Abstract
The antioxidant properties and the flavonoid composition of extracts of different hydrophobicity prepared from the entire edible flowers of Agave durangensis were evaluated. Separately, total extracts of tepals and anthers–pollen were analyzed in the same manner. The high performance liquid chromatography with photodiode array detection (HPLC–DAD) analysis revealed a total of eight flavonols (five quercetin glycosides and three kaempferol glycosides), varying in number and concentration in the different extracts. The total extracts of the entire flowers showed the highest flavonoid content (1210.4 µg/g dry extract) and the most complex flavonoid profile (eight compounds). All the extracts showed important antioxidant activity, which was not evidently associated with their flavonoid content. The total extracts of tepals showed the highest antioxidant properties (total antioxidant capacity, free radical scavenging activity, and iron reducing capacity: 30.2 mg ascorbic acid equivalents, EC50 = 0.074 µg/mL, and IC50 = 43.28 µg/mL, respectively). The flowers of A. durangensis represent an important source of antioxidant flavonols.
Se evaluaron las propiedades antioxidantes y composición fenólica de extractos de diferente hidrofobicidad de flores comestibles enteras de Agave durangensis. Separadamente, se analizaron extractos crudos de tépalos y anteras–polen. El análisis de HPLC–DAD reveló en total ocho flavonoles (cinco glicósidos de quercetina y tres glicósidos de canferol), que variaron en número y concentración en los extractos. Los extractos totales de las flores enteras tuvieron el contenido más alto de flavonoides (1210,4 µg/g extracto seco) y el perfil de flavonoides más complejo (ocho compuestos). Todos los extractos mostraron importante actividad antioxidante, no claramente asociada al contenido de flavonoides. Los extractos crudos de tépalos mostraron las propiedades antioxidantes más altas (capacidad antioxidante total, capacidad bloqueadora de radicales libres, y capacidad reductora de fierro: 30,2 mg de equivalentes de ácido ascórbico, EC50 = 0,074 µg/mL, e IC50 = 43,28 µg/mL, respectivamente). Las flores de A. durangensis representan una fuente importante de flavonoles antioxidantes.
Introduction
Mexico is the center of origin and diversity of the family Agavaceae (Garcia-Mendoza, 1995), to which the genus Agave belongs. One hundred and twenty-five, among the 166 species of the genus Agave, naturally occur in the arid and semiarid regions of Mexico (Good-Ávila, Souza, Gaut, & Eguiarte, Citation2006); however, at present, some species of that genus grow as introduced species in several countries around the world. Agave durangensis is one of the 29 species reported for Durango, Mexico, being important because it supports a regional industry of mescal production, based in the recollection from its wild populations (Ávila-Reyes et al., Citation2010).
Most species of Agave are predominantly monocarpic rosettes reaching the blooming state at the age of around seven to ten years, dying a little after. The inflorescences of those species are among the largest ones in the plant kingdom, reaching up to 10 m above the ground (Gentry, Citation1982). The flowers of A. durangensis are yellow, reach 8 cm long, and like other species of Agave, produce high quantities of pollen and nectar.
Practically, all species of Agave have provided construction material, medicinal substances, ritual elements, and edible products to the different ancient and present cultures of Mesoamerica (Gentry, Citation1982). Currently, among the most important economic products manufactured from Agave are the alcoholic distilled beverages like mescal, tequila, and bacanora (Almaraz-Abarca et al., Citation2011; Castillo & Coelho, Citation2007; Gutiérrez, Acedo, & Valenzuela, Citation2007).
Before maize become the essential food, the agaves were the main source of carbohydrates to the Native American Continent cultures (Zizumbo-Villareal & Colunga-GarcíaMarín, Citation2008). In many regions of Mexico, the inflorescence peduncles of several species of Agave are still cooked and consumed as sweet food (Gentry, Citation1982) and some studies on their edible properties have been done (Colunga-GarcíaMarín, Coello-Coello, Espejo-Peniche, & Fuente-Moreno, Citation1993). The flowers of most species of this genus are also edible (Gentry, Citation1982), and were consumed by the primitive cultures of the American Continent, as was revealed by Vaughn (Citation1974), who reported species of Agave among the floral remains in coprolites found in American archeological sites.
Flavonoids are phenolic compounds ubiquitously occurring in practically all parts of plants. Many of them have several biological properties with medical implications (Mouren, Caillard, & Schwartz, Citation1994; Zhang & Cui, Citation2005).
Pollen is considered as an important source of flavonol glycosides and phenolic acids (Almaraz-Abarca et al., Citation2007; Wiermann & Vieth, Citation1983). Both classes of compounds, providing significant antioxidant properties (Campos, Da Cunha, Navarro, & Utrilla, Citation1994), vary in composition among different groups of plants with a species-specific tendency (Almaraz-Abarca et al., Citation2004, 2006). Other floral structures, like petals and tepals, are rich in phenolic compounds (Bartnik, Gtowniak, & Gromek, Citation2007; Miller, Citation1988) and can also have important antioxidant properties (Vidyalakshimi et al., Citation2006). Studies on the phenolic composition of the flowers or floral structures of the species of Agave are few, that performed by Subramanian and Nair (Citation1970), reporting flavonol glycosides (kaempferol-3-O-glucoside and kaempferol-3-O-rutinoside) in the flowers of Agave americana, and that performed by Almaraz-Abarca et al. (Citation2009), reporting profiles composed of different kaempferol glycosides, in the pollen of a natural population of A. durangensis, are some of them. However, to our knowledge, no screening of the antioxidant potential relative to the phenol composition of the edible flowers of Agave has been carried out. The great deal of pollen and nectar those flowers produce (and the importance of this for apiculture), the abundance of the Agave species throughout the North American Continent, and the significance of their flowers as emergency food in periods of scarcity in arid and semiarid zones, where traditional crops are difficult to establish, should prompt to carry out exploratory analysis of the phenolic compounds and the antioxidant properties of Agave flowers. In this paper, the antioxidant activities of extracts of different hydrophobicity, determined by the formation of a phosphomolybdenum complex, by the free radical scavenging (DPPH*), and the iron reducing capacity methods, of flowers of A. durangensis of Durango, Mexico are discussed in terms of their flavonoid composition, which were determined by HPLC–DAD. Separately, total extracts of tepals and anthers–pollen were also analyzed.
