Abstract
The Box-Behnken design combined with response surface methodology was used to optimize ultrasonic-assisted extraction of polysaccharides from Cyclina sinensis. A high-extraction yield of polysaccharides, 34.63 ± 1.52 g/kg using an ultrasonic power of 500 W, liquid to material ratio of 22:1 (mL/g), with an extraction temperature of 77°C for 31 min was achieved. The polysaccharides exhibited quite a strong reducing property, the total antioxidant capacity, as well as excellent inhibitions of DPPH radical, hydroxyl radical, and superoxide radical. Meanwhile, it was found that the bioactivities appeared to be dose-dependent on polysaccharides.
Resumen: El diseño de Box-Behnken combinado con metodología de superficie de respuesta se utilizó para optimizar la extracción asistida por ultrasonidos de polisacáridos de Cyclina sinensis. Se logró un alto rendimiento de extracción de polisacáridos, 34,63 ± 1,52 g/kg utilizando energía ultrasónica de 500 W, relación de líquido a materiales de 22:01 (ml/g), con una temperatura de extracción de 77°C durante 31 min. Los polisacáridos mostraron una fuerte propiedad reductora, la capacidad antioxidante total, así como excelentes inhibiciones de radical DPPH, radical hidroxilo y radicales superóxido. Mientras tanto, se encontró que las bioactividades parecía ser dependiente de la dosis de polisacáridos.
Introduction
Cyclina sinensis is a bivalve mollusk belonging to Veneridae, commonly known as Chinese venus, black clam and iron clam (Zhao, Li, Kong, & Mao, Citation2009). It is a commonly and mainly edible bivalve along the coast of China and one of the main bivalve species in Chinese aquaculture (Liu, Ma, Hu, Miao, & Li, Citation2002). C. sinensis displays rich nutrients, such as protein, polysaccharides, essential amino acids and fatty acids which may be conducive to the bioactivity of anti-inflammation, anti-tumor and antioxidant (Gu, Yu, & Cai, Citation2006; Li et al., Citation2010; Jiang, Wang, Liu, Gan, & Zeng, Citation2011). In addition, it has been reported that C. sinensis can be used for the treatment of asthma, inflammation and dental ulcer in traditional Chinese medicine (Wang et al., Citation2007). The latest study reported that the heat-extracted polysaccharides from C. sinensis (PCS) had potential hepatoprotective effects in vivo (Jiang, et al., Citation2013). However, to the best of our knowledge, there were no reports of systematic studies on ultrasonic-assisted extraction and biological activities of PCS compared with those of some other bivalvia species such as Mactra veneriformis, Mytilus coruscus and Hyriopsis cumingii (Xu et al., Citation2008; Qiao et al., Citation2009; Luan, Wang, Wu, Jin, & Ji, Citation2011).
Ultrasonic-assisted extraction technology has been employed for extracting polysaccharides from different biological materials in the past decades (Hromadkova & Ebringerova, Citation2003). The enhanced extraction efficiency by ultrasonic treatment is mainly attributed to its mechanical effects, which significantly facilitate mass transfer between immiscible phases through super agitation (Vinatoru et al., Citation1997). Ultrasonic-assisted extraction has been proved as an alternative means of increasing the speed of sample extraction (Zhang et al., Citation2009).
Response surface methodology (RSM) is a statistical method that is useful for mathematical modeling and simultaneously solving multivariate equation (Liu et al., Citation2010). In the present study, ultrasound-assisted extraction for PCS was investigated and the operational conditions were optimized using Box-Behnken Design (BBD) combined with RSM. Furthermore, the antioxidant activities of PCS were also determined in multiple test systems in vitro. The objective of this study is to establish the optimized process of ultrasonic-assisted extraction for PCS and provide bioactivity information about PCS for further development and application of the resource.
Materials and methods
Materials and instruments
C. sinensis was purchased from the local market in Lianyungang city, Jiangsu Province, China.
Glucose, sulfuric acid, phenol, Coomassie brilliant blue G-250, bovine serum albumin, potassium ferricyanide, trichloroacetic acid, ferrous sulfate, hydrogen peroxide, EDTA and pyrogallol etc. were purchased from National Medicine Group Shanghai Co. (Shanghai, China). DPPH were purchased from Sigma (St. Louis, MO, USA). Total antioxidant capacity (TAC) assay kit was purchased from Nanjing Jiancheng bioengineering Institute (Nanjing, China). All other chemicals used were of analytical grade.
