Abstract
Agave syrup is a fermentable by-product from the Agave industry that is used for pulque production, a typical Mexican fermented beverage. However, to date, the information available on its physicochemical composition is scarce, with this study being one of the first contributions on the subject. Here the influence of thermal treatment at 121ºC/15 min on the physicochemical composition of agave syrup was studied. The chemical composition based on sugar content was evaluated by thin-layer chromatography and high-performance liquid chromatography. In addition, the mass spectrum is also presented. Results showed that thermal treatment promoted a change in the chemical composition of the agave syrup, particularly in sugar concentration favoring a high sucrose concentration after the sterilization process. Fructose, glucose in particular, and kestose (GF2) were detected in both samples. The presence of prebiotics such as GF2 suggests that agave syrup may be used in food and nutraceutical industries as a functional beverage.
El jarabe de Agave es un subproducto típico fermentable de la industria del Agave, que es usado para la producción del pulque, una bebida fermentada mexicana. Sin embargo, hasta la fecha, la información disponible sobre su composición fisicoquímica es escasa, siendo esta una de las primeras contribuciones. Aquí, la influencia del tratamiento térmico a 15 libras/121ºC/15 min en la composición fisicoquímica del jarabe de Agave fue estudiada. La composición química basada en el contenido de azúcares fue evaluada por cromatografía en capa fina (TLC) y cromatografía líquida de alta resolución (HPLC). Además, el espectro de masas también es presentado.
Los resultados muestran que el tratamiento térmico cambió la composición química del jarabe de Agave, particularmente en la concentración de azúcares. La sacarosa fue el carbohidrato presente en alta concentración después del proceso de esterilización. La fructosa, glucosa y en particular la kestosa (GF2) fueron detectadas en ambas muestras. La presencia de prebióticos tales como GF2 sugiere que el jarabe de Agave podría ser usado en las industrias de alimentos y farmacéuticas como una bebida funcional.
Introduction
Agave plants are abundant in the semi-desert zones of Mexico where they have been sources of foods (Colunga, Coello, Espejo, & Fuente, Citation1993). Some species of agaves known as “magueyes pulqueros” (Agave salmiana, A. mapisaga, A. atrovirens, A. Americana, A. ferox) are mainly used to produce pulque, one of the oldest alcoholic beverages on the American continent (Ortiz-Basurto et al., Citation2008). This alcoholic beverage is produced by fermentation of the agave syrup by natural microflora, in a short process (12–24 h) with a final alcohol concentration ranging from 3% to 6% (Valadez, Bravo, Santos, Velasco, & Montville, Citation2012).
Some studies have reported that agave syrup is a product with functional properties due to the presence of bioactive compounds such as amino acids and sugars (Ortiz-Basurto et al., Citation2008). One advantage of the use of agave syrup is that it can be consumed quickly after collection without prior pre-treatment. However to date, very few reports have been published on the chemical composition of agave syrup and therefore the chemical changes after thermal treatment are yet unknown.
Few studies regarding the bromatological composition of agave syrup from different species of agaves have been conducted (Ortiz-Basurto et al., Citation2008; Sanchez & Hope Citation1953; Valadez et al., Citation2012). However, the chemical characterization of this typical Mexican beverage is important to establish quality markers and nutritional composition which are relevant aspects in the production of high-quality products for the food industry. Therefore, the aim of this study is to determine the composition of agave syrup and to study the influence of thermal treatment (sterilization) on its composition focusing on sugar content.
Materials and methods
Chemicals and standards
Chromatographic silica gel 60 F254 aluminum sheets (20 × 20 cm) were obtained from Merck (KGaA Darmstadt, Germany). Fructose (F), glucose (G), and sucrose (S) were purchased from Analytika Company. Fructooligosaccharides standards 1-kestose (GF2) 1-nystose (GF3) and 1-β-fructofuranosyl nystose (GF4) were obtained from Wako Chemicals GmbH Company. Acetonitrile and water were HPLC grade. Other chemicals used in this study were analytical grade.
