Abstract
Nowadays, there exists a major concern about the requirement of decreasing the environmental impact of industrial activities. In general, the food industry produces high amount of effluents that must be treated. There are two ways of focusing on the treatment of effluents and residues from food industry. One interesting strategy consists of the revalorization of residues in order to obtain valuable products, and the other approach is the treatment of these effluents using eco-friendly technologies. In this work, a residue of winery industry consisting of grape marc was entrapped in calcium alginate beads in order to obtain a valuable product that could be used as an adsorbent to remove micronutrients from winery effluents in order to avoid eutrophication. Biodegraded grape marc entrapped in calcium alginate beads was able to remove most of the total nitrogen, NH4, and NO3 present in the water and around 60% of Mg, P, K, and total carbon.
Actualmente existe una gran preocupación sobre la necesidad de reducir el impacto medioambiental derivado de actividades industriales. En general, la industria alimentaria produce una gran cantidad de efluentes que deben ser tratados. Existen dos formas de enfocar el tratamiento de estos efluentes y residuos. Una estrategia interesante consiste en revalorizar estos residuos con la finalidad de obtener productos de valor. Otro enfoque se centra en su tratamiento utilizando tecnologías amigables con el medioambiente. En este trabajo, el bagazo de uva – residuo de la industria vitivinícola – ha sido inmovilizado en esferas de alginato cálcico para obtener un producto de valor utilizable como adsorbente, que elimine micronutrientes de los efluentes vitivinícolas evitando así la eutrofización. El bagazo biodegradado, inmovilizado en esferas de alginato, ha eliminado la mayoría del nitrógeno total, NH4 y NO3 existente en el agua y alrededor de un 60% de Mg, P, K y carbono total.
Introduction
Many biodegradable organic wastes, like grape marc from winery industry, can be biodegraded in a convenient and economical way (Moldes, Vázquez, Domínguez, Díaz-Fierros, & Barral, Citation2007; Paradelo, Moldes, & Barral, Citation2009) in order to reduce the environmental impact caused by these kind of residues and, at the same time, obtain a valuable product. Thus, the spontaneous biodegradation of organic matter is a simple and efficient method of transforming agro-industrial wastes into suitable products for use as soil conditioners or fertilizers by increasing the amount of nutrients and humus or humic acids in the soil (Moldes et al., Citation2007).
On the other hand, some authors have demonstrated that grape marc compost could be an effective adsorbent for the adsorption of pollutants from wastewater (Arvanitoyannis, Ladas, & Mavromatis, Citation2006; Paradelo et al., Citation2009). Paradelo et al. (Citation2009), showed that grape marc vermicompost can be used as an adsorbent to remove colored compounds from winery wastewater, although its utilization increased the electrical conductivity of the treated water.
In addition, Villaescusa et al. (Citation2004) and Yuan-Shen, Cheng-Chung, and Chyow-San (Citation2004) evaluated the application of grape stalk waste and wine processing waste sludge as an adsorbent. Previously, for the elimination of Cu, Cr and Ni from wastewater, grape stalk waste and wine processing waste sludge were dried in an oven at 105–110°C; following which, the adsorbent was cut, sieved into several particle sizes and desiccated. Using this eco-friendly adsorbent, these authors got to remove 70–80% of the tested micronutrients; the adsorption process being very dependent on pH, the best results were obtained at pH 5.5.
In comparison with other adsorbents, like activated carbon, grape marc has several benefits, mainly based on the fact that grape marc is considered a residue that must be treated in order to reduce the environmental impact produced by the winery industry (Devesa-Rey, Bustos, Cruz, & Moldes, Citation2011; Vecino, Devesa-Rey, Moldes, & Cruz, Citation2012); whereas activated carbon, one of the most common adsorbents used at the industrial scale, is a non-renewable adsorbent.
The biodegradation of grape marc produces a more stable product, and increases the concentration of humic and fulvic substances that can improve its adsorbent properties (Paradelo et al., Citation2009). Moreover, Arvanitoyannis et al. (Citation2006) suggest that the sorption of metals by winery wastes might be attributed to their proteins, carbohydrates and phenolic compounds that have carboxyl, hydroxyl, sulfate, phosphate and amino groups that can bind metal ions.
