Abstract
The subject of present study was to evaluate the effect of chitosan coating combined with green tea extract (GTE) on the melanosis formation and quality of Pacific white shrimp (Litopenaeus vannamei) during 9 days of storage in ice. The melanosis formation was significantly inhibited and sensory quality was significantly improved in shrimp treated with chitosan coating, GTE and chitosan coating combined with GTE, compared with the control. The increase in the total bacteria amounts, pH and total volatile basic nitrogen were significantly inhibited in shrimp treated with chitosan coating, GTE and chitosan coating combined with GTE. The melanosis score and total volatile basic nitrogen of shrimp treated by chitosan coating combined with GTE was less than that treated by chitosan coating or GTE alone. These results suggested that chitosan coating combined with GTE could be used as an effective natural alternative to synthetic antimelanosic agents to inhibit post-mortem melanosis and improve the quality of shrimp during storage in ice.
El foco del presente estudio fue evaluar el efecto del recubrimiento con chitosán combinado con el extracto de té verde (GTE) en la formación de melanosis y la calidad de la gamba blanca del Pacífico (Litopenaeus vannamei) durante 9 días de almacenamiento con hielo. La formación de melanosis fue significativamente inhibida y la calidad sensorial fue mejorada de forma significativa en las gambas tratadas con recubrimiento con chitosán, extracto de té verde y recubrimiento con chitosán combinado con extracto de té verde, en comparación con la muestra control. El aumento en la cantidad total de bacterias, pH y total de volátiles de nitrógeno básico fue inhibido significativamente en las gambas tratadas con recubrimiento con chitosán, extracto de té verde y recubrimiento con chitosán combinado con extracto de té verde. Los resultados de melanosis y total de volátiles de nitrógeno básico de las gambas tratadas con recubrimiento con chitosán combinado con extracto de té verde fueron menores que los de las gambas tratadas con recubrimiento con chitosán o extracto de té verde únicamente. Estos resultados sugirieron que el recubrimiento con chitosán combinado con extracto de té verde podría ser utilizado como una alternativa natural eficaz de los agentes antimelanósicos sintéticos para inhibir la melanosis postmortem y mejorar la calidad de las gambas durante el almacenamiento con hielo.
Introduction
Due to its high market value and nutritional value, shrimp is a very important marine resource all over the world. However, the shelf life of shrimp is limited due to melanosis and microbiological deterioration during storage. Melanosis or blackening, the formation of black spots in crustaceans such as shrimp and crabs during post-mortem storage, can severely damage the market value and usually makes these seafoods unprofitable (Kim, Marshall, & Wei, Citation2002).
To retard melanosis in crustaceans, and ensure perishables have a longer shelf life, antimelanosic agents, such as 4-hexyl-1,3-benzenediol (4-hexylresorcinol), sulphite-based compounds, and phosphates, have been intensively studied and proved to be effective in inhibiting melanosis (Martínez-Álvarez, López-Caballero, Montero, & Gómez-Guillén, Citation2005; Thepnuan, Benjakul, & Visessanguan, Citation2008). However, the use of synthetic compounds to inhibit melanosis in seafood is limited due to increasing regulatory attention and their potential toxicity (McEvily, Iyengar, & Otwell, Citation1991). Due to the potential health hazards of chemical additives, natural products, especially natural antioxidants and antimicrobial agents, have been intensively examined as safe alternatives to synthetic compounds (Encarnación, Fagutao, Hirono, Ushio, & Ohshima, Citation2010; Maqsood, Benjakul, & Shahidi, Citation2013). Recently, a series of studies has been conducted on the utilization of natural extracts to delay melanosis formation and extend the shelf life of seafood (Fang, Sun, Huang, & Yuan, Citation2013; Nirmal & Benjakul, Citation2011a, Citation2011b, Citation2011c; Sun, Lv, Yuan, & Fang, Citation2014). Green tea extract (GTE), rich in polyphenolic compounds, has been reported to have many favorable physiological effects on health, including anticarcinogenic, regulation of body fat and cardiovascular protection effects in both animals and humans (Jung, Seong, Kim, Myong, & Chang, Citation2013; Nagao, Hase, & Tokimitsu, Citation2007; Tsao et al., Citation2009). In addition, GTE can be used as a natural food additive and active food packaging material due to high antimicrobial and antioxidant activity (Carrizo, Gullo, Bosetti, & Nerin, Citation2014; Perumalla & Hettiarachchy, Citation2011).
