Abstract
Milk fat content is an important factor affecting the flavor of fermented dairy products. The effects of milk fat concentration (0–90) g·L−1 on the changes of flavor-related compounds in the milk base fermented by Lactobacillus casei GBHM-21 were investigated. The amount of some of the free fatty acids (FFAs) and free amino acids (FFAs) increased as the fat concentration increased, accompanied by a concomitant increase of the quantities and intensities of volatile compounds (VCs). A substantial increase in long-chain fatty acids was observed, whereas no significant effect on short-chain fatty acids was observed. The amount of the total FFA was linearly and positively correlated with the total FAA. The most abundant group of VCs was ketones for low-fat samples and carbonyl acids for high-fat samples. The level of FFA-derived ketones increased dramatically first and then reached a plateau, while FFA-derived carbonyl acids continuously increased. These results provided valuable informative utilization of milk fat in the fermented industry.
El nivel de grasa en la leche es un factor importante en el sabor de los productos lácteos fermentados. Se investigaron los efectos del nivel de grasa en la leche (0–90 g·L−1) en los cambios en los compuestos relacionados con el sabor en bases de leche fermentadas con Lactobacillus casei GBHM-21 durante 72 h. Los ácidos no grasos (FFA) y los aminoácidos no grasos (FAA) de forma selectiva, además de la calidad e intensidad de los compuestos volátiles (VC) aumentaron significativamente con el aumento de la concentración de grasa. No se observó ningún efecto significativo en los ácidos grasos de cadena corta, mientras que sí se observó un aumento sustancial en los ácidos grasos de cadena larga. Se encontró que la relación positiva entre el total de ácidos no grasos y aminoácidos no grasos era lineal. El grupo más abundante de compuestos volátiles resultó ser las cetonas para las muestras bajas en grasa, mientras que los ácidos carbonilos para las muestras altas en grasa. El aumento en las cetonas derivadas de ácidos no grasos primero fue dramático y después cónico, mientras que el aumento en los ácidos carbonilos derivados de ácidos no grasos fue lineal. Estos resultados resultarían útiles para una mayor expansión de las aplicaciones de la grasa en la industria de la fermentación.
1. Introduction
Fermented milk bases are widely used as ingredients in a variety of foods, such as fermented dairy beverages (Gomes et al., Citation2013) and bakery products(Hassan, El-Shazly, Sakr, & Ragab, Citation2013), for flavor enhancement. Milk fat content is an important factor influencing the flavor of fermented dairy products. Increasing fat content for better taste and flavor has been demonstrated in cultured buttermilk, which was usually made from skim milk (White, Citation2013). The flavor-related compounds in fermented dairy products, such as those associated with the taste and aroma, are primarily derived from microorganism-mediated glycolysis, proteolysis, and lipolysis (Collins, McSweeney, & Wilkinson, Citation2003; Hassan, Mona, El-Gawad, & Enab, Citation2013; Smit, Smit, & Engel, Citation2005). The addition of milk fat could enhance the flavor of fermented milk by affecting the metabolism of the microorganisms.
However, there is only limited report concerning the effect of milk fat concentration on fermented dairy products. Researchers (Shakerian et al., Citation2014, Citation2013) investigated the effects of different levels of fat on characteristic indices in yogurt and found that different fat levels significantly affected the production of organic acids but not the cell counts of probiotic bacteria or the degree of proteolysis. Additionally, lipolysis leads to the formation of free fatty acids (FFAs) and their oxidation products such as ketones, and therefore contributes to the development of a favored fermented aroma (Hassan, Mona, et al., Citation2013). Meanwhile, proteolysis leads to the production of free amino acids (FAAs), which are crucial for the characteristic taste of fermented milk and also are important precursors in the synthesis of essential volatile compounds (Bassoli, Borgonovo, Caremoli, & Mancuso, Citation2014; Mikulec, Habuš, Antunac, Vitale, & Havranek, Citation2010). However, the interrelation among the concentrations of these flavor-related compounds is relatively less studied.
The LAB L. casei has a multi-enzyme system including esterases, cell-envelope proteinases, peptidases, and amino acid-converting enzymes (Choi & Lee, Citation2001; Martínez-Cuesta, Fernández de Palencia, Requena, & Peláez, Citation2001). All those enzymes could contribute to the generation of flavor-related compounds during fermentation. Therefore, the strain plays a prominent role in the flavor of fermented dairy products and exhibits great potential for the fermentation of milk bases.
In this study, the changes in microbial growth and flavor-related compounds in milk bases fermented with L. casei GBHM-21 following the addition of anhydrous butterfat (ABF) were studied. The aim of this work was to determine whether the fat level significantly affects microbial metabolism and flavor profile and to better expand the application of fat. Changes in levels of FFAs, FAAs, and volatile compounds during fermentation were studied to evaluate the effects of the fat level on flavor-related compounds and on the relationships among these compounds.
