ABSTRACT
The study examined the leaf extracts of Moringa oleifera and Bidens pilosa for presence of bioactive phytochemicals and their antioxidant activities on pH and lipid oxidation of fresh ground beef during 6 days cold storage. The results revealed that B. pilosa leaf extract contained higher amount of bioactive compounds and antioxidant contents (p < 0.05) than M. oleifera leaf extract. The extract of B. pilosa leaf exhibited higher antiradical activity against 2,2-Diphenyl-2-picrylhydrazyl and 2,2íazino- bis-3-ethylbenzothiazoline-6-sulfonic acid radicals than M. oleifera leaf extract and standard butylated hydroxytoluene (BHT) (p < 0.05). Addition of M. oleifera and B. pilosa leaf extracts (0.5 and 1 g/kg) to fresh ground beef were found to lower the pH and thiobarbituric acid-reactive substances values compared with control and BHT treatments (0.2 g/kg) during the storage period (p > 0.05). The antioxidant activities of the extracts indicate that M. oleifera and B. pilosa leaf can be used as nutraceuticals or preservative agents in food industry.
RESUMEN
Este estudio examinó extractos de hoja de Moringa oleifera y Bidens pilosa para detectar la presencia de fitoquímicos bioactivos y sus actividades antioxidantes en el pH y la oxidación lipídica de ternera fresca picada durante 6 días de almacenamiento en frío. Los resultados revelaron que el extracto de B. pilosa contenía una mayor cantidad de compuestos bioactivos y contenido antioxidante (p < 0,05) que el extracto de M. oleifera. El extracto de B. pilosa exhibió una mayor actividad antiradical frente a los radicales DPPH y ABTS que el extracto de M. oleifera y BHT estándar (p < 0,05). La adición de extracto de hoja de M. oleifera y B. pilosa (0,5 y 1 g/kg) a la ternera fresca picada se encontró que obtuvo unos valores menores de pH y TBARS en comparación con la muestra control y los tratamientos BHT (0,2 g/kg) durante el periodo de almacenamiento (p > 0,05). Las actividades antioxidantes de los extractos indican que las hojas de M. oleifera y B. pilosa pueden ser utilizadas como agente nutracéutico o conservante en la industria alimentaria.
PALABRAS CLAVE:
Introduction
Application of plant extract in food industry as natural antioxidant and preservative agents has continued to receive a considerable attention in recent times due to their ability to prolong shelf life and enhance consumer health. As natural antioxidant, plant extract can donate hydrogen ions to inhibit free radical formation and/or interrupt propagation of autoxidation in muscle food (Falowo, Fayemi, & Muchenje, Citation2014). As potential preservative agents, plant extract possesses huge bioactive compounds which are capable of disrupting and degrading the cytoplasmic membrane and cell wall of spoilage microorganisms (Kim, Cho, & Han, Citation2013; Radha Krishnan et al., Citation2014) and also improve the physicochemical qualities of processed meat products (Shah, Don Bosco, & Mir, Citation2015; Velasco & Williams, Citation2011).
Presently, processed meats represent a large percentage of muscle foods consumed in the Western world (Soladoye, Juárez, Aalhus, Shand, & Estévez, Citation2015) because they are easily accessible and relatively inexpensive compared with traditional fresh meat cuts (de Oliveiraa et al., Citation2012). However, due to production process, almost all processed meats including ground or minced beef are easily susceptible to lipid and pigment oxidation. Recent studies have shown that the grinding of meat usually disrupt the muscle cell membranes and expose the lipid membranes to metal ions which in turn act as pro-oxidants to initiate oxidation (Kim et al., Citation2013). The initiation of oxidation process in ground meat limits their shelf life and compromises the physical and nutritional quality of meat by generating rancid flavour and oxidized compounds (aldehydes, ketones and organic acids) which are detrimental to consumer health (Falowo et al., Citation2014).
To deal with these undesirable changes and reduce the use of synthetic preservatives, extracts from plant sources are added to meat and meat products as natural additives (Falowo et al., Citation2014; Velasco & Williams, Citation2011). Interestingly, extract from Moringaceae (Moringa oleifera Lam.) and Asteraceae (Bidens pilosa Linn.) plant families are known to contain rich antioxidant compounds (Adedapo, Jimoh, & Afolayan, Citation2011; Moyo, Masika, Hugo, & Muchenje, Citation2012). The leave of these plants have been used for centuries as dietary ingredients or supplements (Bartolome, Villaseñor, & Yang, Citation2013; Hazra, Biswas, Bhattacharyya, Das, & Khan, Citation2012). Recent studies on their application have showed that they possess great biological activities such as anti-diabetes, antitumor, anti-inflammation, anticancer and antibacterial (Bartolome et al., Citation2013; Dai & Mumper, Citation2010). Reports on their nutritional contents have also showed that they are rich in proteins (including essential amino acids), vitamins, beta-carotene, minerals and low in fat and carbohydrates (Adedapo et al., Citation2011; Moyo et al., Citation2012; Bartolome et al., Citation2013). The antioxidant and biological activities of these plants have been attributed to the presence of phytochemicals including flavonoids and other phenolics in their leaves extract (Al-Owaisi, Al-Hadiwi, & Khan, Citation2014; Falowo, Muchenje, Hugo, & Charimba, Citation2016).
