Abstract
Kale (Brassica oleracea Acephala group) is a green leafy vegetable with high content of nutraceuticals. However, the content of bioactive compounds of kale is affected by the cultivar, and thus it is necessary to identify kale cultivars that have the highest nutritional content, to promote their consumption. The present project’s objective was to characterize the bioactive compounds (phenolic compounds, vitamin C, glucosinolates and individual carotenoids) and the antioxidant capacity of two kale cultivars (Winterbor and Maribor). The Winterbor cultivar presented higher content of phenolic compounds, carotenoids and higher antioxidant capacity than the Maribor, whereas the Maribor cultivar showed higher levels of vitamin C and glucosinolates. One serving size of kale provides more than 100% of the recommended daily intake (RDI) of vitamin A and more than 40% of the RDI of vitamin C. Therefore, kale can be considered an excellent source of antioxidants.
El kale (Brassica oleracea Acephala group) es un vegetal de hoja verde con alto contenido de nutracéuticos. Sin embargo, el contenido de compuestos bioactivos del kale se ve afectado por el cultivar, por lo tanto es necesario determinar cuál cultivar tiene el más alto contenido nutricional para promover su consumo. El objetivo del presente proyecto fue caracterizar los compuestos bioactivos (compuestos fenólicos, vitamina C, glucosinolatos, y carotenoides individuales) y la capacidad antioxidante de dos cultivares de kale (Winterbor and Maribor). El cultivar Winterbor presentó mayor contenido de compuestos fenólicos, carotenoides y mayor capacidad antioxidante que el Maribor, mientras que el cultivar Maribor presentó mayor cotenido de vitamina C y glucosinolatos. Un tamaño de porción de kale brinda 100% de la ingesta diaria recomendada (IDR) de vitamina A y más del 40% de la IDR de vitamina C. Por lo tanto, el kale puede ser considerado una excelente fuente de antioxidantes.
Introduction
Kale (Brassica oleracea Acephala group) is a leafy green vegetable belonging to the Brassicaceae family. In recent years, kale has gained the attention of the scientific community due to its high content of bioactive compounds such as vitamin C, pro-vitamin A, glucosinolates, phenolic antioxidants, dietary fiber, micronutrients (iron, zinc and manganese) and macronutrients (calcium and magnesium) (Ayaz et al., Citation2006; Cartea, Velasco, Obregón, Padilla, & de Haro, Citation2008; Khachik, Beecher, & Goli, Citation1991; Olsen, Aaby, & Borge, Citation2009). Likewise, in vitro and in vivo studies suggest that kale have a positive impact on the prevention of chronic diseases such as cardiovascular diseases (Kahlon, Chapman, & Smith, Citation2007; Kim, Yoon, Kwon, Park, & Lee-Kim, Citation2008; Kural, Küçük, Yücesan, & Örem, Citation2011) and cancer (Chung, Lee, & Sung, Citation2002).
The extensive growth and commercialization of kale can be a realistic approach to increase the dietary intake of antioxidants in the population. However, research is needed to determine variations in the content of bioactive compounds amoung kale cultivars, in order to promote the production of those with the highest nutraceutical content. Therefore, the present project’s objective was to evaluate the concentration of phenolic compounds (PC), vitamin C, glucosinolates, individual carotenoids and the antioxidant capacity (ORAC value) of two kale cultivars (Winterbor and Maribor).