Sample collection and treatment
Agave syrup obtained from Agave salmiana (green maguey) was collected in February 2013 in the Mangas locality, which is close to Saltillo Coahuila, Mexico. Agave syrup was first collected and filtered with a strainer, then stored at a cold temperature and transported. It was then filtered through a fine pore filter paper and stored at −18ºC. For physicochemical characterization, the stored agave syrup was unfrozen and treated by thermal process at 121ºC during 15 min for comparison with untreated samples. Evaluation of the thermal process was performed in triplicate.
Physicochemical analysis
The analysis of untreated and thermally treated agave syrup consisted of the determination of total sugars (TS) content by the Anthrone method (Campana-Torres, Martinez-Cordova, Villarreal-Colmenares, & Civera-Cerecedo, Citation2006; Dreywood, Citation1946; Pearson, Maita, & Lalli, Citation1984), reducing sugars (RS) content by the method previously reported by Miller (Citation1959), using 3-amino-5-dinitrosalicylic acid reagent with some modifications (Gonçalves, Rodríguez, Gomes, Teixeira, & Belo, Citation2010), protein content by the method of Bradford (Bradford Citation1976), lipids, ashes (i.e. mineral content), total solids, moisture and pH using the AOAC methods (AOAC, Citation1990) and soluble solids (°brix) and density with a densitometer (Anton Paar, DMA 35 N, Austria). All analyses were carried out in triplicate.
Detection of sugars by TLC analysis
Thin-layer chromatography analysis (TLC) was used for the qualitative identification of sugars of untreated and thermally treated agave syrup. One microliter of each standard solution of fructose, glucose, sucrose, 1-kestose, 1-nystose, 1-β-fructofuranosyl-nystose, and agave syrup samples were applied to the plates. TLC plates were placed in a mixture of propanol-water-butanol (12:4:3 v/v) used as mobile phase at 30ºC to mark the final plate. After elution, the TLC plates were dried. Chromatograms of the compounds of interest were revealed with a solution of diphenylamine-aniline-phosphoric acid in 4% acetone heated at 100 ºC for 3–5 min in accordance with the methodology by Reiffová and Nemcová (Citation2006). The characteristic color was produced for each compound: pink spots for fructose, 1-kestose, 1-nystose, and 1-β-fructofuranosyl-nystose, blue spots for glucose, and brown spots for sucrose.
Quantification of sugars by HPLC analysis
High-performance liquid chromatography (HPLC) analysis of the untreated and thermally treated agave syrup assisted in the identification and quantification of fructose, glucose, sucrose, GF2, GF3, and GF4 using a Perkin Elmer Series 200 HPLC. Before injection, the samples were filtered through 0.45-µm nylon filters. Separation was carried out at room temperature with a Prevail Carbohydrate ES column (5 µm, 250 × 4.6 mm, Grace). The analytical conditions were an isocratic elution with a mixture of acetonitrile–water (70:30 v/v) at a flow rate of 1 mL min−1 during 18 min. The injection volume used was of 20 µL. Refractive index detector was employed to obtain the data which were recorded and integrated using the Total Chrom Workstation software (Perkin Elmer) (Mussatto, Aguilar, Rodrigues, & Teixeira, Citation2009).
Electrospray ionization-mass spectrometry (ESI-MS) parameters
In this study, a liquid chromatography ion trap mass spectrometer (Varian 500-MS IT Mass Spectrometer, USA) equipped with an electrospray ion source was used. Manual injection of samples at an infusion flow rate of 10 µL min−1 was conducted into the mass spectrometer source during 7 min. All MS analyses were carried out in the negative mode [M-H]−1. Nitrogen was used as a nebulizing gas and helium as a damping gas. The ion source parameters were spray voltage 3.0 kV, capillary voltage and temperature were 90.0 V and 250°C, respectively. Data were collected and processed using MS Workstation software (V 6.9). Full scan spectra were acquired in the m/z range 50–2000. All samples were analyzed in full scan mode.
Statistical analysis
Analysis of variance (ANOVA) was conducted using the software SAS (V 9.0). The Tukey test was used for the multiple comparisons in each individual assay. The differences between mean values were considered significant at p < 0.05.
