ABSTRACT
Camel milk is a good nutritional source for people living in the arid and urban areas. This study aim to identify ACE-inhibitory peptides from dromedary camel milk produced using Lactobacillus helveticus or Lactobacillus acidophilus. Ten ACE-inhibitory peptides were identified using HPLC-MALDI-TOF MS. L. helveticus strain was found superior in respect to production of ACE-inhibitory peptides, compared with L. acidophilus due to having high proteolytic activity. However, all identified amino acid sequences were corresponding to β-casein of camel milk (Camelus dromedarius). Furthermore, molecular mass of identified peptides were below 1200 Da. Some ACE-inhibitory peptides were found to remain stable for up to 15 d of storage.
RESUMEN
La leche de camello se trata de una rica fuente nutricional para las personas que viven en áreas áridas y urbanas. Este estudio pretende identificar los péptidos inhibidores de ACE en la leche de dromedario producida utilizando Lactobacillus helveticus o Lactobacillus acidophilus. Se identificaron diez péptidos inhibidores de ACE utilizando HPLC-MALDI-TOF MS. Se encontró que la cepa de L. helveticus era superior respecto a la producción de péptidos inhibidores de ACE, en comparación con L. acidophilus, debido a su mayor actividad proteolítica. Sin embargo, todas las secuencias de aminoácidos que se identificaron se correspondieron a Caseína β de leche de camello (Camelus dromedarius). Además, la masa molecular de los péptidos identificados resultó por debajo de 1200 Da. Se encontró que algunos péptidos inhibidores de ACE se mantuvieron estables hasta 15 días de almacenamiento.
Introduction
Saudi Arabia is the world’s second biggest camel milk producer with 89,000 tons per year (FAO, Citation2008). Camel milk is important nutritional source in Saudi Arabia (Al Haj & Al Kanhal, Citation2010). The functional and nutritional properties of camel milk are discussed and reviewed by Al Haj and Al Kanhal (Citation2010). Hypertension is recognized as a serious risk factor for cardiovascular diseases, whereas one-third of the developed countries population is estimated to have a risk factor for cardiovascular disease and stroke (López-Fandiño, Gὸmez-Ruiz, Amigo, Recio, Citation2006). Furthermore, the current ACE-inhibitory drugs include Captopril and Enalapril have some adverse side effects on patients including coughing, skin rashes, hypotension and increased potassium levels (Meisel, Walsh, Murray, FitzGerald, Citation2006).
ACE-inhibitory peptides were found to present in the primary structure of many food protein sources including camel milk proteins (Meisel et al., Citation2006; Moslehishad et al., Citation2013; Quan, Tsuda, Miyamoto, Citation2008). The addition of Lactobacillus rhamnosus to camel milk was found to exhibit ACE-I activity (Moslehishad et al., Citation2013). Furthermore, ACE-inhibitory peptide such as Ala-Ile-Pro-Pro-Lys-Lys-Asn-Gln-Asp from Mongolia camel milk proteins was identified using Lactobacillus helveticus 130B4 (Quan et al., Citation2008). The oral ingestion of these low molecular peptides has neither side effect nor causes significant changes in the blood pressure of normotensive subjects (Meisel et al., Citation2006; Takano, Citation2002). It has been reported (Li, Le, Shi, Shrestha, Citation2004) that there is a relationship between structure–activity of ACE-inhibitory peptide and ACE-inhibitory activity. Only ACE-inhibitory peptides that able to bind to the active site of ACE enzyme are that inhibit ACE activity. According to studies, peptides of up to 23 amino acids are expected to accommodate onto the active site of ACE and play role in ACE-inhibitory activity (Meisel et al., Citation2006; Otte, Shalaby, Zakora, Pripp, El-Shabrawy, Citation2007). Furthermore, C-terminal sequence of ACE-inhibitory peptides plays a predominant role in binding to ACE enzyme (López-Fandiño et al., Citation2006). ACE enzyme is only known to have weak binding to peptides having C-terminal dicarboxylic amino acids such as Glutamic acid (E) and Aspartic acid (D) (Cheung, Wang, Ondetti, Sabo, Cushman, Citation1980). On the other hand, amino acids having hydrophobic properties such as tryptophan (W), phenylalanine (F), tyrosine (Y) and especially proline (P) were found to be favorable and more effective when present at each of the three C-terminal positions (Li et al., Citation2004; López-Expósito et al., Citation2006). Moreover, ACE-inhibitory peptides with a positively charged amino acid at the ultimate C-terminal residue such as arginine (guanidine group) and lysine (ε-amino group) would appear to contribute substantially to inhibitory potency (Li et al., Citation2004). The information about the ACE-inhibitory sequence of peptides generated after hydrolysis of camel milk with L. helveticus or Lactobacillus acidophilus in the literature is not abundant. Therefore, the aim of this research is to identify ACE-inhibitory peptides from dromedary camel milk produced by L. helveticus or L. acidophilus.