Ultrasonic cleaner (SK8210LHC, KUDOS Ultrasonic Equipment Co., Shanghai, China, 40 kHz) was used for ultrasonic extraction of polysaccharides, HH-4 thermostatic water bath boiler (Jiangnan Equipment Co., Jintan, China) for heating extraction of polysaccharides, Synergy HT Absorbance Microplate Reader (BioTek Co., USA) for total polysaccharides analysis of sample, RE-52A rotary evaporator (Shanghai Yarong Biochemistry Instrument Factory, Shanghai, China) for concentration of sample and QJ32W1000A high speed disintegrator (Tianjing TST Equipment Co., Tianjing, China) for homogenate of the sample.
Ultrasonic-assisted extraction of PCS
The procedure for PCS extraction was carried out consulting the literature on this subject (Qiao et al., Citation2009). C. sinensis were collected and washed carefully with cold water. After removing the shells and impurities, the fresh flesh was centrifuged to remove the moisture, crushed by a high speed disintegrator and stored at −20°C. Two grams of wet flesh and a certain ratio of water were used for each extraction. The extraction was performed in an ultrasonic cleaner using selected ultrasonic power and temperature for various times. After treatment, the mixture was centrifuged at 3500g for 20 min, and the insoluble residue was treated again as mentioned above. The proteins in the extract were removed by the Sevag reagent. Sevag reagent (100 mL: 80 mL of CHCl3 and 20 mL of butanol) was added to the concentrated extract. The bulk was shaken for 20 min at 25°C. After centrifugation at 3500g for 10 min, the supernatant was collected and subjected to this step 10 times. Precipitated by the addition of three times volume of absolute ethanol for 1 d at 4°C, PCS were collected by centrifugation at 3500g for 20 min, air-drying at 50°C to a constant weight, affording the crude PCS. The extraction yield was calculated according to the following formula: extraction yield (g/kg) = W1*103/W0, where W1 is the weight of crude PCS and W0 is the flesh weight. The total sugar content in PCS was determined by phenol–sulfuric acid method, using glucose for the standard curve. The protein content was determined as previously described (Bradford, Citation1976), using bovine serum albumin for the standard curve.
Experimental design
The extraction parameters were optimized using RSM, data analysis and model sbuilding with software Design Expert (Trial Version 7.0.0, Stat-Ease Inc., Minneapolis, Minnesota, USA). The BBD was employed in this regard. The range and center point values of three independent variables shown in were based on the results of single-factor experiments. BBD in this design consists of twelve factorial points and five replicates of the central point. Liquid to material ratio (X1), extraction time (X2) and extraction temperature (X3) were chosen for independent variables. The extraction yield of PCS was selected as the response for the combination of the independent variables given in Table 1. Experimental runs were randomized to minimize the effects of unexpected variability in the observed responses. The behavior of the system was explained by the following quadratic equation:
Table 1. Box-Behnken arrangement and the response for the yield of PCS.
Table 1. Disposición Box-Behnken y la respuesta para el rendimiento de PCS.
where Y is the estimated response (extraction yield of PCS) and β0, βi, βii and βij are regression coefficients in the intercept, linear, quadratic and interaction terms, respectively, while Xi and Xj are levels of the independent variables.
Reducing power
The determination of reducing power was performed as previously described (Vaquero, Serravalle, de Nadra, & de Saad, Citation2010) with some modifications. A total volume of 1.0 mL sample (varying concentration) was mixed with phosphate buffer (1.0 mL, 0.2 mol/L, pH 6.6) and potassium ferricyanide (1.0 mL, 10 g/L). After the mixture was incubated at 50°C for 20 min and rapidly cooled, 1.0 mL of 100 g/L trichloroacetic acid was added, and the mixture was centrifuged at 5000 rpm for 5 min. The supernatant solution (1.0 mL) was mixed with distilled water (1.0 mL) and ferric chloride (0.5 mL, 1 g/L) for 10 min, and then the absorbance was measured at 700 nm against a blank. Increasing absorbance of the reaction mixture indicates increasing reducing power. The experiment was performed three times and averaged.