Materials and methods
Plant material and chemicals
Kale cultivars (Winterbor and Maribor) were produced by Frigorizados La Huerta, S.A de C.V. (San Francisco de los Romo, Aguascalientes, Mexico). Methanol (HPLC grade), tert-Butyl methyl ether (tBME; HPLC grade), acetone (HPLC grade), isopropyl alcohol (HPLC grade), perchloric acid (HClO4) and phosphoric acid (H3PO4) were purchased from Desarrollo de Especialidades Químicas, S.A. de C.V. (San Nicolás de los Garza, Nuevo León, México). Gallic acid (GA), L-ascorbic acid (AA), 6-hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid (Trolox), trichloroacetic acid (TCA), trifluoroacetic acid (TFA), HEPES, thioglucosidase from Sinapis alba, sinigrin hydrate, glucose oxidase/peroxidase (GOP) assay kit, DL-dithiothreitol (DTT), N-ethylmaleimide (NEM), 2, 2´-bipyridyl and iron (III) chloride (FeCl3) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Butylated hydroxytoluene (BHT) was obtained from Spectrum Quality Products (New Brunswick, NJ, USA).
Extraction and quantification of total phenolic compounds and determination of total antioxidant capacity
Kale tissue (5 g) was homogenized with methanol (20 mL) using a tissuemizer (Advanced homogenizing system, VWR, Radnor, PA, USA). Subsequently, the homogenates were stored overnight (~12 h at 4ºC) and centrifuged (10,000×g, 15 min, 4°C). The clear supernatant (methanol extract) was used for the analyses of total PC and antioxidant capacity.
The total PC was determined with the method described by Singleton and Rossi (Citation1965), adapted to 96-well micro-plate format (Jacobo-Velázquez, Martínez-Hernández, Rodríguez, Cao, & Cisneros-Zevallos, Citation2011). The concentration of total PC was expressed as mg of GA equivalents per kg of kale fresh weight (FW).
The antioxidant capacity of the methanol extract was determined with the oxygen radical absorbance capacity (ORAC) assay. The ORAC value was obtained using the procedure described by Wu et al. (Citation2004) for hydrophilic ORAC. Results were expressed as mg of trolox equivalents (TE) per kg of kale FW.
Extraction and quantification of total glucosinolates
The extraction and quantification of total glucosinolates was performed as described by Miranda Rossetto, Shiga, Vianello, and Pereira Lima (Citation2013) with slight changes. Briefly, kale tissue (3 g of mature leaves) was homogenized with 70% methanol (5 mL) and TFA (1 mL) using a tissuemizer (advanced homogenizing system, VWR, Radnor, PA, USA). Subsequently, the homogenates were stored in an incubator (30 min, 70ºC, 150 rpm) and centrifuged (10,000×g, 30 min, 4°C). The clear supernatant was microfiltered using nylon membranes (0.45 μm, VWR, Radnor, PA, USA). Thereafter, the extracts were evaporated to dryness using a continuous flow of nitrogen. Finally, the extract was resuspended in HEPES-KOH (1 mL, 0.2 M, pH 7). The re-suspended extract (further referred as re-constituted extract) was used for the quantification of total glucosinolates.
For total glucosinolates determinations, an enzymatic assay was performed. The method consisted of the enzymatic hydrolysis of glucosinolates by a thioglucosidase from Sinapis alba, and the subsequent quantification of glucose by the GOP assay kit. Briefly, the re-constituted extracts (50 µL) were incubated (37°C, 24 h) with thioglucosidase solution (25 µL, 0.12 U, HEPES-KOH 0.2 M, pH 7). The enzymatic reaction was stopped by the addition of HClO4 (25 µL, 18 mM). Total glucosinolates content was calculated according to stoichiometry, which states that 1 mol of released glucose is equivalent to 1 mol of glucosinolate. As blank, the levels of glucose in the re-constituted extract non-treated with thioglucosidase were determined. Finally, the glucose released by thioglucosidase was determined with the GOP assay kit according to manufacturer’s instructions. Sinigrin hydrate was used as a positive control. Results were expressed as µmol equivalents of glucosinolates per kg of kale FW.
