Abstract
The proximate composition, functional properties, and proportion of protein fractions in a mamey sapote defatted meal (MSDM) were investigated. Additionally, a mamey sapote protein isolate (MSPI) was obtained by isoelectric precipitation from MSDM and partially characterized. MSDM had a protein content of 240.6 g/kg with the following protein distribution: glutelins (57.25%), prolamins (18.65%), albumins (17.85%) and globulins (6.25%). The maximum solubility and precipitation of MSDM proteins were found at pH 9.8 and 3.5, respectively. MSPI had a protein content of 950.9 g/kg and an in vitro digestibility of 73.6%. MSPI exhibited a lower water absorption capacity than MSDM but higher oil absorption and emulsifying capacities. The highest foam capacity (76.79%), foam (29.73%) and emulsifying (70.0%) stabilities of MSPI were obtained at pH 10. These results demonstrate that MSDM is a suitable material for the production of a high-purity protein isolate that could be used as an ingredient in meat or bakery products.
Se determinó la composición proximal, fracciones proteínicas y propiedades funcionales de la harina desengrasada de semilla de zapote mamey (HDSZM). Asimismo, se obtuvo un aislado proteínico (APZM) a partir de la HDSZM por precipitación isoeléctrica y fue caracterizado parcialmente. La HDSZM presentó un contenido de proteínas de 240,6 g/kg con la siguiente distribución de fracciones proteínicas: glutelinas (57,25%), prolaminas (18,65%), albúminas (17,85%) y globulinas (6,25%). La solubilidad y precipitación máximas de las proteínas de la HDSZM se encontró a pH 9,8 y 3,5, respectivamente. El APZM tuvo un contenido de proteínas de 950,9 g/kg y una digestibilidad in-vitro de 73,6%. El APZM mostró menor capacidad de absorción de agua que la HDSZM pero una mayor capacidad de absorción de aceite y emulsificante. El APSM mostró la mayor capacidad espumante (76,79%), estabilidad a la espuma (29,73%) y emulsificante (70,0%) a pH 10. Estos resultados demostraron que la HADSZM es una materia prima adecuada para la producción de un aislado proteínico de alta pureza que podría utilizarse como ingrediente en productos cárnicos o panadería.
Introduction
Mamey sapote (Pouteria sapota Jacq. H. E. Moore & Stearn) is a tropical fruit tree of the Pouteria genus belonging to the Sapotaceae family. This species is native to southern Mexico and most of Central America and has been introduced as a crop to northern South America, Florida, Hawaii, the Bahamas, the Caribbean islands, the Philippines, and Vietnam (Bayuelo & Ochoa, Citation2006). Mamey sapote fruit are ovoid berries (20 cm in length, 7–12 cm in diameter) with a thick, rough brown peel and soft, sweet pulp varying in color from dark coffee-brown to deep red to light pink. The pulp’s sweet distinctive flavor makes it popular among consumers and is eaten fresh, frozen, or dehydrated as a food ingredient (Azurdia, Citation2006; Téllez, Saucedo, Arévalo, & Valle, Citation2009). Seeds account for 15–25% of the whole fruit, representing a substantial processing by-product, and have several applications. Mamey sapote seed kernels are reported to have high protein (16.9%) and oil (35–49% dry matter) contents. Oil from the kernel is used in traditional medicine systems and is industrially produced for its use in cosmetic and pharmaceutical products (Solís, Tapia, & Durán, Citation2001). Some data have been published on mamey sapote seed oil extraction conditions, its fatty acid composition and the physical characteristics of the crude and refined seed oils (Solís & Durán, Citation2003; Solís et al., Citation2001).
