Abstract
The effect of lintnerization on the banana starch of the Roatán cultivar from acid hydrolysis at 3, 5, and 7 days on the starch digestibility and morphological and thermal features was evaluated. Acid treatment produced an increase in the rapidly digestible starch (RDS) (20.2%) and slowly digestible starch (29.7%), but a decrease in the resistant starch (36.3%) content, as compared with its native counterpart. However, over the longest acid-treatment time, a decrease in the RDS over 5 days was observed. The lintnerized banana starch can be used in food products without additional cooking. The temperature (77–80°C) and enthalpy (11.6–14.1 J/g) of gelatinization increased significantly when the acid-treatment time increased, indicating an arrangement of the crystalline lamella by the double helices of the amylopectin chains. The acid treatment increased the retrogradation rate of the lintnerized banana starch gels. The lintnerization of banana starch for different lengths of time modified its morphology, starch digestibility, and thermal features, indicating changes in the crystalline lamella due to the arrangement of the double helices of amylopectin.
Se evaluó el efecto de la lintnerización del almidón de plátano variedad Roatán a 3, 5 y 7 días sobre sus características de digestibilidad, morfológicas y térmicas. El tratamiento ácido produjo un incremento en el contenido de almidón de digestión rápida (ARD) (20,2%) y digestión lenta (ALD) (29,7%), así como una disminución en el contenido de almidón resistente (AR) (36,3%), en comparación con su contraparte nativa. Sin embargo, a mayor tiempo de hidrólisis ácida se observó una disminución en el contenido de ARD después de los 5 días. El almidón de plátano lintnerizado puede ser utilizado en alimentos donde no se necesite calentamiento. La temperatura (77–80°C) y entalpía (11,6–14,1 J/g) de gelatinización incrementaron significativamente cuando el tiempo de tratamiento ácido aumentó, indicando un rearreglo de las zonas cristalinas debido a las dobles hélices de las cadenas de la amilopectina. El tratamiento ácido incrementó la velocidad de retrogradación en los geles de almidón de plátano. La Lintnerización del almidón de plátano a diferentes tiempos modificó sus características morfológicas, de digestibilidad y térmicas, indicando cambios en las zonas cristalinas debido a un rearreglo de las dobles hélices de las cadenas de la amilopectina.
Introduction
Currently, there is great interest in developing foods with improved nutritional characteristics that are capable of preventing disease. This is because many countries may be associated with the consumption of high amount of digestible carbohydrates and saturated fat. For example, Mexico occupies one of the first places in the world for a population that is developing several chronic degenerative diseases such as obesity, diabetes, and cardiovascular disease. Therefore, the food industry is seeking strategies to develop new products where bioactive compounds such as antioxidants, fatty acid (omega 3 and 6), dietary fiber, and resistant starch (RS) are included to obtain a final product with two main properties from regular ingestion: the traditional nutritional aspects of any food and additional health benefits (Aparicio-Saguilán et al., Citation2005). It has been reported that there is a fraction of starch present in foods that resist enzymatic digestion and it is called RS (Asp, Citation1992). Therefore, the RS is not digested or absorbed in the small intestine, but reaches the colon and once it is there the intestinal bacteria use it as a substrate for fermentation, making short-chain fatty acids such as acetic, propionic, and butyric acids. These acids generate beneficial effects for human health as they can prevent colon cancer and cardiovascular disease and may also regulate the absorption and secretion of water and ions in the intestinal epithelium (Lutz & Scharrer, Citation1991). It has also been reported that a starch fraction of slow digestion has important implications for health. For this reason, starch is classified from the digestibility point of view as rapidly digestible starch (RDS), slowly digestible starch (SDS), and RS (Englyst, Kingman, & Cummings, Citation1992). SDS is fully digested in the small intestine, but is digested slowly. This type of starch can provide a substantial and sustained release of glucose in the blood. The nutritional properties of SDS have potential for use in the diet and in the prevention of diseases such as diabetes. SDS and RS can be obtained from the physical modification of native starch using repeated autoclaving and cooling cycles (González-Soto, Agama-Acevedo, Solorza-Feria, Rendón-Villalobos, & Bello-Pérez, Citation2004; Ranhotra, Gelroth, & Glaser, Citation1996), extrusion (Hasjim & Jane, Citation2009), and enzymatic (Polesi & Silveira-Sarmento, Citation2011) or chemical modification (Woo & Seib, Citation2002). Lintnerization has been the most widely used chemical method to modify the starch structure and generate crystalline structures that resist enzymatic hydrolysis. Some authors have conducted studies on the formation of RS using different conditions of acid hydrolysis to obtain linear chains with lengths that favor the phenomenon of retrogradation. For example, Lehmann, Rössler, Schmiedl, and Jacobasch (Citation2003) lintnerized pea starch during two different periods of time over 1 and 7 days. They reported a higher value of RS after 1 day of hydrolysis (51.3%), which was more than after 7 days (17.2%) of treatment. After 7 days, the molecular degradation was very high, generating short chain lengths which did not favor chain retrogradation. Espinosa-Solis, Sanchez-Ambriz, Hamaker, and Bello-Pérez (Citation2011) reported increases in the RS content of banana (Macho cultivar) and mango starch through increasing the acid concentration. However, the SDS content increased only to about 30% in banana starch. In previous studies, Aparicio-Saguilán et al. (Citation2005) reported a high content of RS for banana starch (Macho cultivar) using lintnerization and lintnerization-autoclaving processes. Autoclave-cooling cycles of lintnerized starch increased the RS content up to 19.3%.