Extraction and quantification of vitamin C
To extract vitamin C from kale, the tissue (2 g) was first homogenized under liquid nitrogen using a chilled mortar and pestle. Thereafter, the tissue was homogenized with TCA (6%, 15 mL), transferred to a 50 mL tube, centrifuged (15,000×g, 25 min, 4 ºC) and maintained on ice until needed for the assay (approximately 1 h). Total vitamin C content was determined in the supernatant by using the 2, 2´-bipyridyl method (Gillespie & Ainsworth, Citation2007; Okamura, Citation1980). Briefly, the extract (100 μL) was placed in a 2-mL tube and mixed with DTT solution (20 mM, 100 μL). The mixture was incubated for 10 min at room temperature in the dark. Then, NEM solution (0.5%, 100 μL) was added to the mixture and incubated for 30 s. Subsequently, TCA (10%, 500 μL), H3PO4 (43%, 400 μL), 2, 2′-bipyridyl (4%, 400 μL) and FeCl3 (3%, 200 μL) solutions were added to the assay tubes. The assay tubes were incubated at 37 ºC for 1 h. Then, 200 μL of the reaction solutions from the assay tubes were placed in a well of a clear 96-well microplate and absorbance readings were collected at 525 nm. Absorbance values were compared against an AA standard curve (0.15–10 mM) prepared in TCA (6%). Results were expressed as mg of vitamin C per kg of kale FW.
Extraction, identification and quantification of kale carotenoids by HPLC-PDA
The extraction of carotenoids from kale tissue was performed as described by Jacobo-Velázquez and Hernández-Brenes (Citation2012) with slight modifications. Briefly, kale tissue (1 g) was homogenized with 0.1% BHT acetone solution (10 mL) using a tissuemizer. Subsequently, the homogenates were filtered under vacuum through Whatman No. 1 filter papers (Piscataway, NJ, USA) to obtain the acetone extracts. This procedure was repeated twice to ensure the complete extraction of carotenoids. The acetone extracts were pooled and concentrated in a rotary evaporator (BÜCHI Labortechnik, AG, Flawil, Switzerland) operating at 35°C, 60 rpm and 250 mbar until acetone was completely evaporated. The remaining water in the flask was removed by evaporating the extracts to dryness using a continuous flow of nitrogen. The dried samples were re-dissolved in isopropyl alcohol (1 mL) and filtered through nylon membranes (0.45 μm) prior to injection to the HPLC system.
Carotenoids were identified and quantified by HPLC with photodiode array detection (PDA). The HPLC system used was composed of two 515 binary pumps, a 717-plus autosampler and a 996-PDA (Waters Corp, Mildford, MA, USA). Carotenoids were separated on a 4.6 mm × 150 mm, 3 μm, C30 reverse phase column (YMC Carotenoid, Waters Corp, Mildford, MA, USA). The mobile phases were methanol/water (96:4, v/v, phase A) and tBME (phase B). The gradient solvent system was 0/95, 10/90, 40/55, 45/25, 50/0, 55/0 and 57/95 (min/% phase A) at a constant flow rate of 0.75 mL/min. Chromatographic data was processed with the Millenium software V3.1 (Waters Corp, Mildford, MA, USA).
The tentative identification of each chromatographic peak was achieved by comparing their retention time and UV/Visible (UV/Vis) absorption spectra characteristics with those of commercial carotenoid standards. In addition, the order of elution and the UV/Vis spectra characteristic from carotenoids reported in previous studies were used as additional parameters of identification. For the quantification of individual carotenoids, standard curves of lutein and all-trans-β-carotene were prepared at a range of 0.4–6.0 ppm (mg/L). The concentration of carotenoids was expressed as mg of each individual compound per kg of kale FW.
Statistical analysis
Statistical analyses were performed using three replicates. Data represent the mean values of the three replicates and bars indicate their standard error of the mean. Analyses of variance (ANOVA) were conducted using JMP software version 5.0 (SAS Institute Inc. Cary, NC, USA) and mean separations performed using LSD test (p < 0.05).
