Abstract
Coconut skim milk (CSM) is a major by-product of the virgin coconut oil industry. It is rich in protein, carbohydrate and minerals but is currently not utilized and is being let out to the environment as waste. The objective of the present work is value addition to this by-product. Different dehydration methods such as drum drying, spray drying and freeze drying were employed for drying CSM. The proximate analysis of dehydrated CSM using different drying methods indicated high protein and sugar content. The CSM powder obtained by these methods was evaluated for the functional properties and sensory attributes. The freeze-dried CSM powder was observed to have superior functional properties, while spray-dried CSM powder had more desirable sensory attributes. Results indicate that spray drying is technically the most feasible method for drying of CSM compared to the other methods based on product overall quality aspects.
La leche de coco descremada es uno de los principales subproductos de la industria del aceite de coco virgen. Es rica en proteínas, carbohidratos y minerales a pesar de lo cual actualmente no se utiliza y es descargada al medio ambiente como desperdicio. El presente artículo tiene como objetivo agregar valor a este subproducto. Se utilizaron distintos métodos para deshidratar la leche de coco descremada (LCD): deshidratación en tambor, deshidratación por aspersión y liofilización. El análisis químico de la LCD deshidratada mediante distintos métodos indicó un alto contenido de proteína y de azúcar. Se realizó una evaluación de las propiedades funcionales y de los atributos sensoriales de la LCD en polvo obtenida por dichos métodos, constatándose que aquella que se obtuvo por liofilización contaba con propiedades funcionales superiores, mientras que la que se obtuvo por aspersión tenía atributos sensoriales más deseables. Tomando en cuenta los aspectos generales de calidad del producto, los resultados obtenidos indican que, en comparación con los otros métodos, la liofilización es el método técnicamente más viable para la deshidratación de la LCD.
1. Introduction
Upward trending world population and increasing costs for traditional food proteins provide many incentives for utilization of oilseed proteins directly in human diet (Cater et al., Citation1977). Coconut and other oilseed plants are considered to be possible sources of dietary proteins. Although fresh coconut meat contains only 4% protein by weight, it is a potentially important source of protein because of the high production of coconut throughout the world (around 60 million tons/year) (http://faostat.fao.org). Coconut could be a valuable source of food grade protein if a suitable method of extraction could be employed for the separation of oil, the major component. Coconut oil is traditionally produced from dried coconut (copra) by expelling. It is possible to obtain oil and protein from fresh coconut without subjecting it to long periods of drying or high temperature. The oil obtained by this process is known as virgin coconut oil (VCO) and it has been gaining popularity in recent times (Marina, Che Man, & Amin, Citation2009a). A process was developed for the production of VCO by wet processing using fresh coconut, without employing any thermal treatment (Marina, Che Man, Nazimah, & Amin, Citation2009b; Raghavendra & Raghavarao, Citation2011).During wet processing, coconut residue (left after expelling of coconut milk), coconut skim milk (CSM) and insoluble protein are the major by-products. Spent coconut residue finds application as dietary fibre due to its high water-holding and swelling capacities compared to any other dietary fibres (Raghavendra et al., Citation2006). CSM and insoluble protein have been utilized to obtain a value-added product, namely coconut protein powder (Naik, Raghavendra, & Raghavarao, Citation2012). Many methods are reported for the separation and concentration of coconut proteins from CSM, such as heat coagulation, isoelectric precipitation, salt precipitation, centrifugation, ultrafiltration (Kwon, Bae, Park, & Rhee, Citation1996) and drying (Hagenmaier, Mattil, & Carter, Citation1974). However, the aqueous phase (CSM) is let out to the environment. It would be of great benefit to convert it into a potential value-added food ingredient.
To integrate protein into food systems, it is essential to look for its functional properties such as solubility, emulsifying and foaming properties. The method of dehydration and processing conditions will have significant effect on the functional properties. Any change in a process or ingredient, that affects protein solubility, also may alter the emulsification and foaming capacities. Freezing, heating, shearing and other processes can influence the functional properties of proteins in food systems (Nielsen, Citation2010). The objective of the present work is to produce and characterize dehydrated CSM powder using different drying methods such as drum drying, spray drying and freeze drying, and compare the functional properties and sensory attributes of powders obtained using the same. This study is relevant to convert CSM into a value-added product in the best possible way, which otherwise is discarded as waste at present.
