Abstract
The objective of this study was to create an optimized culture medium that would maximize the productivity of both γ-aminobutyric acid (GABA) and nattokinase in bacteria. First, Bacillus subtilis was screened from rice straw. Different media compositions were then optimized for GABA and nattokinase production using the Taguchi experimental design method, and the effects of different nitrogen and carbon sources were investigated. Of the various key ingredients tested in the media, fructose and yeast extract were found to have the most significant effect on GABA and nattokinase production. The optimal pH value and temperature for GABA production were found to be 7.0 and 37°C, respectively, after 72 h. The maximum GABA yield from B. subtilis under flask culture was 15.40 mg/mL. For nattokinase production, the optimal pH and temperature were found to be 8.0 and 30°C, respectively, after 48 h, which yielded approximately 130.96 units/mL of nattokinase.
El objetivo del presente estudio se orientó a crear un medio de cultivo optimizado que maximizara la productividad de GABA [ácido aminobutírico gama] y de nattokinasa en bacterias. El primer paso fue cerner Bacillus subtilis de paja de arroz. Luego, se optimizaron distintas composiciones de medios para la producción de GABA y de nattokinasa, para lo que se usó el método de diseño experimental Taguchi, investigándose los efectos de distintas fuentes de nitrógeno y de carbono. En lo que respecta a los diferentes ingredientes clave probados en dichos medios, se encontró que los extractos de fructosa y de levadura tuvieron el efecto más significativo en la producción de GABA y de nattokinasa. Asimismo, después de 72 horas, se observó que el valor de pH y la temperatura óptimos para la producción de GABA fueron 7,0 y 37° C, respectivamente, constatándose que el máximo rendimiento de GABA de B. subtilis en cultivo de matraz fue de 15,40 mg/mL. En cuanto a la producción de nattokinasa, después de 48 horas se encontró que el valor de pH y la temperatura óptimos fueron 8,0 y 30° C, respectivamente, mostrando un rendimiento aproximado de 130,96 unidades/mL de nattokinasa.
Introduction
In recent years, γ-aminobutyric acid (GABA) has drawn more attention from researchers because of its important physiological functions in the mammalian central nervous system, induction of hypotensive, diuretic effects, and tranquilizer effects. Due to its physiological functions, development of functional food containing GABA at a high concentration has been actively pursued.
Microbial fibrinolytic enzymes have dramatically attracted medical interest in the past decades. Nattokinase is a potent fibrinolytic enzyme that is considered to be a promising agent for thrombosis therapy. The enzyme can be found in various sources, including Japanese natto, Korean chungkook-jang soy sauce, and Korean doen-jang. It is also present in various microorganisms, the most important being of the genus Bacillus.
Therefore, many researchers had focused on the isolation and screening of microorganisms for enzyme production with high fibrinolytic activity, purifying and characterizing newly found enzyme. In contrast, there were few reports concerning culture medium optimization. In biotechnology-based industrial processes, the formulation of culture media is of critical importance; its composition affects the product concentration, yield, and volumetric productivity. Cost reduction is another important aspect of medium optimization. The complexities and uncertainties associated with large-scale fermentation usually arise from a lack of knowledge regarding the sophisticated interactions among the different variables (Montgomery, Citation1991). Conventional practice, by single variable optimization (while others are kept constant), does not elucidate the combined effects of all the variables involved (Haaland, Citation1989). The limitations of single variable optimization can be eliminated by optimizing all the affecting parameters collectively using an experimental method with a Taguchi methodology. This is designed using statistical methods to yield the most information in the minimum number of experiments. It has been successfully applied to the optimization of medium composition (Dean & Voss, Citation1999), conditions of enzymatic hydrolysis (Myers & Montgomery, Citation1995), parameters of food preservation (Almeidae, Lima, & Taqueda, Citation2003), and fermentation processes (Sato, Yamada, & Ohtani, Citation2001).
The aim of this study was to optimize the fermentation conditions to maximize GABA and nattokinase activity. Stepwise optimization was performed as follows: (1) selection of the optimal carbon and nitrogen source via the one-variable-at-a-time method; (2) searching the optimal conditions of the significant variables using the Taguchi experimental design; and (3) a confirmation experiment to verify the optimal conditions.
Materials and methods
Isolation and identification of Bacillus subtilis
Over 100 different bacterial strains were isolated. A variety of samples were collected and freezed until being processed. In the laboratory, the samples were homogenized, and the microbes were isolated using the pour plate and streaking methods. Isolated strains were identified based on their morphological and biochemical characteristics, according to Bergey’s Manual of Determinative Bacteriology (Buchanan & Gibbons, Citation1974).
Cultivation
Flask cultures were carried out in a 250 mL flask with a working volume of 50 mL to investigate the effects that the type and initial concentration of the selected complex media would have on cell growth, as well as the production of GABA and nattokinase. The cells were grown aerobically at 37°C in a shaking incubator.