Results and discussion
Total phenolics, vitamin C, glucosinolates and antioxidant capacity content of kale cultivars
The total PC, vitamin C, glucosinolates and antioxidant capacity values of the Winterbor and Maribor kale cultivars are shown in . The Winterbor cultivar showed ~42% higher concentration of PC and ~94% higher antioxidant capacity (ORAC value) than the Maribor, whereas the Maribor cultivar had ~141% higher vitamin C and ~159% higher total glucosinolates content than the Winterbor. It is interesting to observe that although the Maribor cultivar had higher glucosinolates and vitamin C content than the Winterbor, the antioxidant capacity of the Winterbor cultivar was higher than the Maribor. Jacobo-Velázquez and Cisneros-Zevallos (Citation2009) proposed that the antioxidant capacity of plant foods is mainly attributed to their PC content. In addition, the authors suggested that the antioxidant activity value is not only affected by the total PC content but also by the type of individual PC present in the plant food (phenolic profiles). Therefore, Jacobo-Velázquez and Cisneros-Zevallos (Citation2009) applied the concept of specific antioxidant capacity (AOXs) value to establish the effectiveness of a mixture of phenolic compounds to neutralize free radicals. The AOXs value is calculated by dividing the total antioxidant activity of a plant food by its total PC content. Taking this into consideration, the AOXs value in the Winterbor cultivar was ~17 mg TE/mg of PC, whereas the AOXs of the Maribor cultivar was ~13 mg TE/mg PC. These results indicate that the phenolic profile in the Winterbor cultivar is more effective to neutralize free radicals than the PC present in the Maribor cultivar. The values obtained for total PC and ORAC are similar to those previously reported for kale (Zhou & Yu, Citation2006) when the values reported herein are calculated in dry weight basis (considering 85% of moisture). Although the total PC values in kale are relatively low compared to other commonly consumed vegetable, the ORAC value of the Winterbor cultivar is high and comparable to the antioxidant capacity reported for berries such as raspberry (11920 mg TE/kg) and strawberry (8860 mg TE/kg) (Wu et al., Citation2004).
Table 1. Total phenolic compounds, vitamin C, glucosinolates and antioxidant capacity (ORAC value) of Winterbor and Maribor kale cultivars.
Tabla 1. Compuestos fenólicos totales, vitamina C, glucosinolatos y capacidad antioxidante (valor ORAC) de los cultivares Winterbor y Maribor de kale.
The concentration of vitamin C observed in the kale varieties evaluated herein () are similar to values previously reported (Sikora & Bodziarczyk, Citation2012). Vitamin C content in kale is high, especially in the Maribor cultivar (). Indeed, based on the definition of serving size for raw leafy vegetables established by the USDA (1 cup = 67 g), the Maribor cultivar can be considered an excellent source of vitamin C since one serving size provides ~40% of the RDI (recommended daily intake) for men (RDI = 90 mg/day) and ~50% of the RDI for women (RDI = 75 mg/day). On the other hand, the Winterbor cultivar is a good source of vitamin C, since one serving size provides more than 10% of the RDI for men and women. Therefore, nutrient claims such as “good source of vitamin C antioxidant” and “high in vitamin C antioxidant” could be used in the label of fresh Winterbor and Maribor kale cultivars, respectively. Compared to other green leafy vegetables, the concentration of vitamin C in kale is higher than lettuce (92 mg/kg), and similar to those reported for Swiss chard (300 mg/kg) and spinach (750 mg/kg) (USDA, 2013).
