 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Plant oils are sources of unsaturated fatty acids (FA) and antioxidant compounds. Herbs, spices, and fruit seeds are potential source of oils which may be used as functional food and dietary supplements. FA profile and oxidative quality of oils (n = 28) were investigated by the chromatographic and titration methods. Peroxidability index (PI) was calculated and cluster analysis was performed. Differences in FA content show which of them is responsible for clusters allotment and also for similarities within each cluster. Oils from cluster S1 were characterized by high content of C18:2 n-6, whereas oils from cluster S2 by higher content of C16:1 and C18:1 n-9. Oils from S3 cluster were distinguished by higher amount of C18:3 n-3. A total of 4 of 17 FAs detected in all samples significantly differed among clusters. Novel oils may be an interesting functional food component, but detailed analysis is needed to confirm their applicability and human health influence.
RESUMEN
Los aceites vegetales son una fuente de ácidos grasos no saturados (FA) y compuestos antioxidantes. Las hierbas, las especies y las semillas de frutos son una fuente potencial de aceites que pueden ser utilizados como alimentos funcionales y suplementos alimentarios. Se investigaron el perfil de FA y la calidad oxidativa de los aceites (n = 28) mediante métodos de cromatografía y valoraciones. Se calculó el índice de peroxidabilidad (PI) y se realizó un análisis de conglomerados. Las diferencias en el contenido de FA mostraron aquellos que eran los responsables de la distribución de conglomerados aunque también de las similitudes en cada conglomerado. Los aceites del conglomerado S1 se caracterizaron por un alto contenido de C18:2 n-6, mientras que los aceites del conglomerado S2 por su mayor contenido de C16:1 y C18:1 n-9. Los aceites del conglomerado S3 se distinguieron por una mayor cantidad de C18:3 n-3. Cuatro de los 17 ácidos grasos que se detectaron en todas las muestras difirieron significativamente entre los conglomerados. los nuevos aceites podrían tratarse de un interesante componente alimentario funcional, sin embargo es necesario un análisis detallado para confirmar su aplicabilidad y su influencia en la salud humana.
Introduction
Dietary fat is one of the most important macronutrient in human diet. It is the most concentrated source of energy, acts as the medium of fat-soluble vitamins and essential fatty acids (EFA), and plays other important physiological functions in organism. According to the Codex Alimentarius, the amount of energy from fats in well-balanced diet should be 25–30%. Biological significance of fat is determined mainly by the type and proportion of fatty acids (FA). Gas chromatography seems to be the most popular method to investigate the FA profile of different fats and oils. The presence of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA), of which vegetable oils are good sources, appears particularly desirable. Edible oils are a group of important food ingredients, not only as a source of unsaturated FAs but also as a source of natural antioxidants (e.g. tocopherols, carotenoids, and phenolic compounds). Those compounds are of consumers’ awareness due to their proven beneficial influence on human health (Bendini et al., Citation2007; Nikokavouraa, Christodouleas, Yannakopouloua, Papadopoulos, & Calokerinos, Citation2011; Ramadan & Moersel, Citation2006). However, fats with high content of unsaturated FAs are labile food ingredients, which may undergo chemical changes during storage and thermal treatment, e.g. oxidation. During this process, the amount of unsaturated FAs decreases and simultaneously a number of off-flavor and toxic compounds arises. This leads to deterioration of both nutritional value and sensory attributes of oils and food products containing them. Taking into account that the offer of edible oils from herbs, spices, and fruit seeds has grown and many of them are used as functional food, dietary supplements, and personal care products, as well as that the nutritional information concerning these food products is still limited, the investigation of the FA profile of commercially available edible oils is of great demand.
The main aim of this study was to evaluate the FAs’ profile of selected edible oils commercially available in polish market in order to improve understanding of their nutritional and oxidative quality as well as applicability for human nutrition.
Materials and methods
Materials
Samples of different vegetable oils (n = 28) were purchased from local grocery shops in Warsaw during the period between June 2014 and September 2014. Detailed characteristic of examined oils is presented in . Examined oils were cold-pressed and extra virgin oils with expiration date ≥6 months, which were stored in original containers at 4°C or in room temperature. Before analyzes, they were stored according to the manufacturer’s recommendation in dark. All analyzes were performed immediately after opening from fresh oil.
Table 1. Characteristics of examined oils.
Tabla 1. Características de los aceites examinados.
