Abstract
A glycated and cross-linked soybean protein isolate (GC-SPI) with the glucosamine content of 19.40 g/kg protein was prepared by using SPI, transglutaminase, and a degraded chitosan under fixed conditions, aiming to assess modified properties and potential application of GC-SPI. GC-SPI has less reactable –NH2 groups than SPI (0.38 versus 0.48 mol/kg protein), and is verified by electrophoretic analysis to be a glycated and cross-linked protein product. Infrared spectroscopy and circular dichroism analyses demonstrate that GC-SPI contains more –OH groups, and has a more open secondary structure. In comparison with SPI, GC-SPI has higher surface hydrophobicity but lower in vitro digestibility due to protein cross-linking, and exhibits greater water- and oil-binding capacities (9.9 versus 6.4 and 3.7 versus 2.0 kg/kg protein). It is concluded that the glycation and cross-linking confer an open structure and modified properties of SPI, and GC-SPI is a potential ingredient with better hydration and oil-binding than SPI.
Se preparó aislado de proteína de soja glicada y reticulada (GC-SPI) con un contenido de glucosamina de 19,40 g/kg de proteína utilizando SPI, transglutaminasa y chitosán degradado sometido a condiciones determinadas con el objetivo de estudiar las propiedades modificadas y las aplicaciones potenciales de GC-SPI. GC-SPI obtuvo menos grupos reactivos de tipo NH2 en comparación con SPI (0,38 frente a 0,48 mol/kg de proteína) y se verificó mediante análisis electroforético para ser un producto proteínico glicado y reticulado. La espectroscopía infrarroja y el análisis de dicroísmo circular mostraron que el GC-SPI contiene más grupos OH y tiene una estructura secundaria más abierta. En comparación con SPI, GC-SPI tiene una mayor superficie de hidrofobia aunque una menor digestibilidad in vitro debido a la reticulación proteínica, además exhibe una mayor capacidad de retención de agua y de aceite (9,9 frente a 6,4 y 3,7 frente a 2,0 kg/kg de proteína). En conclusión, la glicación y la reticulación conceden una estructura abierta y unas propiedades modificadas en SPI. Además GC-SPI se trata de un ingrediente potencial con una mejor hidratación y capacidad de retención que SPI.
Introduction
Soybean protein, because of its good nutritive value and functional properties, has been widely used in the food industry as one of the protein ingredients (Tang, Wu, Chen, & Yang, Citation2006). Among these commercial soybean protein products is the soybean protein isolate (SPI). SPI contains at least 90% protein, and is usually used in meat, bread, and other foods. However, functional properties of food proteins can be enhanced by physical, chemical, and enzymatic treatments. Application of thermal treatments to soybean protein is the oldest method to enhance protein aggregation (Hermansson, Citation1986). High-pressure treatment also has been used to modify the properties of milk proteins, conferring casein micelles with different sizes, light-scattering properties, and hydration (Huppertz, Fox, De Kruif, & Kelly, Citation2006). The well-known chemical modifications of proteins include acetylation, succinylation, esterification, amidation, and other reactions, have been used in the past studies, and are not introduced here. In addition, protein glycation has become an area of considerable research interest in recent years (Oliver, Melton, & Stanley, Citation2006). The well-known Maillard reaction is able to glycate proteins (Martins, Jongen, & Van Boekel, Citation2000), via the formation of protein–saccharide conjugates. Protein glycation modifies protein properties including solubility, emulsification, and secondary structure (Kato, Mifuru, Matsudomi, & Kobayashi, Citation1992; Li, Enomoto, Ohki, Ohtomo, & Aoki, Citation2005; Xue, Li, Zhu, Wang, & Pan, Citation2013). Unfortunately, protein glycation via the Maillard reaction also has some disadvantages; for example, the formation of undesired browning and potential toxic compounds (Zhang, Ames, Smith, Baynes, & Metz, Citation2009). Protein glycation via enzymatic approaches requires milder conditions, therefore, it might be more suitable than that via the Maillard reaction.
Transglutaminase (TGase, EC 2.3.2.13) induces an acyl-transfer reaction between the glutamine and lysine residues of the proteins to produce intra- and inter-molecular iso-peptide bonds. TGase is thus capable of catalyzing protein cross-linking, which does not reduce nutritional quality but modify properties of the proteins (Seguro, Kumazawa, Kuraishi, Sakamoto, & Motoki, Citation1996). TGase is used to enhance emulsion stability and viscosity of whey and soybean proteins (Gauche, Vieira, Ogliari, & Bordignon-Luiz, Citation2008; Liu & Damodaran, Citation1999), and to generate biodegradable films from soybean and whey proteins (Schmid, Sängerlaub, Wege, & Stäbler, Citation2014; Tang, Jiang, Wen, & Yang, Citation2005). Some saccharides, such as glucosamine and oligochitosan with free –NH2 groups, can also be conjugated into food proteins by TGase to generate glycated and cross-linked products with higher apparent viscosity and better emulsion stability (Jiang & Zhao, Citation2010; Song & Zhao, 2014). However, conjugation of other amino-containing saccharides into food proteins via TGase and the corresponding property changes of the products have not been well-investigated so far.