Experimental
Plant material
Mature flowers of adult plants of A. durangensis were collected from a wild population of La Parrilla, Durango, Mexico (23° 40′ 49″ N, 104° 7′ 17″ W) in July 2010. The plants were growing on the gravel soil of an open grassland, with semiarid climate. The flowers of five individuals, randomly sampled, were combined and three pools of samples were formed and separately analyzed. The entire flowers and, independently, the tepals, and the anthers–pollen were dried in a ventilated oven at 40°C to constant weight, and then ground in a domestic blender. The dry, ground tissues were kept in paper bags, in darkness, at room temperature until analysis. Voucher specimens were deposited at the Herbarium CIIDIR.
Reagents and standards
Acetonitrile (HPLC grade), water (HPLC grade), ethanol (HPLC grade), ethyl acetate (analytical grade), hexane (analytical grade), sulfuric acid (analytical grade), and sodium phosphate were purchased from J. T. Baker (Xalostoc, Mexico). 2,2-Diphenyl-1-picrylhydrazyl (DPPH*), aluminum chloride, ammonium molybdate, and the references quercetin, quercitrin (quercetin-3-rhamnoside), caffeic acid, and ascorbic acid were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). The standards kaempferol-3,7-O-diglucoside, quercetin-3-O-[rhamnosyl-(1-6)-galactoside], kaempferol-3-O-[rhamnosyl-(1-6)-glucoside] came from Apin Chemicals Limited (Abingdon, Oxon, UK). Trichloroacetic acid and ferric chloride were obtained from Merck (Darmstadt, Germany). Potassium ferricyanide was purchased from Fermont (Monterrey, Mexico).
Preparation of extracts
Flavonoids were extracted from dry ground flowers (15.3 g) by maceration in 150 mL of 60% ethanol (v/v), for 24 h, in darkness, and at room temperature. The extracts were centrifuged (5000 rpm) for 10 min, at room temperature, and the supernatants separated. The pellets were re-extracted in 100 mL of 30% ethanol (v/v) for 3 h, centrifuged under the same conditions, and the supernatans decanted. Both supernatants were combined to form the total extracts; an aliquot (50 mL) of each extract was used for further analysis. The total extracts were concentrated to half the volume and then fractioned twice with 125 mL ethyl acetate. The pellets were also macerated in 100 mL hexane for 24 h in darkness. The two organic fractions (ethyl acetate and hexane), the aqueous fractions, and the separated aliquots of the total extracts, were individually concentrated under vacuum to dryness and then re-dissolved in 5 mL ethanol; aliquots were taken to be used in the determination of flavonoid content, in the HPLC–DAD analysis, and in the evaluation of antioxidant capacity. To determine which floral structures provided the major diversity and concentration of flavonoids and the highest antioxidant properties, total extracts of tepals (9.96 g) and anthers–pollen (11.37 g) were prepared as described for the whole flowers, and individually analyzed. The phenolic extractions were prepared from three different pools of samples.
Determination of flavonoid content
Flavonoid content was determined according to Lauranson-Broyer and Lebreton (Citation1993) by linear regression analysis from the following standard curve of quercitrin: Abs425nm = 0.1059 + 0.0002 [Quercitrin], correlation coefficient r = 0.998. The curve was registered after the addition of 60 µL of a freshly prepared 5% (w/v) aluminum chloride solution to 1 mL of quercitrin solution (four different concentrations in the range of 100 to 1400 µg/mL). The absorbances were immediately registered after the addition of aluminum chloride, at 425 nm, using a Spectronic Genesys 2 espectrophotometer (Rochester, New York, USA). The flavonoid content in each sample was also registered after the addition of aluminum chloride and expressed as µg of quercitrin equivalents/g dry extract. The addition of aluminum chloride produces bathochromic shifts (which can be perceived by a yellow coloration) in flavonoids containing orthodihydroxyl groups, due to the formation of complexes between the aluminum and C-4 keto group and either the C-3 or C-5 hydroxyl group of flavones and flavonols; complexes also are formed between the aluminum and the orthodihydroxyl groups in A- or B-ring of flavonoids (Mabry, Markham, & Thomas, Citation1970). The addition of aluminum chloride represents a standard procedure for reproducibility (Lauranson-Broyer & Lebreton, Citation1993). The determination of flavonoid content was estimated individually for three pools of samples.
HPLC-DAD analysis
To determine the flavonoid profiles, aliquots (100 µL) of each extract (concentrated to dryness and re-dissolved in 5 mL ethanol, as mentioned in the section Preparation of extracts) were analyzed as previously described (Campos & Markham, Citation2007) on a Perkin Elmer Series 200 HPLC system (Shelton, Connecticut, USA) and a Perkin Elmer Brownlee Analytical C18 column (4.6 × 250 mm, 5µm) (Shelton, Connecticut, USA), by an acidified acetonitrile–water gradient. Water adjusted to pH 2.5 with orthophosphoric acid was the solvent A, and acetonitrile was the solvent B, mixed according to the following gradient: starting with 100% A, decreasing to 91% over the next 12 min, to 87% over the next 8 min, to 67% over the next 12 min, to 57% over the next 10 min, and held at this level until the end of the 60 min analysis. Standard chromatograms were plotted at 260 and 340 nm. Spectral data for all peaks were accumulated in the range 220–400 nm using diode array detection (Perkin Elmer Series 200). Structural identification was obtained by direct comparisons of retention times and UV spectra of resolved compounds with those of standards; two kaempferol glycosides, identified as kaempferol-3,7-O-diglucoside (compound 2) and kaempferol-3-O[rhamnosyl-(1-6)-glucoside] (compound 6), and one quercetin glycoside, identified as quercetin-3-O-[rhamnosyl-(1-6)-galactoside] (compound 5) ( and ). The structural information of compounds, for which standards were not available, was obtained from their spectral parameters according to the compilations of Mabry, Markham, and Thomas (Citation1970) and Campos and Markham (Citation2007). Quantitative determinations were made by an external standard method, with the commercial reference (quercitrin), by area measurements, using the following standard curve: Area = −0.0046 + 0.0278 [Quercitrin], correlation coefficient r = 0.992. The content of each compound was expressed as µg of quercitrin equivalents/g dry extract. The HPLC–DAD profiles were individually obtained and analyzed for the extracts from three pools of samples.
Figure 1. Structures of compounds 2: kaempferol-3,7-O-diglucoside, 5: quercetin-3-O-[rhamnosyl-(1-6)-galactoside], and 6: kaempferol-3-O-[rhamnosyl-(1-6)-glucoside], found in the flowers of A. durangensis.
Estructuras de los compuestos 2: canferol-3,7-O-diglucósido, 5: quercetina-3-O-[ramnosil-(1-6)-galactósido], y 6: canferol-3-O-[ramnosil-(1-6)-glucósido], encontrados en las flores de Agave durangneis.