2. Materials and methods
2.1. Materials
Fresh and mature coconuts (10–12 months) were purchased from the local market. The chemicals like petroleum ether, diethyl ether, ethanol, phenol, sulphuric acid (H2SO4), hydrochloric acid (HCl) and ammonia used were of analytical grade procured from Merck chemicals, Mumbai, Maharashtra, India. Sodium dodecyl sulphate (SDS) of extra pure grade was procured from HiMedia laboratories, Mumbai, India.
2.2. Preparation of CSM
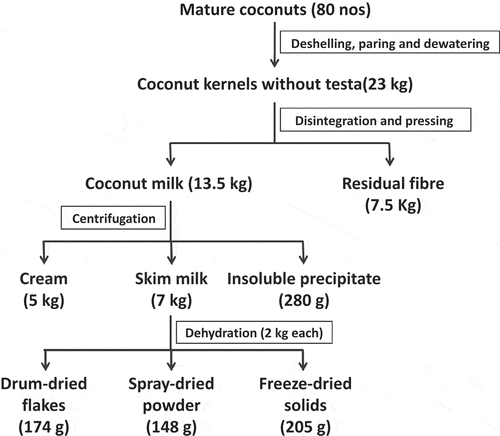
Fresh, mature and pared coconuts (80 numbers) were subjected to disintegration using a rotary wedge cutter (Krauss maffei, Germany) and milk was expelled using screw press. The coconut milk (13.5 kg) was centrifuged to obtain cream, aqueous phase (CSM) and protein precipitate. CSM (7 kg) thus obtained was subjected to different dehydration methods such as spray drying, drum drying and freeze drying. The process flow chart for the production of CSM powder by different drying methods is presented .
2.3. Dehydration methods
2.3.1. Drum drying
CSM (2 L) at ambient temperature (25°C ± 1°C) was fed manually to the heated rolling drums of double drum dryer (Type: MASC 231, P.I.V Stufenlos, Homburg) (heated internally by steam) in small amounts. The drum surface temperature was maintained at 110°C by monitoring the steam pressure. The flakes obtained after drying were collected, ground into powder and stored in an airtight container at 4°C.
2.3.2. Spray drying
CSM (2 L) at ambient temperature (25°C ± 1°C) was fed to the spray dryer (Model: BE1216, Bowen, USA), by a peristaltic pump at a flow rate of 30 mL/min. Nozzle-type atomizer (2 mm diameter) was employed at 3 bar air pressure in a co-current mode air flow system. The inlet air temperature was set at 150°C ± 2°C and the outlet air temperature was about 110°C ± 2°C. The powder was collected, through a cyclone, in the collection chamber. The dried product was stored at 4°C.
2.3.3. Freeze drying
CSM (2 L) at ambient temperature (25°C ± 1°C) was distributed evenly in the trays (4 nos., 60 cm × 29 cm) of the freeze drier (model LT5S, Lyophilisation Systems Inc., USA). During the freeze drying process, the product was first frozen by lowering the temperature to –30°C. The coolants used were R404A (44% w/w pentafluoroethane, 52% w/w 1,1,1-trifluoroethane and 4% w/w 1,1,1,2-tetrafluoroethane) and R508B (54% w/w hexafluoroethane and 46% w/w trifluoromethane) at 150 psi and 200 psi, respectively. The pressure was lowered to 3.3 × 10−4 bar for primary drying and 3.3 × 10−5 bar for secondary drying. Heat was supplied and maintained at 25°C to help the ice sublimate into vapour. After 16 h of drying, the powder was collected from the trays and the powder was stored in an airtight container at 4°C.
The CSM powder obtained by these dehydration methods was analysed for their composition, functional properties such as solubility, emulsification and foaming properties and subjected to colour and sensory analysis.