Total antioxidant capacity
TAC was determined using TAC assay commercial kit (Nanjing Jianchen bioengineering Institute, Nanjing, China). The absorbance of the sample was measured at 520 nm. One unit of TAC was defined as per milliliter of sample that caused an increase of 0.01 in the absorbance per min at 37°C. The experiment was performed three times and averaged. TAC was calculated by the following equation:
DPPH radical-scavenging activity
The DPPH radical-scavenging activity of PCS was estimated as previously described (Guo & Liu, Citation2012). DPPH solution (0.2 mmol/L) in 50 µL of ethanol was added to 100 µL of the sample (varying concentration) and vibrated enough. The absorbance was measured at 517 nm after 20 min of incubation in a 96-well plate in the dark. The experiment was conducted three times and averaged. The activity for scavenge DPPH radical was calculated by the following equation:
Hydroxyl radical-scavenging activity
The hydroxyl radical-scavenging assay was carried out using the method as described by (Smirnof & Cumbes, Citation1989) with some modifications. Both, 50 µL sample and 50 µL FeSO4 (9 mmol/L) were thoroughly mixed with 50 µL H2O2 (8.8 mmol/L) and 50 µL alicylic acid–ethanol solution (10 mmol/L). The reaction mixture was incubated at 37°C for 20 min, and the absorbance was measured at 508 nm. The activity for scavenge hydroxyl radical was calculated using the following equation:
Superoxide radical-scavenging activity
The activity of the scavenge superoxide radical was measured at 25°C using the spectrophotometric monitoring of the inhibition of pyrogallol autoxidation as described (Huang, Xue, Niu, Jia, & Wang, Citation2009) with some modifications. Pyrogallol solution of 50 µL (60 mmol/L) was added into a tube containing 50 µL sample and 100 µL of Tris-HCl-EDTA buffer (0.1 mol/L, pH 7.3). The absorbance at 319 nm was measured each 50 s for 5 min using Synergy HT microplate reader. The antioxidant activity was determined as the percentage of inhibiting pyrogallol autoxidation. The scavenging activity of superoxide radical was calculated using the following equation:
Statistical analysis
All experimental results were centered using three parallel measurements. Analysis of variance was performed by ANOVA procedure. P values of less than 0.05 were regarded as significant and P values of less than 0.01 as very significant.
Results and discussion
Model fitting
In the present study, ultrasound-assisted extraction was employed for polysaccharides from C. sinensis (PCS). The operational conditions were optimized using BBD combined with RSM. Before the optimized experiment, the main parameters independently influencing the yield of PCS by ultrasonic-assisted extraction were investigated as ultrasonic power (varying from 200 to 500 W), extraction temperature (varying from 40°C to 80°C), extraction time (varying from 5 to 40 min) and liquid to material ratio (varying from 10/1 to 50/1).
Extraction power is a factor which can influence the extraction efficiency. The yield of PCS increased gradually over the experimental power and achieved maximum using the ultrasonic power 500 W (100%) as shown in , which is in accordance with the results of previous reports (Guo & Chen, Citation2010; Yue, Shao, Yuan, Wang, & Qiang, Citation2012). Thus, the experimental power was determined as 500 W in this study.
Figure 1. The effects of extraction parameters on the extraction yield of PCS. (a) Effect of ultrasonic power on the extraction yield, the other extract conditions were extraction temperature of 60°C, extraction time of 20 min and liquid/material ratio of 20:1 mL/g; (b) Effect of extraction temperature on the extraction yield, the other extraction conditions were ultrasonic power of 500 W, extraction time of 20 min and liquid/material ratio of 20:1 mL/g; (c) Effect of extraction time on the extraction yield, the other extraction conditions were ultrasonic power of 500 W, extraction temperature of 70°C and liquid/material ratio of 20:1 mL/g; (d) Effect of liquid/material ratio on the extraction yield, the other extraction conditions were ultrasonic power of 500 W, extraction temperature of 70°C and extraction time of 30 min.
Figure 1. Los efectos de los parámetros de extracción sobre el rendimiento de extracción de PCS. (a) Efecto de potencia ultrasónica en el rendimiento de la extracción, las otras condiciones de extracción de 60 °C, el tiempo de extracción de 20 min y la relación de líquido / material de 20:1 mL/g (b) Efecto de la temperatura de extracción en el rendimiento de la extracción las otras condiciones de extracción fueron potencia ultrasónica de 500 W, tiempo de extracción de 20 min y la relación de líquido / material de 20:1 mL/g; (c) Efecto del tiempo de extracción en el rendimiento de la extracción, las otras condiciones de extracción, las otras condiciones de extracción fueron potencia ultrasónica de 500 W, temperatura de extracción de 70 °C y la relación de líquido / material de 20:1 mL/g (d) Efecto de la relación líquido / material en el rendimiento de la extracción de 70 °C y tiempo de extracción de 30 min.
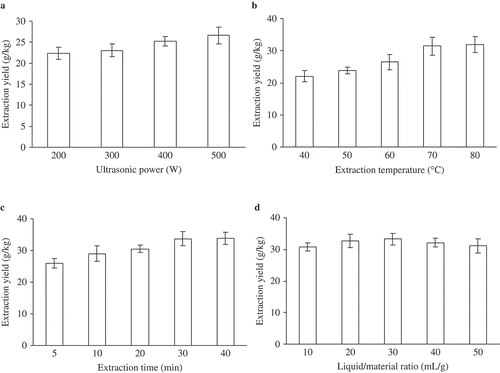
The liquid density and viscosity decreased, resulting in a faster mass transfer, with the increasing of extraction temperature (Hemwimol, Pavasant, & Shotipruk, Citation2006). Furthermore, high-extraction temperature can lead to an increase in the number of cavitations within these tissues and surface contact areas (Palma & Barroso, Citation2002). Thus, application of appropriate high-extraction temperature resulted in enhanced-extraction efficiency. As can be seen from , the yield of PCS significantly increased from 22.02 ± 1.72 g/kg to 31.95 ± 2.45 g/kg as the temperature increased from 40°C to 80°C.
