ABSTRACT
This study investigated the effect of different levels of Moringa oleifera leaf powder (MLP) on physicochemical, microbiological, and sensory characteristics of chicken patties during cold storage. For MLP extracts, methanolic extract exhibited flavonoid and total phenolic contents of 48.0 and 2010.3 mg/kg, respectively. The aqueous extract of MLP showed high (16.7 mm) and mild (8.7 mm) antimicrobial activity (inhibition zones) against Escherichia coli and Staphylococcus aureus, respectively. In chicken patties, the pH, peroxide value (PV), sensory properties, and microbiological characteristics were all affected (P ≤ 0.05) by the incorporation of MLP into the patty formula. The PV and bacterial counts were decreased (P ≤ 0.05) with the increase in MLP concentration; however, their values were increased (P ≤ 0.05) with the progress of storage period. Moderate level (50 g/kg) of MLP was good (P ≤ 0.05) in retarding lipid peroxidation and inhibiting microbial growth without affecting the sensory quality of stored chicken patties.
RESUMEN
Este estudio investigó el efecto de diferentes niveles de Moringa oleifera en polvo (MLP) en las características fisicoquímicas, microbiológicas y sensoriales de hamburguesas de pollo durante su almacenamiento en frío. En los extractos de MLP, el extracto de metanol exhibió contenidos de flavonoides y total fenólico de 48,0 mg/kg y 2010,3 mg/kg, respectivamente. El extracto acuoso de MLP mostró niveles altos (16,7 mm) y medianos (8,7 mm) de actividad antimicrobiana (zonas de inhibición) frente a Escherichia coli y Staphylococcus aureus, respectivamente. En las hamburguesas de pollo, el pH, el índice de peróxido (PV), las propiedades sensoriales y las características microbiológicas se vieron todos afectados (P ≤ 0,05) por la incorporación de MLP en la fórmula de la hamburguesa. El PV y los recuentos bacterianos disminuyeron (P ≤ 0,05) con el aumento de la concentración de MLP, sin embargo, sus valores aumentaron (P ≤ 0,05) con el progreso del periodo de almacenamiento. El nivel moderado (50 g/kg) de MLP fue favorable (P ≤ 0,05) para retardar la peroxidación lipídica e inhibir el crecimiento microbiano sin afectar la calidad sensorial de las hamburguesas de pollo almacenadas.
1. Introduction
Rapid growth in consumer demand for poultry and poultry products over the last decade and increased international trade in these foods have focused attention on objective measures of food safety and quality. Poultry meat and its products have a vast consumer market and are making a significant contribution to the supply of quality protein (Mothershaw et al., Citation2009), vitamins, and minerals (Waskar et al., Citation2009). The incidence of several common metabolic diseases associated with deficiencies of critical dietary minerals, vitamins, and amino acids can be reduced by consuming poultry products because they are rich in all essential nutrients (Cherian, Seena, Bullock, & Antony, Citation2005). Chicken meat products can be considered an important component in healthy diets (Jahan, Paterson, & Spickett, Citation2004), as they contain a higher proportion of polyunsaturated fatty acids compared with meat from other species (Berzaghi, Dalle Zotte, Jansson, & Andrighetto, Citation2005), high nutritional value, and distinct flavor (Patsias, Badeka, Savvaidis, & Kontominas, Citation2008). However, poultry meat products are perishable foods as they deteriorate within 4–10 days, even when stored at refrigeration temperature (Jimenez et al., Citation1997). The time for deterioration also depends on the condition of the poultry carcasses on the date of slaughter, the type of packaging materials, and storage conditions used (Jimenez et al., Citation1997). Amongst meat products, poultry meat is considered to be more prone to the development of oxidative rancidity compared to red meat (Ali & Zahran, Citation2010) due to the higher content of phospholipids in poultry meat. Lipid peroxidation and microbial spoilage are the leading causes of meat quality deterioration affecting color, flavor, texture, and nutritional value (Giannenas et al., Citation2010; Jayawardana, Liyanage, Lalantha, Iddamalgoda, & Weththasinghe, Citation2015). Thus, controlling microbial contamination and oxidative peroxidation during poultry meat product preparation and processing is of great importance for both consumers and meat processors. Synthetic antioxidants and antimicrobials have been discovered to be efficient in diminishing lipid oxidation and microbial growth in meat products (Honikel, Citation2014). However, these synthetic materials are considered unsafe by both human health professionals and consumers (Tang, Kerry, Sheehan, Buckley, & Morrissey, Citation2001). Recently, the use of natural antioxidants and antimicrobials, as preservatives of meat products, has attracted the consumer’s attention due to their potential health benefits and safety (Jung et al., Citation2010). Natural antioxidants, especially those from plant sources, also have the ability to increase the antioxidant capacity of the plasma and reduce the risk of certain diseases such as cancer, stroke, and cardiovascular diseases (Chanda & Dave, Citation2009). In addition, it has greater application potential for consumer’s acceptability, palatability, stability, and shelf life of meat products (Jung et al., Citation2010). One such plant with a potential to be used as a source of natural antioxidants and antimicrobials is Moringa (Moringa oleifera). The leaves and seeds of this plant have important medicinal properties that include antioxidant, antibacterial, and antifungal activities (Bukar, Uba, & Oyeyi, Citation2010) and have been assessed as natural preservative for various meat products (Al-Juhaimi, Ghafoor, Hawashin, Alsawmahi, & Babiker, Citation2016a; Falowo, Muchenje, Hugo, & Charimba, Citation2016; Jayawardana et al., Citation2015; Najeeb, Mandal, & Pal, Citation2015; Shah, Bosco, & Mir, Citation2015). However, the application of Moringa oleifera leaves as a natural preservative in chicken patties has not been adequately studied. Therefore, the main aim of this study was to determine the influence of different levels of M. oleifera leaf powder on the physicochemical, microbiological, and sensory attributes of chicken patties during cold storage.