Banana is a climacteric fruit that is consumed when it is maturity; however, black Sigatoka disease produces a great loss of this fruit in Mexico, mainly in the states of Oaxaca and Veracruz. Black Sigatoka is a disease caused by the ascomycete fungus Mycosphaerella fijiensis, which is the main phytopathological problem of bananas and plantains on the American, Asian, and African continents (Marín, Romero, Guzmán, & Sutton, Citation2003). The pathogen rapidly destroys the leaf tissue, resulting in the photosynthesis being reduced and affecting the plant growth, the fruit quality (thickness and length), and the production, thus causing losses of up to 100%. The fruit from the banana trees with Black Sigatoka does not meet the quality for commercialization as dessert bananas, but it can be used for starch isolation (Bello-Pérez, Agama-Acevedo, Sánchez-Hernández, & Paredes-López, Citation1999).
Banana is a food rich in indigestible compounds such as RS and non-starch polysaccharides such as dietary fiber. Several studies have suggested that consumption of green banana, in particular, has a beneficial effect on human health. However, when the fruit is ripe, the starch hydrolyzes to single sugars such as glucose, fructose, and sucrose; additionally, cooking of some varieties changes the RS to digestible starch. Since the banana variety, Roatán, is one of the foods with high starch content and is perishable, the aim of this work was to study the effect of lintnerization over different hydrolysis times of banana starch on the morphological, thermal, and functional properties, and to relate this with the SDS and RS content.
Materials and methods
Materials
The unripe banana was a gift from the farm “Mundo Nuevo” located in Tuxtepec, Oaxaca, Mexico. The fruit does not meet the quality for direct commercialization. The enzymes acquired from Sigma Aldrich used to determine the properties of digestibility were: Amyloglucosidase (A7095) from Aspergillius niger (300 U/mL), pepsin (P7000) from porcine stomach mucosa (1:10,000 U/mg), pancreatin (P7545) from porcine pancreas (8× USP), invertase (I4504) grade VII from bakers’ yeast (401 U/mg); while the guar gum was purchased from Sigma Aldrich Co. (St. Louis, MO, USA).
Isolation of banana starch
To obtain the starch from unripe banana, the methodology reported by Flores-Gorosquera, García-Suárez, Flores-Huicochea, Nuñez Santiago, and Bello-Pérez (Citation2004) was followed. After the banana peel was removed the fruit was ground in an industrial type blender, (Waring Laboratory, model CB 15, USA). The fruit (3.6 kg of peeled banana) was milled with 6 L of 3 g/L citric acid solution to prevent oxidation of the fruit. The resulting mixture was filtered using an electrical sieve (Retsch, model AS 200, Germany) and different mesh sizes: No. 40 (0.425 mm), 100 (0.15 mm), and 270 (0.053 mm). At each step of sieving, the product was washed with sufficient water until the aqueous solution showed no apparent starch residues. At the end of the sieving operation, the starch was allowed to precipitate overnight, Thereafter, the supernatant was decanted and the starch was washed with distilled water, this process was repeated three times. Finally, the product was dried in a tray dryer (manufactured by SUSESA) at 40°C overnight. The powder obtained with a moisture content of 10% was sieved using a 100-mesh screen, weighed and stored in a container until use.
Lintnerization of starch
Starch lintnerization was conducted by the method proposed by Shin, Woo, and Seib (Citation2003). Banana starch (500 g) was added to 0.4 L of HCl (1.6 M) at different reaction times (3, 5, and 7 days), using constant agitation of 50 rpm at 35°C. After each hydrolysis time, the reaction was neutralized with NaOH (1.6 M) to adjust to pH 7. Starch solids were washed six times with distilled water. The product was dried at 40°C for 24 h. Subsequently, it was ground and sieved with a 100-mesh screen. Finally, the starch obtained was dried at 40°C for 24 h.