2.4. Analytical methods
2.4.1. Moisture
The moisture of coconut milk, CSM and CSM powder samples was determined according to the AOAC (Citation2007) method. A quantity of 5–6 g sample was oven-dried at 100–105°C until constant weight. The difference in weight of the sample before and after drying was measured as moisture content and expressed as g/kg as follows:
2.4.2. Fat
Fat in coconut milk and CSM samples was determined by Mojonnier procedure of AOAC (Citation2007). Ten grams of coconut milk or CSM was weighed into Mojonnier fat extraction flask and 1.5 mL NH4OH was added and shaken vigorously. Three drops of phenolphthalein indicator were added. Ten millilitres of 95% ethanol was added and mixed. Twenty-five millilitres of petroleum ether was added and shaken vigorously and allowed to stand for 30 min for phase separation. The ether phase was decanted. The second extraction was done with 5 mL ethanol and 15 mL each of ethyl ether and petroleum ether. The ether phase was allowed to separate and decanted. Third extraction was carried out using 15 mL each of ethyl ether and petroleum ether. The ether phases were combined and evaporated. The residual fat was weighed and expressed as g/kg.
Soxhlet method (AOAC, Citation2007) was used to estimate the fat content in CSM powder samples with few modifications. Sample was weighed (~5 g) and transferred into cellulose extraction thimble. The thimble was placed in a Soxhlet extractor and extraction was carried out using hexane for 16 h (with heat rate of ~120 drops/min). The solvent was evaporated and the residual oil weight was recorded and expressed as g/kg.
2.4.3. Protein
Protein was estimated by Bradford method (Bradford, Citation1976) using bovine serum albumin (BSA) as standard. Bradford reagent was prepared by dissolving 100 mg coomassie brilliant blue G-250 in 50 mL 95% ethanol. Hundred millilitres 85% (w/v) phosphoric acid was added and volume was made to 1 L with distilled water. After thorough stirring, the solution was filtered through Whatman no. 1 paper and 2 mL of Bradford reagent was added to 1 mL test solution/standard BSA (10–100 µg/mL). The samples were incubated at room temperature (25 ± 2°C) for 15 min and absorbance was recorded at 595 nm using a spectrophotometer (Shimadzu UV 160A, Japan).
For CSM powders, micro-Kjeldahl method (AOAC, Citation2007) was used to determine the total nitrogen content with minor variations. A known quantity (~0.5 g) of sample was digested using concentrated H2SO4 (15 mL) along with digestion mixture (1 g) (consisting of potassium sulphate, selenium dioxide and copper sulphate) in a digestion flask until a clear solution was formed. The acid hydrolysate was neutralized with NaOH and steam distilled. The distillate was collected in 10 mL of 2% Boric acid (containing two drops of mixed indicator, methyl red and bromocresol green). The distillate was titrated against 0.01N HCl until colour changed to colourless and the titre value was recorded. A blank (all reagents and no sample) was digested and distilled to obtain the blank titre value. These titre values were used calculate nitrogen content using the equation
where 1.4007 is the nitrogen correction value. Protein content was calculated by multiplying 6.25 (nitrogen–protein conversion factor) with nitrogen content and expressed as g/kg.
2.4.4. Total sugars and carbohydrate
Total sugars in coconut milk and CSM were determined by Dubois method (Dubois, Gilles, Hamilton, Reber, & Smith, Citation1956) using D-glucose as standard. To 0.5 mL sample/standard solutions (10–100 µg/mL), 1.8 mL concentrated H2SO4 and 300 µl of 5% phenol solution was added and incubated for 15 min at room temperature (25 ± 2°C). Absorbance was recorded at 490 nm using spectrophotometer (Shimadzu UV spectrophotometer, model 160A).
For powder samples, total carbohydrate content was calculated by difference (i.e. balance left after subtracting moisture, ash, fat and protein) and expressed as g/kg.
2.4.5. Ash
Ash content in coconut milk, CSM and CSM powders was estimated by a procedure described by AOAC, Citation2007. Known amount of samples were placed in porcelain crucibles and charred on hot plate till fumes were no longer produced. The crucibles were then placed in furnace at 550°C overnight. The leftover ash was weighed after cooling. Ash content (expressed as g/kg) was calculated as the ratio of weight of ash and weight of sample.