2. Materials and methods
2.1 Strain and reagents
The GBHM-21 bacterial strain has been widely identified as L. casei by the China General Microbiological Culture Collection Center (CGMCC). Bacteria were freeze-dried at a concentration of 1.66 ± 0.24 × 1010 cfu·g−1. The skim milk powder (SMP), containing 342.5 ± 5.2 g·kg−1 protein and 32.7 ± 0.3 g·kg−1 water, was purchased from Fonterra (Auckland, New Zealand). ABF, containing 998.0 g·kg−1 fat, less than 3.0 g·kg−1 FFAs, and less than 1.0 g·kg−1 water, was purchased from Nestle (Shuangcheng, China).
2.2 Preparation of the fermented milk base
Five standardized milk bases (1–5) were prepared. Milk base 1–milk base 5 (CK1–CK5) were standardized according to SMP and ABF levels to contain 29 g·L−1 protein and 0, 15, 30, 60, and 90 g·L−1 fat, respectively. The standardized milk bases were hydrated by stirring at 60 C for 20 min, homogenized at 20–25 MPa, heated at 95 C for 5 min, and finally cooled to 37 C in a water bath before inoculation. The inoculation rate was 0.04% (w/w). The milk bases were incubated separately at 37 C for 72 h. The resulting samples were termed sample 1–sample 5 (S1–S5). Fermentation was stopped by rapidly cooling the milk bases to 4 C in an ice-water bath. All samples were prepared in triplicate.
2.3 Acidity
The pH of the milk bases was monitored before (CK) and after (S) fermentation using a digital pH meter (pHS-25; INESA.CC, Shanghai, China). The titration acidity was determined according to the Association of Official Analytical Chemists (AOAC) 920.124. Neutralization titration was performed using standard 0.1 M NaOH with phenolphthalein as an indicator. The result was expressed as lactic acid (g·L−1).
2.4 Viable cell counts
The number of viable probiotic cells was counted before and after fermentation as previously described (Fonteles, Costa, de Jesus, & Rodrigues, Citation2012). Serial decimal dilutions of each sample were plated in triplicate onto MRS (de Man, Rogosa and Sharp) Agar and incubated at 37 C for 72 hours. The results were expressed as cfu·g−1.
2.5 FFAs
Milk base lipid extraction and gas chromatographic analysis were performed as described by Güler and Gürsoy-Balci (Citation2011), with minor modifications. Briefly, 5 g milk base was mixed with 1 mL of 2.5 M H2SO4 and 10 mL internal standard solution, which contained 400.0 mg·L−1 pentanoic (5:0) and heptadecanoic (17:0) acids (Sigma, USA) in diethyl ether. The mixtures were shaken and mixed for 1 min and then centrifuged (Eppendorf, DE, USA) at 1776 × g for 10 min at 0 C, then the samples were allowed to stand for 1 h. The upper layer was transferred to a centrifuge tube containing 3.0 g anhydrous Na2SO4, which was then added to 10 mL hexane.
After centrifugation at 1776 × g for 10 min, the lipid extracts were applied to an aminopropyl-bonded phase column (Strata NH2, Phenomenex, USA) that had been conditioned with 10 mL hexane/diethyl ether (1:1, v/v). One milliliter of extract was then passed through the column (twice). Next, 5 mL hexane/diethyl ether (1:1, v/v) was passed through the column (twice) to remove all triacylglycerols. The FFAs were then eluted using 2 mL diethyl ether containing 2% formic acid and purged with nitrogen to concentrate the samples; 1 μL of the concentrated solution was used for gas chromatography–mass spectrometry (GC–MS; Agilent 7890A, USA) analysis. All extractions and GC analyses of FFAs were performed in duplicate.
FFAs were analyzed using a GC–MS column (30 m × 0.25 mm inner diameter × 0.25 μm film thickness). Under the GC operating conditions, helium was used as the carrier gas at a constant flow rate of 1 mL·min−1. The GC oven temperature was set to 50 C for 5 min, raised to 230 C at a rate of 5 C·min−1, and then held at 230 C for 20 min. The injector temperature was 250 C, and the run time was 58 min. An Agilent 5975 model quadrupole mass selective detector was operated in the scan mode with the mass range of 33–330 m·z−1 and a scan range of 1 scan·s−1. The interface line to the MS was set at 280 C. The MS was operated in the electron impact (EI) mode with an electron energy of 70 eV and was calibrated by auto-tuning. Peak identification was performed by comparing the retention times and ion spectra with those of authentic standards (Aldrich Chemical Co., Steinheim, Germany) and spectra from the mass spectral database (Nist, 2005, Agilent, Wiley Number, USA). The final FFA concentrations were expressed in mg·L−1 milk base.