Polarized light optical microscopy
The analysis of polarized light microscopy was performed using a Zeiss microscope model Axio Scope-A1 equipped with a Minolita camera and using a 40× objective. In order to observe the starch granules, the samples were premixed with 10 µl of a solution of iodine (2 g/L I2 and 20 g/L KI).
In vitro digestibility tests
Starch digestibility properties were determined by the method proposed by Englyst et al. (Citation1992). This method consists of an enzymatic hydrolysis. Before carrying out the determination of digestibility of starch, a solution containing the enzymes pancreatin, amyloglucosidase and invertase was prepared. Starch (200 mg) was mixed with 2 mL of deionized water in a glass tube, the mixtures were adjusted to 37°C, and 4 mL of pepsin/guar gum/hydrochloric acid solution were added to the tubes and reacted for 30 min. Then 2 mL of 0.25 M sodium acetate and six glass beads were added, the tubes were put in slanted position with shaking at 160 rpm for 20 min. Finally, 2 mL of the enzyme cocktail was added in intervals of 1 min by sample, and after 20 min 50 mL was removed from the sample mixture, placed in a microcentrifuge tube containing 950 µL of 80% ethanol, and after 100 min 50 µL was removed. The microcentrifuge tubes were spun at 14 857g for 5 min and 50 mL of the hydrolyzates were analyzed for glucose content using the GOPOD method (Megazyme International Ireland Ltd., Wicklow, Ireland). Each sample was analyzed in triplicate.
Swelling and solubility profile
Swelling (G) and solubility (S) profiles of native and lintnerized starch were determined according to the Schoch method (1964), which was modified by Sathe and Salunkhe (Citation1981). Using a vortex, 400 mg of each starch sample was dispersed in 40 mL of distilled water. Subsequently, the dispersion was heated to a temperature profile of 50, 70, 80, and 90°C, leaving the stabilized sample at each temperature for 30 min. Each solution at the specified temperature was centrifuged at 3000g for 10 min. The supernatant was decanted and the settled solid (swollen granules) was weighed. Ten milliliter of the supernatant were taken and placed in a previously tared aluminum pan. This was subsequently dried at 120°C for 4 h. G and S were determined as: S = sedimented solid weight/dry weight of the sample; S = (weight of dissolved solids in the supernatant/dry sample weight) × 100.
Thermal analysis
Thermal properties of the starches were studied using a TA Instruments (model 2010, USA) differential scanning calorimeter. Thermal transitions were determined by the method proposed by Paredes-López, Bello-Pérez, and López (Citation1994). Two milligram of each sample (dry basis) were weighed and placed in an aluminum pan, and then 7 μl of deionized water was added. The pan was hermetically sealed and allowed to equilibrate for 1 h before analysis. An empty pan was used as a reference. The sample was subjected to a heating program in a temperature range of 30–140°C and at a rate of 10°C/min. The onset temperature (Tonset), peak temperature (Tp), final temperature (Tf), and gelatinization enthalpy (ΔH) were analyzed using the TA Instruments software OS/2 version 2.1. The gelatinized samples were stored at 4°C for 7 days to evaluate the starch retrogradation. After completion of the storage time, the pans were allowed to stand for 1 h at room temperature and then analyzed in the differential scanning calorimeter (DSC), using the same conditions described previously to starch gelatinization.
Statistical analysis
The results obtained for thermal analysis and digestible test were analyzed using one-way Analysis of Variance procedures. Where analysis showed significant differences (p < 0.05), means were compared using Tukey’s tests at a level of significance of 0.05. Statistical analyses were run using the computer SPSS V. 6.0 (software SPSS Institute Inc., Cary, NC, USA).