2.4.6. Protein solubility
Protein solubility was determined by the method described by Zidani, Fahloul, and Bacha (Citation2012). CSM powder was dispersed in distilled water (2% w/v) for 1 h using a stirrer and centrifuged at 2500g for 10 min at room temperature (25°C ± 1°C). Protein, insoluble under these conditions, separates out as a pellet while soluble protein remains in the supernatant. Protein solubility was measured as the ratio of the protein in the supernatant (soluble protein) to the total protein and expressed as percentage.
2.4.7. Functional properties
2.4.7.1.1. Emulsifying properties.
Emulsifying activity index (EAI) was determined according to the method of Pearce and Kinsella (Citation1978). The emulsion was prepared by taking 40 mL of 0.1% (w/v) protein solution in 0.1M phosphate buffer (pH 7) and 10 mL of oil and homogenizing (Ika labotechnik, model T25 basic) at 10,000 rpm for 1 min. Aliquots of emulsion of 100 µl were pipetted out immediately after homogenization (0 min) and at 10 min and diluted with 10 mL of 0.1% SDS. Absorbance of the diluted emulsion was measured at 500 nm against 0.1% SDS as blank in a spectrophotometer (Unico, SQ 4802). Emulsifying activity was expressed as the EAI and calculated as
where ‘C’ is the weight of protein per unit volume of the aqueous phase before emulsion formation, A500 is the absorbance at 500 nm and ‘V’ is the oil volume fraction of the emulsion.
Emulsion stability index (ESI) was calculated as
where A0 is the absorbance at time 0 min and ΔA is the difference in absorbance over the time interval (10 min).
2.4.7.2.1. Foaming capacity.
Foaming capacity of the samples was determined according to the method of Coffmann and Garcia (Citation1977). Eight grams of sample was added to 100 mL distilled water, and the pH of the solution was adjusted to pH 7 with dilute NaOH (0.1N). Vigorous whipping in a blender was carried out for 1 min and then the solution was poured into a 250 mL measuring cylinder. Volumes were recorded before and after whipping, and the percentage volume increase indicated the foaming capacity. The later was calculated according to the following equation.
2.5. Colour
Commission Internationale de L’Eclairage (CIE) L*, a* and b* values of CSM powder obtained after drying using different methods were measured using a colorimeter (Konica Minolta CM-5, Japan). The values were measured using illuminant D65 and 10° observer angle. The instrument was calibrated using a standard white reflector plate. Hue angle [tan − 1(b*/a*)] and chroma (a*2 + b*2)1/2 were also determined.
2.6. Sensory analysis
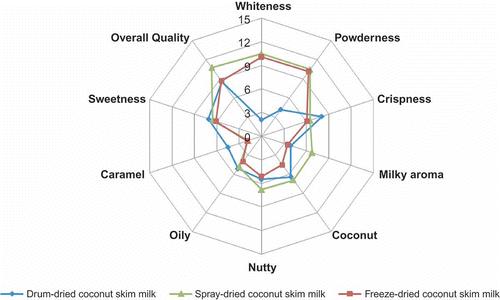
Sensory analysis for dehydrated CSM obtained by different methods was carried out as follows. A group of 12 panellists aged 25–50 years were trained for quantitative descriptive analysis (QDA). The members of the panel were drawn from scientific staff familiar with sensory analysis techniques and who had earlier experience in sensory evaluation of food products. The samples were evaluated in a sensory booth room maintained at a temperature of 22 ± 2°C under fluorescent lighting equivalent to daylight. Descriptors typical to the product were generated in the initial evaluations using free choice profiling. Sensory attributes such as colour (whiteness), texture, aroma (milky, coconut, nutty, oily), sweetness, caramel flavour and overall quality were evaluated by the panellists. The samples were served in petri dishes with three digit coded numbers to avoid bias. QDA method of intensity scaling was used (Stone & Sidel, Citation1998). The score card consisted of a 15 cm scale, where 1.25 cm was anchored as “low’ and 13.75 cm as ‘high’. The panel was asked to mark the intensity of the attribute by drawing a vertical line on the scale and writing the code. The mean scores of individual attributes were calculated and profile was drawn. Significant difference among samples was tested using Ducan’s multiple range test (Duncan, Citation1955). Significance was tested at a probability level of p ≤ 0.05.