Batch cultures were then carried out in a 5 L fermenter (Major Science, USA). The strain culture was prepared at 37°C for 18 h in a 250 mL flask, which contained a 50 mL working volume of optimized media without agar. Three hundred milliliters of strain culture was used to inoculate the fermenter for GABA production, while 450 mL was used for nattokinase production. The initial volume of the culture was 3 L. The temperature and pH were maintained at 37°C and 7 for GABA and at 30°C and pH 8 for nattokinase, respectively. In this study, 5 N HCl and 5 N NaOH were used. The dissolved oxygen (DO) level was uncontrolled.
Optimization of medium compositions for GABA and nattokinase production
Bacillus subtilis had been previously isolated. The strain was cultured at 37°C in a starter culture medium containing 2% (w/v) peptone, 0.25% (w/v) glucose, 0.5% (w/v) NaCl, and 0.25% (w/v) K2HPO4. In this study, the medium was optimized using 9 types of nitrogen sources and 11 types of carbon sources. The nitrogen sources tested were soy peptone, soy protein isolate, peptone from meat, potassium nitrate, sodium nitrate, wheat bran, yeast extract, white egg protein, and monosodium glutamate (MSG). The carbon sources were glucose, sucrose, fructose, maltose, lactose, potato starch, soluble starch, mannitol, xylose, malt extract, and glycerol. The culture medium was placed in a 250 mL Erlenmeyer flask and was aerobically cultured at 37°C for 24 h in a shaking incubator.
Colorimetric determination of GABA levels
In this study, we slightly modified the colorimetric determination method described by Kitaoka and Nakano (Citation1969). First, 0.1 mL of a sample solution was placed in a test tube. Then, 0.2 mL of borate buffer and 1 mL of a phenol reagent were added to the solution, in that order. After mixing the resultant liquid thoroughly and cooling it in ice water, 0.4 mL of a sodium hypochlorite reagent was added. The tube was shaken vigorously and placed in a boiling water bath for 10 min. It was then cooled immediately by immersion in ice water for 5 min. When the volume of the reaction mixture was not enough to fill the photometer cell (approximately 1.7–1.9 mL), it was diluted to a suitable volume with 2 mL of 60% ethanol. The optical density was read at 630 nm.
Fibrinolytic activity screening using a fibrin plate
Based on the criteria of the culture, fibrin plates were used to screen for fibrinolytic activity, as described by the Japan Nattokinase Association (Citation2000). The fibrinogen solution was mixed with an agar solution at 50°C, and 20 mL of this mixture was poured into Petri dishes (15 cm in diameter). The plates were allowed to stand for 30 min at room temperature to form fibrin clots, and holes were made on the fibrin plates using a capillary glass tube (8 mm in diameter). Then, 50 µL of the sample solution was placed in each hole. After incubation at 37°C for 24 h, the diameters of the clear zones around the wells were measured.
Determination of the fibrinolytic nattokinase activity
The fibrinolytic activity was assayed according to the protocols established by the Japan Nattokinase Association (Citation2000). In brief, 1.4 mL of 0.05 mol/L borate buffer at pH 8.5 and 0.4 mL of 0.72% (w/v) fibrinogen solution were mixed and incubated at 37°C for 5 min. Then, 0.1 mL (20 U/mL) of thrombin was added and the mixture was incubated at 37°C for another 10 min. Finally, 0.1 mL of the sample was added, and this final mixture was incubated for an additional 60 min. The reaction was stopped by the addition of 0.2 M trichloroacetic acid. After centrifugation (at 16,000 g for 5 min), the absorbance of the supernatant at 275 nm was read and recorded. One unit of fibrinolytic (FU) activity is defined as the amount of enzyme required to produce an absorbance increase equal to 0.01 in 1 min at 275 nm.
Polymerase chain reaction (PCR) and sequencing
A DNA fragment was amplified by PCR using the paired primers 5′-cgcctgcagatggcgcaatctgttcct-3′ and 5′-cgcctgcagatggtgagaagcaaa-3′ for the forward direction and 5′-gcggaattcttactattattgtgcagctgcttg-3′ for the reverse, with respect to the N-terminal sequence of the B060 enzyme and the 3′-terminal sequence of the aprN gene encoding subtilisin NAT (Ju, Yan, Zhu, & Qi, Citation2004), respectively. The aprN gene consisted of 1473 nucleotides with an open reading frame of 1143 nt, from nt 181 to nt 1326.
The reaction mixture (40 µL) consisted of 2.5 µL of 10× PCR buffer, 2 µL of 25 µM d NTP, 1 µL of 1 U/mL Taq polymerase, 5 µL of genomic DNA, and 0.4 µL of each of the primers (10 µM). PCR runs were performed via 35 cycles of denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 2 min.
Nucleotide sequence determination
To confirm the fidelity of the B060 enzyme DNA sequence, two independent PCR products were sequenced in both directions. Sequence comparisons were made with the databases using BLAST through the NCBI server.