Results and discussion
In this study, the physicochemical composition of agave syrup was evaluated (). Results showed that agave syrup is mainly composed of sugars. Interestingly, only RS profile was modified after the thermal processing, whereas the other components were not modified with the thermal effect. Sanchez-Marroquin and Hope (Citation1953) reported the amount of total RS (100 g L−1), ashes (3.1 g L−1), protein (1.7 g L−1), and Brix degrees (11.0) in samples of sterile agave syrup. These results are different from our work as he evaluated a different spice; however, to date there are no reports that confirm a complete chemical composition of agave syrup, in contrast to the study regarding natural microbial profiles that has been extensively investigated in the samples of pulque and sometimes in samples of agave syrup (Valadez et al., Citation2012).
Table 1. Physicochemical composition of agave syrup.
Tabla 1. Composición fisicoquímica del jarabe de Agave.
Valadez et al. (Citation2012) studied the chemical composition of samples of unfermented agave syrup obtained from three farms. The authors reported differences in contents of protein, carbohydrates, RS, ashes and total acidity. Interestingly, the total content of TS in our samples was higher than that found by these authors; they obtained 6.60% (Arches farm) and 4.48% (San Geronimo farm) while in our study, the content of TS was 10.2% and 10.8% for untreated and thermally treated agave syrup, respectively.
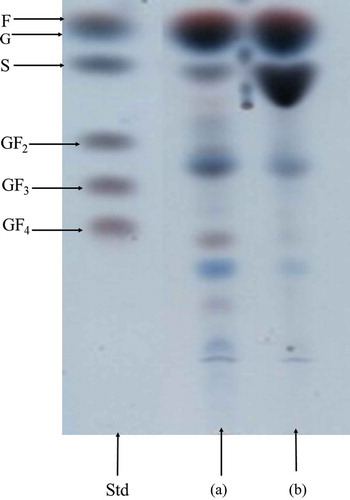
As mentioned above, sugars were the main compounds present in the agave syrup; therefore, a more in-depth study was conducted in order to provide more knowledge about the properties of the available sugars. Agave syrup was characterized using TLC, HPLC, and ESI-MS. TLC chromatography allowed the identification of mono-, di-, oligo- and some polysaccharides in the untreated and treated samples. These analyses of sugars showed high differences between both samples, where the untreated agave syrup presented fructose, glucose, and kestose, while sucrose was also found but in a lower concentration (). TLC is a semi-quantitative technique, where concentration of one compound is proportional to spot size in the plate. Additionally, monosaccharides: fructose and glucose could be easily identified with this technique using the color obtained during reaction allowing differentiation between aldoses (blue) and kestoses (pink) (Reiffová & Nemcová, Citation2006). Moreover, the TLC technique revealed the existence of different sugars in an oligomeric form and others with a higher degree of polymerization. According to the presented color, they could be aldoses and kestoses polymers. Treated agave syrup also contained fructose and glucose, while surprisingly sucrose was present at a higher concentration compared to the untreated agave syrup. Furthermore, a decrease in the content of kestose and oligomeric and polymerized compounds was observed in the treated agave. This could be explained by the presence of fructan oligomers or polymers composed by fructoses linked to the sucrose molecules which can be easily hydrolyzed by thermal treatments leading to free sugars mainly mono- or disaccharides (Apolinário et al., Citation2014). Our results could be related with the presence of inulin or levans in the agave syrup because these compounds have been reported in agave plants. Inulin-type fructans are fructose polymers that have linkages β-(2–1) fructosyl fructose mostly, while levan-type fructans have linkages β-(2–6) fructosyl in their structures (Apolinário et al., Citation2014). Glibowski and Bukowska (Citation2011) studied the effect of pH, temperature and heating time on inulin, and they reported that inulin breakdown occurred when temperature reached 80ºC at pH 4.0 which may have occurred in the agave syrup due to their pH. Several works also published the presence of fructans in Agave plants (Andrews & Urias, Citation2012; Bautista, García, Salcedo, & Parra, Citation2001; Mancilla & López, Citation2002; Ravenscroft et al., Citation2009), supporting the presence of some fructans in the agave syrup (Valadez et al., Citation2012); however, there are no reports that reveal the presence of inulin or levans in the agave syrup.