![Figure 1. Structures of compounds 2: kaempferol-3,7-O-diglucoside, 5: quercetin-3-O-[rhamnosyl-(1-6)-galactoside], and 6: kaempferol-3-O-[rhamnosyl-(1-6)-glucoside], found in the flowers of A. durangensis.Estructuras de los compuestos 2: canferol-3,7-O-diglucósido, 5: quercetina-3-O-[ramnosil-(1-6)-galactósido], y 6: canferol-3-O-[ramnosil-(1-6)-glucósido], encontrados en las flores de Agave durangneis.](/cms/asset/99410bbd-5fbe-4ad9-9fb6-beea926178e1/tcyt_a_801037_f0001_b.gif)
Figure 2. Chromatograms obtained at 260 nm by HPLC–DAD for the total extract of the entire flowers of Agave durangensis, and the standards A: kaempferol-3,7-O-diglucoside (RT: 28.00 min, λmax: 242sh, 265, 322sh, 345), B: quercetin-3-O-[rhamnosyl-(1-6)-galactoside] (RT: 34.10, λmax: 255, 266sh, 295sh, 355), and C: kaempferol-3-O-[rhamnosyl-(1-6)-glucoside] (RT: 35.62, λmax: 265, 290sh, 320sh, 349).
Cromatograma obtenido a 260 nm por HPLC-DAD para el extracto crudo de las flores enteras de Agave durangensis, y para los estándares A: canferol-3,7-O-diglucósido (RT: 28.00 min, λmax: 242sh, 265, 322sh, 345), B: quercetina-3-O-[ramnosil-(1-6)galactósido] (RT: 34.10, λmax: 255, 266sh, 295sh, 355), y C: canferol-3-O-[ramnosil(1-6)glucósido] (RT: 35.62, λmax: 265, 290sh, 320sh, 349).
![Figure 2. Chromatograms obtained at 260 nm by HPLC–DAD for the total extract of the entire flowers of Agave durangensis, and the standards A: kaempferol-3,7-O-diglucoside (RT: 28.00 min, λmax: 242sh, 265, 322sh, 345), B: quercetin-3-O-[rhamnosyl-(1-6)-galactoside] (RT: 34.10, λmax: 255, 266sh, 295sh, 355), and C: kaempferol-3-O-[rhamnosyl-(1-6)-glucoside] (RT: 35.62, λmax: 265, 290sh, 320sh, 349).Cromatograma obtenido a 260 nm por HPLC-DAD para el extracto crudo de las flores enteras de Agave durangensis, y para los estándares A: canferol-3,7-O-diglucósido (RT: 28.00 min, λmax: 242sh, 265, 322sh, 345), B: quercetina-3-O-[ramnosil-(1-6)galactósido] (RT: 34.10, λmax: 255, 266sh, 295sh, 355), y C: canferol-3-O-[ramnosil(1-6)glucósido] (RT: 35.62, λmax: 265, 290sh, 320sh, 349).](/cms/asset/79f7431f-e326-440c-b670-ef48525f7d74/tcyt_a_801037_f0002_oc.jpg)
Total antioxidant capacity
The total antioxidant capacity (TAC) of every type of extract was evaluated by the method developed by Prieto, Pineda, and Aguilar (Citation1999), in which the reduction of Mo (VI) to Mo (V) is carried out by the antioxidant, forming a green phosphate/Mo(V) complex at acidic pH. Aliquots (100 µL) of each sample (containing 100 µg/mL of flavonols, respective concentrations of flavonols calculated from the standard curve of quercitrin) were prepared and combined with 1 mL of a solution containing sulfuric acid (0.6 M), sodium phosphate (28 mM), and ammonium molybdate (4 mM), and incubated at 95°C for 90 min. After reaching room temperature, the absorbance of samples was registered at 695 nm against a blank prepared as indicated for the samples, but by adding ethanol instead of the sample. The reference quercitrin was analyzed in the same manner. TAC was expressed as mg ascorbic acid equivalents. Ascorbic acid equivalents were calculated using the following calibration curve: A695 = 0.1519 + 0.063 [ascorbic acid], correlation coefficient r = 0.991, constructed with ascorbic acid between 1.0 and 30.0 mg/mL. The analysis was done for independent aliquots of the extracts from three pools of samples.
Free radical scavenging activity
The DPPH* method reported by Campos, Da Cunha, Navarro, and Utrilla (Citation1994) was used to evaluate the free radical scavenging activity. Four to five flavonoid concentrations of each sample (10–400 µL; respective concentrations of flavonoids calculated from the standard curve of quercitrin) were individually added to a DPPH* solution (40 µg/mL in ethanol) in such a way so as to maintain a final volume of 1 mL. The decrease in absorbance was determined at 523 nm after 30 min. The DPPH* concentrations in the reaction medium against the flavonol concentrations of samples were plotted to determine, by linear regression, the efficient concentration at 50%, defined as the amount of antioxidant (for the present study, the amount of flavonoids in each extract) needed to decrease by 50% the initial DPPH* concentration (EC50). The following calibration curve, made with DPPH* between 6.25 and 100 µg/mL, was used to calculate the DPPH* concentration (µg/mL) in the reaction medium: A523 = −0.0217 + 0.0159 [DPPH*], correlation coefficient r = 0.9974. Antiradical activities were expressed in terms of EC50 in µg/mL. Four concentrations of reference substances (quercetin, quercitrin, and caffeic acid, used as control samples) were assayed in the same manner. The analysis was separately done for the extracts from three pools of samples.
Iron reducing power
The reducing power (RP) method reported by Yang, Guo, and Yuan (Citation2008) was used to evaluate the iron reducing power of flower extracts of A. durangensis. Aliquots (1 mL) of each sample were combined with 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL potassium ferricyanide (30 mM) and incubated at 50°C for 20 min. Then, 2.5 mL trichloroacetic acid (0.6 M) was added and the mixture was centrifuged (2000 rpm for 10 min). From the upper layer, 2.5 mL of solution was removed and distilled water (2.5 mL) and ferric chloride (0.5 mL, 6 mM) were added to it. The absorbance at 700 nm of the formation of ferrous ions (Fe2+) was registered after 10 min. The highest absorbance values indicated the greatest capacity of reducing ferric (Fe3+) to ferrous (Fe2+) ions. Four flavonol concentrations (10–400 µL combined with the proper volume of ethanol to reach 1 mL as final volume) of each sample (respective concentrations of flavonols calculated from standard curve of quercitrin) were evaluated. The references quercetin, quercitrin, and ascorbic acid were analyzed in the same form. The reducing power was expressed in terms of IC50 (µg/mL), defined as the amount of antioxidant (for the present study, the amount of flavonoids in each extract) needed to reach a value of absorbance of 0.5, which was calculated by linear regression analysis. The evaluation was separately done for the extracts from three pools of samples.