Experimental design
The Taguchi method, a well-known experimental design technique, was employed to study the effects of eight different parameters. The primary tool used in the Taguchi method is the orthogonal array (OA), a matrix of numbers arranged in columns and rows. The Taguchi method applies a generic signal-to-noise (S/N) ratio to quantify the variation under consideration. These S/N ratios are used as measurements of the effects that noise factors have on both the amount of variability in the response data and the closeness of the average response to the target.
In each experimental run, the response of the GABA and nattokinase productions was recorded, and a corresponding S/N ratio was calculated using Equation (1). The overall objective was to estimate the effects of various parameters on the production of GABA and the nattokinase enzyme, and a large S/N ratio is preferred.
Where Y is the response and n is the number of experimental runs. For each selected process parameter, the optimal conditions are those that give the largest S/N ratio.
In this study, we classified the factors into two groups: internal factors and external factors. Internal factors relate to major media compositions, such as the inoculation size, the concentration of the carbon source, and the concentration of the nitrogen source, as well as the concentration of either MSG for GABA production or CaCl2 for nattokinase production. External factors may also affect our productivity goals, including the initial pH, the rotation speed, the incubation temperature, and the incubation time.
and show the four internal factors and four external factors tested in this study. If three levels are assigned to each of these factors and a factorial experimental design was employed using each of these values, the number of permutations would be 34. The fractional factorial design reduced the number of experiments to nine. An L9 type OA was used. This design requires nine experiments, with four parameters having three levels each.
Table 1. Taguchi’s L9 (34) orthogonal array experimental design used to optimize the internal factors.
Tabla 1. Diseño experimental de matriz ortogonal L9 (34) de Taguchi usado para optimizar los factores internos.
Table 2. Taguchi’s L9 (34) orthogonal array experimental design used to optimize the external factors.
Tabla 2. Diseño experimental de matriz ortogonal L9 (34) de Taguchi usado para optimizar los factores externos.
Results and discussion
Screening and identification of the isolated B. subtilis strains
Many fermented foods can enhance the GABA and fibrinolytic activities of bacteria, including Korean chungkook-jang (Kim et al., Citation1996), Chinese douche (Wang et al., Citation2006), Japanese natto (Sumi, Hamada, Tsushima, Mihara, & Muroki, Citation1987), Japanese shiokara (Sumi, Nakajima, & Yatagai, Citation1995), Korea doen-jang (Kim & Choi, Citation2000), Asian fermented shrimp paste (Wong & Mine, Citation2004), and Indonesian tempeh (Kim et al., Citation2006). In this study, the B. subtilis strain with the greatest ability to produce GABA and nattokinase, strain B060, was screened and identified from rice straw.
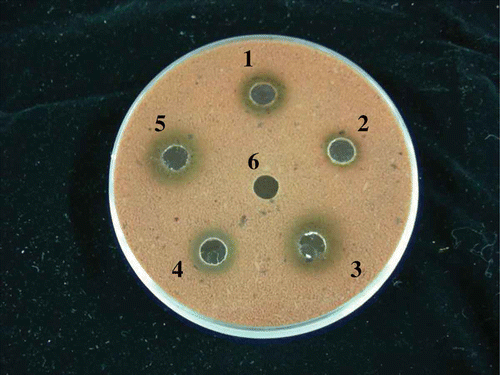
Among the screened B. subtilis strains, the microbes with a higher capacity for GABA and nattokinase production were grown in tubes containing tryptic soy broth (TSB). In this study, 112 strains were obtained in the initial screening, and their levels of GABA and nattokinase were analyzed using a spectrophotometer and a fibrin plate, respectively (). Strain B060 exhibited the greatest capacity for both GABA and nattokinase production. In a preliminary morphological observation, this strain was identified as rod-shaped Gram-positive bacteria. The colony morphology displayed aerial structures. Physiological and biochemical tests did not indicate any interference from KOH but showed catalase-positive results in the presence of H2O2. Strain B060 was further identified as a B. subtilis species based on its 16S rRNA sequence, which exhibited a 99% similarity with B. subtilis F3-7 (). Overall, the results found for this isolated strain were in agreement with the characteristics of B. subtilis.
Table 3. The 16S rRNA similarity between Bacillus subtilis strain B060 and 10 other strains in the Bacillus genus.
Tabla 3. La similitud 16S rRNA entre la cepa B060 de Bacillus subtilis y 10 cepas adicionales del género Bacillus.
Figure 1. The fibrinolytic activity of various bacterial strains, as determined on a fibrin plate. Each strain was isolated from a different source: (1) black soybeans, (2) lotus leaves, (3) commercial natto, (4) rice bran, (5) rice straw, and (6) TSB.
Figura 1. Actividad fibrinolítica de varias cepas de bacteria determinada en una plancha de fibrina. Cada cepa fue aislada de una fuente diferente: (1) frijol de soya negro, (2) hojas de loto, (3) natto comercial, (4) salvado de arroz, (5) paja de arroz, y (6) TSB.