Chitosan is a cationic polysaccharide consisting of (1,4)-linked-2-amino-deoxy-b-D-glucan, and is the deacetylated form of chitin. Chitosan has attracted attention as a potential food preservative of natural origin and has been classified as a GRAS by the US FDA in 2001 (Sagoo, Board, & Roller, Citation2002). Chitosan has been broadly applied to the preservation of seafood products including Atlantic cod, fresh herring fillets, oysters, sardines and salmon due to its non-toxic features, antimicrobial and antifungal activities, biodegradability and biocompatibility, and its film-forming property (Cao, Xue, & Liu, Citation2009; Jeon, Kamil, & Shahidi, Citation2002; Mohan, Ravishankar, Lalitha, & Gopal, Citation2012; Souza et al., Citation2010). Also, it has been discovered that chitosan could be effective in enhancing the quality and shelf life of shrimp (Huang, Chen, Qiu, & Li, Citation2012; Wu, Citation2014). However, to our knowledge, there is no literature reporting the influence of chitosan coating combined with GTE on shelf life and quality of seafood such as shrimps and crabs. The subject of the present study was to evaluate the effect of GTE combined with chitosan coating on the melanosis formation and quality of Pacific white shrimp (Litopenaeus vannamei) during storage in ice by determining microbiological, physicochemical and sensory parameters.
Materials and methods
Preparation of green tea extract and coating solution
Two-hundred gram portions of finely-powdered tea were treated with chloroform using a powder/solvent ratio of 1:10 (w/v) to remove chlorophyll. Then, the mixture was blended with 50% ethanol for 2 h at 40°C in a shaking water bath. The ratio green tea powder: solvent was 1:10 (w/v). The extracts were filtered (Waterman No. 1) and concentrated under vacuum with a rotary evaporator (Eyela, Rikakikai, Tokio, Japan). The concentrate was dried overnight in an oven at 40°C until the solvent was completely evaporated. The extract powder obtained was stored at 4°C until further use. The 1% of GTE dipping solution was prepared by dissolving GTE in distilled water.
Chitosan were purchased from Shanghai Jinsui Biotechnology Company (Shanghai, China). The degree of deacetylation of chitosan was 90%. Chitosan (10 g) was added to 1000 mL of 1% acetic acid and stirred for 1 h at room temperature to obtain 1% w/w chitosan /acetic acid solutions.
Shrimp collection and treatments
Pacific white shrimp measuring 50–55 shrimps/kg were purchased from a local market in Zhoushan, China. The Pacific white shrimps were kept alive and transported to the College of Food and Medicine, Zhejiang Ocean University, Zhoushan. The Pacific white shrimp were randomly assigned into five groups including the control (uncoated) group, GTE group, chitosan group, GTE + chitosan coating group and sodium metabisulfite (SMS) group. The method of treatment with GTE and chitosan coating was based on that of Nirmal and Benjakul (Citation2011a) and Huang et al. (Citation2012). The shrimps in the GTE group and chitosan coating group were dipped into the 1% GTE and 1% chitosan solution at a shrimp/solution ratio of 1:2 (w/v) at 4°C for 30 min, respectively. After dipping, the Pacific white shrimps were drained at ambient temperature for 3 min. The shrimps in the GTE + chitosan coating group were immersed into the 1% GTE at a shrimp/solution ratio of 1:2 (w/v) at 4°C for 30 min. After that, they were dipped into 1% chitosan solution at a shrimp/solution ratio of 1:2 (w/v) at 4°C for 30 min, and then were drained at ambient temperature for 3 min. Another portion of shrimps was treated in 1.25% SMS dissolved in distilled water at a ratio of 1:2 (w/v) for 1 min at 4°C. The samples from each treatment were covered in plastic bags and stored in ice using a shrimp/ice ratio of 1:2 (w/w). To maintain the shrimp/ice ratio, molten ice was removed and replaced with an equal amount of ice every 3 d.
Melanosis assessment
Melanosis assessment of Pacific white shrimp was conducted through visual inspection by six panelists using ten-point scoring using the method of Montero, Ávalos, and Pérez-Mateos (Citation2001). Panelists were asked to give the melanosis score (0 to 10) for shrimp, where 0 = absent; 2 = slight (up to 20% of shrimp’s surface affected); 4 = moderate (20% to 40% of shrimp’s surface affected); 6 = notable (40% to 60% of shrimp’s surface affected); 8 = severe (60% to 80% of shrimps’ surface affected); 10 = extremely heavy (80% to 100% of shrimp’s surface affected). Samples were taken for each treatment every 3 d up to 9 d for melanosis assessment.
Sensory evaluation
Sensory evaluation of shrimp samples was performed by a group of six trained panelists according to Chinese National Standard (Citation1994). Panelists were regular consumers of shrimps and had no allergies to shrimps. They were asked to evaluate appearance, odor, texture, flavor, and overall acceptability of shrimp samples. A rating was assigned separately for each parameter on a 1 to 9 descriptive hedonic scale, with 9 as the highest-quality sample.