Chitosan is a natural bio-macromolecular product abundant in the crustaceans (Muzzarelli et al., Citation2012). Degraded chitosan (i.e. chitooligomers) with the degree of polymerization less than 20 is nontoxic, biodegradable, and biocompatible (Batista, Pinto, Gomes, & Gomes, Citation2006; Tian et al., Citation2010), has many applications in food, medicine, and other fields (Kim, Citation2011), and can be obtained easily by oxidative depolymerization of chitosan with H2O2 (Tian et al., Citation2010). Degraded chitosan contains free –NH2 groups in molecules, and also has a chance to be conjugated into proteins by TGase. A previous study has applied a degraded chitosan to glycate caseinate successfully, resulting in the product with better water-binding and rheological properties (Zhu, Wang, & Zhao, Citation2015). Whether the degraded chitosan is applicable to glycate other proteins for property modification is still an unrevealed topic so far. The effect of the TGase-induced glycation and cross-linking on protein structure is also not assessed.
In the present study, SPI was used as a target protein substrate and subjected to glycation and cross-linking by TGase and the degraded chitosan. Structural features of the glycated and cross-linked SPI (GC-SPI) were assessed by the classic Fourier transform infrared (FT-IR) and circular dichroism (CD) analyses. Some properties of the generated GC-SPI including surface hydrophobicity, in vitro digestibility, hydration, and oil-binding were also evaluated. The aim of the present study was to assess the effects of glycation and cross-linking on SPI properties, and potential of the generated GC-SPI as a new protein ingredient.
Materials and methods
Materials and chemicals
Defatted soybean flour used for SPI preparation was purchased from Harbin Hi-Tech Soybean Food Co. Ltd. (Harbin, China). Chitosan was obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China), with a declared degree of deacetylation of 84% and the viscosity average molecular weight of 860 kDa. Microbial TGase preparation was gifted by Jiangsu Yiming Fine Chemical Industry Co. Ltd. (Jiangsu, China) with a measured activity of 110 units (U) per gram. All chemical reagents used in high performance liquid chromatography (HPLC) analysis were of chromatographic grade. The buffers and solutions were prepared with highly purified water generated from Milli-Q PLUS (Millipore Corporation, New York, NY, USA) and filtered using a 0.45 μm Millipore HA membrane. Other chemicals used were of analytical grade.
Sample preparation
SPI was extracted from the defatted soybean flour according to the reported method (Petruccelli & Anon, Citation1994). An alkaline extract (pH 8.0) separated from the defatted soybean flour was adjusted to an isoelectric precipitation (pH 4.5) at ambient temperature by adding 2 mol/L HCl. The precipitate was collected by centrifugation at 4000g for 20 min, re-suspended in water, continuously stirred at ambient temperature for 1 h, and centrifuged again to remove residual acid. The precipitate was resolved in water and neutralized to pH 7.0 by using 2 mol/L NaOH. The obtained solution was lyophilized at a beginning temperature of –24°C and a pressure of 10 Pa. The obtained lyophilized solution was dried at 60°C for 12 h to ensure complete drying without excessive protein denaturation, and then ground into fine particles (less than 50 μm) to obtain SPI.
The degraded chitosan was prepared according to a reported method (Tian et al., Citation2010). Chitosan of 20 g was dissolved in 2% acetic acid of 800 mL; after that, H2O2 solution (30%, v/v) of 30 mL was added. The mixture was stirred at 70°C for 2 h, cooled to ambient temperature, neutralized to pH 7.0 with NaOH solution of 2 mol/L, and filtered using a Whitman filter to remove insoluble particles. The filtered solution was precipitated by ethanol with final concentrations of 75% (v/v) for 24 h. The obtained precipitate (degraded chitosan) was lyophilized, dried at 50°C for 12 h to ensure further drying without potential decomposition, and then ground into fine particles less than 50 μm.
GC-SPI was prepared as following: the reaction was carried out at pH 7.5 and 37°C for 4 h. Other reaction conditions used in GC-SPI preparation included a protein concentration of 50 g/L, the molar ratio of the acyl donor to the degraded chitosan acceptor of 1:3, and a TGase amount of 10 kU/kg protein. After the reaction, the mixture was quickly heated to 85°C for 5 min to inactivate the TGase, and then cooled to ambient temperature. The pH value of the mixture was adjusted to 4.5 by using 1 mol/L HCl. The obtained precipitate was washed twice with water of pH 4.5, re-dispersed in water, adjusted to pH 7.0 by using 2 mol/L NaOH, and then lyophilized. At the same time, a cross-linked SPI (CSPI) was prepared as GC-SPI but without the addition of the degraded chitosan, and then used as a control. After that, SPI was subjected to the same procedure as GC-SPI but without TGase and the degraded chitosan addition, and then used as another control in forthcoming evaluation. Three lyophilized protein samples were dried at 60°C for 12 h to achieve further drying, ground into fine particles less than 50 μm, sealed in plastic bags, and then stored at –20°C before evaluation.