Statistical analysis
Data were subjected to an analysis of variance (p ≤ 0.05) and means separated by Duncan´s multiple range test. The results were processed by COSTAT computer program (1982).
Results and discussion
Flavonoid content
The flavonoid content in each extract (hexane, ethyl acetate, aqueous fractions, total extracts of entire flower, total extracts of tepals, and total extracts of anthers–pollen of A. durangensis flowers) is displayed in . Significant differences were found in the contents of flavonoids in each extract. The fractions showing the highest contents were those of ethyl acetate and aqueous fractions, with 580 and 303 µg/g dry extract, respectively. A lower quantity of flavonoids could be detected in the hexane fraction (120 µg/g dry extract). The flavonoid content of the total extract of the entire flowers was 1210 µg/g dry extract, the principal supply coming from the anthers–pollen (369 µg/g dry extract). The significant differences in the flavonoid contents and in the flavonoid profiles (section Flavonoid composition of this paper) between tepals and anthers–pollen of the flowers of A. durangensis could be associated with the different physiological roles these compounds play in each of those flower structures, as has been reported for phenolic compounds by several authors (Hernández, Alegre, Van Breusegem, & Munné-Bosch, Citation2009).
Table 1. Flavonoid content, free radical scavenging activity, TAC, and RP of the several analyzed fractions and total extracts of entire flowers and flower structures of Agave durangensis. The free radical scavenging activity, TAC, and RP of reference substances are also shown. The values represent the mean and standard deviation for three independent samples. Different letters in the same column mean significant differences (p ≤ 0.5).
Contenido de flavonoides, actividad bloqueadora de radicals libres, TAC, y RP de las diferentes fracciones y extractos totals de las flores enteras y estructuras florales de Agave durangensis. La actividad bloqueadora de radicales libres, TAC, y RP de las substancias de referencia se muestran también. Los valores representan la media y la desviación estándar de tres muestras independientes. Diferentes letras en una misma columna indican diferencias significativas (p ≤ 0,5).
The flavonoid content estimated here for the anthers–pollen of A. durangensis was higher than that reported by Almaraz-Abarca et al. (Citation2004) for the flavonol content of Bidens odorata pollen (183 µg/g dry pollen), but lower than the contents found in the pollen of Zea mays (402.8 µg/g dry pollen), Solanum rostratum (448 µg/g dry pollen), Amaranthus hybridus (506 µg/g dry pollen), Tagetes sp. (534 µg/g dry pollen, and Ranunculus petiolaris (1648 µg/g dry pollen), all those species with apicultural importance, reported by Almaraz-Abarca et al. (Citation2004).
The flavonoid content of the entire flowers of A. durangensis was lower than that reported by Barreira, Ferreira, Oliveira, and Pereira (Citation2008) for Castanea sativa (chestnut) flowers (160 mg/g extract), by Liu et al. (Citation2009) for Litchi chinensis (lychee) flowers (273 mg/g extract), and by Tai et al. (Citation2011) for Sophora viciifolia flowers (133.8 mg/g dry extract). The flavonoid content of the entire flowers of A. durangensis was also lower than the levels reported for Ginkgo biloba extracts (240 mg/g), a species considered to be rich in flavonoids (Mouren, Caillard, & Schwartz, Citation1994), but was higher than that reported by Zhang and Cui (Citation2005) for Hippophae rhamnoides extracts (10–439 µg/g). A high flavonoid content is an important feature to consider a given plant extract as a worthy source of antioxidant phenols; however, the antioxidant capacities that a particular combination of those flavonoids shows in extracts of different hydrophobicity, representing singular interactions between them, could be an indicator as important as the content of those compounds for selecting extracts of plant species as sources of those natural antioxidants.
The accumulation of flavonoids, and in general of any phenolic compound, in the different plant species is strongly affected by environmental conditions like UV radiation and water supply, causing quantitative variations while the patterns of accumulated phenolics remained stable (Veit et al., Citation1995). The developmental conditions, like the maturation process also have effects on the accumulations of phenols (Fu et al., Citation2009). The assessment of the effects of variations of those factors in the flower flavonoid contents of A. durangensis should be the aim of future studies.
Flavonoid composition
Under the experimental conditions in which the HPLC flavonoid profile of the flowers of A. durangensis was obtained, five quercetin glycosides and three kaempferol glycosides were found. The chromatogram of the total extract of the entire flowers, containing the eight flavonoids found, is shown in . The UV spectra of the eight flavonoids found are shown in . The retention times and spectral features of the same flavonoids are described in . The flavonoid profiles were variable among the different extracts. Seven of the eight flavonoids were present in the total extract of tepals (compounds 1, 3, 4, 5, 6, 7, and 8), while five were detected in the total extract of anthers–pollen (compounds 3, 4, 5, 6, and 7).
Figure 3. UV spectra of the eight flavonols found in the edible flowers of Agave durangensis. The numbers of compounds correspond to those in .
Espectro UV de los ocho flavonoles encontrados en las flores comestibles de Agave durangensis. Los números de los compuestos corresponden a los de la Tabla 2.
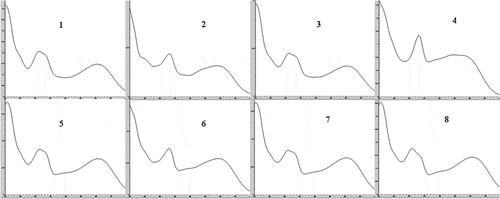
Table 2. Chromatographic and spectral data for the flavonoids found in the flowers of A. durangensis. RT values represent the mean and standard deviation calculated from 6 to 18 independent chromatograms.
Datos cromatográficos y espectrales de los flavonoides encontrados en las flores de Agave durangensis. Los valores de RT representan la media y la desviación estándar calculados a partir de 6 a 18 cromatogramas.
Significant differences were found in the relative concentrations in which each compound was present in several extracts. The compound 7 (quercetin-3-O-glycoside) was the most abundant in the total extract of the entire flowers, and it was among the most abundant in the other extracts, except in the hexane fraction, in which amounts lower than 10 µg/g dry extract were detected for the same compound ().