FAs analysis
The FA profile of examined oils was determined by gas chromatography. FA were transformed and analyzed as FA methyl esters (FAME). From each single oil, three parallel samples were prepared. To one drop of examined oil (c.a. 200 μL), 2.5 mL of 0.5 mol/L natrium hydroxide solution in methanol was added. The mixture was vigorously shaken mechanically. After that, the mixture was heated at 80°C for 10 min and afterwards it was cooled to the room temperature. Subsequently, 1.0 mL of 14% BF3 methanol solution was added. It was vigorously shaken mechanically and heated at 80°C for 15 min. To stop the reaction after cooling to the room temperature, 1.0 mL of water was added. The extraction of FAME was performed with hexane (2 × 0.5 mL). The combined organic extracts were dried with anhydrous sodium sulfate and 100 μL of purified extract was dissolved in 900 μL of hexane. Until further analysis obtained, samples were stored in −20°C. Separation of FAME was performed on gas chromatograph (Shimadzu GC-17, Kyoto, Japan) with BPX 70 capillary column (30 m × 0.25 mm ID × 0.2 μm film; SGE, Ringwood, Australia) and flame-ionization detector. Helium was used as carrier gas. Flame ionization detector temperature was 270°C and injector temperature was set at 250°C. The column temperature program started from 140°C for 5 min and then increased to 240°C at 4°C per min. Individual FA methyl esters were identified by comparing to the retention times of FAME standards (Supelco 37 FAME Mix No. 47885-U standard (Sigma Aldrich)). Results are expressed as percentage of total FA [%FA].
Oxidative quality determination
The content of hydroperoxides was determined as peroxide value (PV) according to ISO 3960:Citation2012. Results are expressed as meq O/kg oil. The content of free FAs was determined as acidic value (AV) according to ISO 660:Citation2010. Results are expressed as mg KOH/g oil.
Peroxidability index (PI) of investigated oils was calculated on the basis of their FA composition, according to the following formula (Yun & Surh, Citation2012):
Statistical analyses
All chemical analyzes were carried out in triplicate. Data are expressed as mean values ± standard deviation. Obtained results were evaluated with Statistica 10.0 (StatSoft, Tulsa, Oklahoma, USA). Cluster analysis of FA profiles in examined oils used data for only those FA which were detected in all examined oils (myristic – C14:0, palmitic – C16:0, palmitoleic – C16:1, heptadecanoic – C17:0, stearic – C18:0, oleic – C18:1 n-9, linolelaidic – C18:2 n-6 trans, linoleic – C18:2 n-6 cis, γ-linolenic -C18:3 n-6, α-linolenic – C18:3 n-3, arachidic – C20:0, eicosenoic – C20:1, eicosadienoic – C20:2, behenic – C22:0, docosadienoic – C22:2, tricosanoic- C23:0, lignoceric – C24:0). Ward agglomeration procedure as a grouping method and Euclidean distance as a function of the distance were used. Mojena’s rate equaled d = 142.75 (for k = 1.25). Differences in FAs’ profile among clusters were verified with non-parametric Kruskal–Wallis test followed by post-hoc multiple comparison test and with U Mann–Whitney test. p-Value ≤0.05 was considered significant.
Results
Applied analytical procedure enabled measurement of 31 FA in oils samples. The detailed FAs’ composition of examined oils is shown in –. All examined oils contained very low amount of saturated fatty acids (SFA), from 7.0% in apricot oil (APR) to 25.5% in poppy seed (POP), whereas level of unsaturated FA was much higher, but differed among examined oil. The lowest content of MUFA (only 1.0%) was detected in evening primrose (EPRIM), while the highest in Hazelnut (HAZ) – 76.0%. PUFA constituted from 6.6% in olive (OL) to 77.9% in hemp (HEM).
Table 2. Fatty acid profile of oils creating sub-cluster A of Cluster S1.
Tabla 2. Perfil de ácidos grasos de los aceites que crearon subconglomerados A del conglomerado S1.
Table 3. Fatty acid profile of oils creating sub-cluster B of Cluster S1.
Tabla 3. Perfil de ácidos grasos de los aceites que han creado el subconglomerado B del conglomerado S1.
Table 4. Fatty acid profile of oils creating Cluster S2.
Tabla 4. Perfil de ácidos grasos de los aceites que crearon el conglomerado S2.
Table 5. Fatty acid profile of oils creating Cluster S3.
Tabla 5. Perfil de ácidos grasos de los aceites que crearon el conglomerado S3.