Microbiological analysis
Microbiological analysis was performed according to the Chinese National Standard (Citation2010) by measurement of the total bacteria amounts indicated as total aerobic plate counts (TPC). Twenty-five grams of shrimps were homogenized with 225 mL of 0.85% sterile saline water at 8000 rpm for 1 min. Samples were serially diluted logarithmically in 0.85% sterile saline water. TPC were determined by spread plating samples on nutrient agar and incubating at 36°C for 48 h. Microbial colonies were counted and reported as log CFU/g of fresh weight.
pH determination
The pH values were measured in shrimp according to the method of López-Caballero, Martínez-Alvarez, Gómez-Guillén, and Montero (Citation2007).
Determination of total volatile basic nitrogen
Total volatile basic nitrogen (TVB-N) was determined according to the method of our previous study (Sun et al., Citation2014). TVB-N was determined by steam distillation of trichloroacetic acid-shrimp extract in triplicates, using an automatic Kjeldahl apparatus (Kjeltec-8400, Foss, Sweden). The TVB-N values were expressed as mg N/100 g shrimp meat.
Statistical analysis
Statistical analysis was performed using the SPSS package program version 11.5 (SPSS inc. Chicago, IL, USA). Data was analyzed by one-way ANOVA, followed by Turkey’s HSD multiple comparison test. The values are reported as means with their standard error for all results. Differences were considered significant at p < 0.05.
Results and discussion
Effect of chitosan coating combined with green tea extract on the melanosis of Pacific white shrimp during storage in ice
The effect of chitosan coating combined with GTE on the melanosis formation of Pacific white shrimp during storage in ice was investigated in the current study. The melanosis score of Pacific white shrimp is shown in . The melanosis formation in all treatments was significantly increased during storage in ice (p < 0.05). This result showed that there was rapid loss in visual quality of Pacific white shrimp during storage in ice, which is similar to our previous studies (Fang et al., Citation2013; Sun et al., Citation2014). The melanosis formation in Pacific white shrimp treated by chitosan coating, GTE, and GTE + chitosan coating was significantly inhibited (p < 0.05), compared with the control. However, the melanosis formation in the shrimps treated with SMS was lowest among the five groups.
Figure 1. Combined effect of chitosan and green tea extract on melanosis score of Pacific white shrimp during storage in. Each item of data is the mean value per treatment and time point (mean ± standard error). SMS, sodium metabisulfite; GTE, green tea extract.
Figura 1. Efecto combinado de chitosán y extracto de té verde en los resultados de melanosis de las gambas blancas del Pacífico durante el almacenamiento con hielo. Cada dato es el valor promedio por tratamiento y tiempo (promedio ± error estándar). SMS, metabisulfito sódico; GTE, extracto de té verde.
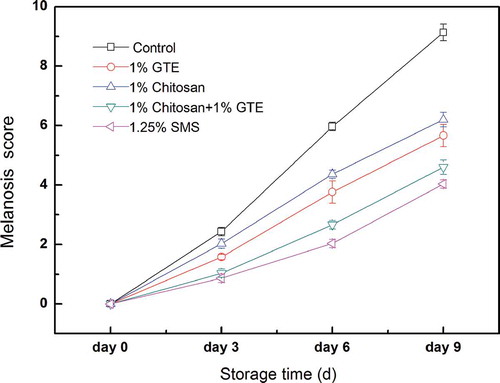
Previous studies showed that natural extracts or natural compounds including catechin, ferulic acid, extracts from Punica granatum, Leucaena leucocephal and Flammulina velutipes could inhibit the melanogenesis of shrimp during storage in ice (Encarnación et al., Citation2010, Citation2011; Fang et al., Citation2013; Nirmal & Benjakul, Citation2009b, Citation2010, Citation2011b). The melanogenesis was significantly inhibited and visual quality was significantly improved in Pacific white shrimp treated with GTE in the present study. This result agrees with those obtained for Nirmal and Benjakul (Citation2011c, Citation2011d, Citation2012), who found that GTE had an inhibition effect on the formation of melanosis of Pacific white shrimp. Phenolic compounds in the GTE could significantly inhibit polyphenoloxidase from cephalothorax of Pacific white shrimp (Nirmal & Benjakul, Citation2011d). In addition, GTE exhibited both copper chelating activity as well as reducing power (Nirmal & Benjakul, Citation2011d). As a result, the inhibition of melanosis in Pacific white shrimp treated with GTE was possibly due to the combined effect between polyphenoloxidase inhibition as well as the reduction of quinone formed during storage in ice (Nirmal & Benjakul, Citation2011d).