Table 3. Relative contents of the individual flavonoids of total extracts and ethyl acetate, aqueous, and hexane fractions of flowers of A. durangensis. The values represent the mean and standard deviation for three independent samples. Different letters in the same line mean significant differences (p ≤ 0.5).
Contenidos relativos de los flavonoides individuales de los extractos crudos y las fracciones de acetato de etilo, acuosa, y de hexano de las flores de Agave durangensis. Los valores representan la media y la desviación estándar de tres muestras independientes. Diferentes letras en una misma línea indican diferencias significativas (p ≤ 0,5).
Kaempferol glycosides were also detected in the flowers of A. americana by Subramanian and Nair (Citation1970); however, its flavonoid profile, composed of kaempferol-3-glucoside and kaempferol-3-rutinoside, was different from that detected here for A. durangensis flowers (). This agrees with previous reports informing about the species-specific tendency of flavonoid profiles (Fiasson et al., Citation1997; Almaraz-Abarca et al., Citation2004; Almaraz-Abarca et al., Citation2009).
Almaraz-Abarca et al. (Citation2009) did not report quercetin derivatives in the pollen of A. durangensis flowers; the detection, in the present study, of three glycoside derivatives of this flavonol (compounds 3, 5, and 7) in the anthers–pollen of that species suggests that they are synthesized and accumulated majorly in the anthers.
Quercetin is a flavonoid with a broad spectrum of biological activity (Rice-Evans, Citation1999). Glycoside derivatives of this flavonol are among the major flavonoids found in extracts of plant species recognized by their favorable effect on human health, like Gingko biloba (Mouren, Caillard, & Schwartz, Citation1994) and Hippophae rhamnoides (Zhang & Cui, Citation2005). According to Rice-Evans (Citation1999), kaempferol with a ring B, having a single 4′-hydroxyl group, has a decreased antioxidant activity, this suggests that the contribution of the three glycoside derivatives of this flavonol to the antioxidant properties of the flower extracts of A. durangensis could be lower than the contribution of the five quercetin derivatives detected in them.
The complexity of the extracts of the flowers of A. durangensis, although important, did not reach that of other human beverages and foods, like that of wines (Goldberg & Soleas, Citation1999) and that of the tropical root crop Colocasia esculenta (Champagne, Hilbert, Legendre, & Lebot, Citation2011), both formed by a broader diversity of phenols. However, the simpler flavonoid profile (in which five quercetin glycosides were present) found in the flowers of A. durangensis can confer on those flowers, important antioxidant properties like the results of the further sections (Total antioxidant capacity, Free radical scavenging activity, and Iron reducing power) of the present paper indicate.
Total antioxidant capacity
presents the TAC for each kind of extract. The total extract of tepals of A. durangensis showed the highest TAC value (30.2 mg ascorbic acid equivalents). This activity was higher than that displayed by the flavonol quercitrin (22.6 ascorbic acid equivalents). The order of activity for the rest of extracts was anthers–pollen total extract (15.9) > ethyl acetate fraction (10.8) > aqueous fraction (7.5) > hexane fraction (6.1) > entire flower total extract (4.6). A clear correlation between the flavonoid content in extracts and the TAC was not found, since the entire flower total extract, having the highest flavonoid content (1210.48 µg/g dry extract) showed the lowest TAC value (4.646 mg ascorbic acid equivalents), and the hexane fraction, having the lowest flavonoid content (120.84 µg/g dry extract), showed one value of TAC (6.113 mg ascorbic acid equivalents) higher than that of the entire flower total extract (4.646 mg ascorbic acid equivalents). The flavonoid profile complexity does not seem to be clearly related to TAC, since the hexane fraction, with three flavonols forming its flavonoid profile, showed a higher level of TAC than the total extracts of the entire flowers, which had the most complex flavonoid profile (). Two out of the three flavonols forming the flavonoid profile of hexane fraction were quercetin derivatives (compounds 5 and 7), which majorly could be involved in conferring antioxidant capacities to this extract even at low concentrations (), since it has been reported that compounds possessing reactive phenolic hydroxyl groups, particularly flavonoids with O-dihydroxyl structure in 3′ and 4′, like the quercetin derivatives, are endowed with an important antioxidant property (Zhang & Shen, Citation1997; Terao, Citation1999). Our results suggest that the particular combination of flavonols and their interactions, determined by their molecular structures, could define the level of TAC in each of the extracts analyzed here. However, some antagonistic effect could be manifested in the total extract of entire flowers, revealing a lower TAC than that of any other extracts.
The TAC values calculated for the different hydrophobicity fractions and for the total extracts of entire flowers, anthers–pollen, and tepals of A. durangensis were lower than those reported by Falleh et al. (Citation2011) for the extracts of leaves, stems, and roots of Mesembryanthemum edule (a medicinal and edible species) determined by the same method. Falleh et al. (Citation2011) also detected important variations in the combination of phenolic compounds present in each of the extracts they analyzed.
Free radical scavenging activity
A linear reduction of DPPH* concentration associated with increasing flavonol concentration in the fractions (aqueous fraction: y = 93.844 − 1.360x, r = −0.998; ethyl acetate fraction: y = 91.215 − 1.343x, r = −0.995; and hexane fraction: y = 86.416 − 4.330x, r = −0.999), entire flower total extract (y = 122.860 − 0.682x, r = −0.999), tepal total extract (y = 82.851 − 33.925x, r = −0.995), and anthers–pollen total extract (y = 81.964 – 10.409x, r = −0.989) of flowers of A. durangensis was observed. These results show a clear correlation between the flavonoid concentration in the extracts and the kinetic behavior of DPPH* disappearance, as was reported for phenolic compounds from several sources, like vegetable oils (Espín, Soler-Rivas, & Wichers, 2000) and peanut (Win et al., Citation2011). The ethyl acetate fraction, the total extract of anthers–pollen, the total extract of tepals, and the total extract of the entire flowers reached a steady state before 5 min, the aqueous fraction at 10 min, and the hexane fraction at 30 min. According to the classification suggested by Brand-Williams, Cuvelier, and Berset (Citation1995), the kinetic behavior of every extract was intermediate.