The predominant SFA were palmitic acid (C16:0) and stearic acid (C18:0). The content of C16:0 was the highest (19.9 ± 0.1%) in rice (RIC), whereas its lowest level was detected in Parsley seed (PARS) (4.7 ± 2.5%). The C18:0 content ranged between 1.3 ± 0.1% (APR) and 13.9 ± 0.6% (POP).
Oleic acid (C18:1 n-9) was the major detected MUFA. It constituted 75.6 ± 0.8% of all FA in HAZ, while in EPRIM only 0.8 ± 0.0% was detected. In macadamia nut (MACA) oil the predominant MUFA was palmitoleic acid (C16:1); the content was 18.2 ± 0.5%. Moreover, high levels of eicosenoic acid (C20:1) were determined in Camelina (CAM) and in Borage (BOR) (12.8 ± 0.0% and 3.9 ± 0.0% of FA, respectively).
As PUFA are considered, linoleic acid (C18:2 n-6) was detected in all examined oils, but its content differed among them. The highest concentration of C18:2 n-6 was observed in EPRIM (74.4 ± 0.7%) whereas the lowest – in OL (5.9 ± 0.1%). In 3 of 28 analyzed oils, α-linolenic acid (ALA, C18:3 n-3) was the predominant FA: Black horehound (BHOR) (61.6 ± 0.2%), Linseed (LN) (50.2 ± 1.3%), and CAM (35.3 ± 0.1%) and its content was much higher than in other samples, where ALA was present in less than 1%. The γ-linolenic acid (GLA, C18:3 n-6) was detected in 17 examined oils. The richest sources of C18:3 n-6 appeared to be BOR (19.9 ± 0.2%), EPRIM (10.0 ± 0.1%), HEM (4.2 ± 0.1%), and PARS (2.2 ± 0.1%). Long-chain polyunsaturated FA (LC PUFA) – arachidonic acid (AA, C20:4 n-6), eicosapentaenoic acid (EPA, C20:5 n-3), and docosahexaenoic acid (DHA, C22:6 n-3) were detected only in few of analyzed oils in minimal amounts. The highest content of C20:2 (eicosadienoic acid) was detected in black cumin (BCUM) (2.3 ± 0.0%), also CAM was the rich source of this FA (1.8 ± 0.0%).
All examined oils contained mainly cis isomers of unsaturated FA and only two trans isomers were detected: elaidic acid (C18:1 n-9 trans) and linolelaidic acid (C18:2 n-6 trans), but its content was below 1%. Higher level of C18:2 n-6 trans was observed only in MACA (4.2 ± 0.1%).
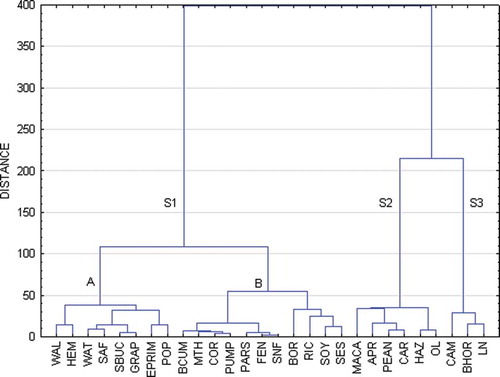
Cluster analysis of FA profiles of oils revealed three clusters: S1, S2, and S3 (). Cluster S1 includes oils with high concentration of C18:2 n-6: Walnut (WAL), HEM, watermelon (WAT), safflower (SAF), sea buckthorn (SBUC), grape seed (GRAP), EPRIM, POP, BCUM, cardo mariano (MTH), corn (COR), pumpkin seed (PUMP), PARS, fennel seed (FEN), sunflower (SNF), BOR, RIC, soybean (SOY), sesame (SES). Cluster S2 includes MACA, APR, peanut (PEAN), Carotino (Canola and red palm fruit) (CAR), HAZ and OL oils, in which higher content of C16:1 and C18:1 n-9 was detected. As far as Cluster S3 is concerned, all oils from this cluster (CAM, BHOR and LN) contained more C18:3 n-3 than other examined oils. Detailed examination of Cluster S1 showed that this cluster consists of two sub-clusters. Oils from sub-cluster A (WAL, HEM, WAT, SAF, SBUC, GRAP, EPRIM, POP) were distinguished by significantly higher (p = 0.0020) content of C18:2 n-6 and lower content of C14:0 (p = 0.0431), C17:0 (p = 0.0093), and C18:1 n-9 (p = 0.0006) in comparison to oils from sub-cluster B (BCUM, MTH, COR, PUMP, PARS, FEN, SNF, BOR, RIC, SOY, SES). Only 4 of 17 FA detected in all samples significantly differed among clusters (). Significant differences in FA content show which of them are responsible for clusters’ allotment and also for similarities within each cluster. Moreover, FA profiles of Clusters S2 and S3 are more similar to each other than each of them to Cluster S1 ().