The shelf life of seafood including Atlantic cod, fresh herring fillets, oysters, and salmon can be increased by using chitosan as an edible protective film (Cao et al., Citation2009; Jeon et al., Citation2002; Mohan et al., Citation2012). The present results are in agreement with those of Huang et al. (Citation2012) who reported that the application of chitosan coatings was effective in retarding melanosis of shrimp during storage. The retarding of melanosis in shrimp treated by chitosan coating may be attributed to certain chitosan functional properties, such as being an antioxidant, an antimicrobial agent, and an oxygen barrier (Huang et al., Citation2012). Additionally, GTE + chitosan coating was more effective than chitosan coating or GTE alone in retarding of melanosis in shrimp, suggesting that there was a synergistic effect between chitosan coating and GTE. The synergistic inhibition of melanosis in Pacific white shrimp by GTE and chitosan coating was plausibly due to the combined effect of PPO inhibition by GTE as well as the antioxidant and antimicrobial, and oxygen barrier activity of chitosan during storage in ice.
Effect of chitosan coating combined with green tea extract on sensory quality of Pacific white shrimp during storage in ice
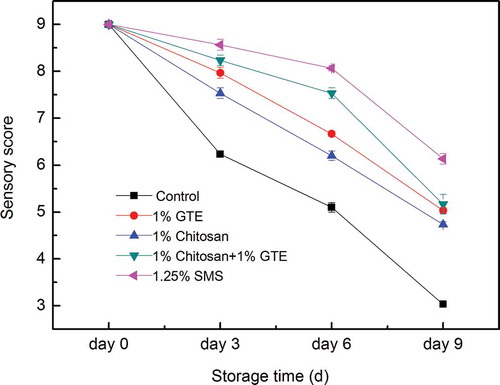
shows the changes in sensory quality of Pacific white shrimp. All samples had the score higher than 9 at the 0th day of storage in ice, and no differences in likeness of samples were found between all treatments (p > 0.05). In general, the sensory scores of Pacific white shrimp showed a tendency to decrease during storage in ice. However, the decrease rate of sensory scores in Pacific white shrimp varied with different treatments, which was fastest in the control group for all the sampling days (p < 0.05). The sensory score of the control shrimp was 5.4 at the 6th day of storage in ice, which reached an unacceptable level according to the Chinese National Standard (GB2741-94). The decrease rate of sensory scores in Pacific white shrimp treated by GTE, chitosan coating, GTE + chitosan coating was significantly lower than the control shrimp (p < 0.05). In addition, the decrease rate of sensory score in shrimp treated by GTE + chitosan coating was significantly lower than that treated by GTE or chitosan coating alone (p < 0.05). These results suggested that treatment with GTE + chitosan coating could improve the sensory properties of shrimp. This was more likely to be due to the lowered microbial spoilage of shrimp, chemical decomposition and melanosis formation.
Figure 2. Combined effect of chitosan and green tea extract on sensory score of Pacific white shrimp during storage in ice. Each item of data is the mean value per treatment and time point (mean ± standard error). SMS, sodium metabisulfite; GTE, green tea extract.
Figura 2. Efecto combinado de chitosán y extracto de té verde en los resultados sensoriales de las gambas blancas del Pacífico durante el almacenamiento con hielo. Cada dato es el valor promedio por tratamiento y tiempo (promedio ± error estándar). SMS, metabisulfito sódico; GTE, extracto de té verde.
Effect of chitosan coating combined with green tea extract on the changes in pH of Pacific white shrimp during storage in ice
shows the changes in pH of Pacific white shrimp. pH of the fresh Pacific white shrimp at the 0th day was 7.28. In general, the rise of pH of shrimp in all treatments was observed during storage in ice. The pH change in shrimp was probably due to the accumulation of basic compounds caused by theactivity of bacteria or enzymatic actions (López-Caballero et al., Citation2007).
Figure 3. Combined effect of chitosan and green tea extract on pH of Pacific white shrimp during storage in ice. Each item of data is the mean of three replicates per treatment and time point (mean ± standard error). SMS, sodium metabisulfite; GTE, green tea extract.
Figura 3. Efecto combinado de chitosán y extracto de té verde en el pH de las gambas blancas del Pacífico durante el almacenamiento con hielo. Cada dato es el promedio de tres réplicas por tratamiento y tiempo (promedio ± error estándar). SMS, metabisulfito sódico; GTE, extracto de té verde.