2. Materials and methods
2.1. Preparation of moringa leaf powder (MLP) and extracts
Moringa leaf samples were obtained from the Faculty of Forestry, University of Khartoum, Khartoum, Sudan. The leaves were cleaned from dust and foreign materials by hand and freed from other parts of the plant, washed with distilled water, and dried at room temperature. The dried leaves were ground by an electrical grinder, sieved through a fine mesh, and kept in polyethylene bags at 4°C. The fine MLP was divided into three portions; the first portion was used for preparing a methanolic extract, the second portion was used for preparing a water extract, and the third portion was incorporated into chicken patties. For extract preparation, 20 g of MLP was either extracted with 300 mL of methanol-water (4:1, v/v) or extracted with 150 mL of distilled water at room temperature (30°C) for 5 h using an orbital shaker. The extract was then filtered and centrifuged (Hettich Zentrifugen, Tuttlingen, Germany) at 4000×g, for 10 min. The supernatant of the methanolic extract was concentrated under reduced pressure at 40°C for 3 h using a rotary evaporator (IKA WERKE RV06ML) to obtain the moringa crude methanolic extract. While the supernatant of the water extract was dried using a rotary evaporator under reduced pressure at 40°C for 8 h and then dissolved in water to get the crude water extract. The extracts were stored in a refrigerator at 4°C until use for analysis of flavonoids, total phenolic contents, and antibacterial activity. All analyses were done in triplicate.
2.2. Proximate composition
Moisture, crude protein, fat, fiber, and ash contents of MLP and MLP-incorporated chicken patties were determined according to the official standard method (AOAC, Citation2003).
2.3. Determination of total flavonoids of MLP extract
The determination of flavonoids was performed according to the colorimetric assay described by Kim, Jeong, and Lee (Citation2003). Briefly, 4 mL distilled water was added to 1 mL of methanolic extract of MLP. Then 0.3 mL of sodium nitrite solution (50 g/L, w/v) was added, followed by 0.3 mL of aluminum chloride solution (100 g/L). The mixture was incubated at ambient temperature for 5 min, and then 2 mL of 1 M sodium hydroxide was added. Immediately, the volume of reaction mixture was made to 10 mL with distilled water. The mixture was vortexed and the absorbance of the pink color developed was determined at 510 nm. A calibration curve was prepared with catechin, and the results were expressed as milligram catechin equivalents/kg sample (mg CE/kg).
2.4. Determination of total phenolic contents of MLP extract
The total phenolic content of methanolic extract of MLP was determined according to Prussian Blue Spectrophotometric method (Price & Butler, Citation1977), where gallic acid was used as an external standard, and the results were expressed as milligram gallic acid equivalents/kg sample (mg GAE/kg).
2.5. Determination of antibacterial activity of MLP aqueous extract
The antibacterial activity of MLP water extract against pure cultures of Escherichia coli and Staphylococcus aureus was measured following the disk diffusion method (Kilani, Abdelwahed, Ammar, & Hyder, Citation2005). Briefly, the surface of the agar plate was flooded with 6–18 h broth culture of the bacteria. The inverted plate was left to dry on the bench for 1 h or in the incubator for 30 min. The impregnated filter paper disks with the moringa extract were suitably placed on the surface of the plate. Subsequently, the plates were incubated at 37°C for 18–24 h, and they were then examined for the presence of inhibition zones of bacterial growth around disks, which indicates susceptibility of the organism to the extract.