Table 6. Statistically significant (p ≤ 0.05) differences in FA among clusters.
Tabla 6. Diferencias estadísticamente significativas (P ≤ 0,05) en FA entre los conglomerados.
Figure 1. Dendrogram of similarity in fatty acid profile in examined oils (method of grouping; Ward agglomeration procedure, function of the distance: Euclidean distance, Mojena’s rate: d = 142.75).S1 – Cluster 1, S2 – Cluster 2, S3 – Cluster 3, WAL – Calnut; HEM – Hemp; WAT – Watermelon; SAF – Safflower; SBUC – Sea buckthorn; GRAP – Grape seed; EPRIM – Evening primrose; POP – Poppy seed; BCUM – Black cumin; MTH – Milk thistle; COR – Corn; PUMP – Pumpkin seed; PARS – Parsley seed; FEN – Pennel seed; SNF – Sunflower; BOR – Borage; RIC – Rice; SOY – Soybean; SES – Sesame; APR – Apricot kernel; CAR – Carotino: canola and red palm fruit; OL – Olive; PEAN – Peanut; MACA – Macadamia nut; HAZ – Hazelnut; BHOR – Black horehound; CAM – Camelina; LN – Linseed.
Figura 1. Dendrograma de similitud en el perfil de ácidos grasos en los aceites examinados (método de agrupación; procedimiento de aglomeración de Ward, función de la distancia: distancia euclidiana, índice de Mojena: d = 142.75).S1 – Conglomerado 1, S2 – Conglomerado 2, S3 – Conglomerado 3, WAL – Nuez; HEM – Cáñamo; WAT – Sandía; SAF – Cártamo; SBUC – Baya marítima; GRAP – Semilla de uva; EPRIM – Onagra; POP – Semilla de amapola; BCUM – Comino negro; MTH – Cardo mariano; COR – Maíz; PUMP – Semilla de calabaza; PARS – Semilla de perejil; FEN – Semilla de hinojo; SNF – Girasol; BOR – Borraja; RIC – Arroz; SOY – Soja; SES – Sésamo; APR – Semilla de albaricoque; CAR – Caroteno: canola y fruto rojo de palma; OL – Aceituna; PEAN – Cacahuete; MACA – Nuez de macadamia; HAZ – Avellana; BHOR – Marrubio fétido; CAM – Camelina; LN – Semilla de lino.
Analyzed oils were characterized by different oxidative quality (). PI calculated based on the FA composition ranged from 9.3 in OL to 140.2 in BHOR. BHOR oil has the lowest PV (3.07 ± 1.76 meqO/kg oil), whereas the highest PV was measured for PARS (42.8 ± 1.68 meqO/kg oil). Content of free FA measured as AV ranged in examined oils from 0.13 ± 0.03 mg KOH/g oil in PEAN to 5.91 ± 0.08 mg KOH/g oil in BCUM ().
Discussion
There are convincing evidences that consumption of vegetable oils have beneficial effects on human health (Barceló-Coblijn et al., Citation2008; Foster, Hardy, & Alany, Citation2010; Schwab et al., Citation2006). It is mainly due to their FAs composition, with unsaturated FA as predominant. The main dietary sources of these nutritionally favorable compounds are oils obtained from raw materials typical for particular country or region, e.g. Turkey, Saudi Arabia, Sudan, Romania, etc. (Dulf, Citation2012; Matthäus & Özcan, Citation2015; Rezig, Chouaibi, Msaada, & Hamdi, Citation2012; Sabahelkhier, Ishag, & Sabir Ali, Citation2011; Salmanizadeh & Moazzezi, Citation2013). FA profile of oil may significantly vary regarding the region of origin, plant variety, etc., and because of that, determination of FA composition can provide a good indicator of product authenticity (Aparicio & Aparicio-Ruiz, Citation2000). The assortment of edible vegetable oils is constantly growing, because they are derived from novel sources, such as nuts, spices, fruit, and vegetable seeds (Koyuncu, Islam, & Küçük, Citation2005; Matthäus & Özcan, Citation2015; Sabahelkhier et al., Citation2011). Nowadays, the availability of these oils is not restricted only to the country of origin, but it spreads around the world. That is why we made an attempt to assess the FA composition of selected oils commercially available in Poland.