The values obtained for total glucosinolates in the Maribor cultivar are higher than those earlier reported for Brassica vegetables such as broccoli (624 µmol/kg), and both kale cultivars showed higher concentration of glucosinolates than those previously reported for Brussels sprouts (172 µmol/kg) and cauliflower (135 µmol/kg) (Song & Thornalley, Citation2007). Glucosinolates are converted into isothiocyanates in the human body by the action of the enzyme myrosinase (Fahey, Zhang, & Talalay, Citation1997). It is well known that isothiocyanates are potent phase II enzymes inducers that protects against chemical carcinogens (Aires, Carvalho, Rosa, & Saavedra, 2013; Fahey et al., Citation1997). Therefore, the consumption of kale would be beneficial for the prevention of cancer, as it has been suggested for prostate cancer (Steinbrecher, Nimptsch, Hüsing, Rohrmann, & Linseisen, Citation2009). However, it would be interesting to determine the glucosinolates profile of kale since individual compounds differ with respect to impact on health (Dinkova-Kostova & Kostov, Citation2012).
Carotenoid analysis
The typical HPLC-PDA kale carotenoid chromatograms (shown at 450 nm) obtained from Winterbor and Maribor cultivars is shown in . No difference was observed between the compounds identified in both cultivars. The tentative identifications of the individual carotenoids present in both cultivars are reported in . The identity of each chromatographic peak was assigned based on the absorption maxima of each chromatographic peak by comparison with the absorption maxima of commercial standards and prior literature data (Chandrika, Jansz, & Warnasuriya, Citation2005; de Azevedo & Rodriguez-Amaya, Citation2004; Lee, Castle, & Coates, Citation2001; Mendes-Pinto, Ferreira, Oliveira, & De Pinho, Citation2004; Taylor, Brackenridge, Vivier, & Oberholster, Citation2006). Furthermore, the order of elution of the individual carotenoids was considered as an additional criterion for identity when prior publications used the same chromatographic conditions (Chandrika et al., Citation2005; de Azevedo & Rodriguez-Amaya, Citation2004; Khachik, Beecher, & Whittaker, Citation1986; Lee et al., Citation2001; Mendes-Pinto et al., Citation2004; Taylor et al., Citation2006).
Figure 1. Typical HPLC-PDA kale carotenoid and chlorophyll chromatograms (shown at 450 nm) obtained from Winterbor (A) and Maribor (B) cultivars. Tentative identification of the chromatographic peaks was performed as indicated in . Peak assignments: (1) neoxanthin, (2) violaxanthin, (3) chlorophyll b, (4) lutein, (5) chlorophyll a, (6) all-trans-β-carotene and (7) 9-cis-β-carotene.
Figura 1. Perfil cromatográfico típico obtenido mediante HPLC-PDA de carotenoides y clorofilas de kale (mostrado a 450 nm) presentes en los cultivares Winterbor (A) y Maribor (B). La identificación tentativa de los picos cromatográficos se realizó como se indica en al Tabla 2. Asignación de picos: (1) neoxantina, (2) violaxantina, (3) clorofila b, (4) luteína, (5) clorofila a, (6) all-trans-β-caroteno y (7) 9-cis-β-caroteno.
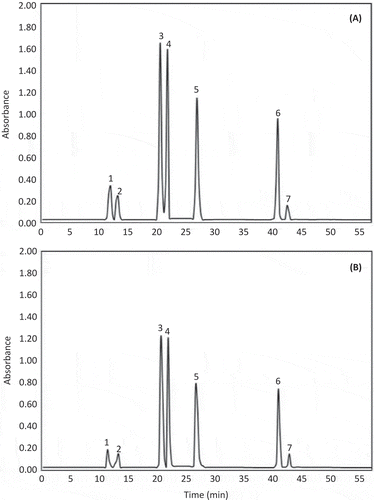
Table 2. Tentative identification of kale (Brassica oleracea Acephala group) carotenoid and chlorophyll chromatographic profiles obtained by HPLC-PDA.
Tabla 2. Identificación tentativa de los perfiles cromatográficos de carotenoides y clorofilas de kale (Brassica oleracea Acephala group) obtenidos mediante HPLC-PDA.