2.6. Chicken patty preparation
Three batches of fresh boneless and skinless chicken thigh and breast meat slices were obtained from an experimental poultry farm (Faculty of Animal Production, University of Khartoum, Khartoum, Sudan) on three different days. The slices were ground through a 0.75-inch plate using a meat grinder. Minced meat, salt, sugar, fat, spices, and calculated ice water were introduced into a Hobart chopper and chopped for about 3 min. The added materials were dispersed uniformly, and then different levels (0, 50, and 100 g/kg) of MLP and calculated amounts of starch were added together with the remainder of the calculated ice water. Patty mix was divided into equal portions of 50 g each and formed into patty disks, using the manual machine, and wrapped with polyvinyl chloride (PVC). Immediately after preparation (day 0), samples from the different treatments were taken for microbiological, physicochemical, and sensory analysis. The rest of the samples were stored refrigerated at 4 ± 1°C for up to 12 days. During the storage period, samples were periodically taken and analyzed for quality attributes. The entire processing and analyses of the chicken patties were replicated three times.
2.7. Measurement of pH and peroxide value of chicken patties extended with MLP
For pH measurements, the patty samples were homogenized in distilled water at a ratio of 1:10 (w/v). The mixture was filtered through Whatman No. 1 filter paper to obtain a clear filtrate. The pH of the filtrate was measured using a digital pH meter (model 210, HANNA instruments microprocessor pH meter, Padova, Italy).
Peroxide value was measured following the official standard method 965.33 (AOAC, Citation2000). Briefly, 50 g of chicken patties were soaked with 200 mL chloroform for 8 h and then filtered through Whatman No. 4 filter paper. Then, a mixture of filtrate and chloroform/acetic acid (2:1.5, v/v) was reacted with a saturated potassium iodide solution in the dark. The released iodine was titrated with a sodium thiosulfate solution, and the peroxide value was then expressed as milliequivalents of active oxygen per kilogram of the sample (meq O2/kg).
2.8. Microbiological analysis
Determination of microbial load, coliform bacteria, E. coli, and S. aureus was carried out using the method described by Harrigan (Citation1998). Briefly, 10 g of each sample was homogenized with 90 mL of sterile peptone water (1.0 g/L). Then, appropriate serial dilutions were prepared, and 1 mL of each dilution was inoculated onto total plate count agar (PCA), MacConkey broth and Brilliant green bile lactose broth, MacConkey agar, and Baired–Parker medium (Oxoid, UK) to obtain the total plate count, coliform count, E. coli, and S. aureus count, respectively, at 37°C for 48 h.
2.9. Sensory evaluation
Sensory analysis was done using a 7-point hedonic scale as described by Trindade et al. (Citation2009). In this assay, the samples were randomly selected, cooked, and kept warm for evaluation. Then, 10 semi-trained panelists (5 males and 5 females, age between 25 and 35 years) were asked to evaluate the samples in terms of color, texture, flavor, juiciness, and overall acceptability. A cut-off score of 4.0 was set for the rejection of the sensorial parameter measured, i.e., any sensory parameter that scores less than 4.0 is rendered unacceptable. Every sample was given a code number and placed under normal light (600 lx). Tap water was provided to the panelists to flush their mouth after each sample testing.
2.10. Statistical analysis
The experiments were designed using a completely randomized experimental block design with three independent blocks (chicken patty batches), in which three treatments (0, 50, and 100 g/kg MLP) were performed, and the measurements were taken on four storage days (0, 4, 8, and 12 days). The effect of treatments and storage periods on the physicochemical, microbial, and sensory characteristics of chicken patties was statistically analyzed using SAS v. 8.1 programs (SAS Institute Inc., Cary, NC). The data of physicochemical properties, microbiological, and sensory attributes from different treatments and storage times was subjected to analysis of variance (two-way ANOVA) using the general linear model (GLM) procedure, which considered the treatments, storage times, and assessors (for sensory data) as a fixed effect, and replications of the experiments as random term. Whenever appropriate the mean separation procedure of LSD was employed, and significances were accepted at P ≤ 0.05.
3. Results and discussion
3.1. Chemical composition and antimicrobial activity of MLP
The results of chemical composition and antimicrobial activity of MLP are presented in . The results of the proximate composition indicated that MLP had a high protein content (293.7 g/kg) with considerable amounts of crude fiber (94.2 g/kg), ash (87.5 g/kg), moisture (82.8 g/kg), fat (70.0 g/kg), total phenolics (2010.3 mg GAE/kg), and flavonoids (48.0 mg CE/kg). These findings are slightly different from those reported previously for moringa leaves of different origin (Das, Rajkumar, Verma, & Swarup, Citation2012; Moyo, Masika, Hugo, & Muchenje, Citation2011; Shah et al., Citation2015). Although the observed differences in chemical composition of moringa leaves between these studies are minor, they could be attributed to the variation in the environmental conditions, mainly temperature and soil fertility, agronomic practices such as irrigation, fertilization, harvest, and postharvest and extraction conditions (methods and solvents) and the maturity stage of moringa leaves. The results of the chemical composition of MLP indicated that the leaves of this plant are a potentially good source of these chemical constituents. Interestingly, the high level of crude protein, fiber, total phenolics, and flavonoids in MLP is of particular nutritional and health significance as it may meet protein and energy requirements and prevent the human body from dangerous diseases arising from malnutrition and carcinogens (Kyriazakis & Houdijk, Citation2006).