Effects of the media composition and culture conditions on the GABA and nattokinase productivity
The effects of various carbon and nitrogen sources on the production of GABA and nattokinase were observed. Due to the screening of different nitrogen sources, soy peptone, soy protein isolate, peptone from meat, potassium nitrate, sodium nitrate, wheat bran, yeast extract, white egg protein, and MSG were used as indicated in the reference media. The complex media was inoculated and cultivated in the fermentation conditions. The GABA and nattokinase activity for each nitrogen source is shown in Of all the nitrogen sources investigated, yeast extract was the most promising, and the corresponding GABA and nattokinase activity was 3.331 g/L and 28.987 FU/mL, respectively. Wang et al. (Citation2009) reported that when inorganic nitrogen sources were used, very poor enzyme activities were achieved much higher activities were obtained with organic nitrogen sources. While, Allagheny, Obanu, and Platt (Citation1996) have reported that reduced nitrogen (as found in ammonium ions, amino groups, or amide groups) is the form utilized in biosynthesis. The organic nitrogen sources in these observations, yeast extract, could extract ammonium and amino acids by enzyme catalysis, all of which are the preferred nitrogen source of B. subtilis (Fisher, Citation1991). Our results showed that the optimal nitrogen source was yeast extract.
Table 4. The effects of various carbon and nitrogen sources on the production levels of GABA and nattokinase.
Tabla 4. Los efectos de varias fuentes de carbono y de nitrógeno en los niveles de producción de GABA y de nattokinasa.
Glucose, sucrose, fructose, maltose, lactose, potato starch, soluble starch, mannitol, xylose, malt extract, and glycerol were screened as main carbon sources, by replacing as indicated in the reference media. The complex media was inoculated and cultivated at the fermentation conditions. Bacillus subtilis uses glucose as the most preferred source for carbon and energy (Stuke & Hillen, Citation2002; Xu, Affleck, & Wangikar, Citation1993) and it was applied as reference. The other carbon source, fructose, gave similarly good results with respect to protease production by strains of B. subtilis. The results showed that, in terms of GABA and nattokinase production, fructose and glucose were the optimal carbon sources. Considering these results, and the productivity, fructose was chosen as the carbon source for further optimization studies. Based on these results, fructose and yeast extract were used in all subsequent experiments.
In previous studies, we were able to increase the GABA yield, but the rate of nattokinase production decreased significantly under the same conditions, indicating that GABA and nattokinase could be produced via the same pathways. Therefore, in this study, we focused on optimizing the conditions that would increase both GABA and nattokinase production. These results show that the production of both GABA and nattokinase is highest at the exponential growth phase.
The optimal media components and culture conditions are shown in The greatest GABA activity occurred in the culture containing 0.1% fructose and 0.3% yeast extract. As shown in , the highest quantity of GABA, 15.40 mg/mL, was reached with the addition of 1% MSG. The reason for this is because MSG acts as a substrate during the catalysis of glutamic acid decarboxylase (GAD) during GABA biotransformation (Zhang et al., Citation2012). In the culture with 4% fructose and 10% yeast extract, the high sugar concentration induced osmosis in the environment around the microorganisms, which was detrimental to metabolite biosynthesis of nattokinase (Fang & Zhong, Citation2002; Wang et al., Citation2009). The addition of 0.3% CaCl2 yielded the highest nattokinase activity level of 130.96 FU/mL (). As reference, Wang et al. (Citation2009) studied the effects of inorganic salts on nattokinase production. The results showed that heavy metal ions, including cupric ion, manganese ion and ferric ion, result in relatively poor fermentation of B. subtilis (Ammar, Matsubara, & Ito, Citation2002; Wei, Hall, & Chang, Citation2001). The use of an inorganic salt source, CaCl2, led to a high rate of nattokinase production.
Figure 2. The effect of various culture conditions on the GABA and nattokinase productivity of B. subtilis strain B060. Tested factors included (A) MSG concentration; (B) CaCl2 concentration; (C) rotation speed; and (D) incubation time. Symbols: (□) GABA content; (■) nattokinase. Values are the mean ± SD of three independent experiments.
Figura 2. Efecto de varias condiciones de cultivo en la productividad de GABA y de nattokinasa de B. subtilis cepa B060. Los factores probados incluyeron (A) concentración de MSG; (B) concentración de CaCl2; (C) velocidad de rotación; y (D) tiempo de incubación. Símbolos: (□) contenido de GABA; (■) nattokinasa. Los valores representan el medio ±DE de los tres experimentos independientes.