Results and discussion
Morphological analysis
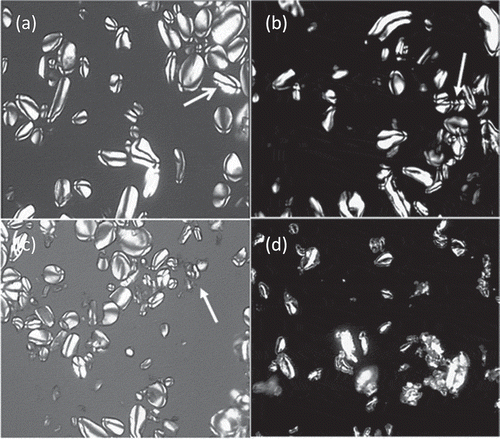
shows photomicrographs obtained by polarized light optical microscopy of native and lintnerized banana starch. In , it is observed that Roatán variety banana starch granules have elliptical shapes. The Maltese cross (indicated with an arrow), which is associated with a molecular order of the starch components (amylose and amylopectin) in the granule, is visible. Millan-Testa, Mendez-Montealvo, Ottenhof, Farhat, and Bello-Pérez (Citation2005) reported a similar morphology for Macho banana starch. However, in other cultivars such as Valery the morphology of the granules has been reported as spherical and elongated shapes (Zhang, Whistler, BeMiller, & Hamaker, Citation2005). The morphological characteristics of the banana starch granules over 3 and 5 days of hydrolysis did not show appreciable differences compared to the control sample ( and c), which was starch granules presenting the Maltese cross, indicating that an ordering of starch components was present even after the acid hydrolysis that produced a perfection of the residual crystallites. However, over the longest acid-treatment time (7 days, ), some granules were destroyed and these were aggregates, others did not show birefringence, and some intact granules were present. The acid-treatment time is an important factor in producing the perfection of the crystallites (this means an increase in the crystallinity level and sharp peaks) and modifying the starch digestibility and its functional properties. It is well known that in the first steps of acid hydrolysis, which were 3 and 5 days of hydrolysis in this study, hydrolysis of amorphous zones of starch granules was produced (Singh & Ali, Citation2009), while after 7 days of acid hydrolysis, the crystalline regions were partially degraded (Espinosa-Solis et al., Citation2011). The crystallinity level in maize starch (A-type X-ray diffraction pattern) after acid hydrolysis over 3 and 7 days increased 5.5% and 7.7%, respectively, but over the highest hydrolysis time (15 days), the granular structure was completely destroyed with a formation of large aggregates (Zhang, Venkatachalam, & Hamaker, Citation2006). Lintnerized potato starch (B-type) over 21 days destroyed the granular structure, but the increment in the crystallinity level was just 8.6% (Zhang et al., Citation2006). The acid not only hydrolyzed the amorphous zones of the starch granules, but also the crystalline regions, indicating that all parts of the starch granules might have been affected. This can be corroborated with the disappearance of the Maltese cross () (Bertoft, Citation2004), and depending on its polymorphism, different structures can be obtained, thus affecting the digestibility and the physicochemical characteristics. The conditions used (acid concentration and time) and the starch source (C-type polymorphism) were important to obtain the morphological features of starch, which reflects the change in the structure after lintnerization.
Figure 1. Microphotograps by polarized light optic microscopy of native and lintnerized starch at different times: (a) native starch, (b) 3 days, (c) 5 days, and (d) 7 days.
Figura 1. Micrografías de microscopía de luz polarizada del almidón nativo y lintnerizado a diferentes tiempos: (a) almidón nativo, (b) 3 días, (c) 5 días, (d) 7 días.
Thermal analysis
shows the thermal transition in excess of water (gelatinization) of native and lintnerized banana starch of the Roatán cultivar on different days, and presents the temperatures and enthalpy of this phase transition. The acid hydrolysis for shorter times (3 and 5 days) produced an increment in the Tp, Tf, and enthalpy values. The arrangement of the starch components and perfection of the crystallites produced for the acid hydrolysis were responsible for this pattern. Acid treatment produced the removal of amorphous regions of the starch granule and the remaining crystalline lamella melted at a higher temperature. At the longest acid-treatment time (7 days), the Tonset and Tf decreased, but the enthalpy values increased as compared with the samples hydrolyzed for 3 and 5 days. The lower Tonset and Tf were due to some crystalline regions of amylopectin being disorganized, and minor temperatures were required to complete the gelatinization process. The wideness of the thermogram over the longest acid hydrolysis times (5 and 7 days) indicated that a mixture of less perfect and/or imperfect crystallites was present, and this was more evident in the sample hydrolyzed for 7 days. The increment in the enthalpy value reflected the energy needed to melt the long double helices of the amylopectin molecule, and double helices formed between amylose and amylopectin, as well as amylose–amylose, during lintnerization (Jayakody & Hoover (Citation2002). The structure produced during lintnerization of the banana starch at different times was due to the fact that, in the first steps, acid hydrolyzes the amorphous regions of the granule and decreases with time. Eventually, the acid hydrolysis continued to partially hydrolyze the crystalline lamella of the granules (Biliaderis, Grant, & Vose, Citation1981). The remaining structure after the acid hydrolysis is a mixture of dextrins, and their proportion as well as arrangement depended on the hydrolysis level (Angellier-Coussy et al., Citation2009; Jacobs, Eerlingen, Rouseu, Colonna, & Delcour, Citation1998; Robin, Mercier, Charbonniere, & Guilbot, Citation1974). These thermal results are associated with the starch digestibility shown by the lintnerized banana starch.
Figure 2. DSC thermograms of lintnerized starch at the indicated times.
Figura 2. Termogramas de DSC del almidón lintnerizado a los tiempos indicados.