Like any oil extraction process, mamey sapote kernel oil extraction yields a defatted meal as a by-product that is generally used as an animal feed (ICUC, 2004). However, its high protein content suggests possible uses in human food through protein extraction using various isolation methods. The low cost and relative technical simplicity of isoelectric precipitation have resulted in its frequent use for protein isolation (Moure, Sineiro, Domínguez, & Parajo, Citation2006). In this process, nonprotein components are eliminated or reduced through protein solubilization, normally followed by recovery at an isoelectric pH; the protein content of the final product usually ranges from 80 to 90% (Vioque, Sánchez-Vioque, Pedroche, Yust, & Millán, Citation2001). A number of recent studies have focused on the preparation of protein isolates by isoelectric precipitation from defatted meals of economically important oilseeds, such as canola and flaxseeds (Can-Karaka, Low, & Nickerson, Citation2011), and from the meals of alternative sources, such as tomato (Solanum lycopersicum) seeds (Liadakis, Tzia, Oreopoulou, & Thompopoulos, Citation1995), guava (Psidium guava) seeds (Bernardino, Ortiz, Martínez, & Dávila, Citation2001), Abyssinian mustard (Brassica carinata) seeds (Pedroche et al., Citation2004) and broad bean (Vicia faba) seeds (Vioque, Alaiz, & Girón-Calle, Citation2012). Most studies address the raw material characteristics and protein content, and describe how various factors (e.g., net charge, electrostatic repulsion, salt concentration, ionic strength, pH, temperature, and processing time) affect protein extraction yield, protein purity, and the functional properties of the protein isolate. The latter include properties such as water/oil absorption, solubility, jellification, emulsifying capacity, and foaming capacity, all of which are technologically important because they determine the potential applications of protein isolates as food ingredients (Moure et al., Citation2006).
The aim of this study was to determine some chemical and functional properties of mamey sapote defatted meal and the proportion of protein fractions based on their solubility. Another aim was to determine the functional properties and in vitro digestibility of a protein isolate extracted by isoelectric precipitation from the meal.
Materials and methods
Raw materials
Mature mamey sapote fruits were harvested (complete redness in the pulp) from March to April, 2011, from Pouteria sapota trees grown in various communities in Santa Maria Jacatepec, Oaxaca State, Mexico. The fruits were weighed, and their lengths and diameters were measured using a manual vernier. Next, the fruits were opened, and the proportions of the peel, pulp, and seed were determined by weighing. The seeds were washed with a 5% chlorine solution and dehulled to extract the kernels.
Preparation of defatted meal
The mamey sapote kernels were cut into slices (approximately 0.2 cm thick) and dried in a dryer with reversal airflow designed and built at the Technological Institute of Tuxtepec (Instituto Tecnologico de Tuxpec) (Herman-Lara, Martínez-Sánchez, Amador-Mendoza, & Ruiz-López, Citation2010). The drying conditions were as follows: 10 cm bed height, 10 h, 60°C, 6 m/s hot air flow and hourly changes of airflow direction. The dried slices were milled and sieved at 0.595 mm (30 mesh, US Sieve size). The resulting flour was mixed with hexane and heated at 60°C for 10 min with stirring. The slurry was allowed to cool to room temperature and then filtered to extract the crude oil. The residue was oven-dried at 40°C for 1 h, milled and sieved through a 0.500-mm mesh to produce a fine powder that was used as the mamey sapote defatted meal (MSDM). This meal was stored in glass containers at room temperature (27 ± 2°C) until use.
The kernel flour and MSDM were analyzed in triplicate for moisture, crude protein (N × 6.25), total fat, crude fiber, and ash, following the 925.10, 920.87, 920.39, 925.08 and 923.03 methods, respectively, of the Association of Official Analytical Chemist (AOAC, Citation1997). Carbohydrates were calculated by subtraction.