2.6 FAAs
FAA extraction was performed as described by Bütikofer, Fuchs, Bosset, and Gmür (Citation1991), with some modifications. All samples were frozen before grinding. About 15 g of grated milk base was weighed, 50 mL n-hexane was added, and the mixture was homogenized for 30 s using a mixer (TM-767II, Haipan Instrument Co. Ltd, Zhongshan, China). The milk base was left to precipitate for 1 min, and the hexane was carefully aspirated off. The degreasing procedure was repeated twice under the same conditions. The degreased residue was transferred to a 250 mL pear-shaped flask in a fume hood, and the residual hexane was removed using a vacuum with an attached water pump for 1 h. FAAs were extracted from the sample by the addition of 1 mL 5% w/v sulfosalicylic acid solution to 1.0 g of each sample; the resulting mixture was centrifuged at 20,000 × g for 10 min at 4 C.
FAAs in the supernatants were quantified by high performance liquid chromatography (HPLC) analysis, as described by Cui, Zhao, Li, Zhao, and Sun (Citation2014). Supernatants were vacuum-dried, derivatized using phenyl isothiocyanate, and then dried again. The derivatized dried samples were dissolved in diluent (phosphoric acid–acetonitrile water solution, pH 7.4) and then centrifuged at 3552 × g. The supernatants were then analyzed using an HPLC system (Waters, Boston, MA, USA) equipped with a Waters Pico-Tag amino acid column (150 m) and a Waters 486UV detector. The parameters used were as follows: column temperature, 38 C; measurement wavelength, 254 nm; mobile phase elutes (binary gradient elutions) of solvent A (sodium acetate–acetic acid buffer solution, pH 6.4, 1.0 mL·min−1) and solvent B (60% acetonitrile solution in pure water, 1.5 mL·min−1). The injection volume was 10 μL. The concentrations of FAAs were calculated using a standard containing 18 different amino acids (Waters).
2.7 Solid-phase microextraction (SPME)-GC–MS
Volatile compounds were extracted and analyzed as described by Feng et al. (Citation2014) with some modifications. An SPME TriPlus automated sampler equipped with a 75 μm carboxen/polydimethylsiloxane fibre (CAR/PDMS, Supelco, Inc., Bellefonte, PA, USA) was employed to extract volatile compounds from the fermented milk bases. Two-gram samples were placed in 20 mL gas-tight vials (Supelco). After the samples were equilibrated at 60 C for 20 min, they were subjected to extraction using CAR/PDMS fibres for 40 min with continuous heating and agitation. After extraction, the fibres were inserted into the GC injector for 3 min to desorb the analytes. Each fermented milk base sample was extracted in duplicate. The fibre was conditioned before use by insertion into the GC injector port for 1 h at 230 C. It was then desorbed for 3 min at 230 C between injections to prevent contamination.
Volatile compounds were analyzed using a Trace GC–MS system equipped with an Ultra GC, a TriPlus automated sampler, and a quadrupole DSQ II MS (Thermo Finnigan, San Jose, CA, USA). Separation was performed using a TR-Wax column (30 m × 0.32 mm × 0.25 μm; J&W Scientific, Folsom, CA, USA). The GC–MS conditions used were consistent with those described by Feng et al. (Citation2013). Briefly, helium was used as the carrier gas with a flow rate of 1.0 mL·min−1 and a split ratio of 10:1. The temperature of the column was maintained at 40 C for 3 min, increased to 120 C at 5 C·min−1, and held for 2 min. It was then raised to 250 C at a rate of 7 C·min−1 and held at 250 C for 5 min. The injection temperature was 230 C, and the ion source temperature was set at 250 C. The mass spectrometer was operated in the EI mode. The ionization energy, detector voltage, scan range, and scan rate applied for analyses were as follows: 70 eV; 350 V; 35–350 m·z−1; and 3.00 scans·s−1, respectively. Chromatograms and mass spectra were evaluated using Xcalibur software version 2.0 (Thermo Finnigan). Volatile compounds were identified by comparing the retention indices (RIs) obtained with those described previously under the same conditions and by comparing the mass spectra with those included in the Nist2005 and Varian libraries. The volatile compounds identified were also compared to previously reported aroma compounds produced during milk fermentation. The volatile compounds were compared as area units (AU × 106) and relative percentages.
2.8 Statistical analysis
The results are shown as means, standard deviations, and relative standard deviations. One-way analysis of variance was used to assess the homogeneity of variances. Statistical analyses of recorded results were performed using the Statistical Program for Social Science for Windows 16.0 (StatSoft. Inc., USA), and p values of less than 0.05 were considered to be statistically significant.
3. Results and discussion
3.1 Fermentation characteristics
The effect of different fat levels on viable cell counts, pH, and titrimetric acidity during fermentation was analyzed (). The viable cell counts were increased consistently for S1–S5 after fermentation, reaching 1–2 × 109 cfu·g−1 (up from 1–2 × 107 cfu·g−1 after inoculation). These increases in viable cell counts suggested that increasing the fat concentration to 90 g·L−1 did not inhibit the proliferation of L. casei GBHM-21. Shakerian et al. (Citation2014) also found no significant effects of fat levels (0.5–5.0%) on cell counts (higher than × 106 cfu·mL−1) of probiotics in yogurts. This was probably due to the concentration and constitution of FFAs, especially short-chain fatty acids (SCFAs) shown in 3.2. The number of viable cells within the five bases remained high, despite the high acidity values, implying that the viability and metabolic activity of L. casei GBHM-21 during the fermentation were considerable.