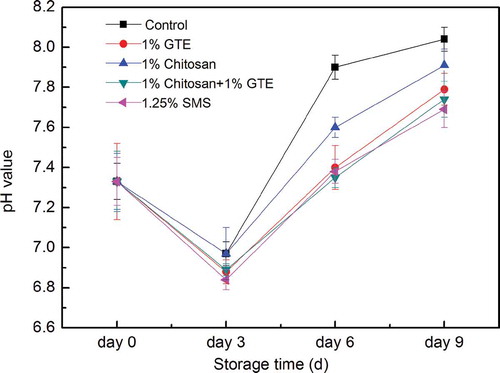
The rate of pH rise varied with different treatments. The rise of pH in Pacific white shrimp treated by GTE, chitosan coating, GTE + chitosan coating was significantly lower than the control shrimp (p < 0.05), which was similar to that of Huang et al. (Citation2012). However, the inhibition in the rise of pH in shrimp was less effective than SMS. These results suggested that GTE + chitosan coating might contribute to retarding quality loss of shrimp, in which spoilage or decomposition could be lowered.
Effect of chitosan coating combined with green tea extract on the changes in TVB-N of Pacific white shrimp during storage in ice
TVB-N is a common and important indicator of the quality of seafood because the rise of TVB-N value in seafood is related to microbial and chemical spoilage. shows the changes of TVB-N values of Pacific white shrimp treated by GTE, chitosan coating, GTE + chitosan coating in comparison with the control. In general, the TVB-N values of Pacific white shrimp in both control and treatments were significantly increased during 9 days of storage in ice (p < 0.05). However, the rise rate of TVB-N values in Pacific white shrimp varied with different treatments, which was highest in the control shrimp for all the sampling days. The rise of TVB-N values in Pacific white shrimp treated by GTE, chitosan coating, GTE + chitosan coating was significantly inhibited, compared with the control and that treated by GTE + chitosan coating was significantly less than those treated by GTE or chitosan coating alone (p < 0.05).
Figure 4. Combined effect of chitosan and green tea extract on the total volatile basic nitrogen content of Pacific white shrimp during storage in ice. Each item of data is the mean of three replicates per treatment and time point (mean ± standard error). SMS, sodium metabisulfite; GTE, green tea extract.
Figura 4. Efecto combinado de chitosán y extracto de té verde en el contenido total de volátiles de nitrógeno básico de las gambas blancas del Pacífico durante el almacenamiento con hielo. Cada dato es el promedio de tres réplicas por tratamiento y tiempo (promedio ± error estándar). SMS, metabisulfito sódico; GTE, extracto de té verde.
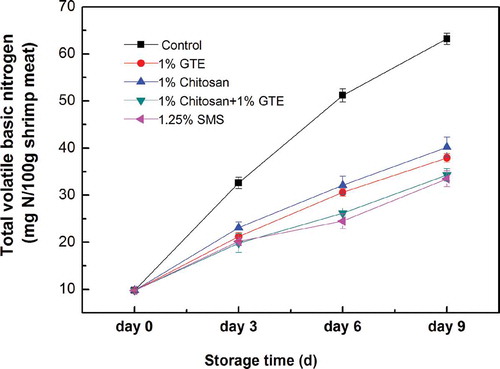
The initial TVB-N value of Pacific white shrimp was 9.8 mg/100 g. For the control sample, the TVB-N value was 51.2 mg/100 g at the 6th day of storage and exceeded the spoilage limit of shrimp. According to the Chinese National Shrimp Sanitary Standard (Citation1994), the TVB-N value of fresh shrimp should be <300 mg/100 g. The TVB-N value of shrimp treated by GTE + chitosan coating was 37.9 mg/100 g at the 9th day of storage in ice. The above results agree with those of previous studies which reported that the TVB-N values of Pacific white shrimp treated with ferulic acid, cinnamaldehyde and grape seed extract were significantly decreased (Mu, Chen, Fang, Mao, & Gao, Citation2012; Nirmal & Benjakul, Citation2009a; Sun et al., Citation2014).
The rise of TVB-N value of shrimp treated by chitosan coating was also inhibited in comparison with control thorough storage. It agrees with those of Huang et al. (Citation2012) and Wu (Citation2014) who reported that a lower increase in TVB-N value was obtained in the shrimps treated by chitosan coating. Additionally, the rise in TVB-N value of shrimps treated by GTE + chitosan coating was significantly lower than those treated by GTE or chitosan coating alone (p < 0.05), suggesting that chitosan coating demonstrated synergism in retarding TVB-N value when used in combination with GTE.