Table 1. Chemical composition and antimicrobial activity of MLP.
Tabla 1. Composición química y actividad antimicrobiana de MLP.
An attempt was made to examine the antibacterial activity of MLP prior to the incorporation of MLP in chicken patty. The results showed that MLP exhibited antibacterial activity against indictor bacteria of both Gram-negative and Gram-positive types such as E. coli and S. aureus (). Water extract showed high antibacterial activity against E. coli (16.7 mm inhibition zone), while it showed low antibacterial activity against S. aureus with an inhibition zone of 8.7 mm. Similarly, Kumar, Pandey, Mohan, and Singh (Citation2012) reported that the aqueous extract of moringa leaves showed antibacterial activity against E. coli and S. aureus with inhibition zones of 14 and 18 mm, respectively. While the report of Thilza et al. (Citation2010) showed that the water extract of M. oleifera leaves had no antibacterial activity against S. aureus and mild activity against E. coli (10 mm). The antimicrobial activity of MLP observed in this study could be attributed to the high total phenolic and flavonoid contents in MLP.
3.2. Effect of MLP on chemical composition of chicken patties
The results of chemical composition (moisture, protein, fat, and ash) of chicken patties incorporated with different concentrations of MLP are shown in . The moisture content of chicken patties decreased with the increase in MLP concentrations, whereas the protein, ash, and fat contents significantly (P ≤ 0.05) increased with the increase in the MLP concentration. Control chicken patty (without moringa) had the highest (P ≤ 0.05) moisture content (760.2 g/kg) compared to samples containing MLP. The results also showed insignificant (P ≥ 0.05) differences in moisture and fat content among chicken patties containing increased concentration of MLP. The increase in protein, fat, and ash contents of chicken patties following the incorporation of MLP may be related to the richness of MLP in these constituents. The reduction of moisture content of MLP-containing chicken patties might be attributed to the increase in the total soluble solids. Similar to our observations, recent reports showed that the moisture content of raw beef patties insignificantly decreased as the percentage of moringa seed flour increased (Al-Juhaimi et al., Citation2016a). The same authors also stated that the incorporation of moringa seed powder showed insignificant increase in the protein, fat, and ash contents. In addition, Hawashin, Al-Juhaimi, Mohamed Ahmed, Ghafoor, and Babiker (Citation2016) reported that the moisture content of raw beef patties gradually decreased as the concentration of defatted olive cake increase, whereas the protein and fat showed a gradual increase. Moreover, Najeeb et al. (Citation2015) reported that incorporation of the moringa leaf powders at 1% level did not show any significant differences in moisture, protein, fat, and ash of the restructured chicken slices compared to both control and reference products during storage. Furthermore, Baldin et al. (Citation2016) indicated that addition of microencapsulated jabuticaba (Myrciaria cauliflora) extract to fresh sausages insignificantly affected the moisture, fat, protein, and ash contents of the product.
Table 2. Effect of addition of MLP on the proximate composition (g/kg) of chicken patty.
Tabla 2. Efecto de la adición de MLP en la composición aproximada (g/kg) de la hamburguesa de pollo.
3.3. Effect of MLP on pH and peroxide values of chicken patties
The pH of the patties was concomitantly (P ≤ 0.05) decreased with the advancement of storage period and MLP concentration ((a)). The highest reduction in the pH of chicken patties during storage was observed in those containing 100 g/kg MLP. The decrease in the pH may be due to the activity of psychrophilic bacteria that ferment the carbohydrate present in the MLP, which might lead to the formation of organic acids, mainly lactic acid. Similar observations on the reduction in pH of moringa leaf-containing herbal sausages have recently been reported (Jayawardana et al., Citation2015). In addition, a recent report showed a reduction in the pH of chicken patties following the addition of pistachio hull water extracts and during cold storage (Al-Juhaimi et al., Citation2016b). Moreover, Karabacak and Bozkurt (Citation2008) and Wenjiao, Yunchuan, Junxiu, and Yongkui (Citation2014) reported a decrease in the pH value during a storage time of Turkish dry fermented sausage and tea polyphenol-treated sausage, respectively. In contrast, other reports indicated increases in the pH of meat products containing moringa leaf and seed extracts (Al-Juhaimi et al., Citation2016a; Muthukumar, Naveena, Vaithiyanathan, Sen, & Sureshkumar, Citation2014; Shah et al., Citation2015) and garlic powder (Bali et al., Citation2011). However, low pH value due to the lactic acid formation is an active character in patty manufacture because it might contribute to the increase in the shelf life due to inhibition of microbial growth at low pH (Østerlie & Lerfall, Citation2005).
Figure 1. pH (a) and peroxide (b) values of chicken patties extended with MLP at different levels (0 g/kg, ◊; 50 g/kg, O; and 100 g/kg, ∆) during storage. Error bars indicate the standard error of three independent replicate samples.