Furthermore, vinasses from winemaking have a high content of micronutrients which may cause eutrophication when freely released into water. Some authors have tested the effectiveness of these effluents as alternative sources of nutrients in biotechnological processes (Salgado et al., Citation2011). Anyway, the management of these wastes must be considered within the winemaking process. In fact, according to the Spanish and European legislation – Law 22/2011 (BOE, Citation2011) and Directive 2008/98/CE (DOUE, Citation2008), respectively – fines and discharging fees are applied to companies for unauthorized discharges into sewerage or vinasse spillage (for instance, Devesa-Rey, Vecino, Varela-Alende, Barral, Cruz & Moldes, Citation2011).
Eutrophication is a widespread problem, since the flux of micronutrients into bodies of water grew by 80% from 1860 to 1990 (Finlayson, D’Cruz, & Davidson, Citation2005), and it is expected to increase in coming decades, mainly due to the upturn in population and the anthropogenic activities such as industrial development, land conversion or use of nitrogen and phosphorus fertilizers in agriculture. It is important, then, to take into account not only the primary sources of soil contamination, but also those responsible for diffuse pollution, like regional planning (Pérez-Rodríguez, de Blas, Soto, Pontevedra-Pombal, & López-Periago, Citation2011).
The major consequence of eutrophication – this excessive plant growth – is the reduction in the amount of oxygen available in water, which results in the death of fish, the loss of leisure areas close to rivers, lakes, or the seaside, and the loss of freshwater suitable for human use (Finlayson et al., Citation2005).
Although some lakes and rivers have recovered from eutrophication after the inputs of micronutrients were reduced, other bodies of water remained eutrophic (Carpenter, Citation2005).
In this work, biodegraded grape marc was entrapped in calcium alginate beads, in order to get a more commercial and manageable adsorbent, and its capability to remove micronutrients from wastewater was evaluated. The over-enrichment of water with micronutrients such as: P, NO3, SO2, or total nitrogen (TN) has emerged as one of the main causes of water quality impairment.
Table 1. Independent and dependent variables used in the study.
Variables independientes y variables dependientes utilizadas en este estudio.
Table 2. Instrumental operating parameters applied for the determination of micronutrients by ICP–OES.
Parámetros instrumentales utilizados para la determinación de micronutrientes por ICP–OES.
Table 3. Operational conditions considered in this study (expressed in terms of the coded independent variables) and experimental results achieved for the dependent variables y1 to y8.
Condiciones experimentales consideradas en este estudio (expresadas como variables independientes codificadas) y resultados experimentales alcanzados para las variables y1 a y8.
Table 4. Regression coefficients and their statistical significance for variables y1 to y8.
Coeficientes de regresión y su significancia estadística para las variables y1 a y8.
Materials and methods
Grape marc
Grape marc is generated after the pressing process in winemaking and it is traditionally considered as a residue (Asselin & Delteil, Citation2003). Grape marc used in these experiments was obtained from local winery industries and it was subjected to a spontaneous biodegradation of the organic matter for a period of 3 months in vessels 29 cm in diameter and 24 cm high, following the protocol carried out in previous works (Moldes et al., Citation2007).
Vinasses
Vinasses were collected from local winery industries. This wastewater had 33 mg/L Mg; 436.3 mg/L K; 52.36 mg/L P; 2.72 mg/L NH4; 0.9 mg/L NO3; 87.18 mg/L SO4; 96.0 mg/L TN, and 6.4 g/L total carbon (TC).
Immobilization of the eco-friendly adsorbent
Entrapped grape marc was prepared according to the procedure described in previous works (Devesa-Rey, Bustos et al., Citation2011; Vecino, Devesa-Rey, Cruz, & Moldes, Citation2013; Vecino et al., Citation2012), by mixing sodium alginate (2%) with biodegraded grape marc (2%). The mixture was then added dropwise from a 1.5 mL pipette (using a Masterflex L/S compact variable-speed pump, dual-channel, 115/230 VAC, Cole-Parmer, Spain) to a solution of calcium chloride (0.58 mol/L), which was used as a cross-linking solution.
