was 1:30; injection temperature, 250°C; scanning scope: 50–550 amu; ionization voltage: 70 eV. Oven temperature was programmed as follows: held at 80°C for 5 min, and then rising to 150°C at 10°C/min, and held for 2 min at 150°C; then continuously rising to 230°C at 5°C/min and held for 10 min. The individual fatty acids were identified and quantified by comparing their retention times with external standards.
2.6. Determination of physicochemical properties of perilla seed oil
The physicochemical properties of the control, hexane, and enzyme extracted perilla seed oils were determined by AOCS standard methods. All the oils were characterized for iodine value, refractive index, unsaponifiable matter, saponification value, peroxide value, and acid value.
2.7. Statistical analysis
Experiments were performed in triplicate and results were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (P < 0.05) was used to compare differences between groups.
3. Results and discussion
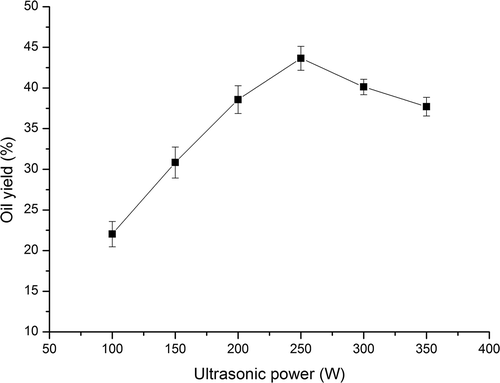
3.1. Effect of ultrasonic power on oil yield
The results in exhibited that the oil yield increased first (from 22.03% to 43.65%) and, then, decreased (from 43.65% to 37.71%) with the increase of ultrasonic power from 100 to 350 W. The ultrasonic power has a significant effect on oil yield by conducting a linear increase of oil yield when ultrasonic power rose from 100 W to 250 W. When larger amount of ultrasound wave passed through solvent, more bubbles would be generated followed by collapse (Hemwimol, Pavasant, & Shotipruk, Citation2006). High-speed jet may therefore generate to disrupt cell walls. Thus the release of oil from perilla seed cells was accordingly improved. The highest oil yield was 43.65% at the ultrasonic power of 250 W. However, the oil yield exhibited a slightly decrease when the power exceeded 250 W. The decrease of oil yield may be due to the free radicals which could lead to the degradation of oils (Lou, Wang, Zhang, & Wang, Citation2010). Therefore, the ultrasonic power of 250 W was selected as the output power in later. Our results are in agreement with the study carried out by Lou et al., in their study the highest oil yield (83.32%) was observed at the ultrasonic power of 250 W (Lou et al., Citation2010). Li et al. and Sivakumar et al. observed the similar increasing trend in oil yield, i.e., appropriate increase in ultrasonic power brought an increase in yield (Li, Pordesimo, & Weiss, Citation2004; Sivakumar, Ravi Verma, Rao, & Swaminathan, Citation2007).
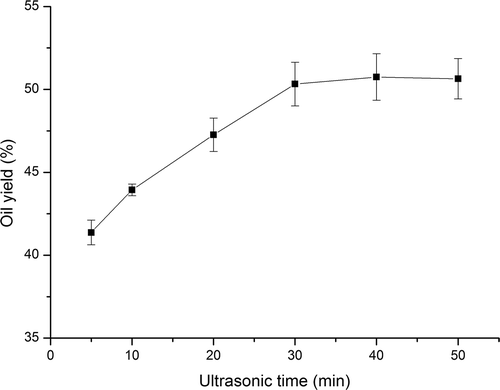
3.2. Effect of ultrasonic time on oil yield
The effect of ultrasonic time on oil yield was shown in . The oil yield was found to increase (from 41.37% to 50.32%) with the increase of time from 5 to 30 min and then level off when the time continuously increased. As the diffusion front moved from the surface of perilla seed powder particles toward the interior, the diffusion area reduced, diffusion distance increased and the diffusion rate would decrease accordingly (Zhao, Kwok, & Liang, Citation2007). Thus, the continuously increase of ultrasonic time would not change much of the oil yield. Similar results have also been shown in the studies of ultrasound-assisted extraction of oil from flaxseed (Zhang et al., Citation2008), tea seed (Shalmashi, Citation2009), and saikosaponins from radix bupleuri (Zhao et al., Citation2007). Zhang et al., Shalmashi et al., and Zhao et al. found that the oil yield was increased with increasing the ultrasonic time, and the increase was then stayed same when sonication was prolonged. Consequently, based on the results obtained so far, 30 min was selected as the ultrasonic time.