Figura 1. Índices de pH (A) y peróxido (B) de hamburguesas de pollo con MLP a diferentes niveles (0 g/kg, ◊; 50 g/kg, O; y 100 g/kg, ∆) durante el almacenamiento. Las barras de error indican el error estándar de tres réplicas independientes.
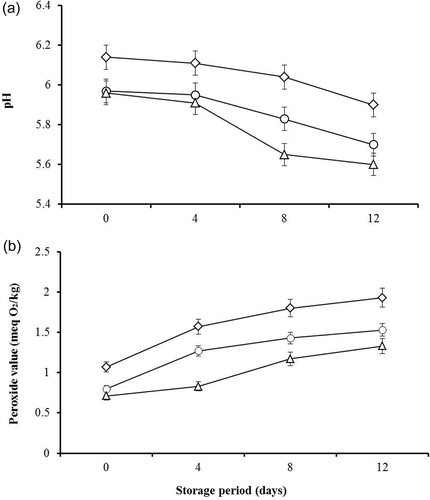
The mean peroxide value was significantly (P ≤ 0.05) increased with the increase storage period, whereas it showed a concomitant (P ≤ 0.05) reduction with the increase in MLP concentration ((b)). On all storage days, patties containing MLP showed significantly (P ≤ 0.05) delayed lipid oxidation compared to the controls. Reduction of peroxide values may be due to inhibition of lipid peroxidation by MLP, which contains polyphenols and flavonoids, which have antioxidant effects (Jayawardana et al., Citation2015). Similarly, several reports revealed that incorporation of moringa leaf and seed extracts in meat products significantly inhibited lipid oxidation (Al-Juhaimi et al., Citation2016a; Das et al., Citation2012; Muthukumar et al., Citation2014; Shah et al., Citation2015). From the results of the present study, total phenols and flavonoids of MLP have antioxidant activity, which could have led to the observed peroxide value reduction of chicken patties during the storage period.
3.4. Effect of MLP on microbial load of chicken patty
The effect of the MLP on the microbiological characteristics of chicken patties during storage is shown in . The microbial load of both control and treated samples of chicken patties had significantly (P ≤ 0.05) increased with increase in storage period but it concomitantly (P ≤ 0.05) decreased with increase in MLP concentration. The total plate count of patty without MLP was the highest compared to that incorporated with MLP. In all patties, the total plate count concomitantly (P ≤ 0.05) increased with the increase in storage period, while incorporation of MLP significantly reduced the total plate count. The low total plate count in MLP-containing chicken patties during storage might be attributed to the lower pH of MLP-containing patties compared to the controls. In addition, the low-weight proteins, peptides, and chemical compounds of Moringa oleifera leaves are known to have antimicrobial properties (Fahey, Citation2005). Thus, these constituents might be responsible for low total plate count in MLP-containing chicken patties. Similarly, incorporation of moringa leaf extracts was reported to reduce the total plate count during the storage of various meat products (Falowo et al., Citation2016; Najeeb et al., Citation2015; Shah et al., Citation2015). In addition, Jayawardana et al. (Citation2015) reported that the total plate count of chicken sausages formulated with 0.5%, 0.75%, and 1% moringa leaves was significantly lower than that of the control during the storage period. Moreover, Al-Juhaimi et al. (Citation2016a) stated that the addition of moringa seed powder to beef patties significantly decreased the aerobic total plate count after 21 days of storage. Furthermore, incorporation of pistachio hull extracts in chicken patties (Al-Juhaimi et al., Citation2016b) and defatted olive cake in beef patties (Hawashin et al., Citation2016) significantly reduced the total plate count and was thus assumed to extend the shelf life of these products. Coliform count of chicken patty significantly decreased (P ≤ 0.05) with the increase of MLP concentration (). Control chicken patty (without MLP) had the highest (P ≤ 0.05) coliform count (2.78 log cfu/g) compared to samples incorporated with MLP and then the values increased subsequently to 2.92, 3.70, and 3.85 log cfu/g during the storage period. Within samples treated with MLP, total coliform count of patties incorporated with 50 g/kg MLP was higher than that treated with 100 g/kg MLP, which showed negligible results throughout storage period. Interestingly, incorporation of 100 g/kg MLP in chicken patties significantly (P ≤ 0.05) eradicated the coliforms, indicating the protective role of MLP that could enhance the safety of these foods during storage. E. coli was detected on day 0 in control patties which was 2.52 log cfu/g and the values increased gradually during storage period (). E. coli was highest in chicken patty without MLP compared to the patty treated with MLP. Within samples treated with MLP, the E. coli count showed negligible results during storage period, but on the 4th day for patty treated with 50 g/kg MLP the count was 2.54 log cfu/g. This could be due to external contamination during the storage and handling of the samples. Thus, strict hygienic practice should be followed during the production and handling process of chicken patties. Similar observations on the protective effect of moringa seed powder against E. coli in beef patties were recently reported (Al-Juhaimi et al., Citation2016a). On the other hand, S. auerus indicated significant (P ≤ 0.05) effects of treatments as well as storage period throughout the observation period. S. auerus for control was significantly higher (2.72 log cfu/g) than treated patties, which increased with the increase in storage period. S. auerus of chicken patty treated with 100 g/kg MLP was found to be lower than that treated with 50 g/kg MLP. With exception to the 12th day, S. auerus was not detected during the storage period in chicken patties incorporated with 100 g/kg MLP. Bukar et al. (Citation2010) have shown the potential of moringa leaves as a sanitizer/preservative that inhibits the growth of the E. coli, S. aureus, Pseudomonas aeruginosa, and Enterobacter aerogenes, which range from food-borne pathogens to spoilage-causing organisms in food. In the current study, the lower total viable count, total coliform, E. coli, and S. auerus counts in chicken patties treated with 50 and 100 g/kg moringa throughout the storage period might be due to the antimicrobial action of MLP. This antimicrobial action might be attributed to the presence of phytochemical compounds such as flavonoids and polyphenols as well as other proteins and peptides.