Protein fractionation
Proteins were extracted from the MSDM based on their solubility, according to the Osborne (Citation1908) fractionation procedure lightly modified. MSDM was first extracted with distilled water (1:10, w/v) using two stirring steps of 1 h at 4°C and then centrifuged at 10,000 g for 30 min at 4°C to recover the albumins in the supernatant. The resulting residue was extracted by magnetic stirring for 4 h with 10% NaCl (1:10, w/v) at 4°C and centrifuged at 10,000 g for 30 min at 4°C. The recovered supernatant was considered as the globulin fraction. The residue from the globulin extraction step was extracted by magnetic stirring for 4 h with 70% aqueous 2-propanol (1:10, w/v) at 4°C and centrifuged at 10,000 g for 30 min at 4°C. The prolamin-containing supernatant was recovered, and the residue was suspended in 0.1 N NaOH at pH 12 by stirring for 4 h at 4°C. The slurry was then centrifuged at 10,000 g for 30 min at 4°C, and the supernatant was recovered to obtain the glutelins. Each protein fraction’s supernatant (i.e., containing albumins, globulins, prolamins, and glutelins) was analyzed for protein content using colorimetry (Bradford, Citation1976). Bovine serum albumin was purchased from Sigma (Sigma-Aldrich Química, Estado de México, Mexico) and used as a protein standard.
Preparation of mamey sapote protein isolate
The method of Bernardino et al. (Citation2001) was applied to prepare the protein isolate from MSDM. Briefly, the pH at which the protein solubility is highest was determined. The MSDM was suspended in deionized water (1:20, w/v) and stirred for 30 min. The pH was adjusted (range: 7–12) and maintained during extraction by adding 0.1 N NaOH. Suspensions were then heated to 45°C for 30 min, and the slurry was centrifuged at 2600 g for 40 min. The pellet was discarded, and the supernatant was filtered for later protein content analysis (Bradford, Citation1976).
The isoelectric point (pI) of the MSDM proteins was determined as the pH at which a maximal precipitation occurred. Defatted meal was mixed with water (1:20 w/v) and stirred for 30 min. The pH of the mixture was then adjusted to that at which maximum protein solubility occurred, and the mixture was heated at 40°C for 30 min. The pI was identified by titrating aliquots of each collected extract to specific pH values and measuring the protein contents (Kjendhal) of the supernatants after centrifuging. The pellet obtained after centrifugation during the maximum protein precipitation process, which constituted that the mamey sapote protein isolate (MSPI) was recovered and freeze-dried (Labconco Lyph Look 6).
MSPI characterization
Proximate chemical composition was measured using AOAC (Citation1997) methods, and the functional properties of the MSDM and MSPI were tested.
Water absorption capacity (WAC) was measured following the method of Kabirullah and Wills (Citation1982). A 0.5 g sample was dispersed in 5 mL of distilled water in a centrifuge tube, stirred for 1 min and allowed to stand for 30 min. The slurry was centrifuged at 1600 g for 20 min, and the absorption was expressed as the percentage increase in the sample weight.
The oil absorption capacity (OAC) was assayed using the method of Lin, Humbert, and Sosulski (Citation1974). Oil (3 mL) was stirred for 1 min with 0.5 g of sample in a centrifuge tube. After standing for 30 min, the suspension was centrifuged at 1600 g for 20 min. Free oil was decanted, and the percentage of absorbed oil was calculated from the difference in weight
The foaming capacity (FC) and foam stability (FS) were tested at various pH values (2, 4, 6, 8, and 10) using the procedure of Kabirullah and Wills (Citation1982). Sample dispersions of 1% (50 mL) were adjusted to the required pH and stirred for 1 min at 25°C using a blender (Oster, mod. 465) at the highest speed. The volume of foam above the liquid surface was measured, and the FC was expressed as the percent increase in volume. FS was expressed as the volume of foam remaining after 30 min.
The emulsifying capacity (EC) was tested at various pH values (2, 4, 6, 8, and 10) following the method of Wang and Kinsella (Citation1976). The sample (0.7 g) was homogenized in 10 mL water for 1 min. After adjusting the pH, 10 mL of olive oil wasadded to the mixture and homogenized at 3000 rpm for 1 min. The resulting emulsion was divided evenly into two 50-mL conical centrifuge tubes and centrifuged at 1100 g for 5 min. The EC (%) was calculated by dividing the volume of the emulsified layer by the volume of emulsion before centrifuging.