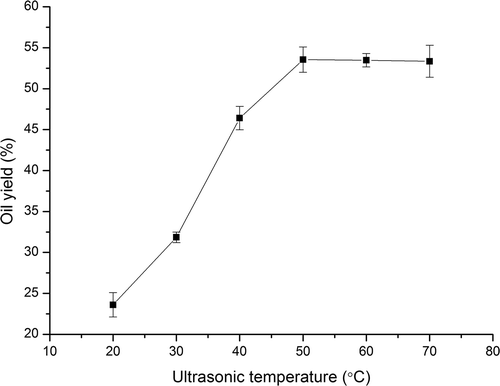
3.3. Effect of ultrasonic temperature on oil yield
The effect of ultrasonic temperature on oil yield was shown in . The oil yield increased from 23.60% to 53.54% with increasing the temperature from 20 to 50°C, and then leveled out after 50°C. Temperature affects the performance of ultrasonic pretreatment by influencing the amount of vapor in ultrasonically generated bubbles. The vapor pressure at high temperatures will help to form vapor-filled bubbles. The explosion of those vapor-filled bubbles had an effect named as cushioning effect (Lou et al., Citation2010). As a result, the cavitational effect would be weakened, and thus the oil yield remained unchanged with continuously increasing the temperature. Therefore, ultrasonic temperature of 50°C was seen to be suitable for ultrasonic pretreatment.
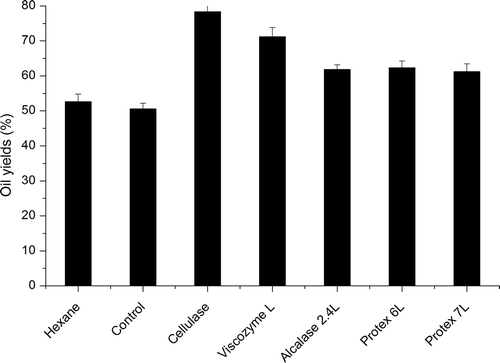
3.4. Effect of enzymes on oil yield
The yields of control, hexane, and enzyme extracted oil were shown in . Enzyme extracted oil exhibited a higher oil yield than the control (without enzyme addition) and hexane groups. The highest oil yield of 81.74% was achieved in cellulase hydrolysis ultrasonic pretreated perilla seed kernel powder. The oil yield of hexane treated samples (52.67%) was close to that of control (50.56%). The oil yields in Alcalase 2.4L (61.84 ± 1.29%), Protex 6L (62.31%), and Protex 7L (61.25%) treated samples were quite close to each other.
Figure 4. Effects of enzymes on oil yield.
Figure 4. Efectos de las enzimas en el rendimiento de aceite.
Plant cell wall is constituted of pectins, hemicelluloses, and micro-fibrils of cellulose cross- linked with protein (Campbell et al., Citation2011). The cell wall is the primary barrier to releasing oil from plant cells; therefore, it must be ruptured as sufficiently as possible for the aim of improving oil yield (Rosenthal, Pyle, & Niranjan, Citation1996). Alcalase 2.4L, Protex 6L, and Protex 7L, were the most effective proteases in the enzymatic extraction of oil from rice bran (Hanmoungjai, Pyle, & Niranjan, Citation2002), soybean (de Moura et al., Citation2008), peanut (Jiang et al., Citation2010), and tea seed (Zhang, Zhang, & Chen, Citation2012). However, in the present study, cellulase was the most effective enzyme. This may be due to the capacity of cellulase on cell wall hydrolysis. In the studies that proteases were the most effective enzymes, the pretreatment method was extrusion, whereas the pretreatment method was sonication in the present study. Lamsal et al. tested the effect of cellulase hydrolysis on oil yield from extruded soybean, and found no obvious increase in oil yield (Lamsal, Murphy, & Johnson, Citation2006). Campbell et al. (Citation2011) also concluded that extrusion pretreatment achieves a complete cellular disruption. Compared to extrusion, the degree of cell disruption in ultrasonic pretreatment is much lower. The addition of cellulase improved the degree of cell disruption by hydrolyzing the remaining intact cell walls. Thus, the oil yield in cellulase treated samples was the highest one. Similarly, cellulase was also reported as the most effective enzyme in the studies of aqueous enzymatic extraction of sunflower oil (Latif & Anwar, Citation2009) and corn germ oil (Moreau et al., Citation2004).