It was reported that an approximate long extraction time favors the production of polysaccharides (Wang, Cheng, Mao, Fan, & Wu, Citation2009). When extraction time varied from 5 min to 30 min, the variance of extraction yield was relatively rapid, and polysaccharides production reached a maximum at 30 min as shown in .
As the shows that the yield of PCS significantly increased from 30.79 ± 1.51 g/kg to 33.33 ± 1.83 g/kg as the ratio increased from 10:1 to 30:1 mL/g due to the increase of the driving force for the mass transfer of the polysaccharides (Bendahou, Dufresne, Kaddami, & Habibi, Citation2007). However, when the ratio continued to increase, the extraction yield no longer changed.
Therefore, the three parameters, liquid to material ratio, extraction time and extraction temperature were involved in the optimized experiment conducted by a BBD (Table 1). All experimental data obtained from 17-run-experiment from response surface analysis model established based on the experimental data were shown in Table 1.
The values of response (extraction yield of PCS) at different experimental combination for coded variables as well as their interactions are shown in Table 1 and . As in the case of polysaccharides extract, liquid to material ratio (X1), extraction time (X2) and extraction temperature (X3) all had positive impacts on the polysaccharides production, the order of influencing the extraction of polysaccharide was extraction temperature (X3) > liquid to material ratio (X1) > extraction time (X2). The extraction yield ranged from 20.33 g/kg to 34.11 g/kg. The application of RSM offers, based on parameter estimates, an empirical relationship between the response variables (extraction yield of PCS) and the test variables under consideration. By applying multiple regression analysis on the experimental data, the response variables and the test variables are related by the following second-order polynomial equation:
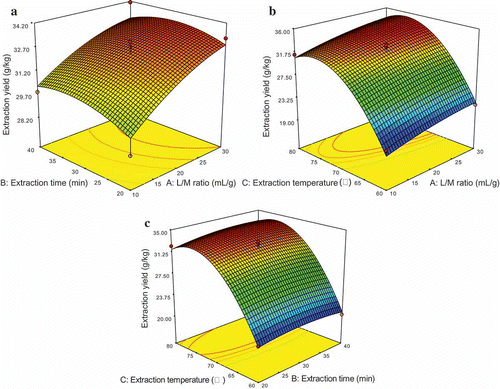
Figure 2. Response surface plots showing effects of pairwise factors on the extraction yield of PCS and their interaction. (a) Liquid to material ratio and extraction time (The extraction temperature was constant at 70°C). (b) Liquid to material ratio and extraction temperature (The extraction time was constant at 30 min). (c) Extraction time and temperature (Liquid to material ratio was constant at 20: 1).
Figure 2. Gráficas de superficie de respuesta mostrando los efectos de pares de factores en el rendimiento de la extracción y sus interacciones. (a) relación líquido/material y tiempo de extracción (La temperatura de extracción fue constante a 70 º C). (b) relación líquido a material y la temperatura de extracción (El tiempo de extracción fue constante a 30 min). (c) Tiempo de extracción y temperatura (relación de líquido a material fue constante a 20:1).
The correlation measure for testing the goodness of fit of the regression equation is the adjusted determination coefficient (R2Adj). The value of R2Adj (0.9616) for Equation (6) is reasonably close to 1, which indicates the regression models defined well the true behavior of the system (Pujari & Chandra, Citation2000). A very low value of coefficient of the variation (C.V.) (3.39%) clearly indicated a very high degree of precision and a good deal of reliability of the experimental values. Statistical testing of the model was performed in the form of analysis of variance (ANOVA), which is required to test the significance and adequacy of the model. The data showed a good fit with the Equation (6), which was statistically acceptable at P < 0.01 level (P < 0.0001) ( ). In addition, the P value of 0.1238 for lack-of-fit implied the lack-of-fit was not significant relative to the pure error, indicating the model equation was adequate to predict the extraction yield of PCS within the range of experimental variables.
Table 2. Analysis of variance (ANOVA) for the response surface quadratic model of PCS.
Table 2. Análisis de varianza (ANOVA) para la superficie de respuesta del modelo cuadrático de PCS.