The emulsion stability (ES) was measured by heating the above emulsion to 80°C for 30 min, then cooling to 25°C and centrifuging at 1100 g for 5 min. The ES was expressed as the percentage of ES remaining after heating.
The in vitro digestibility was analyzed in the MSDM and MSPI using the multienzyme technique of Hsu, Vavak, Satterlee, and Miller (Citation1977). Pepsin from porcine intestinal, trypsin from porcine pancreas type IX-S and chymotrypsin from bovine pancreas type II were obtained from Sigma (Sigma-Aldrich Quimica, Estado de Mexico, Mexico).
Statistical analyses
The results were analyzed using a one-way analysis of variance (ANOVA), and differences between the means were calculated using a least significant difference test at a 95% confidence level. All analyses were performed using Minitab software, version 8.0 (Minitab Inc. State College, PA, USA).
Results and discussion
Proximate chemical composition
The mamey sapote fruits used in this work had an average length of 16.85 ± 1.56 cm, an equatorial diameter of 7.23 ± 0.61 cm and a weight of 617.13 ± 81.90 g. The proportions of pulp, peel, and seed in the fruit were 70, 15.2 and 14.8%, respectively, by weight. The inner kernel of the seed accounted for 85 ± 2.3%.
Fat was the main component in the mamey sapote kernel flour, followed by carbohydrates, proteins, and crude fiber (). Therefore, mamey sapote seed is a rich source of oil. Moreover, protein content in the mamey sapote kernel flour was 133.7 g/kg (dry basis, d.b.), a value higher than that reported for other seed proteins from fruits such as orange (Citrus sinensis) (30.6 g/kg d.b.) (El-Safy, Salem, & El-Ghany, Citation2012) and guava (Psidium guava) (79 g/kg d.b.) (Bernardino et al., Citation2001) but slightly lower than that reported for prickly pear (Opuntia sp) seeds (166 g/kg d.b.) (El-Safy et al., Citation2012).
Table 1. Proximate composition (dry basis) of mamey sapote kernel flour, MSDM, and MSPI.
Composición proximal (base seca) de la harina de la almendra de zapote mamey, HDSZM y APZM.
Extraction lowered the fat content in the MSDM by 91% compared to the kernel flour, leading to an increase in the other components, particularly proteins (approximately 44.4% of increase) (). Protein content (240.6 g/kg d.b.) in the MSDM was lower than reported (300–600 g/kg d.b.) for defatted meals from some commercial oilseeds such as canola (Can-Karaka et al., Citation2011), soybean (Glycine max), rapeseed (Brassica napus L.), cotton (Gossypium sp.), sunflower (Helianthus annus) and peanut (Arachis hypogaea) (Moure et al., Citation2006). However, it was similar to protein content reported for flaxseed (Linum usitatissimum) defatted meal (238.9 g/kg d.b.s) (Can-Karaka et al., Citation2011) and higher than defatted meals from alternative protein sources such as P. guava seeds (76 g/kg d.b.) (Bernardino et al., Citation2001) and chickpea (Cicer arietinum) (227 g/kg d.b.) (Vioque et al., Citation2001).
Protein fractionation
Of the four protein fractions in the MSDM, glutelins, which were obtained as an insoluble residue, were the dominant fraction (572.5 ± 67.8 g/kg protein), followed by prolamins (186.5 ± 24.3 g/kg protein), albumins (178.5 ± 15.6 g/kg protein) and globulins (625 ± 16.7 g/kg protein). This protein fraction distribution is similar to that found in rice (Oryza sativa), which comprises mostly glutelins (540–690 g/kg), followed by prolamins (150–210 g/kg), and low concentration of globulins (100 g/kg) and albumins (50 g/kg) (Sing & Matta, Citation2008), and P. guava seeds, in which glutelins are the main fraction (Bernardino, Scilingo, Añon, & Davila, Citation2006).