3.5. Fatty acid composition of oils
The fatty acid profiles of oils extracted by hexane and enzymes were shown in . Hexane extracted oil had a significantly (P < 0.05) higher content of C18:0 and lower content of C18:2 than control and enzyme extracted oils. Besides, there was no significant difference (P > 0.05) in C16:0, C18:1, C18:3, and C20:0 contents of the control, hexane, and enzyme extracted oils. Abdulkarim et al. also compared the amount of fatty acids of enzyme- and hexane-extracted oil samples, and similarly they found no significant difference (P > 0.05) (Abdulkarim, Long, Lai, Muhammad, & Ghazali, Citation2005). Extracted perilla oil has a high content (approx. 52%) of alpha-linolenic acids (C18:3). The alpha-linolenic acids belong to omega-3 fatty acids, which have been regarded as being protective against many chronic diseases. The omega-3 fatty acids belong to essential fatty acids, which must be obtained in the diet as human being cannot synthesize them. Omega-3 fatty acids exert their beneficial function through reducing hepatic VLDL-TG (VLDL: very-low-density lipoprotein, TG: triglyceride) synthesis and secretion, and enhancing TG clearance from chylomicrons and VLDL particles (Harris et al., Citation2008). Therefore, the high content of omega-3 fatty acids in the extracted oil makes it desirable and popular under the current healthy eating theme.
Table 1. Fatty acid composition of control, hexane, and enzyme extracted oils*.
Table 1. Composición de ácidos grasos de los aceites control y extraídos con hexano y enzimas.
3.6. Physicochemical properties of extracted perilla seed oil
The physicochemical properties of the control, hexane, and enzyme extracted oil were given in . The iodine value, refractive index, unsaponifiable matter, saponification value, peroxide value, and acid value of the oils were not significantly affected (P > 0.05) by different extraction means and enzymes. Similar results were reported by Latif, Diosady, and Anwar (Citation2008). In their study, the effect of different enzymes on enzymatic aqueous extraction of canola oil were investigated through comparing the quality of extracted oils, and no significant differences were observed in iodine value, refractive index, unsaponifiable matter, and saponification value.
Table 2. Physicochemical properties of extracted perilla seed oils* and other edible oils.
Table 2. Propiedades físico-químicas de los aceites extraídos de semillas de perilla y otros aceites comestibles.
To evaluate the physicochemical properties of extracted perilla seed oils, the measured physicochemical values were compared with the adopted values from CODEX STAN 210 (Citation1999) of soybean oil, sunflower seed oil, and canola oil. It is obviously that the iodine value of perilla oil was much higher than that of soybean oil, sunflower seed oil, and canola oil. The iodine value, used to determine the amount of unsaturation in fatty acids, was measured through iodine compounds reacting with C=C bonds of unsaturated fatty acids. The higher the iodine value, the more unsaturated fatty acids are present in the oil (Thomas, Citation2002). This high iodine value was further confirmed by the fatty acids composition of perilla oil, whose unsaturated fatty acids (C18:1, C18:2, and C18:3) comprised approximately 86%. As to the physicochemical properties of refractive index, unsaponifiable matter, and saponification value, perilla seed oil were close to soybean oil, sunflower seed oil, and canola oil.
4. Conclusions
The results indicated that ultrasound-assisted aqueous enzymatic extraction of perilla seed oil was an efficient extraction method with the highest oil yield of 81.74%. More importantly, ultrasound-assisted aqueous enzymatic extraction was an environmental friendly alternative to conventional solvent extraction methods.
Acknowledgements
We wish to thank the support for this work by the National High-tech R&D Program of China (863 Program) (research grant number: 2013AA102104), the National Natural Science Foundation of China (research grant number: 31071493), the China Postdoctoral Science Foundation (research grant number: 2012M511433), the Northeast Agricultural University, and the National Research Center of Soybean Engineering and Technology.
References
- Abdulkarim, S. M., Long, K., Lai, O. M., Muhammad, S. K. S., & Ghazali, H. M. (2005). Some physico-chemical properties of moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chemistry, 93(2), 253–263.
- Asif, M. (2011). Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Oriental Pharmacy & Experimental Medicine, 11(1), 51–59.
- Calder, P. C. (2006). n − 3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition, 83(6), S1505–1519S.
- Campbell, K., Glatz, C., Johnson, L., Jung, S., de Moura, J., Kapchie, V., & Murphy, P. (2011). Advances in aqueous extraction processing of soybeans. Journal of the American Oil Chemists’ Society, 88(4), 449–465.
- Chemat, F., Zill-e-Huma, & Khan, M. K. (2011). Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry, 18(4), 813–835.