The most pronounced differences between analyzed oils were found for unsaturated FA, with proven beneficial impact on humans’ health. Content of linoleic, oleic, and linolenic FA distinguished oils into three clusters, while content of C14:0 and C17:0 revealed allotment of sub-cluster A and B in S1.
FA profile of oil from black cumin seeds, with content of eicosadienoic acid (C20:2) over 2%, was similar to those reported by other authors (Mankowska & Bylka, Citation2009). Nigella sativa oil traditionally used as medicine (Khan, Chen, Tania, & Zhang, Citation2011) may inhibit the inflammation by the decrease of eicosanoids formation and lipids peroxidation in cell membranes (Mankowska & Bylka, Citation2009; Ramadan, Citation2013). Moreover, it can be useful in treatment of allergic reaction and can decrease the levels of glucose, total cholesterol, and triglycerides, as well as exerts anticarcinogenic effect (Mankowska & Bylka, Citation2009).
As far as the oils of high GLA content are concerned, FA profile of Borago officinalis oil obtained in our study corresponds with results obtained by Foster et al. (Citation2010). High level of eicosenoic FA (C20:1) (3.9%) determined by us in BOR confirmed earlier report of Tso, Caldwell, Lee, Boivin, and De Michele (Citation2012), who also found slightly higher share of GLA. Supplementation of diet with Borage oil enhances the levels of n-6 PUFA (dihomogamma-linolenic and arachidonic acid) in the plasma, liver, and vascular tissue of Wistar Kyoto and spontaneously hypertensive rats, and may be responsible for the observed anti-hypertensive effect (Engler & Engler, Citation1998). It may be also useful in some individual patients with atopic dermatitis, as it is associated with essential FAs metabolism abnormality (Bialek & Rutkowska, Citation2015; Foster et al., Citation2010). Also, application of Evening primrose oil is clinically efficient in atopic dermatitis, as well as in mastalgia, menopause, and premenstrual syndrome (Bayles & Usatine, Citation2009). These potential benefits are mainly due to considerable amounts of γ-linolenic acid. In present study, about 10% of GLA was detected in Evening primrose oil ().
As far as oils obtained from fruit seeds are concerned, grape and watermelon seed oils were classified into the sub-cluster A of Cluster S1. Content of C18:2 n-6 as predominant FA in WAT and GRAP was very similar (66.8 ± 0.1% and 67.5 ± 0.9%, respectively). Results obtained in this study were in accordance with those reported by Lutterodt, Slavin, Whent, Turner, and Yu (Citation2011), who detected LA between 66% and 75%, depending on the origin of the grape seeds. Moreover, an antiproliferative activity against HT-29 colon cancer cells of examined grape oils was observed in his study.
The content of LA in PARS was fivefold higher, whereas content of oleic acid was fourfold lower in comparison to results obtained by Parry et al. (Citation2006). Similar observation was confirmed in case of PUMP oil by Haiyan, Bedgood, Bishop, Prenzler, and Robards (Citation2007), who emphasize higher content of oleic acid and lower content of linoleic acid in both refined and cold-pressed pumpkin oil. Such FA profile together with high content of tocopherols, phenolic acids, and sterols may exert positive impact on oxidative stability and increase the range of PUMP potential use not only in food but also in pharmaceuticals (Nyam, Tan, Lai, Long, & Che Man, Citation2009). Our results are in accordance with earlier observation that in sesame oil the content of two predominant FA (oleic and linoleic acids) was nearly equal (Haiyan et al. (Citation2007), which is also confirmed by the ratio of these FA which in our study was 0.8. We observed similar ratio of C18:1 n-9 and C18:2 n-6 in CAM and BHOR oils, respectively, assigned into S3 cluster, while the predominant FA in those oils was C18:3 n-3. ALA plays an important role in the regulation of biological functions, prevention, and treatment of number of disorders (Kostik, Memeti, & Bauer, Citation2013). We detected the highest amount of C18:3 n-3 in Black horehound oil (BHOR, about 62%), while other authors considered linseed oil as the best source of α-linolenic acid (Kostik et al., Citation2013; Matthäus & Özcan, Citation2015). In analyzed LN this FA consisted only slightly more than 50% of total share of FA ().