Solubility and pI of MSDM proteins
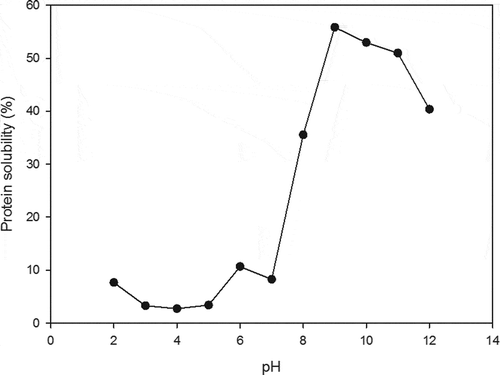
MSDM protein solubility was highest under alkaline conditions (pH 7–12) with the maximum solubility being attained at pH 9.8 (). This suggests that the protein hydrophilic groups were exposed, thereby forming hydrogen bonds with the water and facilitating protein solubility (Moure et al., Citation2006). In contrast, the pI of the MSDM protein was at pH 3.5 (), the same as that reported for proteins from B. carinata seed meal (Pedroche et al., Citation2004). Using these pH values to guide the processing conditions, the protein yield achieved from mamey sapote seeds was 88.45%.
MSPI protein content
The MSPI contained 950.9 g/kg of protein, no carbohydrates and low levels of fiber, fat, and ash (). This protein content is similar to those of protein isolates produced by isoelectric precipitation from defatted P. guava meal (967 g/kg d.b.) (Bernardino et al., Citation2001), B. carinata seed meal (911–926 g/kg d.b.) at different alkaline pH levels (Pedroche et al., Citation2004) and V. faba seeds (924 g/kg d.b.) (Vioque et al., Citation2012). The MSPI protein content was higher than that of defatted canola seed (753.1 g/kg d.b.) and L.usitatissimum seed meal (892.5 g/kg d.b.) (Can-Karaka et al., Citation2011) protein isolates prepared using the same method.
Functional properties of MSPI
WAC
In the MSPI, the WAC (1.27 ± 0.12 mL water/ g material) was lower (p < 0.05) than in the MSDM (1.73 ± 0.23 mL water/g material), most likely because the latter had higher fiber and carbohydrate contents, both of which contribute to the WAC. This same differentiation has been reported between isolates and defatted meals from the seeds of B. carinata) (Pedroche et al., Citation2004) and V. faba (Vioque et al., Citation2012). The WAC for the MSPI was similar to that reported for protein isolates from a P. guava seed (1.5 mL water/g material) (Bernardino et al., Citation2001) and B. carinata seeds (0.99–1.02 g water/g material) (Pedroche et al., Citation2004).
OAC
The OAC value was approximately 41% higher in the MSPI (1.93 mL oil/g material) than in the MSDM (1.13 mL oil/g material), most likely due to the higher protein concentration of the former. This property is largely due to the formation of lipid-protein complexes and the availability of hydrophobic amino acids on the protein’s surface (Chavan, McKenzi, & Shahidi, Citation2001). Protein denaturation during isolation could have promoted these changes (Vioque et al., Citation2012). Protein denaturation during isolation could have promoted these changes. The MSPI OAC value was higher than that reported for a P. guava seed protein isolate (0.6 mL oil/g material) (Bernardino et al., Citation2001) but slightly lower than that reported for a L. maritimus seed protein isolate extracted at pH 12 (2.09 g oil/g material) (Pedroche et al., Citation2004) and a V. faba seed protein isolate (2.31 g oil/g material) (Vioque et al., Citation2012). The high OAC value suggests that the MSPI could function as an extender or substitute in meats to retain the flavor and aroma, and improve a mouthfeel.
Foaming properties
FC is a functional property useful in pastry and some bakery foods (Chavan et al., Citation2001).The MSPI FC values were higher (p < 0.05) than those of the MSDM at all studied pH values (). This can be attributed to the higher protein content of the protein isolate because proteins can be adsorbed at the air–water interface during bubble formation. A rapid conformational change and reorganization reduce the surface tension to form a cohesive viscoelastic film around the bubbles via intermolecular interactions, which allows for a foam formation (Chavan et al., Citation2001).
Table 2. Foaming capacity (FC) and emulsifying capacity (EC) of MSDM and MSPI.