The carotenoids identified in the Winterbor and Maribor kale cultivars included neoxanthin, violaxanthin, lutein, all-trans-β-carotene and 9-cis-β-carotene (, ). In general, the Winterbor cultivar had higher concentration of total carotenoids as compared to the Maribor (). For both cultivars, the carotenoid that showed highest concentration was the lutein (~44% of total). These results are consistent with previous reports on the identification of the main carotenoids present in kale (Khachik et al., Citation1986). The concentration of lutein in the Winterbor cultivar was ~28% higher than the Maribor. Compared to other plant foods rich in lutein, the Winterbor cultivar contains similar amounts as those present in the main sources of lutein (i.e. spinach, turnip greens and collards) of the human diet (Holden, Eldridge, & Beecher, Citation1999). Although currently there is no RDI for lutein, the American Macular Degeneration Foundation (AMDF) recommends daily dosages of 6–30 mg. One serving size of the kale cultivars evaluated herein provides 5.0–7.0 mg of lutein per serving size, and thus kale could be considered an excellence source of lutein.
Table 3. Individual carotenoids and chlorophylls content in Winterbor and Maribor kale cultivars.
Tabla 3. Contenido de carotenoides y clorofilas individuales en los cultivares de Winterbor y Mirabor de kale.
Regarding pro-vitamin A content (all-trans-β-carotene and 9-cis-β-carotene) of kale, the cultivars evaluated contain similar amounts (). The concentration of β-carotene in kale is similar to the content in sweet potato (92 mg/kg), carrot (88 mg/kg), pumpkin (69 mg/kg), spinach (56 mg/kg) and collard (33 mg/kg), which are considered the main sources of β-carotene in the human diet (Holden et al., Citation1999). Converting the all-trans-β-carotene of kale to retinol (Vitamin A) concentration (1 μg of retinol = 6 μg of all-trans-β-carotene) (Haskell et al., Citation2004), each serving size of the Winterbor and Maribor kale cultivars provides 845 μg and 763 μg of retinol, respectively. Therefore, kale can also be considered an excellent souce of vitamin A because it provides more than 100% of its RDI (600 μg). Nutrient claims such as “high in pro-vitamin A antioxidant” could be used in the label of fresh kale. However, although kale is an excellent source of antioxidants, it should be eaten in moderation to prevent hypercarotenenameia (yellow-orange pigmentation in the skin), which is associated with the excessive intake of vegetables rich in pro-vitamin A carotenoids (Nagai et al., Citation1999).
Conclusions
The kale cultivars evaluated in the present study contain high levels of antioxidants. Their content of vitamin C is high (particularly in the Maribor cultivar), and the levels of pro-vitamin A, lutein and glucosinolates are comparables to those present in the main sources of these antioxidants. Therefore, kale can be considered as a functional food since it may be useful for the prevention of different chronic degenerative diseases. The extensive production and comercialization of kale can be a realistic approach to increase the daily intake of antioxidants in the population.
Daniel A. Jacobo-Velázquez
Centro de Biotecnología – FEMSA, Department of Biotechnology and Food Engineering, School of Biotechnology and Food, Tecnológico de Monterrey – Campus Monterrey, E. Garza Sada 2501 Sur, C.P. 64849, Monterrey, NL, México
Corresponding author. Email: [email protected]
Juan Pablo Mora-Mora
Frigorizados La Huerta, S.A. de C.V. Rancho Medio Kilo. Calle 1 número 140, Colonia Medio Kilo, C.P. 20350, San Francisco de los Romo, Aguascalientes, México
José Luis Mora-Nieves
Frigorizados La Huerta, S.A. de C.V. Rancho Medio Kilo. Calle 1 número 140, Colonia Medio Kilo, C.P. 20350, San Francisco de los Romo, Aguascalientes, México
Pedro A. Alanís-Garza
Centro de Biotecnología – FEMSA, Department of Biotechnology and Food Engineering, School of Biotechnology and Food, Tecnológico de Monterrey – Campus Monterrey, E. Garza Sada 2501 Sur, C.P. 64849, Monterrey, NL, México
Alejandro Becerra-Moreno
Centro de Biotecnología – FEMSA, Department of Biotechnology and Food Engineering, School of Biotechnology and Food, Tecnológico de Monterrey – Campus Monterrey, E. Garza Sada 2501 Sur, C.P. 64849, Monterrey, NL, México
Acknowledgements
This study is based upon research supported by research funds from the Tecnológico de Monterrey – Research Chair Initiative (CAT 161). We would also like to thank Frigorizados La Huerta, S.A. de C.V., for providing kale cultivars. The author, A.B.-M., also acknowledges the scholarship (285802) from the Consejo Nacional de Ciencia y Tecnología (CONACYT, México).