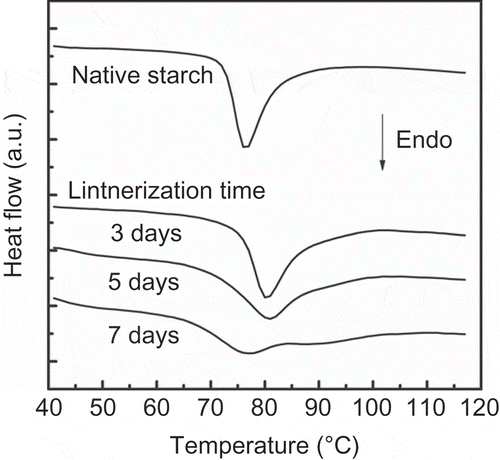
Table 1. Temperatures and enthalpy of gelatinization of lintnerized banana starch at different times.
Tabla 1. Temperaturas y entalpía de gelatinización del almidón de plátano lintnerizado a diferentes tiempos.
shows the endotherms of retrogradation of native and acid-treated banana starch. For all stored gelatinized samples, a wide endotherm was found, indicating heterogeneity of the crystallites produced during starch reorganization. This endotherm of the retrograded gel indicates the melting of the recrystallized starch structure. The temperatures and enthalpy of the retrogradation increased with the hydrolysis time (). This increment might be related to the increment in the mobility of smaller chains produced during the acid hydrolysis as well as the decrement in the branching points of amylopectin (Atichokudomchai, Varavinit, & Chinachoti, Citation2002). The increment in the thermal parameters was more evident over longer hydrolysis times due to the fact that a higher concentration of short chains was produced from the hydrolysis of amylose and external long chains of amylopectin, and it is possible for them to retrograde during storage in a more perfect and/or more stable double helix, resulting in increments in enthalpy value. The retrogradation test of the lintnerized banana starch is important during the development of products that have the addition of this modified starch, where cooking and storage are included.
Figure 3. DSC thermograms of retrograded starch as a function of lintnerization time.
Figura 3. Termogramas por DSC del almidón retrogradado en función del tiempo de lintnerización.
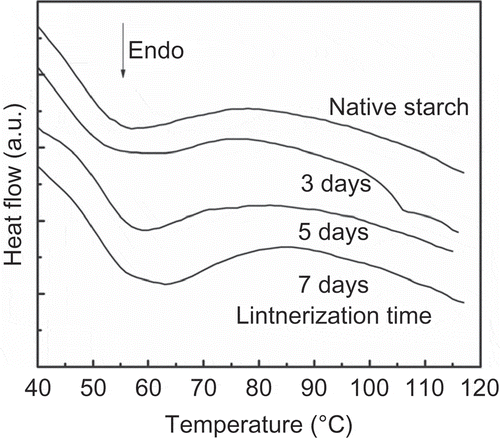
Table 2. Temperatures and enthalpy of retrogradation of lintnerizated banana starch at different times.
Tabla 2. Temperatura y entalpía de retrogradación del almidón de plátano lintnerizado a diferentes tiempos.
Swelling and solubility
To find some functional characteristics of the lintnerized starch powders in some applications, the swelling and solubility were tested. shows the swelling profile of native and lintnerized starch at different temperatures. In general, swelling of all the samples started from 70°C; however, the lintnerized starch over 7 days showed a slight increment in the swelling value at 70°C due to the formation of lintners of small particle size, and those lintners can join more water molecules. This effect was kept at higher temperatures because the lintners over 7 days had the highest swelling values. A similar pattern was found for the starch lintners over 3 and 5 days because after 70°C an increment in the swelling values was shown. The increment in the temperature produced starch disorganization and thus, more water molecules could penetrate to the granules, increasing the swelling values. The difference between lintners of 3 and 5 days at the highest temperature tested is related to the minor change produced by the acid over the shorter hydrolysis time (3 days) as compared with the sample with 5 days of hydrolysis, where more intact granules were observed in the lintners of 3 days. During heating, they retained more water molecules, increasing the swelling value. The decrement in the swelling value of the native starch at the highest temperature indicates the breaking of the starch granules with a loss of water and consequently, a lower swelling value.
Figure 4. Swelling behavior of lintnerized starch at the indicated times.
Figura 4. Comportamiento de hinchamiento del almidón lintnerizado a los tiempos indicados.
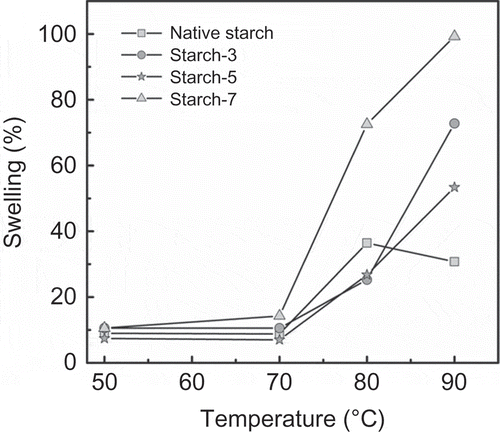
Figure 5. Solubility percentage of lintnerized starch at the indicated times.