Material and methods
Material and cultures
Trifluoroacetic acid (TFA) and n-hexane were purchased from Sigma-Aldrich, St. Louis, MO, U.S.A. Acetonitrile (ACN) and LC-MS water were from Fisher scientific, England, U.K. Amicon filters were from Millipore, Cork, Ireland. MRS broth was from Oxoid, England, U.K. L. helveticus (LMG11445) and L. acidophilus (LMG11430) strains were from Belgian coordinated collections of microorganisms (BCCM-LMG) in freeze dried form.
Growth condition of strains
Freeze dried L. acidophilus and L. helveticus were activated individually in sterile 10 ml MRS broth at 37°C for up to 24 h. Then, 1 ml was inoculated anaerobically in skimmed milk at 37°C for multiple transfers to obtain approximately 108 CFU/ml as a pre-culture. L. helveticus and L. acidophilus strains are selected in the current study because they were previously reported to release ACE-inhibitory peptides from milk (Pihlanto, Virtanen, Korhonen, Citation2010) and cheese (Ong & Shah, Citation2008).
Preparation of camel milk hydrolysate
Whole camel milk from dromedary camels (Majaheim) belongs to Camelus dromedaries (one humped) was collected from Watania Company located in the northern region of Saudi Arabia. Camel milk was sterilized using Arnold method by exposing milk to the steam in open valve autoclave apparatus for 3 successive days at 85°C for 30 min as described by Alhaj, Metwalli, Ismail (Citation2011). The total bacterial count of camel milk after sterilization was negative. Thereafter, samples were inoculated with pre-culture of a total of 3% of L. helveticus and L. acidophilus and labeled as S1 and S2, respectively. Samples were incubated anaerobically at 37°C until pH attained to 4.4 then stored in fridge at 4°C for 15 d (d) to identify ACE-inhibitory peptides during incubation and storage. All samples were lyophilized and stored at −20°C until use.
Separation and identification of the molecular mass and amino acid sequence
Before injecting samples to HPLC, a quantity of 54.7 mg and 58.8 mg of S1 and S2 samples, respectively was vortexed at 1400 rpm with 0.5 ml 5% TFA/8% ACN for 20 min, then vortex at 20,000 g for 15 min to get 450 µl supernatant. This was filtered through 1.5 ml 3-KD cutoff tube (Millipore Amicon) at 10,000 g for 30 min to get 300 µl of water soluble permeates (WSP) using a Hermle centrifuge (Z36HK, Wehingen, Germany). Then, hexane of 200 µl was added and centrifuged at 10,000 g for 5 min. Finally, 100 µl sample loaded to HPLC vial, to use 5 µl sample on HPLC-MALDI-TOF MS. To eliminate sample variations, three experimental replicates were provided for each sample.