The estimation of antiradical capacity of the different extracts was performed by the value of EC50, these are shown in . The order of activity was the following: total extract of tepals (EC50 = 0.074 µg/mL) > aqueous fraction (0.209 µg/mL) > ethyl acetate fraction (0.216 µg/mL) > anthers–pollen total extract (0.231 µg/mL) > hexane fraction (0.614 µg/mL) > entire flower total extract (0.875 µg/mL), although without significant differences among the activities of the ethyl acetate fraction, the total extract of anthers–pollen, and the aqueous fraction. Contrary to the clear correlation between the flavonoid concentration and the kinetic behavior of DPPH* disappearance, an evident correlation between the antiradical activity and the extract flavonoid content or the flavonoid complexity of the extracts was not apparent; as was suggested by the fact that the total extract of the entire flowers, having the highest flavonoid concentration and the most complex profile, showed the lowest antiradical activity, lower than the activity of the hexane fraction, which showed the lowest flavonoid concentration and the most simple flavonoid profile. The correlation between the phenol content in a given sample and the antioxidant activity is, at present, a controversial issue, since while authors like Dobre et al. (Citation2011) have been able to establish that correlation, some others like Negri et al. (Citation2011) have reported no correlations between these two parameters. Our results agree with those reported by Morais, Moreira, Feás, and Estevinho (Citation2011), who did not find a strong relation between phenol content and antioxidant capability. Synergic or antagonistic effects among phenols, or compounds of different chemical nature, as the result of a particular chemical composition, could be defining the antioxidant properties in such a way that not only the phenol concentration is determinant.
also contains the EC50 values of the reference compounds analyzed in the present study. The total extract (0.875 µg/mL) and the hexane fraction (0.614 µg/mL) of the entire flower of A. durangensis showed free radical scavenging activities lower than those of the references quercetin (0.4 µg/mL) and caffeic acid (0.4 µg/mL), but higher than that of quercitrin (1.3 µg/mL). However, the free radical scavenging activity of the total extract of tepals (0.074 µg/mL) was around five-fold higher than that of quercetin and caffeic acid, both potent antioxidants (Rice-Evans, Citation1999; Pietta & Simonetti, Citation1999). The free radical scavenging activity of every extract analyzed here was several folds higher than those of a mixture of honeybee-collected pollen of a semiarid region of Mexico (EC50: 6.4 µg/mL); monofloral bee pollens of Zea mays (EC50: 10.3 µg/mL), Tagetes sp. (EC50: 6.8 µg/mL, Amaranthus hybridus (EC50: 14.0 µg/mL), Solanum rostratum (EC50: 8.4 µg/mL), Bidens odorata (EC50: 9.3 µg/mL), and Ranunculus petiolaris (EC50: 9.9 µg/mL), all reported by Almaraz-Abarca et al. (Citation2004); and also higher than the extracts of roots (11 µg/mL), stems (435 µg/mL), and leaves (5.2 µg/mL) of M. edule, reported by Falleh et al. (Citation2011). These results suggest that the flowers of A. durangensis, majorly the tepals, are important sources of antiradical flavonoids.
The antioxidant activity of flavonoids depends on the part of the molecule with more efficient hydrogen atom or electron-donating properties, which is more frequent in ring B, and relates to the reduction potentials and reactivities of the substituent hydroxyl groups (Rice-Evans, Citation1999). According to Rice-Evans (Citation1999) and Terao (Citation1999), flavonol quercetin has an O-dihydroxy structure in the ring B, an unsaturated ring C, a 5,7-dihydroxy structure in ring A, all giving it a high antioxidant potential. The five glycoside derivatives of quercetin detected at different concentrations and profiles in all the analyzed extracts of the A. durangensis flowers have the structural attributes reported for quercetin, and could explain the high antiradical capacity detected in them. Regardless, some antagonistic effect, probably due to the interactions among other phenols or due to compounds of any other chemical nature, could reduce, at some level, the scavenging capacity in the total extract, while some synergy could be manifested in the tepal total extract, suggesting the importance of the particular combination of flavonoids and their interactions in conferring antiradical capacity.
Iron reducing power
According to Jovanovic and Simic (Citation2000), the reduction potential is the major factor in determining the antioxidant capacity. A clear correlation between the flavonoid concentration in the extracts and the kinetic capacity of reducing ferric (Fe3+) to ferrous (Fe2+) ions was detected (total extract of entire flowers: y = 0.554 + 0.0046x, r = 0.999; ethyl acetate fraction: y = 0.1762 + 0.0066x, r = 0.999; anthers–pollen total extract: y = 0.05 + 0.0093x, r = 0.997; aqueous fraction: y = 0.0254 + 0.0057x, r = 0.998; tepal total extract: y = −0.1269 + 0.0144x, r = 0.999; hexane fraction: y = −0.0398 + 0.0043x, r = 0.999). Similar correlations can be observed in the results reported by Tai et al. (Citation2011) for the edible flowers of S. viciifolia. The estimation of antiradical capacity of several samples was performed by the value of IC50, these are shown in . Significant differences were found in the RP among the different extracts. Those results suggest that the flavonoid composition and concentration in the different extracts confer variable electron-donating properties. The most active was the tepal total extract (43.28 µg/mL), with potency similar to that of ascorbic acid (43.06 µg/mL) (). The order of activity was tepal total extract (43.28 µg/mL) > anthers–pollen total extract (47.03 µg/mL) > ethyl acetate fraction (51.99 µg/mL) > aqueous fraction (85.24 µg/mL) > entire flower total extract (98.58 µg/mL) > hexane fraction (126.52 µg/mL). Contrary to the clear correlation between the flavonoid concentration and the kinetic behavior of reducing ferric to ferrous ions, an evident correlation between the iron reducing power and the extract flavonoid content or the flavonoid complexity in the extracts was not apparent; this fact was similar to that found for the antiradical activity of the extracts of A. durangensis.
Authors like Yildirim, Mavi, and Cara (Citation2001) have revealed that there is a direct correlation between antioxidant activity and reducing power of components of plants. In general, our results showed a tendency which agreed with that proposal since the total extract of the tepals, with the highest reducing potential, also had the highest free radical scavenging capacity and the highest TAC, while the hexane fraction with the lowest reducing power had one of the lowest free radical scavenging capacities and the lowest TAC values. The particular composition of flavonols present in several extracts of the flowers of A. durangensis could exert the antioxidant action by breaking the free radical chain by donating a hydrogen atom to reactive free radicals, converting them into more stable species. The tepal total extract, the total extract of anthers–pollen, and the ethyl acetate fraction showed higher reducing capacities than the extracts of roots, stems, and leaves of M. edule, for which the effective concentrations of the extract to reach the absorbance value of 0.5 were 217, 99, and 62 µg/mL, respectively (Falleh et al., Citation2011), in spite of the fact that the flavonoid concentration of any of the extracts of A. durangensis flowers analyzed here was lower.
The richness in glycoside derivatives of quercetin and kaempferol associated with the high antioxidant properties of extracts the flowers of A. durangensis reveal this food to be an important flavonoid dietary supplement, which also likely has nutritional values as that reported by Sotelo, López-García, and Basurto-Peña (Citation2007) for flowers of Agave salmiana.