References
- Aires, A., Carvalho, R., Rosa, E., & Saavedra, M. J. In press. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CyTA – Journal of Food, doi :10.1080/19476337.2013.769025
- Ayaz, F. A., Glew, R. H., Millson, M., Huang, H. S., Chuang, L. T., Sanz, C., & Hayirlioglu-Ayaz, S. (2006). Nutrient contents of kale (Brassica oleraceae L. var. acephala DC). Food Chemistry, 96(4), 572–579.
- Cartea, M. E., Velasco, P., Obregón, S., Padilla, G., & de Haro, A. (2008). Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry, 69(2), 403–410.
- Chandrika, U. G., Jansz, E. R., & Warnasuriya, N. D. (2005). Identification and HPLC quantification of carotenoids of the fruit pulp of Chrysophyllumroxburghii. Journal of the National Science Foundation of Sri Lanka, 33(2), 93–98.
- Chung, M. J., Lee, S. H., & Sung, N. J. (2002). Inhibitory effect of whole strawberries, garlic juice or kale juice on endogenous formation of N-nitrosodimethylamine in humans. Cancer Letters, 182(1), 1–10.
- deAzevedo, C. H., & Rodriguez-Amaya, D. B. (2004). Carotenoid composition of kale as influenced by maturity, season and minimal processing. Journal of the Science of Food and Agriculture, 85(4), 591–597.
- Dinkova-Kostova, A. T., & Kostov, R. V. (2012). Glucosinolates and isothiocyanates in health and disease. Trends in Molecular Medicine, 18(6), 337–347.
- Fahey, J. W., Zhang, Y., & Talalay, P. (1997). Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proceedings of the National Academy of Sciences, 94(19), 10367–10372.
- Gillespie, K. M., & Ainsworth, E. A. (2007). Measurement of reduced, oxidized and total ascorbate content in plants. Natural Protocols, 2(4), 871–874.
- Haskell, M. J., Jamil, K. M., Hassan, F., Peerson, J. M., Hossain, M. I., Fuchs, G. J., & Brown, K. H. (2004). Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. The American Journal of Clinical Nutrition, 80(3), 705–714.
- Holden, J. M., Eldridge, A. L., Beecher, G. R., Marilyn Buzzard, I. M., Bhagwat, S., Davis, C. S.…, Schakel, S. (1999). Carotenoid content of U.S. foods: An update of the database. Journal of Food Composition and Analysis, 12(3), 169–196.
- Jacobo-Velázquez, D. A., & Cisneros-Zevallos, L. (2009). Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. Journal of Food Science, 74, R107–R113.
- Jacobo-Velázquez, D. A., & Hernández-Brenes, C. (2012). Stability of avocado paste carotenoids as affected by high hydrostatic pressure processing and storage. Innovative Food Science and Emerging Technologies, 16, 121–128.
- Jacobo-Velázquez, D. A., Martínez-Hernández, G. B., Rodríguez, S., Cao, C. -M., & Cisneros-Zevallos, L. (2011). Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. Journal of Agricultural and Food Chemistry, 59(12), 6583–6593.