Adsorption experiments
Adsorption experiments were carried out in 250 mL Erlenmeyer flasks, with different ratios of entrapped grape marc/vinasses, time of extraction, and agitation speed, following the incomplete factorial design described above (see ).
Experimental design and statistical analysis
In order to establish the optimal operational conditions to carry out the adsorption of micronutrients from winery wastewater using entrapped biodegraded grape marc, an incomplete factorial design based on Box–Behnken (Citation1960) factorial design was applied. The independent variables studied consisted of the relationship between ecoadsorbent and wastewater (0.5–1.5), time of operation (30–120) and agitation (75–150); whereas the evaluated dependent variables consisted of percentage reduction of Mg, P, K, NH4, SO4, TN, TC, and NO3 after the treatment with entrapped grape marc.
The experimental data were analyzed by the response surface method with Statistic 7.0 software (Statistica for Windows, StatSoft, Inc, USA) that it is a useful tool to study the synergic effect of independent variables over the selected dependent variables (Bezerra, Santelli, Oliveira, Villar, & Escaleira, Citation2007). shows the range of independent variables studied as well as their standardized (coded) dimensionless values. Coded variables were then assigned values of –1, 0, and +1, corresponding to the lowest, central, and maximum limits of variation for each variable. The response surface obtained from the coded variables is, therefore, not influenced by the magnitude of each variable, which allows the combination of the factors on a dimensionless scale.
Analysis of micronutrients
In order to determine Mg, K, and P, samples were acidified and heated using microwave digestion following the operation conditions showed in . The digested samples were quantitatively analyzed using an Inductively Coupled Plasma–Optical Emission Spectrometry (ICP–OES, Perkin Elmer, Norwalk, USA, Optima 4300 DV).
Samples underwent thermocatalytic digestion (Multi N/C 3100, Analytik Jena, Jena, Germany) and TC and TN were analyzed by non-dispersive IR detector and chemiluminescence detector, respectively.
On the other hand, NO3 and SO4 were analyzed using ion chromatography (709 IC Pump, 732 IC Conductivity Detector, 733 IC Separation Center and Metrosep A Supp 5 as column, Metrohm, Herisau, Switzerland); whereas NH4 was determined by the colorimetric method – Berthelot’s reaction (Glick, Citation1969) – using a segmented continuous flow autoanalyzer (Bran Luebbe AA3 autoanalyzer). The green complex from the reaction was measured at 660 nm.
Results and discussion
Composting is the aerobic biodegradation of organic matter by microorganisms into a more stable product named compost. During composting, microorganisms such as bacteria and fungi break down organic complexes, like polysaccharides, into smaller molecules and produce carbon dioxide, water, minerals, and stabilized organic matter (compost). The process produces heat, which can destroy pathogens and weed seeds. In this work, grape marc was subjected to aerobic biodegradation of the lignocellulose polymers, obtaining a stabilized product, but the temperature reached during this process was lower than 30°C, for this reason, the product obtained is named biodegraded grape marc instead of grape marc compost. Compost can only be obtained when the biodegradation of the organic matter by the microorganisms generates energy, as a product of the microbial metabolism that increases the temperature of the residue over 60°C. Thus, it is very difficult to reach thermophile temperatures using lignocellulose residues, because the biodegradation of the organic matter is carried out by fungus that produces lignocellulose enzymes, which break down the large polymers of cellulose and hemicelluloses, as well as lignin, in order to obtain smaller molecules. The replication of fungus is slower than the replication of bacteria (Lund, Baird-Parker, & Gould, Citation2011), thus it can be speculated that the increase of temperature during composting will be lower in the presence of fungus than in the presence of bacteria.
The commercialization of compost from grape marc could have some inconvenience derived from the low temperatures reached during composting, consequently, it would be interesting to look for new commercial applications for this residue, like its utilization as an adsorbent.