2.7. Statistical analysis
All the physico-chemical analysis and functional property measurements were carried out in triplicate. Results are expressed as mean ± standard deviations. Data was analysed using the analysis of variance (ANOVA) using statistical package for social science (SPSS) 16.0. The differences between mean values were compared using Tukey’s test with level of significance of p ≤ 0.05.
3. Results and discussion
3.1. Composition of coconut milk and CSM
The term ‘coconut milk’ is generically applied to the white, opaque protein–oil–water emulsion obtained from grated or comminuted solid coconut endosperm by expelling (Seow & Gwee, Citation1997). The term ‘CSM’ denotes the aqueous liquid phase that remains after separation of virgin oil from coconut milk (APCC Standards Task Force, 1994). Coconut milk is a natural oil-in-water-type stable emulsion and extra energy (in the form of thermal, centrifugal, pH, chilling and thawing treatments) is required to destabilize this emulsion. During the centrifugation process, phase separation occurs due to the difference in densities and the cream and aqueous phases are collected separately. The composition, in terms of moisture, protein, carbohydrate, fat and ash content of coconut milk and CSM is presented in . The major difference between these two lies in the moisture and fat contents, while the protein and carbohydrate contents remain more or less the same.
Table 1. Composition of coconut milk and coconut skim milk (wet basis).
Tabla 1. Composición de leche de coco y de leche de coco descremada (base húmeda).
3.2. Dehydration of CSM
Drum drying is a common method used for drying of liquids such as milk, breakfast cereals, baby food, instant mashed potatoes, etc. When the CSM is fed to the drum drier, it forms a thin film on the surface of hot drums and during the course of revolution, the material is dried due to heat transfer from steam through metal wall of the drums. As it reaches the other end, the material adhered to the drums is scrapped by a knife. Drum-dried CSM (porous flakes), as shown in , was observed to be light brown in colour. It was observed to have a cooked flavour and caramelization of sugars occurred, which is often the case in drum-dried products due to greater heat exposure.
Figure 2. Pictures of coconut skim milk powder obtained by different methods: A – drum drying, B – spray drying, C – freeze drying.
Figura 2. Fotografías de leche de coco descremada en polvo obtenido por distintos métodos: A – Deshidratación en tambor, B – Deshidratación por aspersión, C – Liofilización.

Spray drying is presently one of the most widely used dehydration methods in food and pharmaceutical industry. This method enables the transformation of feed from a fluid state into dried particulate form by spraying the feed into a hot drying medium (air). It has several advantages like continuous operability, adaptability to full automation and can be designed to virtually any capacity (Gharsallaoui, Roudaut, Chambin, Voilley, & Saurel, Citation2007). The product obtained after spray drying of CSM was found to be a free flowing off-white powder (). Short time of heat exposure, high rate of evaporation and drying taking place at wet bulb temperature are responsible for the production of a high quality product.
Freeze drying is a method, which enables liquid or slurry to be dried under vacuum. Freeze drying is generally known to yield products with near-original colour, negligible loss of nutrients, and excellent rehydration property due to the porous structure of the product (Jiang, Zhang, & Mujumdar, Citation2010). The product obtained after freeze drying was flaky (), but formed lumps due to absorption of atmospheric moisture.
Dehydrated CSM powders obtained by different methods were visually distinctly different from each other. From , it can be observed that 174 g, 148 g and 205 g of CSM powder was obtained by drum, spray and freeze drying of 2 kg CSM, respectively. The highest product yield was observed by freeze drying (68.46 ± 0.09%), followed by drum drying (58.30 ± 0.07%) and the least yield obtained was by spray drying (49.77 ± 0.03%). The low product yield by spray drying is a result of loss due to the adherence of particles to the walls of the spray drier.
3.3. Proximate analysis
Proximate analysis of CSM powders dehydrated by different methods is presented in . Moisture content was found to be significantly different among the samples, lowest being in spray-dried CSM powder. The protein content was about 177 g/kg in spray-dried as well as freeze-dried samples, but low (~159 g/kg) in drum-dried CSM powder. The oil content was observed to be higher in freeze-dried samples compared to drum-dried and spray-dried CSM. Ash and carbohydrate contents are found to be similar in all the CSM powders obtained by the different drying methods.