Figura 5. Porcentaje de solubilidad del almidón lintnerizado a los tiempos indicados.
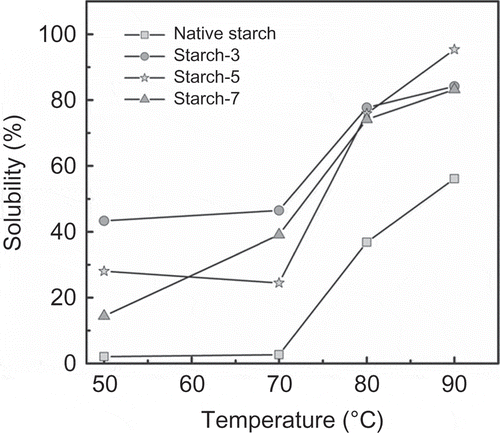
shows solubility values at different temperatures of the lintnerized banana starch. The rapid increment in the solubility of lintnerized banana starch for 7 days was due to the presence of small particles produced by the acid treatment. However, for all lintnerized banana starches (3, 5, and 7 days), a rapid increment was shown after 70°C, a pattern that is related to the higher disorganization of starch granules with the gelatinization temperature obtained in the DSC analysis. Native banana starch showed the lowest solubility values at the different temperatures, which was due to the integrity of the starch granules where restricted swelling and solubility were present.
The degree of packing of double helices in the crystalline lamellae in the lintnerized banana starch may also affect its swelling and solubility (Gao, Vasanthan, Hoover, & Li, Citation2012).
Starch digestibility
The properties of digestion of native and lintnerized starch without cooking are shown in . The RDS and SDS content increased, but the RS content decreased with the acid treatment as compared with the native starch. However, over the longest hydrolysis time (7 days), decrements in RDS and increments in the SDS and RS contents were found as compared to the sample hydrolyzed for 5 days, suggesting a higher degree of lintnerization and consequently a higher perfection of residual crystallites. It was reported that a range of amorphous domains with structural heterogeneity were present in the starch granules (Biliaderis, Citation1991), and that after the acid hydrolysis over different times, perfection of crystallites was produced on different levels, which would affect the enzymatic hydrolysis. A different pattern was reported in lintnerized maize starch because an increment in RDS content was determined in the sample hydrolyzed for 3 days, but longer acid-treatment times (7 and 15 days) showed a decrement, although the RDS values at 7 and 15 days were higher than that in the native sample. It was postulated that disruption of the granular structure with the acid treatment and formation of lintners of small particle size produced an increment in the effective surface area for enzyme adsorption and binding (Kong, Kim, Kim, & Kim, Citation2008). The SDS and RS content decreased with the degree of lintnerization, but both fractions increased in the lintnerized starch for 7 days as compared with its counterpart hydrolyzed for 5 days. This pattern could be related to an extensive hydrolysis, producing lineal chains that are prone to reorganize in a structure that is slowly digestible and resistant. The RS content over 7 days of acid hydrolysis of Roatán banana starch was higher than those values reported for other starch sources, such as mango (20.6%) (Espinosa-Solis et al., Citation2011), plantain (16.2%) (Espinosa-Solis et al., Citation2011), and maize (9.7%) (Zhang et al., Citation2006). A high content of RS of approximately 66% was determined in the native banana starch of Roatán cultivar. This value was higher than previously reported banana starch (40.0%) of the Macho cultivar (Aparicio-Saguilán et al., Citation2005). This is the first report of RS content in the Roatán cultivar. Extensive lintnerization of potato starch for 21 days produced an increment in RDS and a decrement in RS content, with little change in SDS as compared with its native counterpart (Zhang et al., Citation2006). The differences in the digestion properties shown among lintnerized banana, maize, and potato starches could be related to the building block organization of amylopectin, which is hydrolyzed by the acid in different ways (Bertoft, Koch, & Åman, Citation2012) or the distribution of amylose in the amorphous lamella, where amylose could be mixed with amylopectin to a lesser or higher degree depending on the botanical source. Additionally, the location of amylose within the granule might influence the acid hydrolysis of starch components (Wikman, Blennow, & Bertoft, Citation2013).
Table 3. Digestibility values of lintnerized banana starch at the indicated times (%).
Tabla 3. Valores de digestibilidad del almidón de plátano lintnerizado al tiempo indicado (%).