Conclusions
The flavonoid profile of the A. durangensis flowers was formed by five quercetin glycosides and three kaempferol glycosides. The major floral structures accumulating antioxidant flavonols were the anthers–pollen; however, the total extract of the tepals showed the highest antioxidant capacity. Although the ethyl acetate fraction included the highest amounts of flavonoids and possessed the most important antioxidant properties among the extracts of different hydrophobicity, actually, every extract showed a relatively important antioxidant capacity. The particular flavonol profile in each extract is a relevant feature to determine the antioxidant properties. The extracts of the edible flowers of A. durangensis represent a worthy source of flavonols with important antioxidant capacities, which could be regarded as nutraceutical products for application in food and beverages.
The screening of flavonoid composition of plant extracts reveals the diversity, the abundance, and the distribution of those important compounds in the plant kingdom, and also allows the identification of alternative sources, majorly from non-conventional foods, of natural antioxidants. The exploration of the secondary compound composition of the vast non-conventional edible plants all around the world and the creation of databases informing about the distribution, variability, and biological properties of those compounds, besides the establishment of standard methods for their systematic analysis are among the major issues to advance in the understanding of the use and conservation of plants as sources of beneficial chemical compounds for the human being.
Acknowledgments
The authors thank Comisión de Operación y Fomento a las Actividades Académicas, Instituto Politécnico Nacional for the stimuli for research, to Consejo Nacional de Ciencia y Tecnología for the grant to one of the authors, and the reviewers, who made suggestions that certainly improved the quality of the manuscript.
References
- Almaraz-Abarca, N., Campos, M. G., Ávila-Reyes, J. A., Naranjo-Jiménez, N., Herrera-Corral, J., & González-Valdez, L. S. (2004). Variability of antioxidant activity among honeybee-collected pollen of different botanical origin. Interciencia, 29, 574–578.
- Almaraz-Abarca, N., González-Elizondo, M. S., Tena-Flores, J. A., Ávila-Reyes, J. A. Herrera-Corral, J., & Naranjo-Jiménez, N. (2006). Foliar flavonoids distinguish Pinus leiophylla and P. chihuahuana (Coniferales: Pinaceae). Proceedings of the Biological Society of Washington, 119, 426–436.
- Almaraz-Abarca, N., Campos, M. G., Ávila-Reyes, J. A., Naranjo-Jiménez, N., Herrera-Corral, J., & González-Valdez, L. S. (2007). Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). Journal of Food Composition and Analysis, 20, 119–124.
- Almaraz-Abarca, N., Delgado-Alvarado, E. A., Hernández-Vargas, V., Ortega-Chávez, M., Orea-Lara, G., Cifuentes-Díaz de León, A., … Muñiz-Martínez, R. (2009). Profiling of phenolic compounds of somatic and reproductive tissues of Agave durangensis Gentry (Agavaceae). American Journal of Applied Sciences, 6, 1076–1085.
- Almaraz-Abarca, N., Hernández-Vargas, V., Torres-Morán, I., Delgado-Alvarado, A. Orea-Lara, G., Cifuentes-Díaz de León, A., … Naranjo-Jiménez, N. (2011). Agave durangensis. México, D.F., México: IPN-CONACYT-COCYTED.
- Ávila-Reyes, J. A., Almaraz-Abarca, N., Delgado-Alvarado, E. A., González-Valdez, L. S., Valencia-del Toro, G., & Durán-Páramo, E. (2010). Phenol profile and antioxidant capacity of mescal aged in oak wood barrels. Food Research International, 43, 296–300.
- Barreira, J. C. M., Ferreira, I. C. F. R., Oliveira, M. B. P. P., & Pereira, J. A. (2008). Antioxidant activities of the extracts from chestnut flower, leaf skins and fruit. Food Chemistry, 107, 1106–1113.
- Bartnik, M., Gtowniak, K., & Gromek, A. (2007). TLC and HPLC analysis of the flavonoids glycosides in the aerial parts of Peucedanum tauricum Bieb. Journal of Planar Chromatography, 20, 127–130.
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. Lebensmittel Wissenschaft and Technologie, 28, 25–30.
- Campos, M. G., Da Cunha, P. A., Navarro, M. C., & Utrilla, M. P. (1994). Free radical scavenger activity of bee pollen. Polyphenols, 94, 415–426.
- Campos, M. G., & Markham, K. R. (2007). Structure information from HPLC and on-line measured absorption spectra-flavone, flavonols and phenolic acids. Coimbra, Portugal: Coimbra University Press.
- Castillo, G. V. M., & Coelho, A. M. (2007). Dinámica de la cadena agave-tequila: Tendencias y adaptación a la globalización. In J. A. Vázquez-García, M. J. Cházar, G. Hernández-Vera, E. P. Flores-Berrios, & Y. L. Vargas-Rodríguez (Eds.). Agaves del occidente de México (pp. 150–163). Guadalajara, Jal., México: Universidad de Guadalajara-Consejo Regulador del Tequila.
- Champagne, A., Hilbert, G., Legendre, L., & Lebot, V. (2011). Diversity of anthocyanins and other phenolic compounds among tropical root crops from Vanuatu, South Pacific. Journal of Food Composition and Analysis, 24, 315–325.
- Colunga-GarcíaMarín, P., Coello-Coello, J., Espejo-Peniche, L., & Fuente-Moreno, L. (1993). Agave studies in Yucatan, Mexico II. Nutritional values of the inflorescence peduncle and incipient domestication. Economic Botany, 47, 328–334.
- Dobre, I., Dâdei, G., Patrascu, L., Elisei, A. M., & Segal, R. (2011). The antioxidant activity of selected Romanian honeys. The Annals of the University Dunarea de Jos of Galati-Food Technology, 34, 67–73.
- Espin, J. C., Soler-Rivas, C., & Wichers, H. J. (2000). Characterizations of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrilhydrazyl radical. Journal of Agricultural and Food Chemistry, 48, 648–656.
- Falleh, H., Ksouri, R., Medini, F., Guyot, S., Abdelly, C., & Magné, C. (2011). Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Industrial Crops and Products, 34, 1066–1071.
- Fiasson, J., Gluchoff-Fiasson, L. K., & Dahlgren, G. (1997). Flavonoid patterns in European Ranunculus L. subgenus Batrachium (Ranunculaceae). Biochemical Systematics and Ecology, 25, 327–333.
- Fu, M., He, Z., Zhao, Y., Yang, J., & Mao, L. (2009). Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chemistry, 114, 1192–1197.