Chemical and electrophoretic analyses
The amount of the degraded chitosan conjugated into SPI was measured by a reversed phase (RP)-HPLC method, and expressed on the basis of the glucosamine amount conjugated into protein fraction of one kilogram. Sample hydrolysis and glucosamine derivatization followed the two reported methods (El-Saharty & Bary, Citation2002; Guan et al., Citation2011). The mobile phase used in HPLC analysis consisted of methanol (32%, v/v) and acetate buffer of 0.05 mol/L (68%, v/v), and was used at a flow rate of 1 mL/min. The column temperature was set at 35°C. Fluorescence detection was carried out at respective excitation and emission wavelengths of 337 and 454 nm.
Nitrogen content was assayed by the Kjeldahl method (AOAC, Citation1995), and used to calculate protein content with a conversion factor of 6.25. For GC-SPI, the nitrogen amount from the conjugated degraded chitosan was previously assayed by the RP-HPLC method, and then used to correct the measured protein content. The amount of reactable –NH2 groups of the assayed protein samples was assessed as per the method (Church, Swaisgood, Porter, & Catignani, Citation1983), by using o-phthalaldehyde (OPA) to react with free –NH2 groups, and then reported as the moles in protein fraction of one kilogram. For GC-SPI, the –NH2 groups from the conjugated degraded chitosan were also assayed by the RP-HPLC method, and then used for result correction.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was carried out as per the method of Jiang and Zhao (Citation2011), by using running and stacking gels of 120 and 40 g/L, respectively. The gels were stained by Coomassie Brilliant Blue R250 of 25 g/L and the pararosaniline-Schiff reagent to detect protein and saccharide fractions, respectively. SPI and horseradish peroxidase were used as negative and positive controls in SDS-PAGE analysis, respectively.
FT-IR and CD analyses
FT-IR analysis was carried out using a FI-IR instrument (Bruker, Norwalk, CT, USA). Scanning was carried out in a wavenumber range of 900–1800 1/cm, and 64 scans were used for each sample. The classic KBr-disk method was used to prepare analyzed samples, by using pre-dried 1 mg of the samples and 100 mg of KBr to obtain the compressed pellets. At the same time, a Jasco J-815 circular dichroism spectrometer (Jasco Corporation, Tokyo, Japan) was used to perform CD analysis. CD spectra of the samples were recorded at a protein concentration of 0.1 g/L (in a phosphate buffer of 10 mmol/L, pH 7.0), by using a 0.1 cm path length cuvette at ambient temperature, and then expressed as the molar ellipticity (deg cm2/dmol) of the samples at 190–260 nm.
Property evaluation
Surface hydrophobicity was assayed by using 1-anilino-8-naphthalene-sulfonate (ANS) as a fluorescent probe, according to a reference method (Hayakawa & Nakai, Citation1985). Protein samples were prepared at 0.1–0.5 g/L by the phosphate buffer. Aliquots (20 μL) of the ANS solution (8.0 mmol/L in the phosphate buffer) were added into 4 mL of the protein dispersions. Fluorescence intensities of the mixed solutions were detected using a fluorescence spectrophotometer (Type F4500, Hitachi, Kyoto, Japan), by using respective excitation and emission wavelengths of 390 and 470 nm. The initial slope of the plot of fluorescence intensities versus protein concentrations, calculated by a linear regression, was used as an index of the surface hydrophobicity of the assayed protein sample.
In vitro digestibility of the samples was measured by peptic (one-step) and peptic–tryptic (two-step) digestion as per the method of Marciniak-Darmochwal and Kostyra (Citation2009). Trichloroacetic acid (TCA)-soluble nitrogen released into the supernatant was assessed by a spectrophotometer (UV-2401 PC, Shimadzu Co., Kyoto, Japan) at 280 nm, after four-fold dilution of the collected supernatant by water. The detected absorbencies were used to reflect in vitro digestibility of protein samples.
A reported method (Beuchat, Citation1977) was used to assay water- and oil-binding capacities (WBC and OBC) of the protein samples with some modifications. For WBC, water of 5.0 g was added into the samples of 0.5 g, and the contents were held at ambient temperature for 30 min. The contents were centrifuged at 3000 g for 20 min, and the obtained supernatants were weighed. The protein amount in the separated supernatant was assayed to correct WBC calculation. For OBC, refined soybean oil of 3.0 g was added to the samples of 0.5 g, stirred and held at ambient temperature for 30 min. The contents were centrifuged at 3,000 g for 20 min, and the free oil was decanted and weighed. WBC and OBC were expressed as the amount (kg) of water and oil bound into the protein fraction of one kilogram.