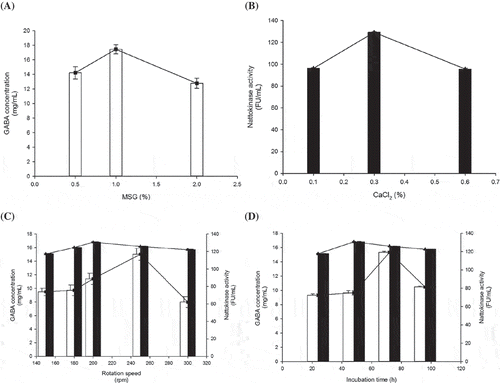
Bacillus subtilis is an aerophilic bacterium; a higher rotational speed provides a greater amount of oxygen, which improves the microorganism’s multiplication and metabolism. Moreover, Unrean and Nguyen (Citation2013) revealed that oxygen availability is simulated as millimoles of oxygen uptake per millimole of substrate consumed. This positive trend between enzyme synthesis and DO content in the culture could be in part due to redox constraints and the large energy requirement of nattokinase production. As shown in , accelerating the flask shaker enhanced the production of GABA and nattokinase. The highest level of GABA production was obtained at 250 rpm after 72 h, and the greatest nattokinase enzyme activity was obtained at 200 rpm after 48 h, as shown in
Optimum pH and temperature for GABA and nattokinase production
The results of the pH optimization test are shown in pH control has been reported to play a key role in GAD activity (Komatsuzaki, Nakamura, Kimura, & Shima, Citation2008). Thus, GABA productivity by B. subtilis B060 was measured at the pH values of 6.0, 7.0, 8.0, and 9.0. The results show that the highest production of GABA was obtained at a pH of 7.0 (15.593 g/L). That was higher than those at pH 6.0 (13.498 g/L), pH 8.0 (13.570 g/L), and pH 9.0 (12.525 g/L).
Figure 3. Optimization of pH (A) and temperature (B) for GABA and nattokinase production. Symbols: (□) GABA content; (■) nattokinase. Values are the mean ± SD of three independent experiments.
Figura 3. Optimización de pH (A) y de temperatura (B) para la producción de GABA y de nattokinasa. Símbolos: (□) contenido de GABA; (■) nattokinasa. Los valores representan el medio ±DE de los tres experimentos independientes.
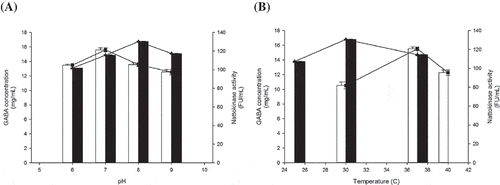
The greatest nattokinase activity occurred at a pH of 8.0 (130.213 FU/mL), which was higher than those at pH 6.0 (101.759 FU/mL), pH 7.0 (115.625 FU/mL), and pH 9.0 (117.469 FU/mL), as shown in The optimized pH value for nattokinase determined in this experiment was higher than that used for many other microbial fibrinolytic enzymes. For example, a pH of 7 was found to be optimal for metalloprotease production in Bacillus sp. KA38 (Kim et al., Citation1997), Bacillus sp. KDO-13 (Lee et al., Citation2001), and B. subtilis A1 (Jeong et al., Citation2004), while a pH of 7.5 was determined for B. subtilis Jin7 nattokinase (Bai, Xu, Han, & Liu, Citation2004).
The optimal fermentation temperature for GABA and nattokinase production was also investigated. The results demonstrated that the optimum temperature was 37°C (15.530 g/L) for GABA and 30°C (114.619 FU/mL) for nattokinase (). Some previous studies have shown that fibrinolytic enzymes are relatively stable at temperatures lower than 40°C (Hsieh et al., Citation2009; Wang et al., Citation2009).
In this study, the GABA and nattokinase production capacities of B. subtilis B060 were investigated using a Taguchi experimental design to determine the optimal substrate concentrations and pH level. Without pH control, the media pH level was found to gradually increase as the B. subtilis B060 cells grew. Because the optimal pH values for B. subtilis growth and its GAD activity are different (Nomura et al., Citation1999; Ueno, Hayakawa, Takahashi, & Oda, Citation1997; Zhang et al., Citation2012), a pH suitable for both cell growth and the biotransformation of the substrate is necessary.
Although elevated temperatures increase the reaction rate, temperatures that are high cause enzyme inactivation and cell aging. Because this situation negatively affects the final yield, temperature control is also necessary for bioconversion (Zhang et al., Citation2012).
The inoculation size also affected the GABA and nattokinase production. The GABA and nattokinase yields both increased as the inoculation size increased. However, their productions decreased dramatically at inoculation concentrations over 15% (7.524 g/L) for GABA and over 10% (86.571 FU/mL) for nattokinase. Therefore, inoculation sizes of 15% and 10% were used in all subsequent experiments on GABA and nattokinase production ability, respectively.