Optimum extraction conditions for the maximum yield of ultrasonic-assisted extraction of PCS
Three-dimensional response surface plots, presented in , are very useful to see interaction effects of the factors on the responses. These types of plots show effects of two factors on the response at a time. In all the presented figures, the other one factor was kept at zero level.
and show the effects of liquid to material ratio interaction with each of the two other factors on the extraction yield of PCS. It is obvious that the lower solvent volume resulted in lower yield (Bendahou, Dufresne, Kaddami, & Habibi, Citation2007), the extraction yield of PCS increased to a certain value with increasing liquid to material ratio from 10:1 to 22:1, which indicated that the liquid to material ratio has remarkable effect on the extraction yield of PCS.
The effects of extraction time with each of the two other factors on the extraction yield of PCS are shown in and . As can be seen from and , it can be implied that the extraction time is less significant than the other two effects. In all situations, when the liquid to material ratio is less than 22:1, the extraction yield of PCS increased with increasing extraction time from 20 min to 30.5 min, while more than 30.5 min appeared to be not in favor of the extraction, which is in accordance with the results of the previous reports (Wang, Cheng, Mao, Fan, & Wu, Citation2009).
and shows the effects of extraction temperature interaction with each of the two other factors on the extraction yield of PCS. As can be seen from and , the effect of extraction temperature was most significant. The extraction yield of PCS increased rapidly when the extraction temperature increased from 60°C to 77°C, and thereafter decreased. This is likely the appropriate increase in temperature which accelerates mass transfer and improves the extraction yield (Palma, & Barroso, Citation2002).
To ensure the predicted results were not biased toward the practical values, experimental rechecking were performed using the deduced optimal parameters by employing the software Design-Expert (within the experimental range): liquid to material ratio of 22:1 mL/g, extraction time 31 min and extraction temperature 77°C, the mean values of extraction yield of PCS was up to 34.63 ± 1.52 g/kg (n = 6), but it was not significantly different to the predicted value (34.27 g/kg), which is similar to the extraction yield of polysaccharides of another bivalvia species Hyriopsis cumingii (Qiao et al., Citation2009). The result suggested that the regression model was accurate and adequate for the prediction of PCS extraction.
The PCS was extracted under the above optimum conditions for various number of extraction (data not shown), the results indicated that extraction times of 1 was enough to extract the PCS. Compared with the highest extraction yield (34.06 ± 0.40 g/kg) optimal conditions of heating extraction is as follows: extraction temperature 95°C, liquid to material ratio 10:1 mL/g, extraction time 240 min and extraction times 2, ultrasonic-assisted extraction has been proved as an effective means of reducing the consumption of thermal energy and decreasing the extraction time. The contents of total sugar and protein were 778.57 ± 6.65 g/kg and 36.27 ± 2.58 g/kg, respectively. PCS was used for the next bioactivity analysis.
Reducing power and total antioxidant capacity
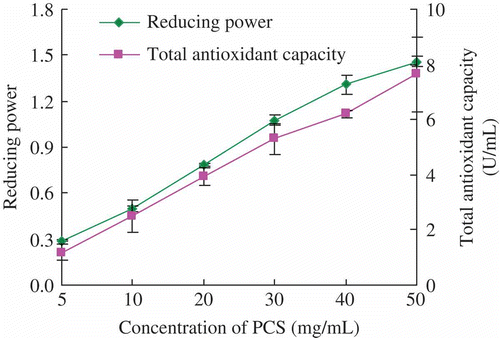
It is well-known that the evaluation of the antioxidant activities on a selected antioxidant required different test systems (Yu et al., Citation2002). During the reducing power assay, the presence of polysaccharides would result in reducing Fe3+/ferricyanide complex to the ferrous form (Fe2+). Therefore, the Fe2+ can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm (Zhang, He & Hu, Citation2011). Total oxidant capacity was determined using the commercial kit. exhibits a strong dependence of reducing power and total antioxidant capacity on the concentration of polysaccharides.
Scavenging activity on DPPH radical
The DPPH radical is a stable-free radical, which had been broadly accepted as a tool for evaluating the free radical-scavenging activity of antioxidant (Nagai, Inoue, Inoue, & Suzuki, Citation2003). In the DPPH assay, the effect of antioxidant on DPPH radical-scavenging was attributed to their hydrogen-donating ability (Chen, Xie, Nie, Li, & Wang, Citation2008).