In Cluster 2 there were oils obtained from various nuts (peanut, hazelnut, macadamia/PEAN, HAZ, MACA) with oleic acid as predominant. The highest content of C18:1 n-9, higher even than in olive oil, was detected in HAZ oil (75.6 ± 0.8%). Our results are similar to those obtained by Koyuncu et al. (Citation2005), who analyzed FA composition of three varieties of hazelnuts, also in terms of major saturated FA content. As far as other reports regarding FA profile of different nuts are concerned (Ryan, Galvin, O’Connor, Maguire, & O’Brien, Citation2006), content of oleic acid was significantly higher, while palmitic and stearic acids were significantly lower in present study. It should be emphasized that oil obtained from macadamia nuts contained markedly higher amount of palmitoleic acid (18.2 ± 0.5%) compared to all other examined samples, where there only traces of C16:1 were identified (). Also, analyzed APR possessed an appreciable proportion of FA in which oleic acid comprised about 67%. Similar results were reported for wild apricot kernel oil (Gupta, Sharma, Tilakratne, & Verma, Citation2012).
The lipid quality depends inter alia on the oxidation degree. That is why detection of lipid oxidation products is of utmost importance. There are many methods applied to oxidative changes in food lipids measurement but there is no ideal method. Determination of PV seems to be the basic method of their assessment and many researchers still use it (Caponio et al., Citation2013; Georgescu, Bratu, & Aram, Citation2013; Maurer, Hatta-Sakoda, Pascual-Chagman, & Rodriguez-Saona, Citation2012). Determination content of hydroperoxides formed during initial stages of the oxidation cascade is of great importance, because from them may arise various secondary lipid oxidation products, with proven detrimental for human health features (Esterbauer, Citation1993; Marquez-Ruiz, Garcia-Martiznez, & Holgado, Citation2008), such as aliphatic aldehydes, alcohols, ketones, and hydrocarbons (Kolakowska, Citation2003). The safe PV for cold-pressed and virgin oils specified by FAO in Codex Alimentarius is ≤15 meqO/kg oil (Codex Alimentarius, Citation2001). In examined oils, in 6 of 28 samples this limit of PV was exceeded () which is alarming, taking into account that all these oils were fresh and their expiration date was ≥6 months.
Hydrolytic fragmentation of fat triacylglycerols (TAG) occurs due to their contact with water, and resulted with free fatty acids (FFAs), mono- and di-glycerides and glycerol arise (Dobarganes, Marquez-Ruiz, & Velasco, Citation2000; Warner, Citation1999). More susceptible to such changes are fats with a high content of short and unsaturated FA (Choe & Min, Citation2007). According to Codex Alimentarius, the recommended AV for both virgin and cold-pressed oils should not exceed 4.0 mg KOH/g oil (Codex Alimentarius, Citation2001). For most of the examined oils, AV was below this value. Only for POP, BCUM, and PARS content of FFA exceed recommended value ().
Summing up, edible oils, especially those obtained from novel, unconventional sources, should be carefully examined to check whether they meet the safety requirements. It is very important for the consumers, who require their health benefits, as well as for the manufacturers, who want to ensure the proper quality of the offered food products.
Conclusions
In conclusion, consumption of the edible oils derived from unconventional sources such as herbs, spices, and fruit seeds may be an interesting way to improve humans’ diet quality, due to its beneficial FAs composition. However, detailed analyzes of their oxidative stability during storage or processing are needed to be conducted.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aparicio, R., & Aparicio-Ruiz, R. (2000). Authentication of vegetable oils by chromatographic techniques. Journal of Chromatography A, 881, 93–104. doi:10.1016/S0021-9673(00)00355-1
- Barceló-Coblijn, G., Murphy, E.J., Othman, R., Moghadasian, M.H., Kashour, T., & Friel, J.K. (2008). Flaxseed oil and fish-oil capsule consumption alters human red blood cell n–3 fatty acid composition: A multiple-dosing trial comparing 2 sources of n–3 fatty acid. American Journal of Clinical Nutrition, 88, 801–809.
- Bayles, B., & Usatine, R. (2009). Evening primrose oil. American Family Physician, 80, 1405–1408.