- García-Mendoza, A. (1995). Riqueza y endemismos de la familia Agavaceae en México. In E. Linares, P. Dávila, F. Chiang, R. Bye, & T. Elias (Eds.), Conservación de plantas en peligro de extinción: Diferentes enfoques (pp. 51–75). México, D. F., México: UNAM.
- Gentry, H. S. (1982). Agaves of continental North America. Tucson, AR: The University of Arizona Press.
- Goldberg, D., & Soleas, G. J. (1999). Analysis of antioxidant wine polyphenols by high performance liquid chromatography. Methods in Enzymology, 299, 122–137.
- Good-Ávila, V. S., Souza, V., Gaut, S. B., & Eguiarte, E. L. (2006). Timing and rate of speciation in Agave (Agavaceae). Proceedings of the National Academy of Science USA, 103, 9124–9129.
- Gutiérrez, C. M. L., Acedo, F. E., & Valenzuela, Q. A. I. (2007). Industria del bacanora y su proceso de elaboración. Ciencia y Tecnología Alimentaria, 5, 394–404.
- Hernández, I., Alegre, L., Van Breusegem, F., & Munné-Bosch, S. (2009). How relevant are flavonoids as antioxidants in plants? Trends in Plant Science, 14, 125–132.
- Jovanovic, S. V., & Simic, M. G. (2000). Antioxidants in nutrition. Annals of the New York Academy of Sciences, 899, 326–334.
- Lauranson-Broyer, J., & Lebreton, P. (1993). Flavonoids and morphological traits of needles, as markers of natural hybridization between Pinus uncinata Ram and Pinus sylvestris L. Biochemical Systematics and Ecology, 21, 241–247.
- Liu, S. C., Lien, J. T., Wang, C. K., Chen, H. Y., & Yang, D. J. (2009). Antioxidant properties of various solvent extracts from lychee (Litchi chinensis Sonn.) flowers. Food Chemistry, 114, 577–581.
- Mabry, T. J., Markham, K. M., & Thomas, M. B. (1970). The Systematic Identification of Flavonoids. New York, NY: Springer-Verlag.
- Miller, J. M. (1988). Floral pigments and phylogeny in Echinocereus (Cactaceae). Systematic Botany, 13, 173–183.
- Morais, M., Moreira, L., Feás, X., & Estevinho, L. M. (2011). Honeybee-collected pollen from five Portuguese natural parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food and Chemical Toxicology, 49, 1096–1101.
- Mouren, X., Caillard, P., & Schwartz, F. (1994). Study of the antiischemic action of EGb 761 in the treatment of peripheral artherial occlusive disease by TcPo2 determination. Angiology, 45, 413–417.
- Negri, G., Teixeira, E. W., Teles, M. M. L., Alves, F., Moretis, A. C. C. C., Pzar, O. I., … Salatino, A. (2011). Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from southeast Brazil. Journal of Agricultural and Food Chemistry, 59, 5516–5522.
- Pietta, P., & Simonetti, P. (1999). Dietary flavonoids and interaction with physiologic antioxidants. In L. Packer, M. Hiramatsu, & T. Yoshikawa (Eds.), Antioxidant Food Supplements in Human Health (pp. 283–308). San Diego, CA: Academic Press.
- Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamine E. Analytical Biochemistry, 269, 337–341.
- Rice-Evans, C. (1999). Screening of phenolics and flavonoids for antioxidant activity. In L. Packer, M. Hiramatsu, & T. Yoshikawa (Eds.), Antioxidant Food Supplements in Human Health (pp. 239–253). San Diego, CA: Academic Press.
- Sotelo, A., López-García, S., & Basurto-Peña, F. (2007). Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods for Human Nutrition, 62, 133–138.
- Subramanian, S. S., & Nair, A. G. R. (1970). Chlorogenin and kaempferol glycosides from the flowers of Agave americana. Phytochemistry, 9, 2582.
- Tai, Z., Cai, L., Dai, L., Dong, L., Wang, M., Yang, Y., … Ding, Z. (2011). Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chemistry, 126, 1648–1654.
- Terao, J. (1999). Dietary flavonoids as plasma antioxidants on lipid peroxidation: Significance of metabolic conversion. In L. Packer, M. Hiramatsu, & T. Yoshikawa (Eds.), Antioxidant food supplements in human health (pp. 255–268). San Diego, CA: Academic Press.
- Vaughn, M. B. Jr. (1974). Prehistoric diet in southwestern Texas: The coprolite evidence. American Antiquity, 39, 407–420.
- Veit, M., Beckert, C., Höhne, C., Bauer, K., & Geiger, H. (1995). Interspecific and intraspecific variation of phenolics in the genus Equisetum subgenus Equisetum. Phytochemistry, 38, 881–891.
- Vidyalakshimi, K. S., Dorni, A. I. C., Vasanthi, H. R., Rajamanickam, G. V., & Sukumar, D. (2006). Free radical scavenging activity of Mussaenda glabra. Journal of Applied Sciences, 6, 2251–2256.
- Wiermann, R., & Vieth, K. (1983). Outer pollen wall, an important accumulation site for flavonoids. Protoplasma, 118, 230–233.
- Win, M. M., Abdul-Hamid, A., Baharin, B. S., Anwar, F., Sabu, M. C., & Pak-Dek, M. S. (2011). Phenolic compounds and antioxidant activity of peanut’s skin, hull, raw kernel and roasted kernel flour. Pakistan Journal of Botany, 43, 1635–1642.
- Yang, J., Guo, J., & Yuan, J. (2008). In vitro antioxidant properties of rutin. LWT-Food Science and Technology, 41, 1060–1066.
- Yildirim, A., Mavi, A., & Cara, A. A. (2001). Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. Journal of Agricultural and Food Chemistry, 49, 4083–4089.
- Zhang, Q., & Cui, H. (2005). Simultaneous determination of quercetin, kaempferol, and isorhamnetin in phytopharmaceuticals of Hippophae rhamnoides L. by high-performance liquid chromatography with chemiluminescence detection. Journal of Separation Science, 28, 1171–1178.
- Zhang, J., & Shen, S. (1997). Antioxidant activities of baicalin, green tea polyphenols and alizarin in vitro and in vivo. Journal of Nutrition and Environmental Medicine, 7, 79–89.
- Zizumbo-Villareal, D., & Colunga-GarcíaMarín, P. (2008). Early coconut distillation and the origins of mezcal and tequila spirits in west-central Mexico. Genetic Resource and Crop Evolution, 55, 493–510.