Table 3. Effect of addition of MLP on microbial load (log cfu/g) of chicken patty during storage.
Tabla 3. Efecto de la adición de MLP en la carga microbiana (log cfu/g) de la hamburguesa de pollo durante el almacenamiento.
Table 4. Effect of addition of MLP on sensory quality of chicken patty.
Tabla 4. Efecto de la adición de MLP en la calidad sensorial de la hamburguesa de pollo.
3.5. Effect of MLP on sensory quality of chicken patty
The incorporation of MLP in chicken patties significantly affected (P ≤ 0.05) the color, flavor, tenderness, and juiciness scores of MLP-formulated patties (). For all sensorial parameters measured, i.e. color, flavor, tenderness, juiciness, and overall acceptability, panelists rated control patty superior to patties treated with MLP. None of the measured parameters of patties treated with 50 g/kg MLP had a score less than 4.0 (the set cutoff score), which means that they were all acceptable to the consumer. Among the patties treated with MLP, panelists rated patties with 100 g/kg MLP inferior to the 50 g/kg MLP-treated patties. Also, it was noted that three of the parameters (color, flavor, and overall acceptability) of 100 g/kg MLP-treated patties had scores that were less than the set cutoff scores, indicating that they were unacceptable to the consumers. The color of the patties was seriously affected by the inclusion of MLP and the effect increased with increase in MLP level in the patties. Our findings are comparable to those reported by Jayawardana et al. (Citation2015), who observed the highest consumer preference for appearance, color, odor, and taste in the control (0.04% BHT), 0.25%, and 0.50% moringa leaf-incorporated chicken sausages and increasing the moringa leaf percentage above 0.5% negatively affected the sensory attributes. In addition, Al-Juhaimi et al. (Citation2016a) formulated beef patties with various concentrations of moringa seed powders and they found that the sensory attributes of cooked patties decreased with increasing the levels of moringa seed powder. However, Das et al. (Citation2012) stated that treating goat patties with 0.1% moringa leaves did not have any negative effect on the sensory attributes. Moreover, Muthukumar et al. (Citation2014) did not observe any changes in the sensory attributes of cooked pork patties following the incorporation of moringa leaf extract. Furthermore, Hawashin et al. (Citation2016) observed that incorporation of defatted olive cake in beef patties at a concentration of up to 4% did not affect the sensory attributes of formulated patties. The variations in these studies might be due to the differences in the percentage of the added amounts of moringa powder and/or extracts, the used parts of the plant (seeds or leaves), the age of the trees, and the procedures of incorporation of moringa in the formulated meat products.
4. Conclusions
This study concludes that using MLP as an ingredient in chicken patty production improves the nutritional value and storage stability of the product. The addition of MLP to chicken patty decreased microbial growth and delayed the oxidative rancidity of the patty. Addition of MLP to chicken patties at concentrations up to 50 g/kg (w/w) did not affect the sensorial properties and overall acceptability of chicken patties as perceived by the consumer. Application of MLP to extend the shelf life of chicken patties could both satisfy the modern consumer’s demands for natural, safe, and healthy food ingredients and add commercial value to this plant.
Acknowledgments
The authors wish to acknowledge King Saud University, Deanship of Scientific Research, College of Food & Agricultural Sciences Research Center (Riyadh, Saudi Arabia), for supporting this study.
Disclosure statement
The authors reported no potential conflict of interest.
Additional information
Funding
References
- Ali, F.H., & Zahran, D.A. (2010). Effect of growth enhancers on quality of chicken meat during cold storage. Advance Journal of Food Science and Technology, 2, 216–219.