Table 1. Effects of different fat concentrations on the viable cell count and acidity of fermented milk bases before (CK) and after (S) 72 h.
Tabla 1. Efectos de las diferentes concentraciones de grasa en el recuento viable de células y acidez de las bases de leche fermentadas antes (CK) y después (S) de 72 h.
Following the increase in fat content, the titrimetric acidity of CK2–CK5 increased slightly compared with that of CK1. We expected that is because a small amount of SCFAs in the ABF (Xu & Qiu, Citation2001) dissolved in the aqueous phase during titrimetric acidity measurements. There were no significant differences (p > 0.05) in pH and titrimetric acidity among S1–S5, which was consistent with the changes in viable cell counts.
3.2 FFAs
shows the effects of different fat concentrations on FFAs in the milk bases before and after L. casei GBHM-21 fermentation. As expected, the total FFA concentrations of CK1–CK5 and S1–S5 all increased along with the increase in fat concentration (the inset figure in ). Obviously, the increase of the fat concentration in the milk base brought more FFAs after fermentation. The highest concentration of an individual FFA in S4 and S5 was C8:0, while that in S2 and S3 was C10:0. Notably, there was no significant difference in SCFAs (C4:0–C6:0), while middle-chain fatty acids (C8:0–C10:0) and long-chain fatty acids (LCFAs, C12:0–C18:0) exhibited the greatest increase along with the fat concentration increase. No significant difference in SCFAs also could be related to no significant effects on the viable cell count because FFAs, particularly SCFAs, inhibited bacterial growth (Nair et al., Citation2005). Furthermore, the composition ratio of LCFAs was higher among samples with high fat concentrations (S4 and S5), while the percentage of SCFAs was higher in samples with low fat concentration. This may be due to the strong esterolytic activity of L. casei for C6:0 (Choi & Lee, Citation2001). And the observation that SCFAs and methyl mercaptan can react to form methyl thioester, which also results in fermented aromas (Collins et al., Citation2003).
Figure 1. Effects of different fat concentrations on FFA concentrations in milk bases fermented with L. casei GBHM-21. C4:0, butanoic acid; C6:0, hexanoic acid; C8:0, octanoic acid; C10:0, decanoic acid; C12:0, dodecanoic acid; C14:0, tetradecanoic acid; C16:1, palmitoleic acid; C16:0, hexadecenoic acid; C18:1, oleic acid; C18:0, octadecanoic acid. S2–S5: milk bases containing 29 g·L−1 protein and 15, 30, 60, or 90 g·L−1 fat, respectively, fermented for 72 h.
Figura 1. Efectos de diferentes concentraciones de grasa en concentraciones de ácidos no grasos en bases de leche fermentadas con L. casei GBHM-21.C4:0, ácido butírico; C6:0, ácido hexanoico; C8:0, ácido octanoico; C10:0, ácido decanoico; C12:0, ácido dodecanoico; C14:0, ácido tetradecanoico; C16:1, ácido palmitoleico; C16:0, ácido hexadecenoico; C18:1, ácido oleico; C18:0, ácido octadecanoico. S2–S5: bases de leche de 29 g·L−1 de proteína y 15, 30, 60, o 90 g·L−1 de grasa, respectivamente, fermentadas durante 72 h.
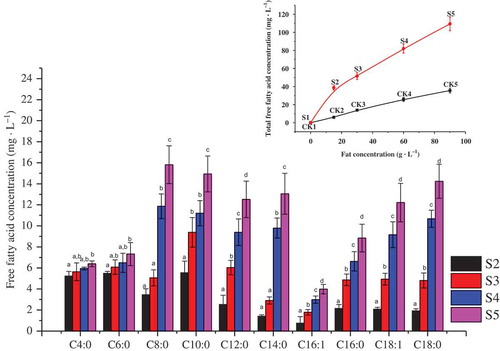
The quantity of unsaturated fatty acids (USFAs) increased, particularly C18:1 (3.70 mg·L−1 in S2 and 12.22 mg·L−1 in S5). The changes in FFA profiles suggested that the increased fat content enhanced the synthesis of lipases, esterases, and other relevant enzymes in the lag phase in L. casei GBHM-21. Therefore, the aroma-related compounds formed from FFA catabolism are important for evaluating the effects of FFAs on the flavor of fermented milk bases.
3.3 FAAs
shows the effects of increasing fat concentrations on FAAs in milk bases fermented with L. casei GBHM-21. The total FAA contents of CK1–CK5 increased from 7.55 ± 0.09 mg·100 g−1 to 10.62 ± 0.02 mg·100 g−1 along with the increase in the fat level.