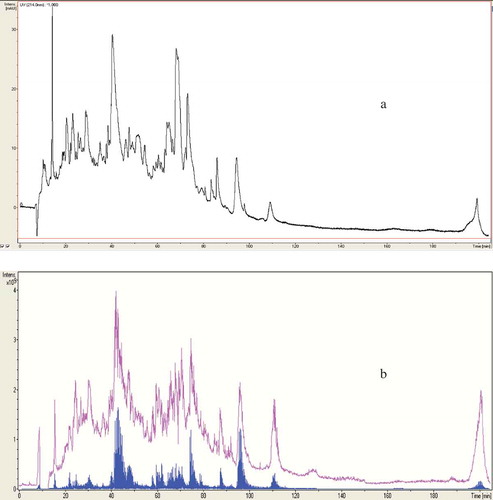
Eluted peptides from SPE column were fractionated on HPLC-MALDI-TOF MS ( and ) using a C18 column (2.1 × 100 mm, 1.8 μm) at 21°C using a flow rate of 0.4 ml/min and monitored at 214–280 nm. Samples were separated using a linear gradient of 98% A and 2% B to 58% A and 42% B over 80 min (solvent A: water with 0.1% (v/v) TFA and solvent B: ACN with 0.1% (v/v) TFA). Positive mode was used to operate the mass spectrometer with a nebulizer pressure of 50 psi using 11 l/min of drying gas flow and a drying temperature of 350°C. Mass spectra were recorded over the mass/charge range of 100–2000 m/z. In order to confirm the presence of identified peptides, three technical replicates were injected for each experimental replicates above. The spectral data were processed and presented as peptide masses for every eluted peak. The peaks profile was identified by matching the peptide masses of peaks against known sequence of β-casein (CASB_CAMDR) using the SwissProt database with the Mascot search algorithm. Identified peptides were accepted if present in at least two experimental replicates from three.
Figure 1. Profile of WSP from camel milk sample fermented with L. helveticus strain, separated by LC chromatography (a), MS chromatography and MS/MS chromatography (b).
Figura 1. Perfil de WSP de la muestra de leche de camello fermentada con cepa de L. helveticus, separada mediante cromatografía LC (A), cromatografía MS y cromatografía MS/MS (B).
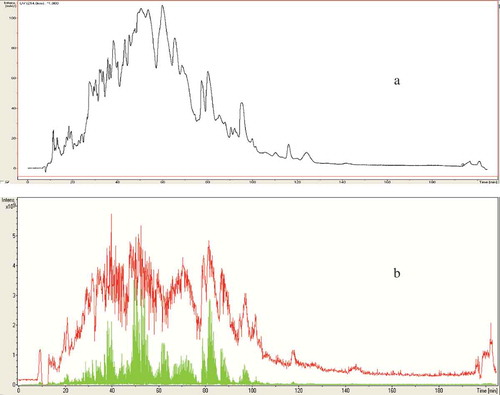
Figure 2. Profile of WSP from camel milk sample fermented with L. acidophilus strain, separated by LC chromatography (a), MS chromatography and MS/MS chromatography (b).
Figura 2. Perfil de WSP de la muestra de leche de camello fermentada con cepa de L. acidophilus, separada mediante cromatografía LC (a), cromatografía MS y cromatografía MS/MS (b).
Results and discussion
Sequences of ACE-inhibitory precursor peptides
The molecular mass of peptides (peptide length) is not the only important factor for exhibiting its functional activities, other factors including ACE-inhibitory activity and amino acid sequence also has an important role to play. In the current study, WSP of camel milk samples fermented with L. helveticus and L. acidophilus was subjected to HPLC-MS ( and ), and the resultant molecular masses were identified and searched against SwissProt database with the Mascot search algorithm. All identified amino acid sequences were originated from β-casein of Camelus dromedarius which represents 65% of total camel milk caseins (Al Haj & Al Kanhal, Citation2010). ACE-inhibitory activity from casein hydrolysates was reported to be superior upon milk casein compared with whey hydrolysates because casein is richer in proline (Otte et al., Citation2007). The finding results in showed that seven ACE-inhibitory peptides were identified from camel milk fermented with L. helveticus strain solely as follows; three peptides of sequences 1 (LSLSQF, SLSQF or SQF)KVLPVPQ, three ACE-inhibitory peptides of the sequences 2 (TDLEN, DLEN or LEN)LHLPLPL, and single peptide of sequence 3 KVLPVPQQMVPYPQ. While, only three ACE-inhibitory peptides were identified from camel milk fermented with L. acidophilus strain due to its slow growth as follows; one peptide of the sequence 4 VLPFQEPVPDPVRG; one peptide of the sequence 5 FQEPFPDPVR and one peptide of the sequence 6 VMVPFLQPK. Based on expasy proteomics server (Bioinformatics Resource Portal), sequence 1 corresponds to amino acid positions 178–191, sequence 2 to positions 144–155 and sequence 3 to positions 185–198 (). Sequence 4 corresponds to positions 206–219; sequence 5 to positions 208–218 and sequence 6 corresponding to position 98–107 in β-casein. Moreover, all identified ACE-inhibitory peptides were corresponding to β-casein of C. dromedarius milk and contain at least one proline (P) residue at the C-terminal position (). This property is favorable and more effective in ACE-inhibitory activity compared to other hydrophobic amino acids as reported by Li et al. (Citation2004) and López-Expósito et al. (Citation2006). On the other hand, tyrosine (Y) and arginine (R) were detected at the ultimate C-terminal position of camel milk fermented with S1 or S2 samples. These amino acids residues are possible candidate for ACE-inhibitory activity of fermented camel milk and contribute substantially to their inhibitory potency. Furthermore, all identified ACE-inhibitory peptides share at least three amino acids at the C-terminal residue of the previously characterized ACE-inhibitory peptides in cow milk reported by Meisel et al. (Citation2006). This type of matching was also reported by Hernández-Ledesma, Amigo, Ramos, Recio (Citation2004). The molecular weights of peptides in the current study were also determined by LC-MS, and were all found to be below 1200 Da. The identified peptides in the current study have amino acid residue of up to 14 amino acids; making them a possible candidate for ACE-inhibitory activity. Small peptides were found to be more easily absorbed (without further hydrolysis by digestive enzymes) through the intestinal wall than long peptides (Meisel & Bockelmann, Citation1999) especially peptides containing proline such as Val-Pro-Pro and Ile-Pro-Pro (Yamamoto, Citation1997).