Statistical analyses
All experiments and evaluations were carried out at least three times. The data were analyzed and reported by using Statistics Package for Social Science (SPSS) 13.0 software (SPSS Inc., Chicago, IL, USA) and MS Excel 2003 (Microsoft Corporation, Redmond, WA, USA). All data were expressed as means ± standard deviations. The differences between the mean values of multiple groups were analyzed by one-way analysis of (ANOVA) with Duncan’s multiple range tests. A value of P < 0.05 was considered significant.
Results and discussion
Preparation and analyses of GC-SPI
SPI and the degraded chitosan were used as respective acyl donors and acceptors to prepare GC-SPI. With the selected preparation conditions (condition selection is not discussed here), GC-SPI was generated, and then subjected to property evaluation.
HPLC analysis results show that GC-SPI contains glucosamine of (19.40 ± 0.41) g/kg protein, but no glucosamine is detected in both SPI and CSPI. This indicates that only GC-SPI is a glycated protein product. Glucosamine content of GC-SPI in the present study is higher than that of another GC-SPI (12.1 g/kg protein) reported in a previous study (Song & Zhao, 2014). Different glucosamine contents might rise from different acyl acceptors (degraded chitosan and oligochitosan) used in the preparation. Using OPA to assay these reactable – NH2 groups of the three protein samples, it is found that SPI, CSPI, and GC-SPI contain reactable –NH2 groups of (0.48 ± 0.01), (0.38 ± 0.01), and (0.39 ± 0.01) mol/kg protein, respectively. Both CSPI and GC-SPI are therefore evidenced to be cross-linked protein products, as they have less –NH2 groups than SPI. Lysine residues in proteins are involved in TGase-induced cross-linking (Hassan, Osman, & Babiker, Citation2007), resulting in the blockage of the ε–NH2 groups (i.e. reactable –NH2 groups). It is expected that the proteins cross-linked by TGase should contain less –NH2 groups. In the present study, content of reactable –NH2 groups of GC-SPI is not different from that of CSPI (P > 0.05), indicating that the occurred SPI glycation had an insignificant effect on another reaction (i.e. SPI cross-linking). Similar result also has been found in another study (Song & Zhao, 2014), in which SPI, cross-linked SPI, and the GC-SPI were observed to have reactable –NH2 groups of 0.47, 0.38, and 0.37 mol/kg protein, respectively. This previous study provides a support to the present study. The two saccharides (degraded chitosan and oligochitosan) used in the present and previous studies have chemical similarity, and the two glycated and cross-linked products prepared have the same content of reactable –NH2 groups. This demonstrates that TGase-induced SPI cross-linking is not clearly impacted by the glycation of two saccharides.
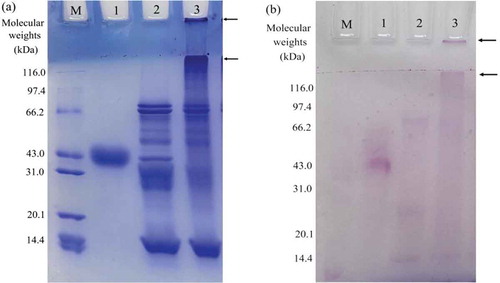
SDS-PAGE analysis has been used to investigate glycated proteins in two past studies (Diftis & Kiosseoglou, Citation2006; Liu, Zhao, Zhao, Ren, & Yang, Citation2012). Chemical features of GC-SPI in the present study were also revealed by this technique. Protein profiles () demonstrate that GC-SPI is glycated and cross-linked protein product, as GC-SPI is observed to contain protein polymers of higher molecular weights () and saccharide fraction (). These electrophoretic results are in agreement with those results reported in another study (Song & Zhao, 2014), in which electrophoretic analysis also declare SPI cross-linking as well as conjugation of an oligochitosan of 1 kDa into SPI.
Figure 1. SDS-PAGE profiles of the assayed samples stained for protein (a) and saccharide (b) detection. Lane M, standard protein markers with molecular weights of 14.4–116.0 kDa; Lane 1, horseradish peroxidase; Lane 2, soybean protein isolate (SPI); Lane 3, glycated and cross-linked SPI. The arrows indicate the bands of protein polymers of higher molecular weights in the samples.
Figura 1. Perfiles de SDS-PAGE de las muestras de ensayos teñidas para la detección de proteína (a) y sacárido (b). Línea M, marcadores proteínicos estándar con pesos moleculares entre 14,4–116,0 kDa; Línea 1, peroxidada de rábano picante; Línea 2, aislado de proteína de soja (SPI); Línea 3, SPI glicado y reticulado. Las flechas indican las bandas de polímeros proteínicos con mayor peso molecular en las muestras.