Figure 2. Effects of different fat concentrations (0, 15, 30, 60, and 90 g·L−1 fat for S1–S5) on FAA concentrations in milk bases fermented with L. casei GBHM-21.Note: a–eMean values ± standard deviation with different letters in the same row that are significantly different (Duncan’s test, p ≤ 0.05). Asp: Asparagine; Glu: glutamic acid; Ser: serine; Gly: glycine; His: histidine; Arg: Arginine; Thr: threonine; Ala: Alanine; Pro: proline; Tyr: tyrosine; Val: valine; Met: methionine; Cys: cystine; Ile: isoleucine; Leu: leucine; Trp: tryptophan; Phe: phenylalanine; Lys: lysine.
Figura 2. Efectos de diferentes concentraciones de grasa (0, 15, 30, 60 y 90 g·L−1 de grasa para S1–S5) en concentraciones de ácidos no grasos en bases de leche fermentadas con L. casei GBHM-21.Nota: a–eLos valores promedio ± desviación estándar con letras distintas en la misma fila son significativamente diferentes (Test Duncan, p ≤ 0,05).Asp: Asparagina; Glu: ácido glutámico; Ser: serina; Gly: glicina; His: histidina; Arg: Arginina; Thr: treonina; Ala: Alanina; Pro: prolina; Tyr: tirosina; Val: valina; Met: metionina; Cys: cistina; Ile: isoleucina; Leu: leucina; Trp: triptófano; Phe: fenilalanina; Lys: lisina.
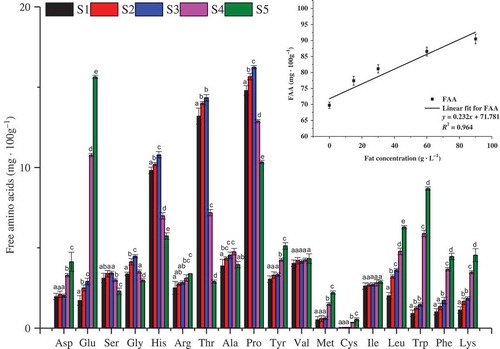
As shown in the inset figure in , there were clearly positive relationships (R2 = 0.964) between the amount of fat in the milk base and the total FAA contents after fermentation. However, there were obviously different trends in the concentrations of individual FAAs (). With the increase in fat concentration, the concentration of some FAAs, such as Glu, Leu, Trp, Phe, and Lys, were significantly increased. In contrast, the levels of Ser, Gly, His, Thr, and Pro were the highest in S3. Additionally, some FAAs, such as Ala and Cys, were not significantly affected by the fat concentration. The different changes in the levels of various FAAs could be attributed to the improved proteolysis and FAA catabolism for L. casei GBHM-21 influenced by the increase of fat.
In S1, S2, and S3, the major FAAs were Pro (sweet), Thr, and His (bitter), while Glu (fresh, sour), Pro, and Trp (bitter) were predominant in S4 and S5. Because FAAs have been shown to be associated with different tastes (Bassoli et al., Citation2014), these results suggested that the taste of the milk base fermented by L. casei GBHM-21 obviously affected when the fat content was increased to a certain extent, i.e., up to 60 g·L−1 in our study. FAAs can also be converted via a variety of pathways due to the catalytic activity of enzymes, contributing to malty, fruity, and sweet flavors (Ardö, Citation2006; Fox, Singh, & McSweeney, Citation1995). Since FAAs significantly changed, the aroma formation was expected to be quite different.
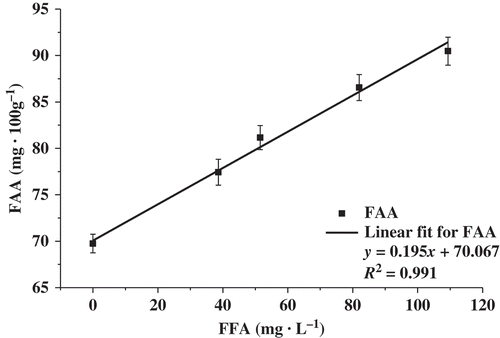
If the total FFA and FAA contents of S1–S5 were studied jointly, a linear, positive relationship (R2 = 0.991) was established (). In contrast, Hernández et al. (Citation2009) found a linear, inverse relationship between the total FFA and FAA contents because the FFAs, particularly SCFAs, hydrolyzed by lipase could inhibit bacterial growth (Nair et al., Citation2005). These data showed that there was no inhibition of bacterial growth () and that both total FAA and total FFA contents increased. This was because the percentage of SCFAs decreased with the increase in fat concentration in our study. Therefore, the content and composition of FFAs should be considered when assessing the effects of FFAs on bacterial growth and FAAs.
Figure 3. Linear relationship between the total FFA concentration and the total FAA concentration in milk bases fermented with L. casei GBHM-21.
Figura 3. Relación lineal entre la concentración total de ácidos no grasos y la concentración total de aminoácidos no grasos en bases de leche fermentadas con L. casei GBHM-21.