Table 2. Proximate analysis of coconut skim milk powders dehydrated by different methods.
Tabla 2. Análisis químico de leche de coco descremada en polvo deshidratada por distintos métodos.
3.4. Functional properties
Protein functionality has been defined as the physical and chemical properties of protein molecules that affect their behaviour in food products during processing, storage and consumption. The functional properties of proteins contribute to the quality attributes, organoleptic properties and processing yields of food. It is often desirable to characterize the functional properties of food proteins to optimize their use in a food product. Three of the most important protein functional properties in foods include protein solubility, emulsification and foaming.
3.4.1. Protein solubility
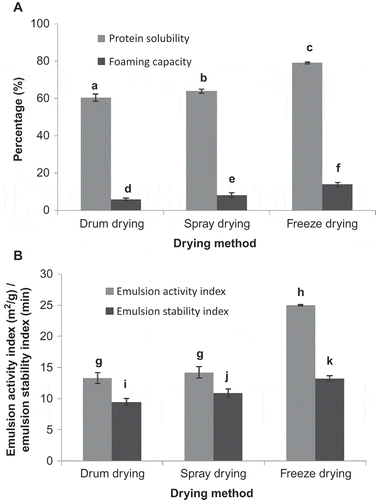
Proteins usually need to be soluble under the conditions of use for optimal functionality in food systems. CSM powders showed significantly different (p ≤ 0.05) protein solubilities when dried by different methods. The CSM powder obtained by the freeze drying method showed the highest solubility (about 80%) when compared to spray-dried product (about 65%) and drum-dried product (about 62%) (). The lower solubility could be attributed to the partial denaturation of the protein during drum and spray drying.
Figure 3. Functional properties (protein solubility, foaming capacity, emulsion activity index and emulsion stability index) of dehydrated coconut skim milk obtained by different dehydration methods.Values are averages ± standard deviation from three replicate analyses. a–c, d–f, g–h, i–k Values followed by same superscripted letters are not significantly different (p ≤ 0.05) for protein solubility, foaming capacity, emulsion activity index and emulsion stability index, respectively.
Figura 3. Propiedades funcionales (solubilidad de proteína, propiedades de espumado, índice de actividad de emulsiones e índice de estabilidad de emulsiones) de leche de coco descremada y deshidratada, obtenida por distintos métodos de deshidratación.Los valores son medias ± desviación estandard de tres replicas”. a–c, d–f, g–h, i–kValores seguidos por la misma letra superíndice no son significativamente diferentes (p ≤ 0,05) para proteina soluble, capacidad antiespumante, índice de actividad emulsionante, índice de estabilidad emulsionante, respectivamente.
3.4.2. Emulsification
Emulsifying properties can be of value when incorporating proteins in mixed systems (water and oil). The emulsion activity index (EAI) for freeze-dried product highest (25.1 m2/g) where as the EAI for spray-dried and drum-dried product were lower and not significantly different (14.86 m2/g and 13.94 m2/g, respectively). EAI indicates the area of interface between aqueous and oil phases stabilized per unit weight of sample. More the denaturation, less is the solubility of protein, accordingly protein migration to the interface reduces making the emulsions inherently unstable. Mc Watters and Holmes (Citation1979) have also shown that solubility and emulsification properties of soy and peanut flours were adversely affected by moist heat treatment. Emulsion stability index was the highest for freeze-dried sample (13.55 min) followed by spray-dried powder (11.36 min) and least for drum-dried powder (9.83 min) (). It indicates that freeze-dried powder can produce more stable emulsions compared to spray-dried and drum-dried samples.
3.4.3. Foaming capacity
Foams are coarse dispersions of gas bubbles in a liquid or semi solid continuous phase. Proteins being in the continuous phase lower the surface tension between the two phases during foam formation and impart stability to films formed around the gas bubbles. CSM dehydrated by different methods exhibited significant difference (p ≤ 0.05) in their foaming capacities. Foaming capacity of the freeze-dried powder was highest (14.75%) and is the indication for the high concentration of quality of protein. Foaming capacity of the drum-dried product and spray-dried CSM powder were 6.6% and 9.26%, respectively (). The negative influence of heat treatment on foaming capacity was observed in cereal foods by Ibanoglu and Ibanoglu (Citation1997).