Structural characteristics of the GC-SPI
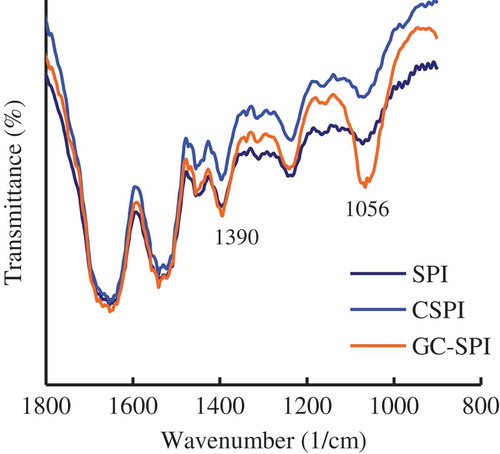
FT-IR analysis was used to show structural characteristics of SPI, CSPI and GC-SPI in their side chains, reflected by the existence of some chemical groups. The obtained FT-IR spectra of SPI, CSPI and GC-SPI with wavenumber range of 900–1,800 1/cm are shown in (). It can be seen form the spectra that GC-SPI has two peaks (around 1390 and 1056 1/cm) with stronger absorption, in comparison with SPI and CSPI; and the same time, CSPI and SPI possess similar peak absorption. These results declare that GC-SPI has higher contents of chemical groups than SPI and CSPI. The absorbance of C–O stretching and –OH deformation vibrations are typically around 1400–1330 and 1100 1/cm (Silverstein, Webster, & Kiemle, Citation2005), respectively. That is, there are more –OH groups in the molecules of GC-SPI other than in those molecules of SPI and CSPI. Protein cross-linking by TGase does not involve formation of –OH groups (i.e. SPI and CSP should have same content of –OH groups), whereas saccharide conjugation into proteins results in more –OH groups as the saccharide fraction contains many –OH groups. Therefore, FT-IR results confirm again that GC-SPI is a glycated protein product with more –OH groups than SPI and CSPI.
Figure 2. FT-IR spectra of soybean protein isolate (SPI), cross-linked SPI (CSPI), and glycated and cross-linked SPI (GC-SPI) in the compressed KBr pellets.
Figura 2. Espectro FT-IR del aislado de proteína de soja (SPI), SPI reticulado (CSPI) y SPI glicado y reticulado (GC-SPI) en los pellets comprimidos de KBr.
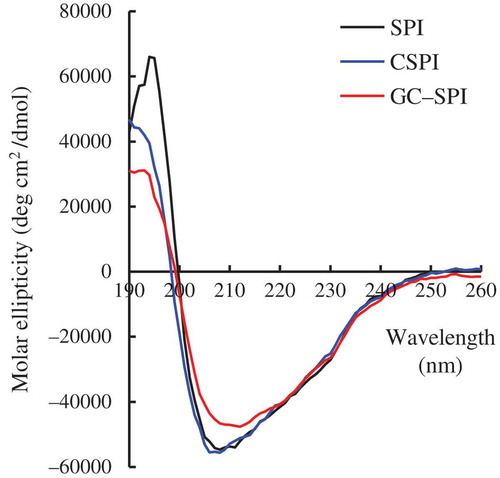
CD spectra of SPI, CSPI and GC-SPI are given in (), in which the three lines give briefly reflection about their secondary structural characteristics. SPI shows the greatest positive molar ellipticity around 192 nm, followed by CSPI and GC-SPI. The molar ellipticity around 192 nm is a reflector of α-helix structure in protein molecules (Silverstein et al., Citation2005); that is, SPI and GC-SPI have more and least α-helix structure, respectively. GC-SPI also has less negative molar ellipticity than SPI and CSPI around 208 and 215 nm, two respective reflectors of the α-helix and the β-sheet structure (Silverstein et al., Citation2005). These results evidence GC-SPI having less α-helix and β-sheet structure. All results suggest that GC-SPI possesses a less ordered but more open secondary structure than SPI and CSPI. Soybean proteins consist of mainly globulins, characteristic in an ordered secondary structure (Tang et al., Citation2006). TGase-induced cross-linking results in soybean proteins more flexible structure (Mizuno, Mitsuiki, Motoki, Ebisawa, & Suzuki, Citation2000), verifying that CSPI and GC-SPI should have a more open secondary structure than SPI. TGase treatment of soybean proteins has been observed to result in decreased α-helix and β-sheet but increased open secondary structure (Song & Zhao, 2014), giving a support to the present result. At the same time, conjugation of the degraded chitosan into SPI suggests that there are some branched fractions in the peptide chains of proteins. These branched fractions will interfere with the formation of an ordered secondary structure in protein molecules, and finally make a contribution to the disordered (i.e. open) secondary structure. As the result, GC-SPI has a more open secondary structure than CSPI.
Figure 3. Circular dichroism spectra of soybean protein isolate (SPI), cross-linked SPI (CSPI), and glycated and cross-linked SPI (GC-SPI) dispersed in a phosphate buffer (10 mmol/L, pH 7.0) at a fixed protein concentration of 0.1 g/L.
Figura 3. Espectro de dicroísmo circular del aislado de proteína de soja (SPI), SPI reticulado (CSPI) y SPI glicado y reticulado (GC-SPI) disperso en un tampón fosfato (10 mmol/L, pH 7,0) con una concentración proteínica determinada de 0,1 g.