Effects of various process parameters on GABA and fibrinolytic enzyme production
L9 OA design was used to study the effects of various process parameters on GABA and fibrinolytic enzyme production. Using this method, the parameters were optimized for their concentrations. Depending upon the combinations of the process variables and their levels, GABA and fibrinolytic enzyme production in each run varied largely, indicating strong influence of the variables on the response. Further, to understand which of these variables affected GABA and fibrinolytic enzyme production in a significant manner, their ranking was performed based on the calculated delta S/N ratio. The response table () represents means (larger is better), S/N ratio, and delta values for S/N ratio obtained with L9 OA. The delta values indicate which factor has the greater effect on the response of interest and are calculated by taking the difference between the highest and lowest characteristic average for a factor. The greater the effect of the component, the higher the delta value for that component. The S/N ratio indicates the factors that have the greatest effect on the response and delta S/N ratio was used as a criterion for ranking of factors (). The factors with higher delta S/N ratio had greater impact on response and were ranked higher in order compared with the factors with lower delta S/N ratio. In the present study, the effects of the individual parameters as internal factors for GABA production can be ranked as MSG > Yeast Extract > Fructose > Inoculation size, and for nattokinase production as Yeast Extract > CaCl2 > Fructose > Inoculation size. Moreover, external factors for GABA production as Incubation time > Incubation temperature > Rotation speed > Initial pH, and for nattokinase production as Incubation time > Incubation temperature > Initial pH > Rotation speed. The final optimized medium produced 15.40 g/L GABA and 130.958 FU/mL nattokinase as compared to 3.876 g/L and 24.317 FU/mL before optimization, respectively. This confirmed the robustness of solution for optimal levels of parameters identified from Taguchi’s OA and implied that the selected conditions were the most suitable in practice.
Table 5. Signal-to-noise (S/N) ratio and delta for S/N ratio.
Tabla 5. La relación de señal/ruido (S/N) y delta para la relación S/N.
Time course study of the GABA and nattokinase levels of B. subtilis
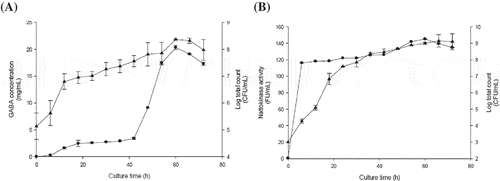
The GABA and nattokinase production capacities of B. subtilis were determined by conducting a time course analysis on the B060 strain in the optimized culture medium when compared with TSB. shows the highest obtained quantities of GABA and nattokinase, which occurred after 72 h and 48 h of cultivation, respectively. In this strain, the GABA content was typically higher during the exponential growth phase (), when the cells are capable of transforming the primary carbon source into biosynthetic precursors. This transformation reduces power and energy, which is generally trapped in the form of adenosine triphosphate (ATP), phosphoenolpyruvate (PEP), or proton gradients. The biosynthetic precursors thus generated are then channeled through various pathways to create monomers, nucleotides, fatty acids, sugars, and amino acids, including GABA.
Figure 4. The changes in GABA (A) and nattokinase (B) production by Bacillus subtilis over the course of 180 h of culture in either Taguchi’s optimization medium or tryptic soy broth (TSB) medium. Symbols: (●) GABA or nattokinase levels when cultured in Taguchi’s optimization medium; (▲) GABA or nattokinase levels when cultured in TSB; (■) Bacillus subtilis counts when cultured in Taguchi’s optimization medium; (×) Bacillus subtilis counts when cultured in TSB. Data are expressed as the mean ± SD from three independent experiments.
Figura 4. Cambios en la producción de GABA (A) y de nattokinasa (B) por Bacillus subtilis durante 180 horas de cultivo en el medio de optimización de Taguchi o en el medio de caldo de tríptico de soya (TSB). Símbolos: (●) niveles de GABA o de nattokinasa cultivados en el medio de optimización de Taguchi; (▲) niveles de GABA o de nattokinasa cultivados en TSB; (■) conteo de Bacillus subtilis cultivada en el medio de optimización de Taguchi; (×) conteo de Bacillus subtilis cultivado en TSB. Los datos son expresados como el medio ±DE de los tres experimentos independientes.
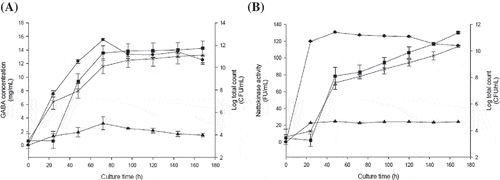
The bacteria’s ability to produce nattokinase is also initialized in the exponential growth phase, as shown in
Based on the results obtained in this study, the GABA and nattokinase yields were greatly improved when the bacteria were cultured in the medium optimized with the Taguchi method. At 15.40 mg/mL after 72 h, the production rate of GABA in this medium was higher than that of the B. subtilis grown in TSB medium. The nattokinase activity was also greatest in the Taguchi medium, at 130.96 FU/mL after 48 h.
Batch cultures in a 5 L fermenter
The data of the flask cultures show that the optimum concentrations of yeast extract and fructose were 15.40 mg/mL and 130.96 units/mL for GABA and nattokinase production, respectively. A batch culture was carried out in a 5 L fermenter to investigate the time courses of GABA and nattokinase production as well as cell growth, as shown in
Figure 5. The GABA (A) and nattokinase (B) production in batch cultures of Bacillus subtilis. Symbols: (●) GABA or nattokinase levels when cultured in a 5 L fermenter; (▲) Bacillus subtilis counts when cultured in a 5 L fermenter. Data are expressed as the mean ± SD from three independent experiments.
Figura 5. Producción de GABA (A) y de nattokinasa (B) en cultivos de lotes de Bacillus subtilis. Símbolos: (●) niveles de GABA o de nattokinasa cultivados en fermentador de 5 L; (▲) conteo de Bacillus subtilis cultivada en un fermentador de 5 L. Los datos son expresados como el medio ±DE de los tres experimentos independientes.