In this work, grape marc was biodegraded for 3 months to achieve a more stable product with high adsorbent properties and, after that, it was immobilized in calcium alginate beads in order to obtain an eco-friendly adsorbent that could be used to remove micronutrients from winery wastewater.
shows the set of adsorption experiments carried under different operational conditions as well as the experimental results achieved for variable y1 to y8 after treatment. Data of are referred to as the percentage of micronutrients removed from the effluent of winery industry after adsorption treatment with entrapped grape marc. From the data obtained in the analysis of samples, and taking into account the composition of the untreated effluent, the reduction percentage for each micronutrient was calculated.
After the statistical treatment of data, a quadratic function, based on Box–Behnken (Citation1960) factorial design, can be obtained for all the dependent variables studied (Equation 1).
The coefficients and the significance of each coefficient (p-values) for the variables y1 to y8, corresponding to the dependent variables tested, are shown in . Replacing these coefficients in Equation 1, different equations can be created in order to determine the values of the dependent variables studied, within the ranges tested.
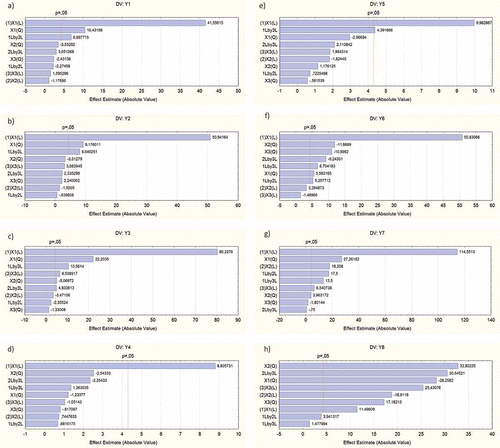
For all the dependent variables studied, the most important independent variable was the eco-adsorbent/wastewater relationship, whereas the time of extraction and the agitation speed had a negligible effect on the adsorption of nutrients. shows the pareto chart of standardized effects for all the dependent variables. In many of these variables (y1, y2, y4, and y5), time of extraction and agitation speed gave coefficients with p > 0.05. Thus, simpler equations could be obtained by including in the equations only those coefficients whose combination of independent variables gave p < 0.05.
Figure 1. Pareto charts of standardized effects of variables y1 (a), y2 (b), y3 (c), y4 (d), y5 (e), y6 (f), y7 (g) and y8 (h).Figura 1. Representación gráfica donde se mide el efecto estandarizado de las variables y1 (a), y2 (b), y3 (c), y4 (d), y5 (e), y6 (f), y7 (g) y y8 (h).
On the other hand, shows the variation of the dependent variables studied with the amount of eco-adsorbent used (x1) and the time of extraction (x2), fixing the agitation speed (x3) at intermediate values.
Figure 2. Variation in y1 to y8 with the ratio of entrapped grape marc/vinasses and time, at a fixed agitation speed of 112 rpm.Figura 2.Variación de y1 a y8 con la relación bagazo inmovilizado/vinaza y el tiempo, a una velocidad de agitación fija de 112 rpm.
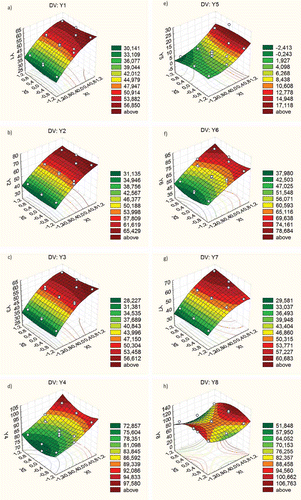
On the other hand, variables y1 to y8 yielded r2 values of 0.97, 0.98, 0.98, 0.96, 0.82, 0.98, 0.96, and 0.79, respectively. The coefficient of determination, r2, is used in the context of statistical models, whose main purpose is to provide a measure of how well future outcomes are likely to be predicted by the model. The theoretical data were calculated by replacing the coefficients of in Equation 1; observing a good agreement between experimental results and the data predicted by the model for all the dependent variables studied.