- Bendini, A., Cerretani, L., Carrasco-Pancorbo, A., Gómez-Caravaca, A.M., Segura-Carretero, A., Fernández-Gutiérrez, A., & Lercker, G. (2007). Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade Alessandra. Molecules, 12, 1679–1719. doi:10.3390/12081679
- Bialek, M., & Rutkowska, J. (2015). The importance of γ-linolenic acid in the prevention and treatment. Advances in Hygiene and Experimental Medicine, 69, 892–904.
- Caponio, F., Giarnetti, M., Summo, C., Paradiso, V., Cosmai, L., & Gomest, T. (2013). A comparative study on oxidative and hydrolytic stability of monovarietal extra virgin olive oil in bakery products. Food Research International, 54, 1995–2000. doi:10.1016/j.foodres.2013.06.022
- Choe, E., & Min, D.B. (2007). Chemistry of deep-fat frying oils. Journal of Food Science, 72, R77–R86. doi:10.1111/j.1750-3841.2007.00352.x
- Codex Alimentarius. (2001). Codex standard for named vegetable oils CX-STAN 210-1999 (Vol. 8, pp. 11–25). Rome: Secretariat of the Joint FAO/WHO Food Standards Programme, FAO.
- Dobarganes, C., Marquez-Ruiz, G., & Velasco, J. (2000). Interactions between fat and food during deep-frying. European Journal of Lipid Science and Technology, 102, 521–528. doi:10.1002/1438-9312(200009)102:8/9<521::AID-EJLT521>3.0.CO;2-A
- Dulf, F.V. (2012). Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chemistry Central Journal, 6, 106–117. doi:10.1186/1752-153X-6-106
- Engler, M.M., & Engler, M.B. (1998). Dietary borage oil alters plasma, hepatic and vascular tissue fatty acid composition in spontaneously hypertensive rats. Prostaglandins, Leukotrienes and Essential Fatty Acids, 59, 11–15. doi:10.1016/S0952-3278(98)90046-1
- Esterbauer, H. (1993). Cytotoxicity and genotoxicity of lipids-oxidation products. American Journal of Clinical Nutrition, 57, 779S–786S.
- Foster, R.H., Hardy, G., & Alany, R.G. (2010). Borage oil in the treatment of atopic dermatitis. Nutrition, 26, 708–718. doi:10.1016/j.nut.2009.10.014
- Georgescu, A.A., Bratu, M.G., & Aram, D. (2013). Influence of natural antioxidants on peroxide value of some vegetable oils. Annals Food Science and Technology, 14, 415–417.
- Gupta, A., Sharma, P.C., Tilakratne, B.M.K.S., & Verma, A.K. (2012). Studies on physico-chemical characteristics and fatty acid composition of wild apricot (Prunus armeniaca Linn.) kernel oil. Indian Journal of Natural Products and Resources, 3, 366–370.
- Haiyan, Z., Bedgood, D.R., Bishop, A.G., Prenzler, P.D., & Robards, K. (2007). Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chemistry, 100, 1544–1551. doi:10.1016/j.foodchem.2005.12.039
- ISO 3960:2012. Animal and vegetable fats and oils. Determination of peroxide value. Geneva: International Organization for Standarization.
- ISO 660:2010. Animal and vegetable fats and oils. Determination of acidic value and acidity. Geneva: International Organization for Standardization.
- Khan, M.A., Chen, H., Tania, M., & Zhang, D. (2011). Anticancer activities of Nigella sativa (Black cumin). African Journal of Traditional, Complementary and Alternative Medicine, 8(S), 226–232. doi:10.4314/ajtcam.v8i5S.10
- Kolakowska, A. (2003). Lipid oxidation in food systems. In Z.E. Sikorski & A. Kolakowska (Eds.), Chemical and functional properties of food lipids (pp. 133–160). Boca Raton, FL: CRC Press.
- Kostik, V., Memeti, S., & Bauer, B. (2013). Fatty acids composition of edible oils and fats. Journal of Hygienic Engineering and Design, 4, 112–116.
- Koyuncu, M.A., Islam, A., & Küçük, M. (2005). Fat and fatty acid composition of hazelnut kernels in vacuum packages during storage. Grasas y Aceites, 65, 263–266.
- Lutterodt, H., Slavin, M., Whent, M., Turner, E., & Yu, L. (2011). Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chemistry, 128, 391–399. doi:10.1016/j.foodchem.2011.03.040
- Mankowska, D., & Bylka, W. (2009). Nigella- sativa L. – active compounds, biological properties. Herba Polonica, 55, 109–125. (in Polish, abstract in English)
- Marquez-Ruiz, G., Garcia-Martiznez, M.C., & Holgado, F. (2008). Changes and effects of dietary oxidized lipids in the gastrointestinal tract. Lipids Insights, 2, 11–19.