3.4 Volatile compounds
Both the relative amounts and types of volatile compounds were significantly affected (p < 0.05) by the increase in fat (). Fat addition brought markedly larger and considerably more complex volatile compounds in S2–S5 than those in S1. The total area and the types of volatile compounds detected increased along with the fat concentration increase; for example, the total area of S5 was 3.6-fold of that of S2, and eight more volatile compounds detected in S5 were found than those in S3. Zaręba, Ziarno, Ścibisz, and Gawron (Citation2014) identified 14 types of volatile compounds in L. casei DN-114 001 fermented milk. Of these 14 compounds, seven were consistent with compounds identified in our study. Changes in the amounts and types of compounds suggested that the concentration of milk fat had a crucial influence on the formation of fragrant aromas in fermented dairy products.
Table 2. Effects of different fat concentrations (0, 15, 30, 60 and 90 g·L−1 fat for S1–S5) on volatile compounds in milk bases fermented with L. casei GBHM-21 for 72 h.
Tabla 2. Efectos de diferentes concentraciones de grasa (0, 15, 30, 60 y 90 g·L−1 de grasa para S1–S5) en los compuestos volátiles en bases de leche fermentadas con L. casei GBHM-21 durante 72 h.
In this study, the identified volatile compounds were divided into ketones, carboxylic acids, aldehydes, and others. The proportion of these different types of compounds was different among samples; the dominant volatile compounds in S1, S2, and S3 were ketones (91.7%, 50.9%, and 50.3%, respectively), while those in S4 and S5 were carbonyl acids (53.1% and 62.4%, respectively). The most common compounds in S2 and S3 were acetic acid, 2-heptanone, and 3-hydroxy-2-butanone, whereas the major compounds in S4 and S5 were isovaleric acid, acetic acid, and 2-heptanone, predominantly metabolites from FFAs.
Acetic acid has contributed a sharp vinegar-like flavor to milk, and butyric acid has an essential role in the formation of basic fermented dairy aromas (Frank, Owen, & Patterson, Citation2004). As the fat content increased, enzymes related to fat metabolism increased, resulting in significant changes in the types and amounts of carboxylic acids, particularly branched-chain (BC) isovaleric acid and isobutyric acid. A number of ketones are important in determining the aroma of fermented dairy products: 2,3-butanedione (sweet buttery), 2-heptanone (musty, sweet, moldy, varnish), and 2-nonanone (floral fruity, peach) (Frank et al., Citation2004; García-Quintáns, Blancato, Repizo, Magni, & López, Citation2008). Methyl ketones are produced by the oxidation of polyunsaturated fatty acids (Hassan, Mona, et al., Citation2013), and methyl ketones generated through this process are generally odd-chain ketones (Collins et al., Citation2003). Consistent with these pathways, the ketone present at the highest concentration in both S2 and S3 was 2-heptanone.
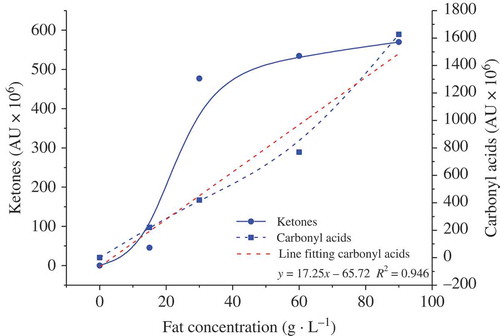
As presented in , the simultaneous increase in relative total amounts of ketones and carbonyl acids was associated with the increases in FFAs and fat. For ketones derived from FFAs, the relative amount increased sharply when the fat concentration increased from 0 to 30 g·L−1 and gently when the fat concentration increased from 30 to 90 g·L−1. However, for carbonyl acids metabolized from FFAs, the relative amount increased linearly (R2 = 0.946). Therefore, increasing the fat content affected the production of volatile ketones and carbonyl acids. From these data, we concluded that the metabolism of FFAs by L. casei GBHM-21 was limited, particularly the production of ketones.
Figure 4. Effects of fat concentrations on the relative total amount of ketones and carbonyl acids derived from FFAs in milk bases fermented with L. casei GBHM-21.Note: Ketones derived from FFAs, including 2-pentanone, 2-hentanone, 2-nonanone, and 2-undecanone; carbonyl acids derived from FFAs, including butyric acid, isobutyric acid, isovaleric acid, hexanoic acid, and octanoic acid.
Figura 4. Efectos de la concentración de grasa en la cantidad total relativa de cetonas y ácidos carbonilos derivados de ácidos no grasos en bases de leche fermentadas con L. casei GBHM-21.Nota: Cetonas derivadas de ácidos no grasos, incluyendo 2-pentanona, 2-hentanona, 2-nonanona y 2-undecanona; ácidos carbonilos derivados de ácidos no grasos, incluyendo ácido butírico, ácido isobutírico, ácido isovalérico, ácido hexanoico y ácido octanoico.