- Al-Juhaimi, F., Adiamo, O.Q., Alsawmahi, O.N., Gahfoor, K., Sarker, Z.I., Mohamed Ahmed, I.A., & Babiker, E.E. (2016b). Effect of pistachio seed hull extracts on quality attributes of chicken burger. CyTA - Journal of Food. doi:10.1080/19476337.2016.1193057
- Al-Juhaimi, F., Ghafoor, K., Hawashin, M.D., Alsawmahi, O.N., & Babiker, E.E. (2016a). Effects of different levels of Moringa (Moringa oleifera) seed flour on quality attributes of beef burgers. CyTA - Journal of Food, 14, 1–9. doi:10.1080/19476337.2015.1034784
- AOAC. (2000). Peroxide value of oils and fats. Official Method 965.33, 41, 12.
- AOAC. (2003). Official method of analysis (17th ed., Vol. 7. pp. 65–68). Washington DC: Association of Official Analytical Chemists.
- Baldin, J.C., Michelin, E.C., Polizer, Y.J., Rodrigues, I., De Godoy, S.H.S., Fregonesi, R.P., … Trindade, M.A. (2016). Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Science, 118, 15–21. doi:10.1016/j.meatsci.2016.03.016
- Bali, A., Das, S.K., Khan, A., Patra, D., Biswas, S., & Bhattacharyya, D. (2011). A comparative study on the antioxidant and antimicrobial properties of garlic and coriander on chicken sausage. International Journal of Meat Science, 1, 108–116. doi:10.3923/ijmeat.2011.108.116
- Berzaghi, P., Dalle Zotte, A., Jansson, L.M., & Andrighetto, I. (2005). Near infrared reflectance spectroscopy as a method to predict chemical composition of breast meat and discriminate between different n-3 feeding sources. Poultry Science, 84, 128–136. doi:10.1093/ps/84.1.128
- Bukar, A., Uba, A., & Oyeyi, T.I. (2010). Antimicrobial profile of Moringa oleifera lam. Extracts against some food – borne microorganisms. Bayero Journal of Pure and Applied Sciences, 3, 43–48. doi:10.4314/bajopas.v3i1.58706
- Chanda, S., & Dave, R. (2009). In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. African Journal of Microbiology Research, 3, 981–996.
- Cherian, A., Seena, S., Bullock, R.K., & Antony, A.C. (2005). Incidence of neural tube defects in the least-developed area of India: A population-based study. The Lancet, 366, 930–931. doi:10.1016/S0140-6736(05)67319-9
- Das, A.K., Rajkumar, V., Verma, A.K., & Swarup, D. (2012). Moringa oleiferia leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. International Journal of Food Science & Technology, 47, 585–591. doi:10.1111/j.1365-2621.2011.02881.x
- Fahey, J.W. (2005). Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees for Life Journal, 1(5). http://www.tfljournal.org/article.php/20051201124931586.Retrieved2008-08-16
- Falowo, A.B., Muchenje, V., Hugo, C.J., & Charimba, G. (2016). In vitro antimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects on ground beef quality during cold storage. Cyta –Journal of Food, 14(4), 541–546. doi:10.1080/19476337.2016.1162847
- Giannenas, I., Pappas, I.I.S., Mavridis, S., Kontopidis, G., Skoufos, J., & Kyriazakis, I. (2010). Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poultry Science, 89, 303–311. doi:10.3382/ps.2009-00207
- Harrigan, W.F. (1998). Laboratory method in microbiology. London: Academic Press.
- Hawashin, M.D., Al-Juhaimi, F., Mohamed Ahmed, I.A., Ghafoor, K., & Babiker, E.E. (2016). Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Science, 122, 32–39. doi:10.1016/j.meatsci.2016.07.017
- Honikel, K.O. (2014). Minced meat. In M. Dikeman & C. Devine (Eds.), Encyclopedia of meat sciences (2nd ed., Vol. 2, pp. 422–424). London: Elsevier Academic Press. 32 Jamestown Road, NW1 7BY, UK.