Effect of chitosan coating combined with green tea extract on the microbiological changes of Pacific white shrimp during storage in ice
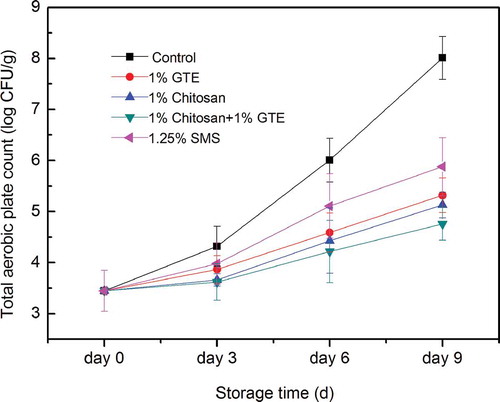
TPC of Pacific white shrimp during storage in ice are shown in . In general, an increasing tendency of TPC in all samples was observed throughout the storage for 9 days. The increase in TPC was highest in the control group for all the sampling days. The increase in TPC of Pacific white shrimp treated by GTE, chitosan coating, GTE + chitosan coating was significantly inhibited, compared with the control and that treated by GTE + chitosan coating was significantly less than that treated by GTE or chitosan coating alone (p < 0.05). The TPC of shrimp in the control group were 3.45 log CFU/g at the 0th day and 8.01 log CFU/g at the 9th day of storage in ice. At the end of storage (9th day), TPC of the control shrimp, those treated with SMS, 1% GTE, 1% chitosan, and 1% GTE + 1% chitosan coating were 5.88, 5.32, 5.13 and 4.76 log CFU/g, respectively.
Figure 5. Combined effect of chitosan and green tea extract on total aerobic plate count of Pacific white shrimp during storage in ice. Each item of data is the mean of three replicates per treatment and time point (mean ± standard error). SMS, sodium metabisulfite; GTE, green tea extract.
Figura 5. Efecto combinado de chitosán y extracto de té verde en el recuento total de aeróbicos en placa de gambas blancas del Pacífico durante el almacenamiento con hielo. Cada dato es el promedio de tres réplicas por tratamiento y tiempo (promedio ± error estándar). SMS, metabisulfito sódico; GTE, extracto de té verde.
GTE are considered rich sources of polyphenolic compounds that show antioxidant or antimicrobial effects (Perumalla & Hettiarachchy, Citation2011). Several studies have proved that GTE prepared with water and 80% ethanol exhibited antimicrobial activity. Bañón, Díaz, Rodríguez, Garrido, and Price (Citation2007) reported that ascorbate and GTE increased the shelf life of beef patties by delaying microbial spoilage. Nirmal and Benjakul (Citation2012) found that GTE, which was the source of catechin, could inhibit some microorganisms in shrimp. Nirmal and Benjakul (Citation2009b) reported that Pacific white shrimp treated with 0.1% catechin had a lower enterobacteriaceae count as compared to the control after 10 days of storage in ice. In the present study, GTE also exerted antimicrobial activity in Pacific white shrimp during storage in ice.
Chitosan is well known for its broad spectrum of antimicrobial activities against both gram-positive and gram-negative bacteria (Kong, Chen, Xing, & Park, Citation2010). The antimicrobial activity of chitosan could be mediated by the interactions between the positively charged chitosan and negatively charged microbial cell membranes, which induces the leakage of cellular proteins and other intracellular constituents (No, Meyers, Prinyawiwatkul, & Xu, Citation2007). Chitosan also inhibits microbial growth by the chelation of nutrients and essential metals, spore components, as well as the penetration of the nuclei of the microorganisms, which leads to the interference with protein synthesis by binding with DNA. Chitosan coatings act as an oxygen barrier and can inhibit the growth of aerobic bacteria (Devlieghere, Vermeulen, & Debevere, Citation2004). Previous studies have reported that the use of chitosan coating improves shrimp quality (Huang et al., Citation2012; Wu, Citation2014). The present finding further confirmed that chitosan coating could inhibit microbial growth in shrimp.
Conclusions
The melanosis formation was significantly inhibited and sensory quality was significantly improved in Pacific white shrimp treated with chitosan coating, GTE and chitosan coating combined with GTE, compared with the control. The increases in the pH, TVB-N and TPC were significantly inhibited in Pacific white shrimp treated with chitosan coating, GTE and chitosan coating combined with GTE. However, the melanosis formation and TVB-N of Pacific white shrimp treated by chitosan coating combined with GTE was lower than that treated by chitosan coating or GTE alone during 9 days of storage in ice. These results suggested that chitosan coating combined with GTE could be used as an effective natural alternative to synthetic antimelanosic agents to inhibit post-mortem melanosis and improve the quality of shrimp during storage in ice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bañón, S., Díaz, P., Rodríguez, M., Garrido, M. D., & Price, A. (2007). Ascorbate, green tea and grape seed extracts increase the shelf life of low sulphite beef patties. Meat Science, 77, 626–633. doi:10.1016/j.meatsci.2007.05.015
- Cao, R., Xue, C. H., & Liu, Q. (2009). Changes in microbial flora of Pacific oysters (Crassostrea gigas) during refrigerated storage and its shelf-life extension by chitosan. International Journal of Food Microbiology, 131, 272–276. doi:10.1016/j.ijfoodmicro.2009.03.004
- Carrizo, D., Gullo, G., Bosetti, O., & Nerín, C. (2014). Development of an active food packaging system with antioxidant properties based on green tea extract. Food Additives and Contaminants Part A: Chemistry Analysis Control Exposure & Risk Assessment, 31, 364–373. doi:10.1080/19440049.2013.869361
- Chinese National Standard (GB2741-94). (1994). Hygienic standard for sea shrimp. Beijing: Chinese National Hygiene Ministry.