The results of functional properties of CSM powders obtained by different dehydration methods are presented in and . The freeze-dried CSM powder exhibited best functional properties compared to spray-dried CSM powder followed by drum-dried CSM powder. Similar effects of different methods of drying on the functional properties of enzyme-treated groundnut flour are also reported by Bhagya and Srinivasan (Citation1989). This is due to the fact that protein in freeze-dried products does not undergo thermal denaturation and hence it is highly reconstitutable. Although freeze-dried CSM powder exhibited best functional properties, the process has certain drawbacks such as relatively long processing time and high capital cost besides the product obtained being hygroscopic in nature. Spray-dried sample had very good product characteristics like free flowing nature and appealing colour with good functional properties.
3.5. Colour analysis
The colour analysis of CSM powders dehydrated by different methods is presented in . High L* value indicates lightness, which is observed in the case of freeze-dried and spray-dried CSM powders. Similarly, a* values are very low for freeze-dried and spray-dried CSM powders. Positive a* indicates redness, which is evident in the drum-dried CSM powder. The positive b* values indicate yellowness, which is found to be low in freeze-dried and spray-dried samples. Similar observations were recorded by Caparino et al. (Citation2012) when mango powder was produced using different drying methods. These results strongly substantiate the perception of colour in sensory evaluation. Hue angle describes the colour perception, which is significantly different (p ≤ 0.05) for all the CSM powders, while chroma, which indicates saturation of colour, is similar for freeze-dried and spray-dried CSM powders (p ≤ 0.05).
Table 3. Colour analysis of coconut skim milk powders dehydrated by different methods.
Tabla 3. Análisis de color de leche de coco descremada y deshidratada por distintos métodos.
3.6. Sensory analysis
Dehydrated CSM powder prepared using spray drying method had characteristic milky, coconut and nutty aroma. Samples prepared by drum drying and freeze drying methods had a low score for these typical and specific aroma notes of the product. Drum-dried CSM powder was less white in colour (p ≤ 0.05) and had a flaky appearance, while it had a strong caramel aroma and crisp texture. There was no significant difference for sweetness among the samples (p ≤ 0.05). Spray-dried CSM powder had more desirable attributes and, therefore, scored highest (10.8 out of 15) for overall quality, as seen in .
4. Conclusion
CSM, which is a by-product of the VCO industry, could be successfully dried using various dehydration methods such as drum drying, spray drying and freeze drying without addition of any additives. Freeze-dried CSM powder was found to have the best functional properties compared to the powder obtained by other dehydration methods. Spray drying yielded CSM powder with good quality in terms of product characteristics and moderately good functional properties. Hence, spray drying was considered to be the most feasible method for the drying of CSM and results indicate that spray-dried CSM powder can be used as a natural food additive and emulsifier.
Acknowledgements
The authors wish to thank the Director, CFTRI, for the infrastructural facilities at the institute. Aduja Naik acknowledges UGC, Government of India, for the UGC-SRF fellowship.
References
- AOAC (2007). Official method of analysis (17th ed.). Washington, DC: Association of Official Analytical Chemists.
- APCC (1994). International codes and standard for aqueous coconut products, 2nd draft. Jakarta: Standards Task Force, Asian and Pacific Coconut Community.
- Bhagya, S., & Srinivasan, I. (1989). Effect of different method of drying on the functional properties of enzyme treated groundnut flour. Lebensmittel-Wissenschaft and Technologie, 22, 329–333.
- Bradford, M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003–2697(76)90527–3
- Caparino, O. A., Tang, J., Nindo, C. I., Sablani, S. S., Powers, J. R., & Fellman, J. K. (2012). Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. Journal of Food Engineering, 111, 135–148. doi:10.1016/j.jfoodeng.2012.01.010
- Cater, C. M., Mattil, K. F., Meinke, W. W., Taranto, M. V., Lawhon, J. T., & Alford, B. B. (1977). Cottonseed protein food products. Journal of the American Oil Chemists’ Society, 54, A90–A93. doi:10.1007/BF02912380
- Coffmann, C. V., & Garcia, V. V. (1977). Functional properties and amino acid content of a protein isolate from Mung bean flour. Journal of Food Technology, 12, 473–484.