Table 1. Selected sequences of (a) ACE-inhibitory peptides of established activity released from milk proteins in comparison to (b) relevant peptides characterized in bacterial fermented milk of camel. Sequences of potentially ACE-inhibitory peptides encrypted in (b) are underlined. Amino acid sequence positions in camel β-casein are given.
Tabla 1. Secuencias seleccionadas de (a) péptidos inhibidores de ACE de la actividad establecida liberada en las proteínas de la leche en comparación con (b) los péptidos relevantes caracterizados en la fermentación bacteriana de la leche de camello. Las secuencias de péptidos inhibidores de ACE potenciales encriptadas en (b) están subrayadas. Se presentan las posiciones de las secuencias de aminoácidos en caseína ß de camello.
Some ACE-inhibitory peptides in the current study were found to be stable during different storage period. For example, (TDLEN, DLEN or LEN)LHLPLPL peptides were identified after 9 d of storage () in S1 samples. These peptides were also identified after 15 d of storage but with further degradation; LHLP, HLPL. The release of shorter peptides (LHLP, HLPL) were found to give higher ACE-inhibitory activity (data not shown) than peptides (TDLEN, DLEN or LEN)LHLPLPL stored for 9 d in S1 samples. These finding reflect the importance of amino acid sequence in ACE-inhibitory activity. Similar results were also reported by Quirós, Dávalos, Lasunción, Ramos and Recio (Citation2008) for bovine milk β-casein. ACE-inhibitory peptides were also reported by Hernández-Ledesma et al. (Citation2004) to remain stable in commercial products and yak milk casein hydrolysate (Mao, Ni, Sun, Hao, Fan, Citation2007). On the other hand, some other ACE-inhibitory peptides were not found to remain stable during entire storage due to further degradation or formation of new ACE-inhibitory peptides. For example, PVPDPVRG peptide was not identified after 9 d () of storage but identified after 15 d. It is suggested that stable ACE-inhibitory peptides can be later recovered and added to food products. These products should be a good carrier for these peptides to possess ACE-inhibitory activity.
Conclusion
In fact, there is little information about the sequence of peptides generated after hydrolysis of camel milk with L. helveticus or L. acidophilus. Identified ACE-inhibitory peptides from dromedary camel milk has shown to have Tyr (Y), Arg (R) and Pro (P) at their ultimate C-terminal position, considering them as a possible candidate for ACE-inhibitory activity. These amino acids within the peptides were previously found to contribute substantially to ACE-inhibitory potency. The strain L. helveticus is superior in respect to production of ACE-inhibitory peptides, compared with L. acidophilus due to having high proteolytic activity as it requires all amino acids to fulfill its exceptional need of amino acids. Regular consumption of lactic fermented camel milk might assist in reducing cardiovascular diseases resulting from hypertension. It is speculated that finding in this study might form the basis for future research for the design and development of fermented camel milk products exerting health benefits.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Al Haj, O.A., & Al Kanhal, H.A. (2010). Compositional, technological and nutritional aspects of dromedary camel milk - a Review. International Dairy Journal, 20, 811–821. doi:10.1016/j.idairyj.2010.04.003
- Alhaj, O.A., Metwalli, A.A., & Ismail, E.A. (2011). Heat stability of camel milk proteins after sterilization process. Journal of Camel Practice and Research, 18, 277–282.