- Matthäus, B., & Özcan, M.M. (2015). Oil content, fatty acid composition and distributions of vitamin-E-active compounds of some fruit seed oils. Antioxidants, 4, 124–133. doi:10.3390/antiox4010124
- Maurer, N.E., Hatta-Sakoda, B., Pascual-Chagman, G., & Rodriguez-Saona, L.E. (2012). Characterization and authentication of a novel vegetable source of omega-3 fatty acids, sacha inchi (Plukenetia volubilis L.) oil. Food Chemistry, 134, 1173–1180. doi:10.1016/j.foodchem.2012.02.143
- Nikokavouraa, A., Christodouleas, D., Yannakopouloua, E., Papadopoulos, K., & Calokerinos, A.C. (2011). Evaluation of antioxidant activity of hydrophilic and lipophilic compounds in edible oils by a novel fluorimetric method. Talanta, 84, 874–880. doi:10.1016/j.talanta.2011.02.007
- Nyam, K.L., Tan, C.P., Lai, O.M., Long, K., & Che Man, Y.B. (2009). Physicochemical properties and bioactive compounds of selected seed oils. LWT – Food Science and Technology, 42, 1396–1403. doi:10.1016/j.lwt.2009.03.006
- Parry, J., Hao, Z., Luther, M., Su, L., Zhou, K., & Yu, L. (2006). Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. Journal of the American Oil Chemists’ Society, 83, 847–854. doi:10.1007/s11746-006-5036-8
- Ramadan, M.F. (2013). Healthy blends of high linoleic sunflower oil with selected cold pressed oils: Functionality, stability and antioxidative characteristics. Industrial Crops and Products, 43, 65–72. doi:10.1016/j.indcrop.2012.07.013
- Ramadan, M.F., & Moersel, J.T. (2006). Screening of the antiradical action of vegetable oils. Journal of Food Composition and Analysis, 19, 838–842. doi:10.1016/j.jfca.2006.02.013
- Rezig, L., Chouaibi, M., Msaada, K., & Hamdi, S. (2012). Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Industrial Crops and Products, 37, 82–87. doi:10.1016/j.indcrop.2011.12.004
- Ryan, E., Galvin, K., O’Connor, T.P., Maguire, A.R., & O’Brien, N.M. (2006). Fatty acid profile, tocopherol, squalene and phytosterol content of brazil, pecan, pine, pistachio and cashew nuts. International Journal of Food Sciences and Nutrition, 57, 219–228. doi:10.1080/09637480600768077
- Sabahelkhier, M.K., Ishag, K.E.A., & Sabir Ali, A.K. (2011). Fatty acid profile, ash composition and oil characteristics of seeds of watermelon grown in Sudan. British Journal of Science, 1, 76–80.
- Salmanizadeh, S., & Moazzezi, S. (2013, February 26–27). The effect of chlorophyll pigments on the oxidative stability of Iranian extra virgin olive oil. 1st International e-Conference on Novel Food Processing (IECFP2013), Mashhad.
- Schwab, U.S., Callaway, J.C., Erkkilä, A.T., Gynther, J., Uusitupa, M.I.J., & Järvinen, T. (2006). Effects of hempseed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factors. European Journal of Nutrition, 45, 470–477. doi:10.1007/s00394-006-0621-z
- Tso, P., Caldwell, J., Lee, D., Boivin, G.P., & De Michele, S.J. (2012). Comparison of growth, serum biochemistries and n-6 fatty acid metabolism in rats fed diets supplemented with high-gamma-linolenic acid safflower oil or borage oil for 90 days. Food and Chemical Toxicology, 50, 1911–1919. doi:10.1016/j.fct.2012.01.001
- Warner, K. (1999). Impact of high-temperature food processing on fats and oils. In L.S. Jackson, M.G. Knize, & J.N. Morgan (Eds.), Impact of processing on food safety (pp. 67–778). New York, NY: Kluwer Academic/Plenum Publishers.
- Yun, J.M., & Surh, J. (2012). Fatty acid composition as a predictor for the oxidation stability of Korean vegetable oils with or without induced oxidative stress. Preventive Nutrition and Food Science, 17, 158–165. doi:10.3746/pnf.2012.17.2.158