- Chinese National Standard (GB4789.2-2010). (2010). Microbiological examination of food hygiene: Detection of aerobic bacterial count. Beijing: Chinese National Hygiene Ministry.
- Devlieghere, F., Vermeulen, A., & Debevere, J. (2004). Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiology, 21, 703–714. doi:10.1016/j.fm.2004.02.008
- Encarnación, A. B., Fagutao, F., Hirayama, J., Terayama, M., Hirono, I., & Ohshima, T. (2011). Edible mushroom (Flammulina velutipes) extract inhibits melanosis in Kuruma shrimp (Marsupenaeus japonicus). Journal of the Science of Food and Agriculture, 76, C52–C58.
- Encarnacion, A. B., Fagutao, F., Hirono, I., Ushio, H., & Ohshima, T. (2010). Effects of ergothioneine from mushrooms (Flammulina velutipes) on melanosis and lipid oxidation of Kuruma shrimp (Marsupenaeus japonicus). Journal of Agricultural and Food Chemistry, 58, 2577–2585. doi:10.1021/jf903944y
- Fang, X. B., Sun, H. Y., Huang, B. Y., & Yuan, G. F. (2013). Effect of pomegranate peel extract on the melanosis of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Journal of Food Agriculture and Environment, 11, 105–109.
- Huang, J. Y., Chen, Q. C., Qiu, M., & Li, S. Q. (2012). Chitosan-based edible coatings for quality preservation of postharvest whiteleg shrimp (Litopenaeus vannamei). Journal of the Science of Food and Agriculture, 77, C491–C496.
- Jeon, Y. J., Kamil, J. Y. V. A., & Shahidi, F. (2002). Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. Journal of Agricultural and Food Chemistry, 50, 5167–5178. doi:10.1021/jf011693l
- Jung, M. H., Seong, P. N., Kim, M. H., Myong, N. H., & Chang, M. J. (2013). Effect of green tea extract microencapsulation on hypertriglyceridemia and cardiovascular tissues in high fructose-fed rats. Nutrition Research and Practice, 7, 366–372.
- Kim, J., Marshall, M. R., & Wei, C. (2002). Polyphenoloxidase in seafood enzymes: Utilization and influence on postharvest seafood quality. In N. Haard & B. Simpson (Eds.), Seafood enzymes (pp. 271–315). New York, NY: Marcel Dekker.
- Kong, M., Chen, X. G., Xing, K., & Park, H. J. (2010). Antimicrobial properties of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology, 144, 51–63. doi:10.1016/j.ijfoodmicro.2010.09.012
- López-Caballero, M. E., Martínez-Álvarez, O., Gómez-Guillén, M. D. C., & Montero, P. (2007). Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. International Journal of Food Science & Technology, 42, 1029–1038. doi:10.1111/ifs.2007.42.issue-9
- Maqsood, S., Benjakul, S., & Shahidi, F. (2013). Emerging role of phenolic compounds as natural food additives in fish and fish products. Critical Reviews in Food Science and Nutrition, 53, 162–179. doi:10.1080/10408398.2010.518775
- Martinez-Alvarez, O., Lopez-Caballero, M. E., Montero, P., & Gomez-Guillen, M. C. (2005). A 4-hexylresorcinol-based formulation to prevent melanosis and microbial growth in chilled tiger prawns (Marsupenaeus japonicus) from aquaculture. Journal of the Science of Food and Agriculture, 70, M415–M422.
- McEvily, A. J., Iyengar, R., & Otwell, S. (1991). Sulfite alternative prevents shrimp melanosis. Food Technology, 45, 80–86.