In the batch cultures of B. subtilis, the highest GABA yield, 20.58 mg/mL, was obtained after 60 h. The nattokinase activity maximum, 145.50 units/mL, was also found at 60 h, after which a slight decrease was observed. Both the maximum GABA concentration and the maximum nattokinase activity were much higher in the fermenter than in flask culture, likely due to the fermenter’s ability to maintain the optimum pH level. And agitation speed is one of the culture parameters responsible for controlling DO content in the culture. Unrean and Nguyen (Citation2013) reported that the increasing trend of nattokinase yield could be improved by increasing the available oxygen. Experimentally, the oxygen sufficient condition can be achieved using oxygen-enriched air or by increasing agitation to control DO% content in the culture above zero.
Thus, media optimization using the L9 OA resulted in enhanced production of GABA and the fibrinolytic enzyme, which were 5.31- and 8.74-fold higher than the levels produced in unoptimized medium, respectively.
The deduced amino acid sequence and restriction enzyme map of the B. subtilis B060 enzyme compared with those of proteases that share the same N-terminal sequences
To compare the gene coding for the B060 enzyme with those of subtilisin NAT, subtilisin J, subtilisin E, subtilisin amylosacchariticus and mesentericopeptidase, the DNA fragment was amplified from the genomic DNA of B. subtilis B060 by PCR. The deduced amino acid sequence of the restriction enzyme site was determined using NCBI BLAST (www.ncbi.nlm.nih.gov/blast.cgi).
The DNA sequence of the aprN gene has an open reading frame encoding 290 nucleotides. The amino acid sequence alignment showed that the highest sequence identity (99%) occurred with the nattokinase (aprN) gene from B. subtilis. These results demonstrated that the B060 enzyme belongs to the subtilisin family; it has high homology to subtilisin NAT and has been designated subtilisin B060.
Conclusion
In this study, B. subtilis was isolated from rice straw. An L9 OA was used to determine the optimal medium composition needed to maximize GABA and nattokinase production. Subsequently, in a culture batch fermenter, the maximum yields of GABA and nattokinase were 20.58 mg/mL and 145.50 units/mL, respectively. Finally, the properties of the purified enzymes were determined to identify the nature of the fibrinolytic enzyme that was produced.
Acknowledgments
This research was supported by the National Science Council of Taiwan (project no. NSC 101-2622-E-020-007-CC3).
References
- Allagheny, N., Obanu, Z. A., & Platt, G. C. (1996). Control of ammonia formation during Bacillus subtilis fermentation of legumes. Food Microbiology, 29, 321–333.
- Almeidae, S., Lima, U. A., & Taqueda, M. (2003). Use of response surface methodology for selection of nutrient levels for culturing Paecilomyces variotii in eucalyptus hemicellulosic hydrolyzate. Bioresource Technology, 87, 45–50.
- Ammar, Y. B., Matsubara, T., & Ito, K. (2002). Some properties of levansucrase of Bacillus natto stabilized with periodate oxidized glucomannan. Enzyme and Microbial Technology, 30, 875–882.
- Bai, Z., Xu, M., Han, S., & Liu, K. (2004). Purification and characterization of nattokinase. Journal of Northeast Agriculture University, 35, 667–673.
- Buchanan, J. R., & Gibbons, N. E. (1974). Bergey’s manual of determinative bacteriology (8th ed.). Baltimore: The Williams and Wilkins Company.
- Dean, A., & Voss, D. (1999). Response surface methodology. In Design and analysis of experiments (547–566). New York: Springer-Verlag.
- Fang, Q. H., & Zhong, J. J. (2002). Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites ganoderic acid and polysaccharide. Biochemical Engineering Journal, 10, 61–65.
- Fisher, S. H. (1991). Control of carbon and nitrogen metabolism in Bacillus subtilis. Annual Review of Microbiology, 45, 107–135.
- Haaland, P. D. (1989). Experimental design in biotechnology. Dekker: New York.
- Hsieh, C. W., Lu, W. C., Hsieh, W. C., Huang, Y. P., Lai, C. H., & Ko, W. C. (2009). Improvement of the stability of nattokinase using γ-polyglutamic acid as a coating material for microencapsulation. LWT – Food Science and Technology, 42, 144–149.
- Japan Nattokinase Association. (2000). Degradation of artificial thrombus by nattokinase. Retrived May 18, 2012, from http://jnattokinase.org/en/jnka_nattou_1.html
- Jeong, Y. K., Kim, J. H., Gal, S. W., Kim, J. E., Park, S. S., & Chung, K. T. (2004). Molecular cloning and characterization of the gene encoding a fibrinolytic enzyme from Bacillus subtilis strain A1. World Journal of Microbiology and Biotechnology, 20, 711–717.
- Ju, H. K., Yan, J. P., Zhu, L., & Qi, Y. P. (2004). Identification of two novel fibrinolytic enzymes from Bacillus subtilis QK02. Comparative Biochemistry and Physiology Part C, 137, 65–74.