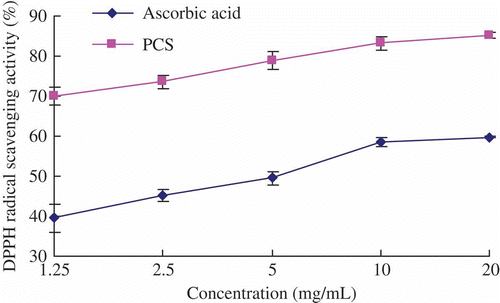
shows that the DPPH radical-scavenging effect of PCS and ascorbic acid increased from 70.58 ± 2.12% and 39.60 ± 3.50% to 85.29 ± 0.78% and 59.80 ± 0.17%, respectively, when the concentration varied from 1.25 to 20 mg/mL. The EC50 were 0.11 and 4.78 mg/mL. It seemed that the scavenging activity of the DPPH radical of PCS was relatively more prominent than that of ascorbic acid at the same concentration, which might be attributed to its other contents – protein, uronic acid and sulfuric radical (Qiao et al., Citation2009). The results suggested that PCS have noticeable DPPH radical-scavenging effect.
Scavenging activity on hydroxyl radical
Hydroxyl radical, well known as the most reactive free radical, can cause the ageing of human body and some diseases (Siddhuraju & Becker, Citation2007). The scavenging capability was not due to the direct scavenging but because of the inhibition of hydroxyl free radical generation by chelating ions such as Fe2+ and Cu2+ (Qi et al., Citation2006). Hydroxyl radical can be generated by the reaction of Fe2+ and H2O2, and the polysaccharides could reduce the generation of hydroxyl radical by chelating Fe2+.
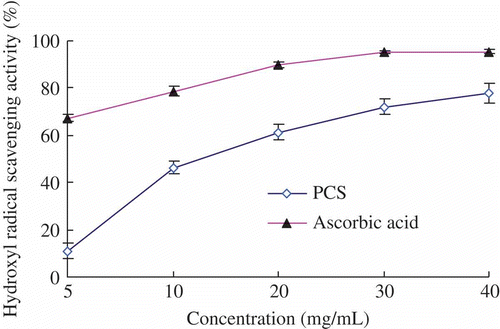
shows the hydroxyl free radical-scavenging ability of PCS and compared it with ascorbic acid as control standards. It seemed that the antioxidant activities of all the tested samples were mostly related to their concentrations. The values of scavenging activities ranged from 10.87 ± 3.39% to 77.80 ± 3.94% when the concentration of PCS varied from 5 to 40 mg/mL, the EC50 of PCS was 12.50 mg/mL. The PCS exhibited a certain extent of hydroxyl free radical-scavenging activity, but with an efficacy lower than that of the reference ascorbic acid (94.68 ± 0.30%) at the same concentration (40 mg/mL), the EC50 of ascorbic acid was 3.02 mg/mL.
Scavenging activity on superoxide radical
Superoxide radical (O2−), well known as another highly toxic species, is related to numerous biological reactions and thus study of this radical-scavenging is also important (Kanatt, Chander & Sharma, Citation2007). shows the superoxide radical-scavenging effect increased from 50.44 ± 3.04% to 89.98 ± 0.79%, when the concentration of PCS varied from 2.5 to 30 mg/mL, the EC50 of PCS was 2.50 mg/mL. Whereas, the efficacy was far lower than that of the reference ascorbic acid (99.54 ± 0.09%) at the concentration (1.0 mg/mL), the EC50 of ascorbic acid was 0.18 mg/mL.
Figure 6. Scavenging activities of sample and control standards on superoxide free radical (mean ± SD, n = 3).
Figure 6. Actividad de eliminación de muestras y control normalizado sobre radicales libres superóxido (media ± DE. n = 3)
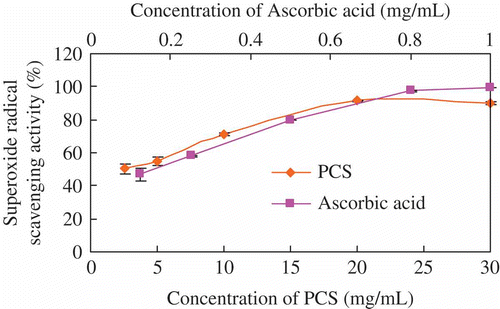
The EC50 of PCS were 0.11, 12.50 and 2.50 mg/mL for DPPH, hydroxyl and superoxide radical, which are similar to the EC50 values of polysaccharides from Inonotus obliquus and Sargassum graminifolium (Mu et al., Citation2012; Zhang, Wu, Wang, & Lan, Citation2012). The results obtained from the test systems suggested that PCS have significant antioxidant activities, and the effects increase with increasing concentration of PCS, which are in accordance with the previous reports (Jiang, Wang, Liu, Gan, & Zeng, Citation2011).