- Cheung, H.S., Wang, F.L., Ondetti, M.A., Sabo, E.F., & Cushman, D.W. (1980). Binding of peptide substrate and inhibition of angiotensin-converting enzyme: Importance of the COOH terminal dipeptides sequence. Journal of Biological Chemistry, 255, 401–407.
- Food and Agriculture organization-FAO. (2008). Camel milk. Retrieved from http://www.fao.org.
- Hernández-Ledesma, B., Amigo, L., Ramos, M., & Recio, I. (2004). Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion. Journal of Agricultural and Food Chemistry, 52, 1504–1510. doi:10.1021/jf034997b
- Li, G.H., Le, G.W., Shi, Y.H., & Shrestha, S. (2004). Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutrition Research, 24, 469–486. doi:10.1016/S0271-5317(04)00058-2
- López-Expósito, I., Gómez-Ruiz, J.Á., Amigo, L., & Recio, I. (2006). Identification of antibacterial peptides from ovine αs2-casein. International Dairy Journal, 16, 1072–1080. doi:10.1016/j.idairyj.2005.10.006
- Mao, X.-Y., Ni, J.-R., Sun, W.-L., Hao, -P.-P., & Fan, L. (2007). Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides. Food Chemistry, 103, 1282–1287. doi:10.1016/j.foodchem.2006.10.041
- Meisel, H., & Bockelmann, W. (1999). Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropho-functional properties. Antonie van Leeuwenhoek, 76, 207–215. doi:10.1023/A:1002063805780
- Meisel, H., Walsh, D.J., Murray, B., & FitzGerald, R.J. (2006). ACE inhibitory peptides. Chapter 13. In Y. Mine & F. Shahidi (Eds.), Nutraceutical proteins and peptides in health and disease Vol. 4 (pp. 269–315). Boca Raton, FL: Taylor and Francis Group Publisher.
- Moslehishad, M., Ehsani, M.R., Salami, M., Mirdamadi, S., Ezzatpanah, H., Naslaji, A.N., & Moosavi-Movahedi, A.A. (2013). The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. International Dairy Journal, 29, 82–87. doi:10.1016/j.idairyj.2012.10.015
- Ong, L., & Shah, N.P. (2008). Influence of Probiotic Lactobacillus acidophilus and L. helveticus on Proteolysis, Organic Acid Profiles, and ACE-Inhibitory Activity of cheddar Cheeses Ripened at 4, 8, and 12°C. Journal of Food Science, 73, M111–120. doi:10.1111/j.1750-3841.2008.00689.x
- Otte, J., Shalaby, S.M., Zakora, M., Pripp, A.H., & El-Shabrawy, S.A. (2007). Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis. International Dairy Journal, 17, 488–503. doi:10.1016/j.idairyj.2006.05.011
- Pihlanto, A., Virtanen, T., & Korhonen, H. (2010). Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. International Dairy Journal, 20, 3–10. doi:10.1016/j.idairyj.2009.07.003
- Quan, S., Tsuda, H., & Miyamoto, T. (2008). Angiotensin I-converting enzyme inhibitory peptides in skim milk fermented with Lactobacillus helveticus 130B4 from camel milk in Inner Mongolia, China. Journal of the Science of Food and Agriculture, 88, 2688–2692. doi:10.1002/jsfa.v88:15
- Quirós, A., Dávalos, A., Lasunción, M.A., Ramos, M., & Recio, I. (2008). Bioavailability of the antihypertensive peptide LHLPLP: Transepithelial flux of HLPLP. International Dairy Journal, 18, 279–286. doi:10.1016/j.idairyj.2007.09.006
- Takano, T. (2002). Anti-hypertensive activity of fermented dairy products containing biogenic peptides. Antonie van Leeuwenhoek, 82, 333–340. doi:10.1023/A:1020600119907
- Yamamoto, N. (1997). Antihypertensive peptides derived from food proteins. Biopolymers, 43, 129–134. doi:10.1002/(SICI)1097-0282(1997)43:2<>1.0.CO;2-6