- Mohan, C. O., Ravishankar, C. N., Lalitha, K. V., & Gopal, T. K. S. (2012). Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocolloids, 26, 167–174. doi:10.1016/j.foodhyd.2011.05.005
- Montero, P., Ávalos, A., & Pérez-Mateos, M. (2001). Characterization of polyphenoloxidase of prawns (Penaeus japonicus). Alternatives to inhibition: Additives and high-pressure treatment. Food Chemistry, 75, 317–324. doi:10.1016/S0308-8146(01)00206-0
- Mu, H., Chen, H., Fang, X., Mao, J., & Gao, H. (2012). Effect of cinnamaldehyde on melanosis and spoilage of Pacific white shrimp (Litopenaeus vannamei) during storage. Journal of the Science of Food and Agriculture, 92, 2177–2182. doi:10.1002/jsfa.v92.10
- Nagao, T., Hase, T., & Tokimitsu, I. (2007). A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity, 15, 1473–1483. doi:10.1038/oby.2007.176
- Nirmal, N. P., & Benjakul, S. (2009a). Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chemistry, 116, 323–331. doi:10.1016/j.foodchem.2009.02.054
- Nirmal, N. P., & Benjakul, S. (2009b). Melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) treated with catechin during iced storage. Journal of Agricultural and Food Chemistry, 57, 3578–3586. doi:10.1021/jf900051e
- Nirmal, N. P., & Benjakul, S. (2010). Effect of catechin and ferulic acid on melanosis and quality of Pacific white shrimp subjected to prior freeze-thawing during refrigerated storage. Food Control, 21, 1263–1271. doi:10.1016/j.foodcont.2010.02.015
- Nirmal, N. P., & Benjakul, S. (2011a). Inhibition of melanosis formation in Pacific white shrimp by the extract of lead (Leucaena leucocephala) seed. Food Chemistry, 128, 427–432. doi:10.1016/j.foodchem.2011.03.048
- Nirmal, N. P., & Benjakul, S. (2011b). Inhibitory effect of mimosine on polyphenoloxidase from cephalothoraxes of Pacific white shrimp (Litopenaeus vannamei). Journal of Agricultural and Food Chemistry, 59, 10256–10260. doi:10.1021/jf201603k
- Nirmal, N. P., & Benjakul, S. (2011c). Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. International Journal of Food Microbiology, 149, 247–253. doi:10.1016/j.ijfoodmicro.2011.07.002
- Nirmal, N. P., & Benjakul, S. (2011d). Use of tea extracts for inhibition of polyphenoloxidase and retardation of quality loss of Pacific white shrimp during iced storage. LWT-Food Science and Technology, 44, 924–932. doi:10.1016/j.lwt.2010.12.007
- Nirmal, N. P., & Benjakul, S. (2012). Effect of green tea extract in combination with ascorbic acid on the retardation of melanosis and quality changes of Pacific white shrimp during iced storage. Food and Bioprocess Technology, 5, 2941–2951. doi:10.1007/s11947-010-0483-5
- No, H. K., Meyers, S. P., Prinyawiwatkul, W., & Xu, Z. (2007). Applications of chitosan for improvement of quality and shelf life of foods: A review. Journal of the Science of Food and Agriculture, 72, R87–R100.
- Perumalla, A. V. S., & Hettiarachchy, N. S. (2011). Green tea and grape seed extracts – Potential applications in food safety and quality. Food Research International, 44, 827–839. doi:10.1016/j.foodres.2011.01.022
- Sagoo, S., Board, R., & Roller, S. (2002). Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiology, 19, 175–182. doi:10.1006/fmic.2001.0474
- Souza, B. W. S., Cerqueira, M. A., Ruiz, H. A., Martins, J. T., Casariego, A., Teixeira, J. A., & Vicente, A. A. (2010). Effect of chitosan-based coatings on the shelf life of salmon (Salmo salar). Journal of Agricultural and Food Chemistry, 58, 11456–11462. doi:10.1021/jf102366k
- Sun, H. Y., Lv, H., Yuan, G. F., & Fang, X. B. (2014). Effect of grape seed extracts on the melanosis and quality of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Science and Technology Research, 20, 671–677. doi:10.3136/fstr.20.671
- Thepnuan, R., Benjakul, S., & Visessanguan, W. (2008). Effect of pyrophosphate and 4-hexylresorcinol pretreatment on quality of refrigerated white shrimp (Litopenaeus vannamei) kept under modified atmosphere packaging. Journal of the Science of Food and Agriculture, 73, S124–S133.
- Tsao, A. S., Liu, D., Martin, J., Tang, X. M., Lee, J. J., El-Naggar, A. K., … Papadimitrakopoulou, V. (2009). Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prevention Research, 2, 931–941. doi:10.1158/1940-6207.CAPR-09-0121
- Wu, S. J. (2014). Effect of chitosan-based edible coating on preservation of white shrimp during partially frozen storage. International Journal of Biological Macromolecules, 65, 325–328. doi:10.1016/j.ijbiomac.2014.01.056