Properties of the GC-SPI
Four properties including surface hydrophobicity, in vitro digestibility, WBC and OBC were assessed for SPI, CSPI and GC-SPI, to clarify if GC-SPI has some properties applicable in food processing. The obtained results () show that the carried out glycation and cross-linking conferred GC-SPI with two enhanced and applicable properties (water- and oil-binding capacities), in comparison with the respective properties of SPI.
Table 1. Some properties of soybean protein isolate (SPI), cross-linked SPI (CSPI), as well as glycated and cross-linked SPI (GC-SPI).
Tabla 1. Algunas propiedades del aislado de proteína de soja (SPI), SPI reticulado (CSPI) y SPI glicado y reticulado (GC-SPI).
GC-SPI and CSPI have higher surface hydrophobicity than SPI (18.38 and 18.42 versus 14.87) () (P < 0.05). The increased surface hydrophobicity of GC-SPI and CSPI is a result of TGase-induced protein cross-linking. It has been found that protein unfolding and exposure of hydrophobic residues previously buried in tertiary structure during cross-linking will bring about enhanced surface hydrophobicity (Hiller & Lorenzen, Citation2008). Two past studies have found that the glycated and cross-linked protein products from caseinate and soybean protein have lower surface hydrophobicity than caseinate and soybean protein (Jiang & Zhao, Citation2011; Song & Zhao, 2014), and thus share inconsistent results with the present study.
Referring to these measured values of in vitro digestibility, both CSPI and GC-SPI show greater resistance to both peptic and peptic–tryptic digestion than SPI (P < 0.05) (). The detected TCA-soluble nitrogen released from CSPI and GC-SPI are 0.259 and 0.241 (peptic digestion) or 0.528 and 0.601 (peptic–tryptic digestion), respectively, while that from SPI is 0.288 (peptic digestion) or 0.622 (peptic–tryptic digestion). Less TCA-soluble nitrogen is released from CSPI or GC-SPI; that is, both CSPI and GC-SPI have lower digestibility than SPI. However, GC-SPI also shows greater digestion (P < 0.05) than CSPI during peptic–tryptic digestion. Roos, Lorenzen, Sick, Schrezenmeir, and Schlimme (Citation2003) stated that protein cross-linking would sterically shield the peptide bonds against proteolysis, and finally results in decreased in vitro digestibility. A glycated and cross-linked caseinate also has been found to give better digestion than the cross-linked caseinate during peptic–tryptic digestion (Song & Zhao, 2014). The present results are consistent to these reported results.
In comparison with SPI, both GC-SPI and CSPI have greatly enhanced WBC (9.9 and 8.5 versus 6.4 kg/kg protein) and OBC (3.7 and 3.5 versus 2.0 kg/kg protein) (P < 0.05) (). TGase-induced cross-linking has a positive effect on WBC of SPI, because Motoki, Nio, and Takinami (Citation1984) have found that TGase treatment could improve hydration of caseinate and soybean protein by increasing the ability to swell and bind water. In addition, attachment of hydrophilic saccharides into protein molecules can also increase WBC of the conjugates in a previous study (Matemu, Kayahara, Murasawa, & Nakamura, Citation2009), as the saccharides usually have many hydrophilic groups helpful to water-binding properties of the conjugates. OBC of protein samples also could be increased after TGase treatment. Ahn, Kim, and Ng (Citation2005) have observed improved OBC of soybean flours treated by TGase. This result provides a support to the present result. It also can be expected that higher surface hydrophobicity of GC-SPI and CSPI than SPI make a contribution towards their greater OBC. It is thus concluded that glycation and cross-linking confer GC-SPI with improved WBC and OBC. GC-SPI thus is potential protein ingredient with better water- and oil-binding capacities.
Conclusion
TGase is capable of inducing glycation and cross-linking of SPI in the presence of a degraded chitosan. The obtained product, a glycated and cross-linked SPI, contains glucosamine fraction and protein polymers; especially, it has more –OH groups and a more open secondary structure. Referring to SPI, the glycated and cross-linked SPI has property changes including higher surface hydrophobicity, lower in vitro digestibility, and more important, much greater water- and oil-binding capacities. These results suggest an interesting enzymatic approach to modify proteins, which can confer SPI with structure and property modification simultaneously. The product thus obtained is potential ingredient with better hydration and oil-binding than SPI. However, other properties of the product should be assessed in future to collect more information for further consideration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors thank the anonymous reviewers and editors for their valuable advice.
ORCID
Xin-Huai Zhao ![]() http://orcid.org/0000-0001-9682-5426
http://orcid.org/0000-0001-9682-5426
Additional information
Funding
References
- Ahn, H. J., Kim, J. H., & Ng, P. K. W. (2005). Functional and thermal properties of wheat, barley, and soy flours and their blends treated with a microbial transglutaminase. Journal of Food Science, 70, c380–c386. doi:10.1111/j.1365-2621.2005.tb11433.x
- AOAC. (1995). Official methods of analysis (16th ed.). Arlington, VA: Association of Official Analytical Chemists.