Capacidad espumante (CE) y capacidad emulsificante (CEm) de la HDSZM y del APZM.
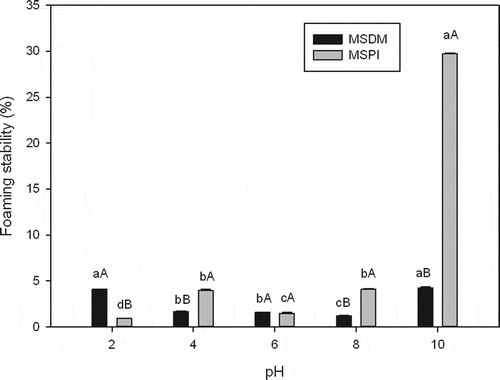
The FC was affected by pH; the MSPI FC was highest (p < 0.05) in an alkaline medium (pH 10), whereas the MSDM FC was highest at pH 2 (). Moreover, the FS was also affected by pH (); the MSDM FS was highest (p < 0.05) at pHs 2 (4.08%) and 10 (4.25%), and the MSPI was also highest (29.73%) at pH 10, although with a higher (p < 0.05) value. This behavior agrees with studies indicating that surface activity is determined by factors such as pH (Moure et al., Citation2006), at pHs above or below the pI (3.5 in the present study), the FC increases due to the greater solubility of protein fractions (Ogunwolu, Henshaw, Mock, Santros, & Awonorin, Citation2009). Compared to other seed protein isolates with similar protein concentrations, the MSPI FC was higher than that of a P. guava seed isolate (50%) (Bernardino et al., Citation2001) but lower than that of a B. carinata seed isolate (181–280%) at alkaline pH (Pedroche et al., Citation2004). These discrepancies arise because the FC is also affected by molecular factors such as conformation, configuration, and flexibility, together with the distribution of hydrophilic and hydrophobic residues in the primary structure (Moure et al., Citation2006).
Figure 2. Foaming stability of mamey sapote defatted meal (MSDM) and mamey sapote protein isolate (MSPI) at various pHs. Means for the same pH having different capital letters are significantly different (p < 0.05). Means for the same sample (MSDM or MSPI) having different lowercase letters are significantly different (p < 0.05).
Estabilidad espumante de la harina desengrasada de semillas de zapote mamey (HDSZM) y aislado proteínico de zapote mamey (APZM) a diferentes pHs. Los promedios para el mismo pH con diferente letra mayúscula son significativamente diferentes (p < 0.05). Los promedios para la misma muestra (HDSZM o APZM) con diferente letra minúscula son significativamente diferentes (p < 0.05).
Emulsifying properties
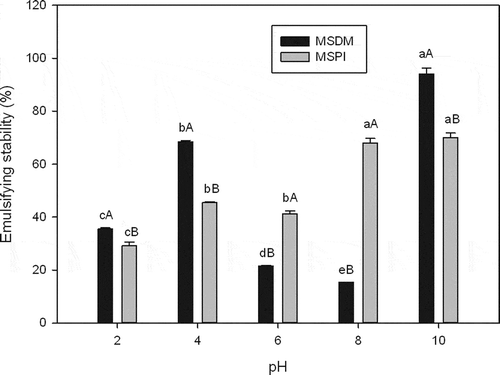
Both EC () and ES of MSPI () were affected by pH. The MSDM EC value was highest at pH 6 (approximately 13% higher than the highest MSPI EC at pH 10). The highest (p < 0.05) value (94.05%) for the MSDM ES was achieved at pH 10, and this was 25% higher than the highest ES values (68.03–70.0%) of MSPI at pHs 8 and 10 (). Similar behaviors have been reported for defatted cashew nut (Anacardium occidentale L.) meal (Ogunwolu et al., Citation2009). A higher EC in a defatted meal than the EC in a protein isolate can arise because at low protein concentrations, protein adsorption at the oil water interface is diffusion controlled. Additionally, at lower protein concentrations, polypeptides are less folded during the shearing involved in the emulsifying process. This is facilitated by the hydrophobic association of the peptide chains with lipid droplets, which greatly increases the available protein surface area and enhances the emulsifying efficiency (Ogunwolu et al., Citation2009).