Besides FFA metabolism, various volatile compounds are also produced from the biochemical pathways leading to FAA metabolism. Ziadi et al. (Citation2010) concluded that aminotransferase could catalyze the conversion from α-ketoglutarate and BC amino acids into BC keto acids. We observed difference in these metabolites concurrent with the elevated FAA levels in the fermented milk base (). Methional, broken down from Met (Fox et al., Citation1995), was present only in S2 and S3. Dimethyl disulfide, the decomposition product of methional (Fox et al., Citation1995), was only found in S5. Although additional volatile compounds were present at relatively low levels, these types of compounds have previously been shown to play important roles in the flavor of the fermented milk base by providing a rich and soft casein aroma (Frank et al., Citation2004). Furthermore, 2-ethylfuran is considered to represent a lipid oxidation product and could evoke a powerful burnt, sweet, and coffee-like flavor. This compound can influence the course of the Maillard reaction with amino acids (Adams, Bouckaert, Lancker, Meulenaer, & Kimpe, Citation2011), which were also enhanced in S4 and S5, as discussed earlier. Therefore, fermented milk bases prepared with increased fat content may have a more fragrant aroma characteristic of fermentation and may be more useful for food applications, such as baking.
4. Conclusions
In conclusion, increasing the fat concentration in L. casei GBHM-21 fermented milk base to 90 g·L−1 had a significant impact on both GBHM-21 fermentation characteristics and the levels of FFAs, FAAs, and volatile compounds. The increase in fat not only enhanced bacterial lipolysis but also modulated proteolysis and the synthesis of volatile compounds by exogenously stimulating the L. casei GBHM-21 enzyme system. Additionally, the relationships among fat concentration, total FFAs, total FAAs, and the volatile compounds derived from FFAs were established. The results of ketones and carbonyl acids are probably two important parameters to explain the difference in volatile compounds. The study could contribute to a better understanding of fat application in the fermented dairy industry. Future studies are required to establish a flavor model, evaluate the flavor of the fermented milk base, and identify the active enzymes in L. casei GBHM-21.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Zhining Bao ![]() http://orcid.org/0000-0002-6120-1119
http://orcid.org/0000-0002-6120-1119
Additional information
Funding
References
- Adams, A., Bouckaert, C., Lancker, F. V., Meulenaer, B. D., & Kimpe, N. D. (2011). Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. Journal of Agricultural and Food Chemistry, 59, 11058–11062. doi:10.1021/jf202448v
- AOAC. (2000). The acidity of cheese. Titrimetric method, Method no 920.124. Washington, DC: Association of Official Analytical Chemists.
- Ardö, Y. (2006). Flavour formation by amino acid catabolism. Biotechnology Advances, 24, 238–242. doi:10.1016/j.biotechadv.2005.11.005
- Bassoli, A., Borgonovo, G., Caremoli, F., & Mancuso, G. (2014). The taste of D- and L-amino acids: In vitro binding assays with cloned human bitter (TAS2Rs) and sweet (TAS1R2/TAS1R3) receptors. Food Chemistry, 150, 27–33. doi:10.1016/j.foodchem.2013.10.106
- Bütikofer, U., Fuchs, D., Bosset, J. O., & Gmür, W. (1991). Automated HPLC-amino acid determination of protein hydrolysates by precolumn derivatization with OPA and FMOC and comparison with classical ion exchange chromatography. Chromatographia, 31, 441–447. doi:10.1007/BF02262386
- Choi, Y. J., & Lee, B. H. (2001). Culture conditions for the production of esterase from Lactobacillus casei CL96. Bioprocess and Biosystems Engineering, 24, 59–63. doi:10.1007/s004490100233
- Collins, Y., McSweeney, P., & Wilkinson, M. (2003). Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. International Dairy Journal, 13, 841–866. doi:10.1016/S0958-6946(03)00109-2
- Cui, C., Zhao, M., Li, D., Zhao, H., & Sun, W. (2014). Biochemical changes of traditional Chinese-type soy sauce produced in four seasons during processing. CyTA-Journal of Food, 12, 166–175. doi:10.1080/19476337.2013.810673
- Feng, Y., Cai, Y., Su, G., Zhao, H., Wang, C., & Zhao, M. (2014). Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chemistry, 145, 126–134. doi:10.1016/j.foodchem.2013.07.072
- Feng, Y., Cui, C., Zhao, H., Gao, X., Zhao, M., & Sun, W. (2013). Effect of koji fermentation on generation of volatile compounds in soy sauce production. International Journal of Food Science & Technology, 48, 609–619. doi:10.1111/ijfs.12006
- Fennema, O. R. (2003). Free amino acids, peptide and protein. In S. Damodran (Ed.), Food chemistry (pp. 267–275). Beijing: China Light Industry Press.
- Fonteles, T. V., Costa, M. G. M., De Jesus, A. L. T., & Rodrigues, S. (2012). Optimization of the fermentation of cantaloupe juice by Lactobacillus casei NRRL B-442. Food Bioprocess Technology, 5, 2819–2826. doi:10.1007/s11947-011-0600-0
- Fox, P., Singh, T., & McSweeney, P. (1995). Biogenesis of flavour compounds in cheese. Advances in Experimental Medicine and Biology, 367, 59–98.