Therefore, using a relationship between entrapped grape marc/vinasses around 1.5, the model predicts that 56% of Mg; 65% of P, and 56% of K can be removed, after 30 min of treatment, from the evaluated wastewater using an intermediate agitation speed (112 rpm). The adsorption of Mg, P, and K by the eco-adsorbent was very fast. Thus, from –c, it can be observed that 30 min were enough to obtain the maxima elimination of Mg, P and K.
These results are in concordance with those stated by Mayer, Gerrity, Rittmann, Reisinger and Brandt-Williams (Citation2013), which indicated that adsorption process appears to have the greatest potential for near-term implementation in order to remove P from wastewater.
Regarding the elimination of NH4 and NO3, and show that the evaluated adsorbent was very effective, removing up to 97% and 100% of NH4 and NO3, respectively.
Contrary to and related with the elimination of SO4, the model predicts that using the maximum relationship of adsorbent/vinasses, at intermediate agitation speed and at any extraction time, in the range tested, only 17% of SO4 can be removed (). This percentage can be improved up to 26%, increasing the agitation speed to 150 rpm.
Moreover, regarding the elimination of TC, the model predicts that 60% of TC can be removed from winery wastewater using 1.5 adsorbent/vinasse ratio and using an extraction time over 75 min ().
Concerning the removal of TN from vinasses, 78% of TN can be removed using the highest concentration of adsorbent studied ().
It is important to point out that, in the literature, there are almost no studies about the elimination of micronutrients from water; thus, it is difficult to compare the results achieved in the present work with those from other authors. Most of the studies found in literature, which use either eco-friendly adsorbents or activated charcoal, focus on the removal of colored compounds and heavy metals in wastewater.
Finally, it is interesting to point out that the micronutrients removed from vinasses could increase the quality of grape marc in order to be used as a soil conditioner, or a fertilizer. Subsequently, after the treatment of vinasse with this adsorbent, it can be speculated that there exist two possibilities for treating the exhausted adsorbent. On one hand, the adsorbent could be regenerated, on the other, it could be used as soil amendment. In order to elucidate which is the best option, future experiments should be carried out.
Conclusion
On the basis of the results achieved in this work, entrapped biodegraded grape marc in calcium alginate beads could be an eco-friendly adsorbent to remove micronutrients existent in wastewater from food industry. This eco-adsorbent was able to remove about 100% of NH4 and NO3; 60% of K and Mg; 65% of TC and P, and 80% of TN from the winery industry wastewater. In future, it will be necessary to carry out more works in order to evaluate the stability and regeneration of this biodegradable adsorbent, in order to be applied in the creation of eco-barriers used in the treatment of water before discharging it into rivers or lakes.
Acknowledgements
The authors wish to thank to Xunta de Galicia (project GPC, ref. CN2012/277) and Vecino X. is grateful to the University of Vigo for her predoctoral contract.
References
- Arvanitoyannis, I. S., Ladas, D., & Mavromatis, A. (2006). Potential uses and applications of treated wine waste: A review. International Journal of Food Science and Technology, 41, 475–478. doi: 10.1111/j.1365-2621.2005.01111.x.
- Asselin, C., & Delteil, D. (2003). Vinificaciones: Principales operaciones unitarias comunes. In: C. Flancy (Ed.), Enología: Fundamentos Científicos y Tecnológicos (pp. 418–442). Madrid: Mundi-Prensa.
- Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S., & Escaleira, L. A. (2007). Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta, 76, 965–977. doi: 10.1016/j.talanta.2008.05.019.
- BOE. (2011). Law 22/2011, de 28 de Julio, de residuos y suelos contaminados.
- Box, G. E. P., & Behnken, D. W. (1960). Simplex-sum designs – A class of 2nd order rotatable designs derivable of those of 1st order. The Annals of Mathematical Statistics, 31, 838–864. doi: 10.1214/aoms/1177705661.
- Carpenter, S. R. (2005). Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proceedings of the National Academy of Sciences of the United States of America, 102, 10002–10005. doi: 10.1073/pnas.0503959102.