Conclusions
The lintnerization of banana starch over different days showed an increment in the RDSs and SDSs, with a decrement in RS content. The length of the acid treatment modified the morphological features of the banana starch granules with the formation of lintners of small particle size that influenced its digestion. The increment in the gelatinization temperature with the lintnerization reflected the increment in the crystalline lamella with the concomitant hydrolysis of the amorphous regions, along with the formation of more stable double helices. The gelatinized gels of lintnerized banana starch were prone to retrograde when the length of the acid treatment was increased. The swelling and solubility values increased with the length of the hydrolysis, indicating structural changes and formation of lintners of small particle size. The lintnerization over the different times modified the digestibility characteristics, thermal properties, and functional properties of Roatán banana starch. Therefore, by controlling the length of the acid treatment, it is possible to obtain starch with specific features that can be used in food products without additional cooking.
Acknowledgments
This project was financed by the PROMEP/103.5/09/4225 in Mexico, which is gratefully acknowledged.
References
- Angellier-Coussy, H., Putaux, J. L., Molina-Boisseau, S., Dufresne, A., Bertoft, E., & Perez, S. (2009). The molecular structure of waxy maize starch nanocrystals. Carbohydrate Research, 344, 1558–1566. doi:10.1016/j.carres.2009.04.002
- Aparicio-Saguilán, A., Flores-Huicochea, E., Tovar, J., García-Suárez, F., Gutiérrez-Meraz, F., & Bello-Pérez, L. A. (2005). Resistant starch-rich powders prepared by autoclaving of native and lintnerized banana starch: Partial characterization. Starch - Stärke, 57, 405–412. doi:10.1002/star.200400386
- Asp, N. G. (1992). Resistant starch. Proceedings from the second plenary meeting of EURESTA. European Journal of Clinical Nutrition, 46(2), SI.
- Atichokudomchai, N., Varavinit, S., & Chinachoti, P. (2002). A study of annealing and freeze-thaw stability of acid-modified tapioca starches by differential scanning calorimetry (DSC). Starch - Stärke, 54, 343–349. doi:10.1002/1521-379X(200208)54:8<343::AID-STAR343>3.0.CO;2-J
- Bello-Pérez, L. A., Agama-Acevedo, E., Sánchez-Hernández, L., & Paredes-López, O. (1999). Isolation and partial characterization of banana starches. Journal of Agricultural and Food Chemistry, 47, 854–857. doi:10.1021/jf980828t
- Bertoft, E. (2004). Lintnerization of two amylose-free starches of A and B-crystalline types, respectively. Starch/Stärke, 56, 167–180. doi:10.1002/star.200300255
- Bertoft, E., Koch, K., & Åman, P. (2012). Building block organisation of clusters in amylopectin from different structural types. International Journal of Biological Macromolecules, 50, 1212–1223. doi:10.1016/j.ijbiomac.2012.03.004
- Biliaderis, C. G. (1991). The structure and interactions of starch with food constituents. Canadian Journal of Physiology and Pharmacology, 69, 60–78. doi:10.1139/y91-011
- Biliaderis, C. G., Grant, D. R., & Vose, J. R. (1981). Structural characterization of legume starches. II. Study on acid-treated starches. Cereal Chemistry, 58, 502–507.
- Englyst, H. N., Kingman, S. M., & Cummings, J. H. (1992). Classification and measurement of nutritional important starch fractions. European Journal of Clinical Nutrition, 46, S33–S50.
- Espinosa-Solis, V., Sanchez-Ambriz, S. L., Hamaker, B. R., & Bello-Pérez, L. A. (2011). Fine structural characteristics related to digestion properties of acid-treated fruit starches. Starch/Stärke, 63, 717–727. doi:10.1002/star.201100050
- Flores-Gorosquera, E., García-Suárez, F. J., Flores-Huicochea, E., Nuñez Santiago, M. C., & Bello-Pérez, L. A. (2004). Yield of starch extraction from plantain (musa paradisiaca). Pilot plant study. Acta Científica Venezolana, 55, 86–90.