- Batista, M. K. S., Pinto, L. F., Gomes, C. A. R., & Gomes, P. (2006). Novel highly-soluble peptide-chitosan polymers: Chemical synthesis and spectral characterization. Carbohydrate Polymers, 64, 299–305. doi:10.1016/j.carbpol.2005.11.040
- Beuchat, L. R. (1977). Functional and electrophoretic characteristics of succinylated peanut flour protein. Journal of Agricultural and Food Chemistry, 25, 258–261. doi:10.1021/jf60210a044
- Church, F. C., Swaisgood, H. E., Porter, D. H., & Catignani, G. L. (1983). Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. Journal of Dairy Science, 66, 1219–1227. doi:10.3168/jds.S0022-0302(83)81926-2
- Diftis, N., & Kiosseoglou, V. (2006). Physicochemical properties of dry-heated soy protein isolate–dextran mixtures. Food Chemistry, 96, 228–233. doi:10.1016/j.foodchem.2005.02.036
- E1-Saharty, Y. S., & Bary, A. A. (2002). High-performance liquid chromatographic determination of neutraceuticals, glucosamine sulphate and chitosan, in raw materials and dosage forms. Analytica Chimica Acta, 462, 125–131. doi:10.1016/S0003-2670(02)00279-9
- Gauche, C., Vieira, J. T. C., Ogliari, P. J., & Bordignon-Luiz, M. T. (2008). Crosslinking of milk whey proteins by transglutaminase. Process Biochemistry, 43, 788–794. doi:10.1016/j.procbio.2008.04.004
- Guan, Y., Tian, Y., Li, Y., Yang, Z. F., Jia, Y. Y., Hang, T. J., & Wen, A. D. (2011). Application of a liquid chromatographic/tandem mass spectrometric method to a kinetic study of derivative glucosamine in healthy human urine. Journal of Pharmaceutical and Biomedical Analysis, 55, 181–186. doi:10.1016/j.jpba.2011.01.012
- Hassan, A. B., Osman, G. A., & Babiker, E. E. (2007). Effect of chymotrypsin digestion followed by polysaccharide conjugation or transglutaminase treatment on functional properties of millet proteins. Food Chemistry, 102, 257–262. doi:10.1016/j.foodchem.2006.04.043
- Hayakawa, S., & Nakai, S. (1985). Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. Journal of Food Science, 50, 486–491. doi:10.1111/j.1365-2621.1985.tb13433.x
- Hermansson, A. M. (1986). Soy protein gelation. Journal of the American Oil Chemists Society, 63, 658–666. doi:10.1007/BF02638232
- Hiller, B., & Lorenzen, P. C. (2008). Surface hydrophobicity of physicochemically and enzymatically treated milk proteins in relation to techno-functional properties. Journal of Agricultural and Food Chemistry, 56, 461–468. doi:10.1021/jf072400c
- Huppertz, T., Fox, P. F., De Kruif, K. G., & Kelly, A. L. (2006). High pressure-induced changes in bovine milk proteins: A review. Biochimica Et Biophysica Acta (Bba)-Proteins and Proteomics, 1764, 593–598. doi:10.1016/j.bbapap.2005.11.010
- Jiang, S.-J., & Zhao, X.-H. (2010). Transglutaminase-induced cross-linking and glucosamine conjugation in soybean protein isolates and its impacts on some functional properties of the products. European Food Research and Technology, 231, 679–689. doi:10.1007/s00217-010-1319-2
- Jiang, S.-J., & Zhao, X.-H. (2011). Transglutaminase-induced cross-linking and glucosamine conjugation of casein and some functional properties of the modified product. International Dairy Journal, 21, 198–205. doi:10.1016/j.idairyj.2010.12.004
- Kato, A., Mifuru, R., Matsudomi, N., & Kobayashi, K. (1992). Functional casein- polysaccharide conjugates prepared by controlled dry heating. Bioscience, Biotechnology and Biochemistry, 56, 567–571. doi:10.1271/bbb.56.567
- Kim, S. K. (2011). Chitin, chitosan, oligosaccharides and their derivatives. Boca Raton, FL: CRC Press.