- Devesa-Rey, R., Bustos, G., Cruz, J. M., & Moldes, A. B. (2011). Optimisation of entrapped activated carbon conditions to remove coloured compounds from winery wastewaters. Bioresource Technology, 102, 6437–6442. doi:10.1016/j.biortech.2011.03.072.
- Devesa-Rey, R., Vecino, X., Varela-Alende, J. L., Barral, M. T., Cruz, J. M., & Moldes, A. B. (2011). Valorization of winery waste vs. the costs of not recycling. Waste Management, 31, 2327–2335. doi:10.1016/j.wasman.2011.06.001.
- DOUE. (2008). Directiva 2008/98/CE del Parlamento Europeo y del Consejo, de 19 de Noviembre de 2008, sobre los residuos.
- Finlayson, C. M., D’Cruz, R., & Davidson, N. (2005). Millennium ecosystem assessment. Ecosystems and human well-being: Wetlands and water. Synthesis. Washington, DC: World Resources Institute.
- Glick, D. (1969). Methods of biochemical analysis, volume 17. New York: Interscience.
- Lund, B., Baird-Parker, A. C., & Gould, G. W. (2011). The microbiological safety and quality of food. Volume I. Gaithersburg, MD: Aspen Publishers, Inc.
- Mayer, B. K., Gerrity, D., Rittmann, B. E., Reisinger, D., & Brandt-Williams, S. (2013). Innovative strategies to achieve low total phosphorus concentrations in high water flows. Critical Reviews in Environmental Science and Technology, 43, 409–441. doi: 10.1080/10643389.2011.604262.
- Moldes, A. B., Vázquez, M., Domínguez, J. M., Díaz-Fierros, F., & Barral, M. T. (2007). Evaluation of mesophilic biodegraded grape marc as soil fertilizer. Applied Biochemistry and Biotechnology, 141, 27–36. doi: 10.1007/s12010-007-9208-2.
- Paradelo, R., Moldes, A. B., & Barral, M. T. (2009). Treatment of red wine vinasses with non-conventional substrates for removing coloured compounds. Water Science and Technology, 59, 1585–1592. doi: 10.2166/wst.2009.166.
- Pérez-Rodríguez, P., de Blas, E., Soto, B., Pontevedra-Pombal, X., & López-Periago, J. E. (2011). El conflicto de uso del suelo y la calidad de los alimentos. The soil use conflict and food quality. CyTA – Journal of Food, 9, 342–350. doi: 10.1080/19476337.2011.615944.
- Salgado, J. M., Rodríguez, N., Max, B., Pérez, B., Rodríguez, R., Cortés, S., & Domínguez, J. M. (2011). Evaluation of wine vinasses as alternative nutrients in biotechnological processes. Evaluación de vinazas como nutriente alternativo en procesos biotecnológicos. CyTA – Journal of Food, 9, 278–281. doi: 10.1080/19476337.2011.597514.
- Vecino, X., Devesa-Rey, R., Cruz, J. M., & Moldes, A. B. (2013). Entrapped peat in alginate beads as green adsorbent for elimination of dye compounds from vinasses. Water, Air and Soil Pollution, 224, 1–9. doi: 10.1007/s11270-013-1448-x.
- Vecino, X., Devesa-Rey, R., Moldes, A. B., & Cruz, J. M. (2012). Optimization of batch operating conditions for the decolourization of vinasses using surface response methodology. Microchemical Journal, 102, 83–90. doi: 10.1016/j.microc.2011.12.004.
- Villaescusa, I., Fiol, N., Martínez, M., Miralles, N., Poch, J., & Serarols, J. (2004). Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Research, 38, 992–1002. doi: 10.1016/j.watres.2003.10.040.
- Yuan-Shen, L., Cheng-Chung, L., & Chyow-San, C. (2004). Adsorption of Cr (III) from wastewater by wine processing waste sludge. Journal of Colloid and Interface Science, 273, 95–101. doi: 10.1016/j.jcis.2003.12.051.