- Jahan, K., Paterson, A., & Spickett, C.M. (2004). Fatty acid composition, antioxidants and lipid oxidation in chicken breasts from different production regimes. International Journal of Food Science and Technology, 39, 443–453. doi:10.1111/j.1365-2621.2004.00799.x
- Jayawardana, B.C., Liyanage, R., Lalantha, N., Iddamalgoda, S., & Weththasinghe, P. (2015). Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT - Food Science and Technology, 64, 1204–1208. doi:10.1016/j.lwt.2015.07.028
- Jimenez, S.M., Salsi, M.S., Tiburzi, M.C., Rafaghelli, R.C., Tessi, M.A., & Coutaz, V.R. (1997). Spoilage microflora in fresh chicken breast stored at 4°C influence of packaging methods. Journal of Applied Microbiology, 83, 613–618. doi:10.1046/j.1365-2672.1997.00276.x
- Jung, S., Choe, J., Kim, B., Yun, H., Kruk, Z.A., & Jo, C. (2010). Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Science, 86, 520–526. doi:10.1016/j.meatsci.2010.06.007
- Karabacak, S., & Bozkurt, H. (2008). Effects of Urtica dioica and Hibiscus sabdariffa on the quality and safety of sucuk (Turkish dryfermented sausage). Meat Science, 78(3), 288–296. doi:10.1016/j.meatsci.2007.06.013
- Kilani, S., Abdelwahed, A., Ammar, R., & Hyder, N. (2005). Chemical composition, antimicrobial and antimutagenic activities of essential oil from Tunisian Cyperus rotundus. Journal of Essential Oil Research, 19, 368–379. doi:10.1080/10412905.2005.9699035
- Kim, D.O., Jeong, S.W., & Lee, C.Y. (2003). Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry, 81, 321–326. doi:10.1016/S0308-8146(02)00423-5
- Kumar, V., Pandey, N., Mohan, N., & Singh, R.P. (2012). Antibacterial and antioxidant activity of different extract of Moringa oleifera Leaves an in vitro study. International Journal of Pharmaceutical Sciences Review and Research, 12, 89–94.
- Kyriazakis, I., & Houdijk, J.G. (2006). Immunonutrition: Nutritional control of parasites. Small Ruminant Research, 62, 79–82. doi:10.1016/j.smallrumres.2005.07.036
- Mothershaw, A.S., Gaffer, T., Kadim, I., Guizani, N., Al-Amri, I., Mahgoub, O., & Al-Bahry, S. (2009). Quality characteristics of broiler chicken meat on salt at different temperatures. International Journal of Food Properties, 12, 681–690. doi:10.1080/10942910801993858
- Moyo, B., Masika, P.J., Hugo, A., & Muchenje, V. (2011). Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology, 10, 12925–12933. doi:10.5897/AJB10.1599
- Muthukumar, M., Naveena, B.M., Vaithiyanathan, S., Sen, A.R., & Sureshkumar, K. (2014). Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. Journal of Food Science and Technology, 51, 3172–3180. doi:10.1007/s13197-012-0831-8
- Najeeb, A.P., Mandal, P.K., & Pal, U.K. (2015). Efficacy of leaves (drumstick, mint and curry leaves) powder as natural preservatives in restructured chicken block. Journal of Food Science and Technology, 52, 3129–3133. doi:10.1007/s13197-014-1316-8
- Østerlie, M., & Lerfall, J. (2005). Lycopene from tomato products added minced meat: Effect on storage quality and colour. Food Research International, 38, 925–929. doi:10.1016/j.foodres.2004.12.003
- Patsias, A., Badeka, A.V., Savvaidis, I.N., & Kontominas, M.G. (2008). Combined effect of freeze chilling and MAP on quality parameters of raw chicken fillets. Food Microbiology, 25, 575–581. doi:10.1016/j.fm.2008.02.008
- Price, M.L., & Butler, L.G. (1977). Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. Journal of Agricultural and Food Chemistry, 25, 1268–1273. doi:10.1021/jf60214a034
- Shah, M.A., Bosco, S.J.D., & Mir, S.A. (2015). Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packaging and Shelf Life, 3, 31–38. doi:10.1016/j.fpsl.2014.10.001
- Tang, S.Z., Kerry, J.P., Sheehan, D., Buckley, D.J., & Morrissey, P.A. (2001). Antioxidant activity of added tea catechins on lipid oxidation of raw minced red meat, poultry and fish muscle. International Journal of Food Science and Technology, 36, 685–692. doi:10.1046/j.1365-2621.2001.00497.x
- Thilza, I.B., Sanni, S., Isah, A.Z., Sanni, F.S., Talle, M., & Joseph, B.M. (2010). In vitro antimicrobial activity of water extract of Moringa oleifera leaf stalk on bacteria normally implicated in eye diseases. Academia Arena, 2, 80–82.
- Trindade, R.A., Lima, A., Andrade-Wartha, E.R., Oliveira E Silva, A.M., Mancini-Filho, J., & Villavicencio, A.L.C.H. (2009). Consumer’s evaluation of the effects of gamma irradiation and natural antioxidants on general acceptance of frozen beef burger. Radiation Physics and Chemistry, 78, 293–300. doi:10.1016/j.radphyschem.2008.12.003
- Waskar, V.S., Devangare, A.A., Gosavi, P.P., Ravikanth, K., Maini, S., & Rekhe, D.S. (2009). Meat quality attributes of broilers supplemented with herbal toxin binder product. Veterinary World, 2, 274–277. doi:10.5455/vetworld.
- Wenjiao, F., Yunchuan, C., Junxiu, S., & Yongkui, Z. (2014). Effects of tea polyphenol on quality and shelf life of pork sausages. Journal of Food Science and Technology, 51, 191–195. doi:10.1007/s13197-013-1076-x