- CODEX STAN 210. (1999). Codex standard for named vegetable oils. Current official standards (Amended 2003, 2005). FAO/WHO Food standards. Codex Alimentarius. Retrieved from: http://www.codexalimentarius.net
- de Moura, J., Campbell, K., Mahfuz, A., Jung, S., Glatz, C., & Johnson, L. (2008). Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. Journal of the American Oil Chemists’ Society, 85(10), 985–995.
- Hanmoungjai, P., Pyle, D. L., & Niranjan, K. (2002). Enzyme-assisted water-extraction of oil and protein from rice bran. Journal of Chemical Technology & Biotechnology, 77(7), 771–776.
- Harris, W. S., Miller, M., Tighe, A. P., Davidson, M. H., & Schaefer, E. J. (2008). Omega-3 fatty acids and coronary heart disease risk: Cinical and mechanistic perspectives. Atherosclerosis, 197(1), 12–24.
- Hemwimol, S., Pavasant, P., & Shotipruk, A. (2006). Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrasonics Sonochemistry, 13(6), 543–548.
- Jiang, L., Hua, D., Wang, Z., & Xu, S. (2010). Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food and Bioproducts Processing, 88(2–3), 233–238.
- Knorr, D., Ade-Omowaye, B. I. O., & Heinz, V. (2002). Nutritional improvement of plant foods by non-thermal processing. Proceedings of the Nutrition Society, 61(02), 311–318.
- Lamsal, B., Murphy, P., & Johnson, L. (2006). Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. Journal of the American Oil Chemists’ Society, 83(11), 973–979.
- Latif, S., & Anwar, F. (2009). Effect of aqueous enzymatic processes on sunflower oil quality. Journal of the American Oil Chemists’ Society, 86(4), 393–400.
- Latif, S., Diosady, L. L., & Anwar, F. (2008). Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. European Journal of Lipid Science and Technology, 110(10), 887–892.
- Li, H., Pordesimo, L., & Weiss, J. (2004). High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International, 37(7), 731–738.
- Li, Y., Zhang, Y., Wang, M., Jiang, L. Z., & Sui, X. N. (2012). Simplex-centroid mixture design applied to the aqueous enzymatic extraction of fatty acid-balanced oil from mixed seeds. Journal of the American Oil Chemists’ Society, 90(3), 349–357.
- Lou, Z., Wang, H., Zhang, M., & Wang, Z. (2010). Improved extraction of oil from chickpea under ultrasound in a dynamic system. Journal of Food Engineering, 98(1), 13–18.
- Moreau, R. A., Johnston, D. B., Powell, M. J., & Hicks, K. B. (2004). A comparison of commercial enzymes for the aqueous enzymatic extraction of corn oil from corn germ. Journal of the American Oil Chemists’ Society, 81(11), 1071–1075.
- Reena, M. B., Reddy, S. R. Y., & Lokesh, B. R. (2009). Changes in triacylglycerol molecular species and thermal properties of blended and interesterified mixtures of coconut oil or palm oil with rice bran oil or sesame oil. European Journal of Lipid Science and Technology, 111(4), 346–357.
- Rosenthal, A., Pyle, D. L., & Niranjan, K. (1996). Aqueous and enzymatic processes for edible oil extraction. Enzyme and Microbial Technology, 19(6), 402–420.
- Shalmashi, A. (2009). Ultrasound-assisted extraction of oil from tea seeds. Journal of Food Lipids, 16(4), 465–474.
- Sivakumar, V., Ravi Verma, V., Rao, P. G., & Swaminathan, G. (2007). Studies on the use of power ultrasound in solid–liquid myrobalan extraction process. Journal of Cleaner Production, 15(18), 1813– 1818.
- Thomas, A. (2002). Fats and fatty oils. Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH.
- Yoon, S., & Noh, S. (2011). Positional distribution of fatty acids in perilla (perilla frutescens) oil. Journal of the American Oil Chemists’ Society, 88(1), 157–158.
- Zhang, Z. S., Wang, L. J., Li, D., Jiao, S. S., Chen, X. D., & Mao, Z. H. (2008). Ultrasound-assisted extraction of oil from flaxseed. Separation and Purification Technology, 62(1), 192–198.
- Zhang, W. G., Zhang, D. C., & Chen, X. Y. (2012). A novel process for extraction of tea oil from Camellia oleifera seed kernels by combination of microwave puffing and aqueous enzymatic oil extraction. European Journal of Lipid Science and Technology, 114(3), 352– 356.
- Zhao, S., Kwok, K. C., & Liang, H. (2007). Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Separation and Purification Technology, 55(3), 307–312.