- Kahlon, T. S., Chapman, M. H., & Smith, G. E. (2007). In vitro binding of bile acids by spinach, kale, Brussels sprouts, broccoli, mustard greens, green bell pepper, cabbage and collards. Food Chemistry, 100(4), 1531–1536.
- Khachik, F., Beecher, G. R., & Goli, M. B. (1991). Separation, identification, and quantification of carotenoids in fruits, vegetables and human plasma by high performance liquid chromatography. Pure and Applied Chemistry, 63(1), 71–80.
- Khachik, F., Beecher, G. R., & Whittaker, N. F. (1986). Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. Journal of Agricultural and Food Chemistry, 34(4), 603–616.
- Kim, S. Y., Yoon, S., Kwon, S. M., Park, K. S., & Lee-Kim, Y. C. (2008). Kale juice improves coronary artery disease risk factors in hypercholesterolemic men. Biomedical and Environmental Sciences, 21(2), 91–97.
- Kural, B. V., Küçük, N., Yücesan, F. B., & Örem, A. (2011). Effects of kale (Brassica oleracea L. var. acephala DC) leaves extracts on the susceptibility of very low and low density lipoproteins to oxidation. Indian Journal of Biochemistry & Biophysics, 48, 361–364.
- Lee, H. S., Castle, W. S., & Coates, G. A. (2001). High-performance liquid chromatography for the characterization of carotenoids in the new sweet orange (Earlygold) grown in Florida, USA. Journal of Chromatography A, 913(1–2), 371–377.
- Mendes-Pinto, M. M., Ferreira, A. C. S., Oliveira, M. B. P. P., & De Pinho, P. G. (2004). Evaluation of some carotenoids in grapes by reversed- and normal-phase liquid chromatography: A qualitative analysis. Journal of Agricultural and Food Chemistry, 52(10), 3182–3188.
- Miranda Rossetto, M. R., Shiga, T. M., Vianello, F., & Pereira Lima, G. P. (2013). Analysis of total glucosinolates and chromatographically purified benzylglucosinolate in organic and conventional vegetables. LWT – Food Science and Technology, 50(1), 247–252.
- Nagai, K., Hosaka, K., Kobo, S., Nakabayashi, T., Amagasaki, Y., & Noriko, N. (1999). Vitamin A toxicity secondary to excessive intake of yellow-green vegetables, liver and laver. Journal of Hepatology, 31(1), 142–148.
- Okamura, M. (1980). An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clinica Chimica Acta, 103(3), 259–268.
- Olsen, H., Aaby, K., & Borge, G. I. (2009). Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. Journal of Agricultural and Food Chemistry, 57, 2816–2825.
- Sikora, E., & Bodziarczyk, I. (2012). Composition and antioxidant activity of kale (Brassica oleracea L. var. acephala) raw and cooked. ACTA Scientiarum Polonorum Technologia Alimentaria, 11(3), 239–248.
- Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158.
- Song, L., & Thornalley, P. J. (2007). Effect of storage, processing and cooking on glucosinolate content of Brassica Vegetables. Food and chemical toxicology, 45(2), 216–224.
- Steinbrecher, A., Nimptsch, K., Hüsing, A., Rohrmann, S., & Linseisen, J. (2009). Dietary glucosinolate intake and risk of prostate cancer in the EPIC-Heidelberg cohort study. International journal of cancer, 125(9), 2179–2186.
- Taylor, K. L., Brackenridge, A. E., Vivier, M. A., & Oberholster, A. (2006). High-performance liquid chromatography profiling of the major carotenoids in Arabidopsis thaliana leaf tissue. Journal of Chromatography A, 1121(1), 83–91.
- Wu, X., Beecher, G. R., Holden, J. M., Haytowitz, D. B., Gebhardt, S. E., & Prior, R. L. (2004). Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. Journal of Agricultural and Food Chemistry, 52(12), 4026–4037.
- Zhou, K., & Yu, L. (2006). Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT – Food Science and Technology, 39(10), 1155–1162.