Figure 1. TLC chromatogram of analyzed agave syrups before and after of thermal treatment. Standards (Std): Fructose (F), glucose (G), sucrose (S), 1-kestose (GF2), 1-nystose (GF3) and 1-β-fructofuranosyl-nystose (GF4). Untreated agave syrup (a) and treated agave syrup (b).
Figura 1. Cromatografía por TLC de los jarabes de agave antes y después del tratamiento térmico.Estándares (Std): Fructosa (F), glucosa (G), sacarosa (S), 1-kestosa (GF2), 1-nistosa (GF3) and 1-β-fructofuranosil-nistosa (GF4). Jarabe de agave sin tratamiento (a) y jarabe de agave tratado (b).
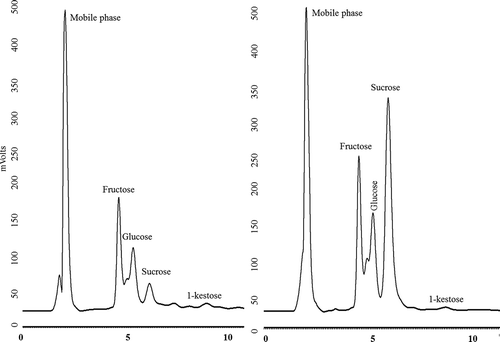
The results obtained by TLC are in accordance with data obtained by HPLC analysis () confirming the significant increase in the amount of sucrose in the thermally treated syrup. (right) shows the chromatogram of untreated agave syrup where the presence of fructose, glucose, sucrose, and kestose is revealed. Identification of kestose in agave syrup is important because it may allow for the agave syrup to be considered as a functional and nutraceutical beverage due to the documented prebiotic effect of kestose and therefore may have beneficial health effects. However, the agave syrup may be supplemented with a major concentration of kestose to potentiate the beneficial properties – an acceptable daily intake of kestose is about 0.8 g per kg of body weight (Yun, Citation1996). Other biological properties have been documented for this compound (kestose), such as laxative effects, involvement in the regulation of lipid metabolism and an important role in absorption of minerals and in cancer prevention (Panesar, Bali, Kumari, Babbar, & Oberoi, Citation2014).
Figure 2. Sugars profile by HPLC of analyzed of agave syrup before and after thermal process.
Figura 2. Perfil de azúcares por HPLC del jarabe de agave antes y después del proceso térmico.
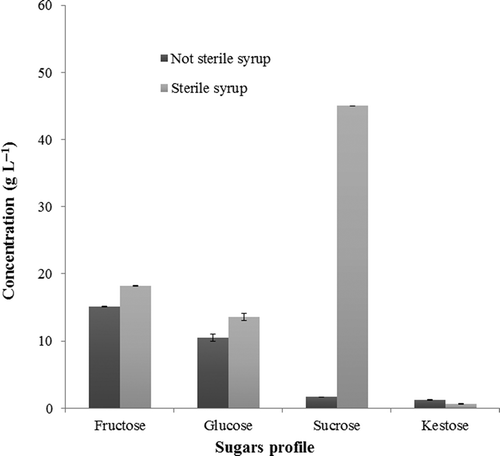
Results presented in (left) clearly show a significant increase in sucrose content in the thermally treated agave. Small amounts of kestose are also observed, confirming agave syrup as a viable alternative for use within the food industry even after thermal sterilization treatment. The application of this thermal process is of the highest importance as agave syrup is rapidly and spontaneously fermented by native microorganisms, resulting in the production of “pulque” a Mexican alcoholic beverage. Thermal treatment could be used to preserve the agave syrup and extend its shelf life.
Quantitative data of major sugars in agave syrups are presented in . Sugars levels are influenced by thermal treatment with significant differences in sucrose content. These results indicate the presence of thermolabile compounds in the samples as monosaccharides such as fructose that could be affected by caramelization reactions that occur above their melting point (example fructose 110ºC), but glucose and sucrose were not affected by this type of reactions under these thermal conditions. Interestingly, sucrose content in agave syrup was increased over 20 times after thermal processing (1.66 g L−1 and 41.39 g L−1 before and after, respectively); in contrast, the amount of kestose, fructose, and glucose was similar in the agave syrup before and after thermal treatment (1.2 and 0.7, 15.21 and 16.12, 10.89 and 12.71 g L−1, respectively). Besides, the fructose could be affected by heat process due to the caramelization reactions that occur when sugars are heated above their melting point.