Conclusions
In this study, BBD was employed to optimize the parameters for the ultrasonic-assisted extraction of PCS. As a result, the highest yield of crude PCS (34.63 ± 1.52 g/kg) was obtained under the following conditions: ultrasonic power 500 W, liquid to material ratio 22:1 mL/g, extraction temperature 77°C and extraction time of 31 min. The results indicated that ultrasonic-assisted extraction process could be used as an effective method to extract PCS. Furthermore, the antioxidant activities in vitro of PCS in multi-test systems were evaluated. The results demonstrated that PCS exhibited quite a strong reducing property total antioxidant capacity as well as excellent scavenging activities on the DPPH radical, hydroxyl radical, and superoxide radical, which could be beneficial to the antioxidant protection system in the food industry and human body. Further works on the comparison of structure and biological activity between ultrasonic-assisted PCS and heating-assisted PCS are fascinating and are in progress.
Acknowledgments
This study was supported by the foundation for Science Research Project of Lianyungang (CG1131) and the Natural Science Research Program of Jiangsu Higher Education Department (10KJB350001).
References
- Bendahou, A., Dufresne, A., Kaddami, H., & Habibi, Y. (2007). Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydrate Polymers, 68, 601–608. doi:10.1016/j.carbpol.2006.10.016
- Bradford, M. M. (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Chen, Y., Xie, M. Y., Nie, S. P., Li, C., & Wang, Y. X. (2008). Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chemistry, 107, 231–241. doi:10.1016/j.foodchem.2007.08.021
- Gu, R. R., Yu, Y. S., & Cai, Y. L. (2006). Analysis and evaluation of the nutritive composition of Cyclina sinensis. Chinese Journal of Zoology, 41, 70–74 (in Chinese). doi:0250-3263(2006)03-70-05
- Guo, L., & Chen, Y. (2010). Optimization of ultrasonic-assisted extraction of polysaccharides from Enteromorpha Prolifera by response surface methodology. Food Science, 31, 117–121. doi:1002-6630(2010)16-0117-05
- Guo, L., & Liu, C. (2012). Extraction and antioxidant activity of ultrasonic-assisted flavonoids from Suaeda salsa. Applied Mechanics and Materials, 140, 343–349. doi:http://10.4028/www.scientific.net/AMM.140.343
- Hemwimol, S., Pavasant, P., & Shotipruk, A. (2006). Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrasonics Sonochemistry, 13, 543–548. doi:10.1016/j.ultsonch.2005.09.009
- Hromadkova, Z., & Ebringerova, A. (2003). Ultrasonic extraction of plant materials-investigation of hemicellulose release from buckwheat hulls. Ultrasonic Sonochemistry, 10, 127–133. doi:10.1016/S1350-4177(03)00094-4
- Huang, W., Xue, A., Niu, H., Jia, Z., & Wang, J. W. (2009). Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chemistry, 114, 1147–1154. doi:10.1016/j.foodchem.2008.10.079
- Jiang, C., Wang, M., Liu, J., Gan, D., & Zeng, X. (2011). Extraction, preliminary characterization, antioxidant and anticancer activities in vitro of polysaccharides from Cyclina sinensis. Carbohydrate Polymers, 84, 851–857. doi:10.1016/j.carbpol.2010.11.027
- Jiang, C., Xiong, Q., Gan, D., Jiao, Y., Liu, J., Ma, L., & Zeng, X. (2013). Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydrate Polymers, 91, 262–268. doi:10.1016/j.carbpol.2012.08.029
- Kanatt, S. R., Chander, R., & Sharma, A. (2007). Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chemistry, 100, 451–458. doi:10.1016/j.foodchem.2005.09.066
- Li, X. Y., Dong, Z. G., Yan, B. L., Cheng, H. L., Meng, X. P., Shen, H. D., & Li, J. L. (2010). Analysis and evaluation of nutritional components in Cyclina sinensis and Meretrix meretrix. Food Science, 3 1, 366–370 (in Chinese). doi:1002-6630(2010)23-0366-05
- Liu, W., Yu, Y., Yang, R., Wan, C., Xu, B., & Cao, S. (2010). Optimization of total flavonoid compound extraction from Gynura medica leaf using response surface methodology and chemical composition analysis. International Journal of Molecular Sciences, 11, 4750–4763. doi:10.3390/ijms11114750
- Liu, W., Ma, Y., Hu, S., Miao, G., & Li, J. (2002). Rearing Venus clam seeds, Cyclina sinensis (Gmelin), on a commercial scale. Aquaculture, 211, 109–114. doi:10.1016/S0044-8486(01)00859-6