- Dubois, M., Gilles, K. A., Hamilton, J. K., Reber, P. A., & Smith, F. (1956). Calorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356. doi:10.1021/ac60111a017
- Duncan, D. B. (1955). Multiple range test and multiple F test. Biometrics, 11, 1–42.
- Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Research International, 40, 1107–1121. doi:10.1016/j.foodres.2007.07.004
- Hagenmaier, R. N., Mattil, K. F., & Carter, C. M. (1974). Dehydrated coconut skimmed milk as a food product: Composition and functionality. Journal of Food Science, 39, 196–199. doi:10.1111/j.1365-2621.1974.tb01021.x
- Ibanoglu, E., & Ibanoglu, S. (1997). The effect of heat treatment on the foaming properties of tarhana, a tradition Turkish cereal food. Food Research International, 30, 799–802. doi:10.1016/s0963-9969(98)00048-9
- Jiang, H., Zhang, M., & Mujumdar, A. S. (2010). Microwave freeze-drying characteristics of banana crisps. Drying Technology, 28, 1377–1384. doi:10.1080/07373937.2010.482702
- Kwon, K. S., Bae, D., Park, K. H., & Rhee, K. C. (1996). Aqueous extraction and membrane techniques improve coconut protein concentrate functionality. Journal of Food Science, 61, 753–756. doi:10.1111/j.1365-2621. 1996.tb12197.x
- Marina, A. M., Che Man, Y. B., & Amin, I. (2009a). Virgin coconut oil: Emerging functional food oil. Trends in Food Science and Technology, 20, 481–487. doi:10.1016/j.tifs.2009.06.003
- Marina, A. M., Che Man, Y. B., Nazimah, S. A. H., & Amin, I. (2009b). Chemical properties of Virgin Coconut Oil. Journal of the American Oil Chemists’ Society, 86, 301–307. doi:10.1007/s11746-009-1351-1
- Mc Watters, K. H., & Holmes, M. R. (1979). Influence of moist heat on solubility and emulsification properties of soy and peanut flours. Journal of Food Science, 44, 774–776. doi:10.11111/j.1365-2621.1979.tb08498.x
- Naik, A., Raghavendra, S. N., & Raghavarao, K. S. M. S. (2012). Production of coconut protein powder from coconut wet processing waste and its characterization. Applied Biochemistry and Biotechnology, 167, 1290–1302. doi:10.1007/s12010-012-9632-9
- Nielsen, S. S. (2010). Protein separation and characterization procedures: Food analysis. New York, USA: Springer.
- Pearce, K. N., & Kinsella, J. E. (1978). Emulsifying properties of proteins: Evaluation of a turbidimetric technique. Journal of Agricultural and Food Chemistry, 26, 716–723.
- Raghavendra, S. N., & Raghavarao, K. S. M. S. (2011). Aqueous extraction and enzymatic destabilization of coconut milk emulsions. Journal of the American Oil Chemists’ Society, 88, 481–487. doi:10.1007/s11746-010-1695-6
- Raghavendra, S. N., Ramachandra Swamy, S. R., Rastogi, N. K., Raghavarao, K. S. M. S., Kumar, S., & Tharanathan, R. N. (2006). Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. Journal of Food Engineering, 72, 281–286. doi:10.1016/j.jfoodeng.2004.12.008
- Seow, C., & Gwee, C. (1997). Coconut milk: Chemistry and technology. International Journal of Food Science and Technology, 32, 189–201. doi:10.1046/j.1365-2621.1997.00400.x
- Stone, H., & Sidel, J. (1998). Quantitative descriptive analysis: Developments, applications, and the future. Food Technology, 52, 48–52.
- Zidani, S., Fahloul, D., & Bacha, A. (2012). Effects of pH, NaCl, ethanol, and drying methods on the solubility of Saccharomyces cerevisiae proteins. Cyta – Journal of Food, 10, 42–47. doi:10.1080/19476337.2010.543472