Figure 3. Emulsifying stability of mamey sapote defatted meal (MSDM) and mamey sapote protein isolate (MSPI) at various pHs. Means for the same pH having different capital letters are significantly different (p < 0.05). Means for the same sample (MSDM or MSPI) having different lowercase letters are significantly different (p < 0.05).
Estabilidad emulsificante de la harina desengrasada de semillas de zapote mamey (HDSZM) y aislado proteínico de zapote mamey (APZM) a diferentes pHs. Los promedios para el mismo pH con diferente letra mayúscula son significativamente diferentes (p < 0.05). Los promedios para la misma muestra (HDSZM o APZM) con diferente letra minúscula son significativamente diferentes (p < 0.05).
The highest MSPI EC achieved (45%) was similar to that reported for a L. usitatissimum protein isolate (49.89%) and higher than that reported for an A. occidentale protein isolate (12.4%) (Ogunwolu et al., Citation2009). The EC was also slightly lower than that reported for a canola protein isolate (51.6%) (Can-Karaca, Low, & Nickerson, Citation2011), and clearly lower than values for a Psidium guava seed protein isolate (60%) extracted at pH 10 (Bernardino et al., Citation2001). These differences between protein isolates of different origins are due to variations in the proteins’ ability to act as emulsifiers and physicochemical factors, which are affected by differences in protein characteristics such as molar mass, hydrophobicity, conformational stability, and charge (Moure et al., Citation2006).
In vitro digestibility
The digestibility of the MSPI (73.6 ± 0.1%) was higher than that of the MSDM (68.71 ± 0.2%). This difference is most likely caused by the isolation process because this process increases the protein concentration, denatures the proteins, and destroys protease inhibitors, thereby increasing protein availability (Bernardino et al., Citation2001). The digestibility observed here was similar to that reported (Chavan et al., Citation2001) for Lathyrus maritimus protein isolates (78.6–79.2%) produced with sodium hydroxide and sodium hexametaphosphate, for which the digestibility was determined using a sequential pepsin-pancreatin system. The relatively high digestibility obtained suggests that MSPI is a promising food ingredient.
Conclusions
The protein content (240.6 g/kg) and distribution of the protein fractions of the MSDM, which was rich in glutelins and prolamins, indicates that it has potential use as a food protein source. Therefore, a protein isolate (MSPI) was prepared from the MSDM by an isoelectric precipitation method that included steps at pH 9.8 for maximal solubilization and pH 3.5 for maximal precipitation of the proteins. The product had a high protein content (950.9 g/kg) and relatively high in vitro digestibility (73.6%). These characteristics of the MSPI and its functional properties (i.e., high oil absorption capacity and high foam capacity and foam stability, and a moderately water absorption and emulsifying capacities) demonstrate that the MSPI has potential as a food ingredient for use as an extender, fat substitute, foam promoter, or emulsifier in meat, pastry, and bakery products.
Acknowledgments
The authors are grateful to DGEST (Direccion General de Educación Superior Tecnológica) for the financial support of the project.
References
- AOAC. (1997). Official methods of analysis (15th ed.). Washington, DC: Association of Official Analytical Chemist.
- Azurdia, C. (2006). Fruits for the future 6: Tres Especies de Zapote en América Tropical (Pouteria spp) [In spanish]. Monograph, 216 p.
- Bayuelo, J. J. S., & Ochoa, I. (2006). Caracterización morfológica de sapote mamey [Pouteria sapota (Jacquin) H. E. Moore & Stearn] del centro occidente de Michoacán, México [In Spanish]. Revista Fitotecnia Mexicana, 29, 9–17.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
- Bernardino, N. A., Ortiz, M. A., Martínez, A. A. L., & Dávila, O. G. (2001). Guava seed protein isolate: Functional and nutritional characterization. Journal of Food Biochemistry, 25, 77–90.