Figure 3. Influence of thermal treatment on sugars composition of agave syrup.
Figura 3. Influencia del tratamiento térmico sobre la composición de azúcares del jarabe de agave.
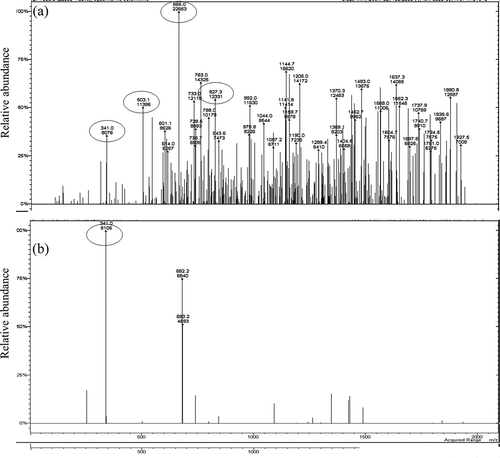
Finally, LC-ESI-MS analysis was carried out as a complementary study to evidence the presence of previously identified sugars. shows the mass spectrum of the untreated and treated agave syrup (a, b respectively) without derivatization. Unfortunately, for both untreated and treated syrups, the masses corresponding to glucose and fructose were not identified under the conditions used. Sucrose, kestose, and oligomeric fructans were unambiguously confirmed in untreated and treated agave syrups ( and ) and the increase in the sucrose content of the treated sample was confirmed. The thermal treatment was shown to degrade oligomeric and polymeric sugars producing a clear mass spectrum although some oligomers are still present. Although further characterization of the syrups is needed, the observed hydrolysis of the oligomers and polymers present in the untreated agave syrup should be related with the observed increase in the sucrose content of the thermally treated agave. An LC-ESI-MS method proved efficient ionization for some sugars present in the agave syrup and therefore this methodology could be applied for other types of samples for the determination of sugar compounds. LC-ESI-MS in comparison with the more commonly employed GC-MS procedures reduces times of sample preparation and the LC-ESI-MS method makes it possible to analyze tri- and tetra-saccharides (Wan & Yu, Citation2006) as shown in . In this figure, the mass corresponding to 1-nystose (GF3) and 1-β-fructofuranosyl-nystose (GF4) is appreciated (665.0 and 827.3 in negative mode), respectively. Interestingly, in the treated agave syrup, , the mass of GF3 and GF4 was not observed and this may well be due to the low concentration in the sample, since a similar case happened with HPLC analysis.
Conclusions
The sugar composition of agave syrup before and after thermal treatment showed broad differences: a significantly higher content of sucrose being detected in the thermally treated samples and the concentration of sucrose increased over 20 times compared with the untreated agave syrup. Similar amounts of fructose, glucose, and 1-kestose were observed in both samples. The mass corresponding to 1-nystose and 1-β-fructofuranosyl-nystose, also considered as prebiotic compounds, was confirmed with the LC-ESI-MS. The formation of these compounds might be attributed to the degradation of other high molecular weight sugars by thermal treatment. Finally, these results suggest that agave syrup is a viable alternative for use in the food industry due to the content of prebiotic molecules in conjunction with the concentration of fermentable sugars.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andrews, H., & Urias, J. E. (2012). Thermal properties of agave fructans (Agave tequilana Weber var. Azul). Carbohydrate Polymers, 87, 2671–2676. doi:10.1016/j.carbpol.2011.11.053
- AOAC. (1990). 3. Plants and 4. Animal feed. In K. Helrich (Ed.), Official methods of analysis of the Association of Official Analytical Chemists. Arlington, TX: Association of Official Analytical Chemists.