- Gao, J., Vasanthan, T., Hoover, R., & Li, J. (2012). Structural modification of waxy regular, and high-amylose maize and hulless barley starches on partial acid hydrolysis and their impact on physicochemical properties and chemical modification. Starch/Stärke, 64, 313–325. doi:10.1002/star.201100128
- González-Soto, R. A., Agama-Acevedo, E., Solorza-Feria, J., Rendón-Villalobos, R., & Bello-Pérez, L. A. (2004). Resistant starch made from banana starch by autoclaving and debranching. Starch-Stärke, 56, 495–499. doi:10.1002/star.200400283
- Hasjim, J., & Jane, J. (2009). Production of resistant starch by extrusion cooking of acid-modified normal-maize starch. Journal of Food Science, 74, C556–562. doi:10.1111/j.1750-3841.2009.01285.x
- Jacobs, H., Eerlingen, R. C., Rouseu, N., Colonna, P., & Delcour, J. A. (1998). Acid hydrolysis of native and annealed wheat, potato and pea starches—DSC melting features and chain length distributions of lintnerised starches. Carbohydrate Research, 308, 359–371. doi:10.1016/S0008-6215(98)00100-1
- Jayakody, L., & Hoover, R. (2002). The effect of lintnerization on cereal starch granules. Food Research International, 35, 665–680. doi:10.1016/S0963-9969(01)00204-6
- Kong, B., Kim, J., Kim, M., & Kim, J. B. (2008). Porcine pancreatic α-amylase hydrolysis of native starch granules as a function of granule surface area. Biotechnology Progress, 19, 1162–1166. doi:10.1021/bp034005m
- Lehmann, U., Rössler, C., Schmiedl, D., & Jacobasch, G. (2003). Production and physicochemical characterization of resistant starch type III derived from pea starch. Nahrung/Food, 47, 60–63. doi:10.1002/food.200390014
- Lutz, T., & Scharrer, E. (1991). The colonic flora, fermentation, and large bowel digestive function. In S. F. Philips (Ed.), The large intestine: Physiology, pathophysiology and disease (pp. 51–92). New York, NY: Mayo foundation, Raven Press.
- Marín, D. H., Romero, R. A., Guzmán, M., & Sutton, T. B. (2003). Black sigatoka: An increasing threat to banana cultivation. Plant Disease, 87, 208–222. doi:10.1094/PDIS.2003.87.3.208
- Millan-Testa, C. E., Mendez-Montealvo, M. G., Ottenhof, M. A., Farhat, I. A., & Bello-Pérez, L. A. (2005). Determination of the molecular and structural characteristics of okenia, mango and banana starches. Journal of Agricultural and Food Chemistry, 53, 495–501. doi:10.1021/jf048862x
- Paredes-López, O., Bello-Pérez, L. A., & López, M. G. (1994). Amylopectin: Structural, gelatinisation and retrogradation studies. Food Chemistry, 50, 411–417. doi:10.1016/0308-8146(94)90215-1
- Polesi, L. F., & Silveira-Sarmento, S. B. (2011). Structural and physicochemical characterization of RS prepared using hydrolysis and heat treatments of chickpea starch. Starch/Stärke, 63, 226–235. doi:10.1002/star.201000114
- Ranhotra, G. S., Gelroth, J. A., & Glaser, B. K. (1996). Energy value of resistant starch. Journal of Food Science, 61, 453–455. doi:10.1111/j.1365-2621.1996.tb14215.x
- Robin, J. P., Mercier, C., Charbonniere, R., & Guilbot, A. (1974). Litnerized starches. Gel filtration and enzymatic studies of insoluble residues from prolonged acid treatment of potato starch. Cereal Chemistry, 51, 389–406.
- Sathe, S. K., & Salunkhe, D. K. (1981). Isolation, partial characterization and modification of the great northern bean (Phaseolus vulgaris L.) starch. Journal of Food Science, 46, 617–621. doi:10.1111/j.1365-2621.1981.tb04924.x
- Schoch, T. J. (1964). Swelling power and solubility of granular starches. In R. L. Whistler (Ed.), Methods in carbohydrate chemistry: Vol. 4 (pp. 106–108). New York: Academic Press.
- Shin, M., Woo, K., & Seib, P. (2003). Hot-water solubilities and water sorptions of resistant starches at 25 °C. Cereal Chemistry, 80, 564–566. doi:10.1094/CCHEM.2003.80.5.564
- Singh, V., & Ali, S. Z. (2009). Studies on acid modified starches – a review. Trends Carbohydrate Research, 1, 1–17.
- Wikman, J. A., Blennow, A., & Bertoft, E. (2013). Effect of amylose deposition on potato tuber starch granule architecture and dynamics as studied by lintnerization. Biopolymers, 99, 73–83. doi:10.1002/bip.22145
- Woo, K. S., & Seib, P. A. (2002). Cross-linked resistant starch: Preparation and properties. Cereal Chemistry, 79, 819–825. doi:10.1094/CCHEM.2002.79.6.819
- Zhang, G., Venkatachalam, M., & Hamaker, B. (2006). Structural basis for the slow digestion property of native cereal starches. Biomacromolecules, 7, 3259–3266. doi:10.1021/bm060343a
- Zhang, P., Whistler, R. L., BeMiller, J. N., & Hamaker, B. R. (2005). Banana starch: Production, physicochemical properties, and digestibility – a review. Carbohydrate Polymers, 59, 443–458. doi:10.1016/j.carbpol.2004.10.014