- Frank, D. C., Owen, C. M., & Patterson, J. (2004). Solid phase microextraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT-Food Science and Technology, 37, 139–154. doi:10.1016/S0023-6438(03)00144-0
- García-Quintáns, N. G., Blancato, V., Repizo, G., Magni, C., & López, P. (2008). Citrate metabolism and aroma compound production in lactic acid bacteria. In B. Mayo, P. López, & G. Pérez-Martínez (Eds.), Molecular aspects of lactic acid bacteria for traditional and new applications (pp. 65–88). Kerala: Research Signpost.
- Gomes, J. J. L., Duarte, A. M., Batista, A. S. M., Figueiredo, R. M. F., Sousa, E. P., Souza, E. L., & do Egypto Queiroga, R. C. R. (2013). Physicochemical and sensory properties of fermented dairy beverages made with goat’s milk, cow’s milk and a mixture of the two milks. Food Science and Technology, 54, 18–24.
- Güler, Z., & Gürsoy-Balci, A. C. (2011). Evaluation of volatile compounds and free fatty acids in set types yogurts made of ewes’, goats’ milk and their mixture using two different commercial starter cultures during refrigerated storage. Food Chemistry, 127, 1065–1071. doi:10.1016/j.foodchem.2011.01.090
- Hassan, A. A., El-Shazly, H. A. M., Sakr, A. M., & Ragab, W. A. (2013). Influence of substituting water with fermented skim milk, acid cheese whey or buttermilk on dough properties and baking quality of pan bread. World Journal of Dairy Food Science, 8, 100–117.
- Hassan, F. A. M., Mona, A. M., El-Gawad, A., & Enab, A. K. (2013). Flavour compounds in cheese (Review). Research on Precision Instrument and Machinery, 2, 15–29.
- Hernández, I., Barrón, L., Virto, M., Pérez-Elortondo, F., Flanagan, C., Rozas, U., … De Renobales, M. (2009). Lipolysis, proteolysis and sensory properties of ewe’s raw milk cheese (Idiazabal) made with lipase addition. Food Chemistry, 116, 158–166. doi:10.1016/j.foodchem.2009.02.026
- Martı́nez-Cuesta, M. C., Fernández de Palencia, P., Requena, T., & Peláez, C. (2001). Enzymatic ability of Lactobacillus casei subsp. casei IFPL731 for flavour development in cheese. International Dairy Journal, 11, 577–585. doi:10.1016/S0958-6946(01)00046-2
- Mikulec, N., Habuš, I., Antunac, N., Vitale, L., & Havranek, J. (2010). Influence of peptide and amino acids on the formation of cheese flavour. Food Technology and Biotechnology, 60, 219–227.
- Nair, M. K. M., Joy, J., Vasudevan, P., Hinckley, L., Hoagland, T. A., & Venkitanarayanan, K. S. (2005). Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. Journal of Dairy Science, 88, 3488–3495. doi:10.3168/jds.S0022-0302(05)73033-2
- Shakerian, M., Razavi, S. H., Khodaiyan, F., Ziai, S. A., Yarmand, M. S., & Moayedi, A. (2014). Effect of different levels of fat and inulin on the microbial growth and metabolites in probiotic yogurt containing nonviable bacteria. International Journal of Food Science & Technology, 49, 261–268. doi:10.1111/ijfs.2013.49.issue-1
- Shakerian, M., Razavi, S. H., Ziai, S. A., Khodaiyan, F., Yarmand, M. S., & Moayedi, A. (2013). Proteolytic and ACE-inhibitory activities of probiotic yogurt containing non-viable bacteria as affected by different levels of fat, inulin and starter culture. Journal of Food Science and Technology, 52, 1–6. doi:10.1007/s13197-013-1202-9
- Smit, G., Smit, B. A., & Engels, W. J. M. (2005). Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiology Reviews, 29, 591–610. doi:10.1016/j.fmrre.2005.04.002
- White, C. H. (2013). Cultured buttermilk. In R. C. Chandan & A. Kilara (Eds.), Manufacturing yogurt and fermented milks (pp. 371). Wese Sussex: John Wiley & Sons.
- Xu, G., & Qiu, A. (2001). (Butter (H. Pingping. Trans)). In L. Ye (Ed.), Bailey’s industrial oil and fat products (pp. 2–7). Beijing, China: China Light Industry Press. (Reprinted from Bailey’s industrial oil and fat products by Hui, Y. H., Ed., 1996, John Wiley & Sons)
- Zaręba, D., Ziarno, M., Ścibisz, I., & Gawron, J. (2014). The importance of volatile compound profile in the assessment of fermentation conducted by Lactobacillus casei DN-114 001. International Dairy Journal, 35, 11–14. doi:10.1016/j.idairyj.2013.09.009
- Ziadi, M., Bergot, G., Courtin, P., Chambellon, E., Hamdi, M., & Yvon, M. (2010). Amino acid catabolism by Lactococcus lactis during milk fermentation. International Dairy Journal, 20, 25–31. doi:10.1016/j.idairyj.2009.07.004