- Bernardino, N. A., Scilingo, A. Añon, M., & Davila, O. G. (2006). Guava seed storage: Fractionation and characterization. Lebensmittel Wissenschaft und-Technologie, 39, 902–910.
- Can-Karaca, A., Low, N., & Nickerson, M. (2011). Emulsifying properties of canola and flaxseed protein isolates produced by isoelectric precipitation and salt extraction. Food Research International, 44, 2991–2998.
- Chavan, U. D., McKenzi, D. B., & Shahidi, F. (2001). Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chemistry, 74, 177–187.
- El-Safy, F. S., Salem, R. H., & El-Ghany, M. E. A. (2012). Chemical and nutritional evaluation of different seed flours as novel sources of protein. World Journal of Dairy & Food Sciences, 7, 59–65.
- Herman-Lara, E., Martínez-Sánchez, C. E., Amador-Mendoza, A., & Ruiz-López, I. I. (2010). Effect of airflow reversal on packed-bed drying of carrots. Journal of Food Process Engineering, 33, 684–700.
- Hsu, W. H., Vavak, D. L., Satterlee, L. D., & Miller, G. A. (1977). Multienzime technique for estimating protein digestibility. Journal of Food Science, 42, 1269–1279.
- Kabirulla, M., & Wills, R. B. H. (1982). Functional properties of aceylated and succinylated sunflower protein isolates. Journal Food Technology, 17, 235–249.
- Liadakis, G. N., Tzia, C., Oreopoulou, V., & Thompopoulos, C. D. (1995). Protein isolation from tomato seed meal, extraction optimization. Journal of Food Science, 60, 477–481.
- Lin, M. J. Y., Humbert, E. S., & Sosulski, F. W. (1974). Certain functional properties of sunflower meal products. Journal of Food Science, 39, 368.
- Moure, A., Sineiro, J., Domínguez, H., & Parajo, J. C. (2006). Functionality of oilseed protein products: A review. Food Research International, 39, 945–963.
- Ogunwolu, S. O., Henshaw, F. O., Mock, H. P., Santros, A., & Awonorin, S. O. (2009). Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chemistry, 115, 852–858.
- Osborne, T. B. (1908). Our present knowledge of plant proteins. Science, 28, 417–427.
- Pedroche, J., Yust, M. M., Lqari, H., Giron-Calle, J., Alaiz, M., Vioque, J., & Millan, F. (2004). Brassica carinata protein isolates: Chemical composition, protein characterization and improvement of functional properties by protein hydrolysis. Food Chemistry, 88, 337–346.
- Sing, A., & Matta, N. K. (2008). Variation in protein fractions and their correlation studies in rice. Indian Journal of Crop Science, 3, 83–86.
- Solís, F., J. A., & Durán, B. C. (2003). Characterization of eutectic mixtures in different natural fat blends by thermal analysis. European Journal of Lipid Science and Technology, 105, 742–748.
- Solís, F. J. A., Tapia, S., M., & Durán, B. M. C. (2001). Aceite de almendra de zapote mamey, un análisis de rendimientos y condiciones de extracción. Información Tecnológica, 12, 23–28.
- Téllez, P. P., Saucedo, V. C., Arévalo, G. M. L., & Valle, G. S. (2009). Maduración de frutos de mamey (Pouteria sapota Jacq.) tratados con 1-methylcyclopropene and refrigerated storage. CyTA-Journal of Food, 7(1), 45–51.
- Vioque, J., Alaiz, M., & Girón-Calle, J. (2012). Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chemistry, 132, 67–72.
- Vioque, J., Sánchez-Vioque, R., Pedroche, J., Yust, M. M., & Millán, F. (2001). Obtención y aplicaciones de concentrados y aislados proteicos [In spanish]. Grasas y aceites, 52, 127–13.
- Wang, J. C., & Kinsella, J. E. (1976). Functional properties of novel protein alfalfa leaf protein. Journal of Food Science, 41, 286–292.