- Kim, S. H., & Choi, N. S. (2000). Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp. strain DJ-4 screened from Doen-Jang. Bioscience, Biotechnology, and Biochemistry, 64, 1722–1725.
- Kim, W., Choi, K., Kim, Y., Park, H., Choi, J., & Lee, Y. (1996). Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11–4 screened from Chungkook-Jang. Applied and Environmental Microbiology, 62, 2482–2488.
- Kim, H. K., Kim, G. T., Kim, D. K., Choi, W. A., Park, S. H., Jeong, Y. K., & Kong, I. S. (1997). Purification and characterization of a novel fibrinolytic enzyme from Bacillus sp. KA38 originated from fermented fish. Journal of Bioscience and Bioengineering, 84(4), 307–312.
- Kim, S. B., Lee, D. W., Cheigh, C. I., Choe, E. A., Lee, S. J., & Hong, Y. H. (2006). Purification and characterization of a fibrinolytic subtilisin-like protease of Bacillus subtilis TP-6 from an Indonesian fermented soybean, Tempeh. Journal of Industrial Microbiology and Biotechnology, 33, 436–444.
- Kitaoka, S., & Nakano, Y. (1969). Colorimetric determination of ω-amino acids. Journal of Biochemistry, 66(1), 87–94.
- Komatsuzaki, N., Nakamura, T., Kimura, T., & Shima, J. (2008). Characterization of glutamate decarboxylase from a high γ-aminobutyric acid (GABA) producer, Lactobacillus paracasei. Bioscience, Biotechnology and Biochemistry, 72, 278–285.
- Lee, S. K., Bae, D. H., Kwon, T. J., Lee, S. B., Lee, H. H., & Park, J. H. (2001). Purification and characterization of a fibrinolytic enzyme from Bacillus sp. KDO-13 isolated from soybean paste. Journal of Microbiology and Biotechnology, 11, 845–852.
- Montgomery, D. C. (1991). Design and analysis of experiments (3rd ed.). New York: Wiley.
- Myers, R. H., & Montgomery, D. C. (1995). Response surface methodology: Process and product optimization using designed experiments. New York: John Wiley & Sons.
- Nomura, M., Nakajima, I., Fujita, Y., Kobayashi, M., Kimoto, H., Suzuki, I., & Aso, H. (1999). Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology, 145, 1375–1380.
- Sato, T., Yamada, Y., & Ohtani, Y. (2001). Production of menaquinone (vitamin K2)-7 by Bacillus subtilis. Journal of Bioscience and Bioengineering, 91, 16–20.
- Stuke, J., & Hillen, W. (2002). Regulation of carbon catabolism in Bacillus species. Annual Review of Microbiology, 54, 849–880.
- Sumi, H., Hamada, H., Tsushima, H., Mihara, H., & Muroki, H. (1987). A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet. Experientia, 43, 1110–1111.
- Sumi, H., Nakajima, N., & Yatagai, C. (1995). A unique strong fibrinolytic enzyme (Katsuwokinase) in shipjack ‘‘Shiokara’’, a Japanese traditional fermented food. Comparative Biochemistry and Physiology – Part B: Biochemistry and Molecular Biology, 112(3), 543–547.
- Ueno, Y., Hayakawa, K., Takahashi, S., & Oda, K. (1997). Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Bioscience, Biotechnology and Biochemistry, 61, 1168–1171.
- Unrean, P., & Nguyen, N. H. A. (2013). Metabolic pathway analysis and kinetic studies for production of nattokinase in Bacillus subtilis. Bioprocess and Biosystems Engineering, 36, 45–56.
- Wang, C., Du, M., Zheng, D., Kong, F., Zu, G., & Feng, Y. (2009). Purification and characterization of nattokinase from Bacillus subtilis Natto B-12. Journal of Agricultural and Food Chemistry, 57, 9722–9729.
- Wang, C. T., Ji, B. P., Li, B., Nout, R., Li, P. L., & Ji, H. (2006). Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from Chinese traditional Douchi. The Journal of Industrial Microbiology and Biotechnology, 33(9), 750–758.
- Wei, Q., Hall, C. W., & Chang, K. C. (2001). Natto characteristics as affected by steaming time, Bacillus strain, and fermentation time. Journal of Food Science, 66, 167–173.
- Wong, A. H. K., & Mine, Y. (2004). Novel fibrinolytic enzyme in fermented shrimp paste, a traditional Asian fermented seasoning. Journal of Agricultural and Food Chemistry, 52, 980–986.
- Xu, Z. F., Affleck, R., & Wangikar, P. (1993). Transition state stabilization of subtilisins in organic media. Biotechnology and Bioengineering, 43, 515–520.
- Zhang, Y., Song, L., Gao, Q., Yu, S. M., Li, L., & Gao, N. F. (2012). The two step biotransformation of monosodium glutamate to GABA by Lactobacillus brevis growing and resting cells. Applied Microbiology and Biotechnology, 94, 1619–1627.