- Li, C. P., Enomoto, H., Ohki, S., Ohtomo, H., & Aoki, T. (2005). Improvement of functional properties of whey protein isolate through glycation and phosphorylation by dry heating. Journal of Dairy Science, 88, 4137–4145. doi:10.3168/jds.S0022-0302(05)73099-X
- Liu, M., & Damodaran, S. (1999). Effect of transglutaminase-catalyzed polymerization of β-casein on its emulsifying properties. Journal of Agricultural and Food Chemistry, 47, 1514–1519. doi:10.1021/jf981030c
- Liu, Y., Zhao, G. L., Zhao, M. M., Ren, J. Y., & Yang, B. (2012). Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chemistry, 131, 901–906. doi:10.1016/j.foodchem.2011.09.074
- Marciniak-Darmochwal, K., & Kostyra, H. (2009). Influence of nonenzymatic glycosylation (glycation) of pea proteins (Pisum sativum) on their susceptibility to enzymatic hydrolysis. Journal of Food Biochemistry, 33, 506–521. doi:10.1111/jfbc.2009.33.issue-4
- Martins, S. I., Jongen, W. M., & Van Boekel, M. A. (2000). A review of Maillard reaction in food and implications to kinetic modelling. Trends in Food Science and Technology, 11, 364–373. doi:10.1016/S0924-2244(01)00022-X
- Matemu, A. O., Kayahara, H., Murasawa, H., & Nakamura, S. (2009). Importance of size and charge of carbohydrate chains in the preparation of functional glycoproteins with excellent emulsifying properties from tofu whey. Food Chemistry, 114, 1328–1334. doi:10.1016/j.foodchem.2008.11.011
- Mizuno, A., Mitsuiki, M., Motoki, M., Ebisawa, K., & Suzuki, E. (2000). Relationship between the glass transition of soy protein and molecular structure. Journal of Agricultural and Food Chemistry, 48, 3292–3297. doi:10.1021/jf991151s
- Motoki, M., Nio, N., & Takinami, K. (1984). Functional properties of food proteins polymerized by transglutaminase. Agricultural and Biological Chemistry, 48, 1257–1261. doi:10.1271/bbb1961.48.1257
- Muzzarelli, R. A. A., Boudrant, J., Meyer, D., Manno, N., DeMarchis, M., & Paoletti, M. G. (2012). Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydrate Polymers, 87, 995–1012. doi:10.1016/j.carbpol.2011.09.063
- Oliver, C. M., Melton, L. D., & Stanley, R. A. (2006). Creating proteins with novel functionality via the Maillard reaction: A review. Critical Reviews in Food Science and Nutrition, 46, 337–350. doi:10.1080/10408690590957250
- Petruccelli, S., & Anon, M. C. (1994). Relationship between the method of obtention and the structural and functional properties of soy proteins isolates. 1. Structural and hydration properties. Journal of Agricultural and Food Chemistry, 42, 2161–2169. doi:10.1021/jf00046a017
- Roos, N., Lorenzen, P. C., Sick, H., Schrezenmeir, J., & Schlimme, E. (2003). Cross-linking by transglutaminase changes neither the in vitro proteolysis nor the in vivo digestibility of caseinate. Kieler Milchwirtschaftliche Forschungsberichte, 55, 261–276.
- Schmid, M., Sängerlaub, S., Wege, L., & Stäbler, A. (2014). Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packaging Technology and Science, 27, 799–817. doi:10.1002/pts.v27.10
- Seguro, K., Kumazawa, Y., Kuraishi, C., Sakamoto, H., & Motoki, M. (1996). The epsilon-(gamma-glutamyl) lysine moiety in crosslinked casein is an available source of lysine for rats. The Journal of Nutrition, 126, 2557–2562.
- Silverstein, P. M., Webster, F. X., & Kiemle, D. J. (2005). Spectrometric identification of organic compounds. Hoboken, NJ: John Wiley & Sons Press.
- Song, C.-L., & Zhao, X.-H. (2014). Structure and property modification of an oligochitosan-glycosylated and crosslinked soybean protein generated by microbial transglutaminase. Food Chemistry, 163, 114–119. doi:10.1016/j.foodchem.2014.04.089
- Tang, C.-H., Jiang, Y., Wen, Q.-B., & Yang, X.-Q. (2005). Effect of transglutaminase treatment on the properties of cast films of soy protein isolates. Journal of Biotechnology, 120, 296–307. doi:10.1016/j.jbiotec.2005.06.020
- Tang, C.-H., Wu, H., Chen, Z., & Yang, X.-Q. (2006). Formation and properties of glycinin-rich and β-conglycinin-rich soy protein isolate gels induced by microbial transglutaminase. Food Research International, 39, 87–97. doi:10.1016/j.foodres.2005.06.004
- Tian, M., Chen, F., Ren, D. W., Yu, X. X., Zhang, X. H., Zhong, R., & Wan, C. X. (2010). Preparation of a series of chitooligomers and their effect on hepatocytes. Carbohydrate Polymers, 79, 137–144. doi:10.1016/j.carbpol.2009.07.039
- Xue, F., Li, C., Zhu, X. W., Wang, L. F., & Pan, S. Y. (2013). Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Research International, 51, 490–495. doi:10.1016/j.foodres.2013.01.012
- Zhang, Q. B., Ames, J. M., Smith, R. D., Baynes, J. W., & Metz, T. O. (2009). A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. Journal of Proteome Research, 8, 754–769. doi:10.1021/pr800858h
- Zhu, C.-Y., Wang, X.-P., & Zhao, X.-H. (2015). Property modification of caseinate responsible to transglutaminase-induced glycosylation and crosslinking in the presence of a degraded chitosan. Food Science and Biotechnology, 24, 843–850. doi:10.1007/s10068-015-0109-9