- Apolinário, A., Damasceno, B. P., Beltrão, N. E., Pessoa, A., Converti, A., & Da Silva, J. A. (2014). Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydrate Polymers, 101, 368–378. doi:10.1016/j.carbpol.2013.09.081
- Bautista, M., García, L., Salcedo, R., & Parra, L. A. (2001). Azúcares en agaves (Agave tequilana Weber) cultivados en el estado de Guanajuato. Acta Universitaria, 11, 33–38.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Campana-Torres, A., Martinez-Cordova, L., Villarreal-Colmenares, H., & Civera-Cerecedo, R. (2006). Carbohydrate and lipid digestibility of animal and vegetal ingredients and diets for juvenile Australian redclaw crayfish, Cherax quadricarinatus. Aquaculture Nutrition, 12, 103–109. doi:10.1111/anu.2006.12.issue-2
- Colunga, P., Coello, J., Espejo, L., & Fuente, L. (1993). Agave studies in Yucatan, Mexico. II. Nutritional value of the inflorescence peduncle and incipient domestication. Economic Botany, 47, 328–334. doi:10.1007/BF02862302
- Dreywood, R. (1946). Qualitative test for carbohydrate material. Industrial and Engineering Chemistry, 18, 499–505.
- Glibowski, P., & Bukowska, A. (2011). The effect of pH, temperature and heating time on inulin chemical stability. Acta Scientiarium Polonorum Technologia Alimentaria, 10, 189–196.
- Gonçalves, C., Rodríguez, R. M., Gomes, N., Teixeira, J. Á., & Belo, I. (2010). Adaptation of dinitrosalicylic acid method to microtiter plates. Analytical Methods, 2, 2046–2048.
- Mancilla, N. A., & López, M. G. (2002). Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber var azul. Journal of Agricultural and Food Chemistry, 50, 806–812. doi:10.1021/jf0110295
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–428. doi:10.1021/ac60147a030
- Mussatto, S. I., Aguilar, C. N., Rodrigues, L. R., & Teixeira, J. A. (2009). Colonization of Aspergillus japonicus on synthetic materials and application to the production of fructooligosaccharides. Carbohydrate Research, 344, 795–800. doi:10.1016/j.carres.2009.01.025
- Ortiz-Basurto, R. I., Pourcelly, G., Doco, T., Williams, P., Dornier, M., & Belleville, M.-P. (2008). Analysis of the main components of the aguamiel produced by the maguey-pulquero (Agave mapisaga) throughout the harvest period. Journal of Agricultural and Food Chemistry, 56, 3682–3687. doi:10.1021/jf072767h
- Panesar, P. S., Bali, V., Kumari, S., Babbar, N., & Oberoi, H. S. (2014). Part III natural functional food products. Chapter 10 prebiotics. In S. K. Brar, G. S. Dhillon, & M. FERNANDES (Eds.), Biotransformation of waste biomass into high value biochemicals (pp. 237–259). New York, NY: Springer-Verlag.
- Pearson, T. R., Maita, Y., & Lalli, C. M. (1984). A manual of chemical and biological methods for seawaters analysis (1st ed.). New York, NY: Pergamon Press.
- Ravenscroft, N., Cescutti, P., Hearshaw, M., Ramsout, R., Rizzo, R., & Timme, E. (2009). Structural analysis of fructans from Agave americana grown in South Africa for spirit production. Journal of Agricultural and Food Chemistry, 57, 3995–4003. doi:10.1021/jf8039389
- Reiffová, K., & Nemcová, R. (2006). Thin-layer chromatography analysis of fructooligosaccharides in biological samples. Journal of Chromatography A, 2, 14–21.
- Sanchez-Marroquin, A., & Hope, P. H. (1953). Agave juice, fermentation and chemical composition studies of some species. Journal of Agricultural and Food Chemistry, 1, 246–249. doi:10.1021/jf60003a007
- Valadez, R., Bravo, G., Santos, N. F., Velasco, S. I., & Montville, T. J. (2012). The artisanal production of pulque, a traditional beverage of the Mexican highlands. Probiotics Antimicrobial Proteins, 4, 140–144. doi:10.1007/s12602-012-9096-9
- Wan, E. C., & Yu, J. (2006). Determination of sugar compounds in atmospheric aerosols by liquid chromatography combined with positive electrospray ionization mass spectrometry. Journal of Chromatography A, 1107, 175–181. doi:10.1016/j.chroma.2005.12.062
- Yun, J. (1996). Fructooligosaccharides- occurrence, preparation and application. Enzyme and Microbial Technology, 19, 107–117. doi:10.1016/0141-0229(95)00188-3