- Luan, H. M., Wang, L. C., Wu, H., Jin, Y., & Ji, J. (2011). Antioxidant activities and antioxidative components in the surf clam, Mactra veneriformis. Natural Product Research, 25, 1838–1848. doi:10.1080/14786419.2010.530268
- Mu, H., Zhang, A., Zhang, W., Cui, G., Wang, S., & Duan, J. (2012). Antioxidative Properties of Crude Polysaccharides from Inonotus obliquus. International Journal of Molecular Science, 13, 9194–9206. doi:10.3390/ijms13079194
- Nagai, T., Inoue, R., Inoue, H., & Suzuki, N. (2003). Preparation and antioxidant properties of water extract of propolis. Food Chemistry, 80, 29–33. doi:10.1016/S0308-8146(02)00231-5
- Palma, M., & Barroso, C. G. (2002). Ultrasound-assisted extraction and determination of tartaric and malic acids from grapes and winemaking by-products. Analytica Chimica Acta, 458, 119–130. doi:10.1016/S0003-2670(01)01527-6
- Pujari, V., & Chandra, T. S. (2000). Statistical optimization of medium components for enhanced riboflavin production by a UV-mutant of Eremothecium ashbyii. Process Biochemistry, 36, 31–37. doi:10.1016/S0032-9592(00)00173-4
- Qi, H., Zhang, Q., Zhao, T., Hu, R., Zhang, K., & Li, Z. (2006). In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorganic & Medicinal Chemisty Letters, 16, 2441–2445. doi:10.1016/j.bmcl.2006.01.076
- Qiao, D., Hu, B., Gan, D., Sun, Y., Ye, H., & Zeng, X. (2009). Extraction optimized by using response surface methodology, purification and preliminary characterization of polysaccharides from Hyriopsis cumingii. Carbohydrate Polymers, 76, 22–429. doi:10.1016/j.carbpol.2008.11.004
- Siddhuraju, P., & Becker, K. (2007). The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chemistry, 101, 10–19. doi:10.1016/j.foodchem.2006.01.004
- Smirnof, N., & Cumbes, Q. J. (1989). Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry, 28, 1057-1060. doi:10.1016/0031-9422(89)80182-7
- Vaquero, M. J. R., Serravalle, L. R. T., de Nadra, M. C. M., & de Saad, A. M. S. (2010). Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Control, 21, 779–785. doi:10.1016/j.foodcont.2009.10.017
- Vinatoru, M., Toma, M., Radu, O., Filip, P. I., Lazurca, D., & Mason, T. J. (1997). The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrasonics Sonochemistry, 4, 135–139. doi:10.1016/S1350-4177(97)83207-5
- Wang, L. P., Geng, R. Q., Liu, Y., Zhang, H. B., Chen, Y., & Xue, F. (2007). Status of research and utilization of Cyclina sinensis germplasm resources in China. Jiangsu Agricultural Science, 4, 254–255 (in Chinese). doi:1002-1302 (2007) 04-0254-02
- Wang, Y., Cheng, Z., Mao, J., Fan, M., & Wu, X. (2009). Optimization of ultrasonic-assisted extraction process of Poria cocos polysaccharides by response surface methodology. Carbohydrate Polymers, 77, 713–717. doi:10.1016/j.carbpol.2009.02.011
- Xu, H., Guo, T., Guo, Y., Zhang, J., Li, Y., Feng, W., & Jiao, B. (2008). Characterization and protection on acute liver injury of a polysaccharide MP-I from Mytilus coruscus. Glycobiology, 18, 97–103. doi:10.1093/glycob/cwm116
- Yue, T., Shao, D., Yuan, Y., Wang, Z., Qiang, C. (2012). Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. Journal of Separation Science, 16, 2138–2145. doi:10.1002/jssc.201200295
- Yu, L., Haley, S., Perret, J., Harris, M., Willson, J., & Qian, M. (2002). Free radical scavenging properties of wheat extracts. Journal of Agricultural and Food Chemistry, 50, 1619–1624. doi:10.1021/jf010964p
- Zhang, C. Y., Wu, W. H., Wang, J., & Lan, M. B. (2012). Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Marine Drugs, 10, 119–130. doi:10.3390/md10102208
- Zhang, G., He, L., & Hu, M. (2011). Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innovative Food Science & Emerging Technologies, 12, 18–25. doi:10.1016/j.ifset.2010.12.003
- Zhang, Q. A., Zhang, Z. Q., Yue, X. F., Fan, X. H., Li, T., & Chen, S. F. (2009). Response surface optimization of ultrasound-assisted oil extraction from autoclaved almond powder. Food Chemistry, 116, 513–518. doi:10.1016/j.foodchem.2009.02.071
- Zhao, Y., Li, Q., Kong, L., & Mao, Y. (2009). Genetic and morphological variation in the venus calm Cyclina sinensis along the coast of China. Hydrobiologia, 635, 227–235. doi:10.1007/s10750-009-9916-4