Despite the above-mentioned qualities, limited studies are available on the efficacy of M. oleifera extracts (Hazra et al., Citation2012; Muthukumar, Naveena, Vaithiyanathan, Sen, & Sureshkumar, Citation2014; Shah et al., Citation2015) and to our knowledge, the preservative effect of extract from the leaves of B. pilosa in meat products as potential antioxidants has not been studied. Therefore, the objective of this study was to investigate the effect of M. oleifera and B. pilosa leaf extracts on the oxidative stability of ground meat from cattle. Prior to application of the extracts in meat samples, the phytochemical constituents and antioxidant activities of the plant leaves were also determined.
Materials and methods
Chemicals
Gallic acid, 2,2-Diphenyl-2-picrylhydrazyl (DPPH), 2,2íazino- bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4ʹ,4ʹ-disulfonic acid, sodium carbonate, butylated hydroxytoluene (BHT) and rutin were purchased from Sigma Chemical Co. (St. Louis, MO, USA), n-hexane, Aluminium chloride (AlCl3), Folin–Ciocalteu phenol reagent and sodium carbonate were from Merck (Damstadt, Germany). All other chemicals used including the solvents, were of analytical grade.
Plant sample and extract preparation
Bidens pilosa and M. oleifera leaf were obtained from the University of Fort Hare farm (South Africa) and Moringa South Africa Ltd, respectively. The M. oleifera was grown commercially by Moringa South Africa, and processed by air-drying while B. pilosa was processed by oven-drying at 40°C for 12 hr, and milled into powder through a 2 mm sieve. The dry plant samples (200 g) were exhaustively macerated with 800 ml of ethanol-water solution (7:3) at room temperature for 2 days. Each extract was separated from the residue by filtration using Whatmann no.1 filter paper and then concentrated under reduced pressure at 55 °C using a rotary evaporator. The extracts were lyophilized with a freeze-drier and the dried extracts were used for the determination of the antioxidant activity at concentration of 1 mg/ml. Determination of the nutritive values of the plants were carried out on the dry samples. All analyses were done in triplicate. The dried powder of plant extracts were then stored at 20° C for further analysis. The phenolic contents of the extracts have been analyzed previously (Falowo et al., Citation2016).
GC-MS analysis of the crude extracts
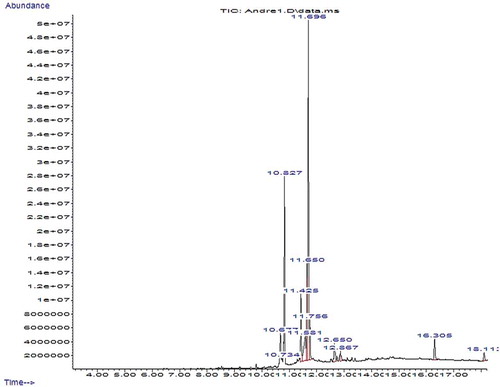
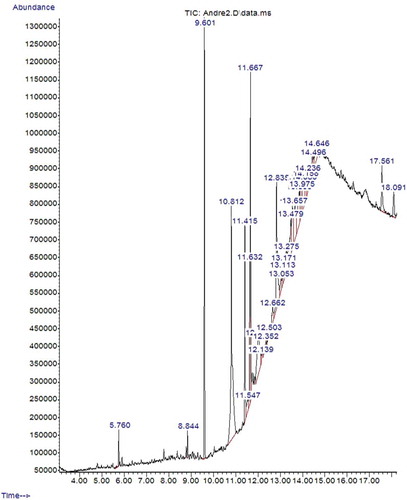
The gas chromatography mass spectrometry (GC-MS) analysis of crude extract of B. pilosa and M. oleifera plant was quantitatively performed using an Agilent 7890B GC system coupled with an Agilent 5977A MSD with a Zebron-5MS column (ZB-5MS 30 m × 0.25 mm × 0.025μm) (5% -phenylmethylpolysiloxane). GC-grade helium was used as carrier gas at a constant flow rate of 2 mL/min. The crude extracts were dissolved with appropriate solvent, filtered and diluted in n-hexane. The samples were diluted at a ratio of 1:50 and injection was achieved through an autosampler. The column temperature was maintained at 50°C and gradually increased at 10°C per minute until a final temperature of 250°C was reached. The time taken for the GC-MS analysis was calculated automatically as 18.23 min. The identification of the components was based on computer matching of the mass spectra with the National Institute of Standards and Technology library (NIST 11 MS library).
Determination of carotenoid and total chlorophyll contents
The content of chlorophylls a and b, and as well as total carotenoids, was determined using the method of Lichtenthaler (Citation1987). Approximately 1 g of dry plant samples (1 g) was extracted with 50 mL of 80% acetone (v/v) solution after incubation in the dark for 24 h at room temperature. After filtration (Whatman no. 1 filter paper), the filtrate volume was adjusted to 100 mL with 80% acetone (v/v). Absorbance was read at 662 nm, 644 nm and 470 nm using spectrophotometer to measure the content of chlorophyll a, chlorophyll b and carotenoids, respectively. Total chlorophyll was calculated as the sum of chlorophylls a and b. Total chlorophyll and total carotenoid contents were analyzed in triplicate and expressed as mg/g on a dry weight basis.
DPPH radical scavenging activities
The free radical scavenging activity of extracts on DPPH radical was estimated using the method described by Liyana-Pathiranan, Shahidi, and Alasalvar (Citation2006). A solution of 0.135 mM DPPH in ethanol was prepared and 1.0 mL of this radical solution was mixed with 1.0 mL of sample solution. The reaction mixture was incubated in the dark for 30 min at room temperature and then the absorbance was measured at 517 nm using spectrophotometer. Rutin and BHT were used as reference standards. The ability of the extract to scavenge DPPH radical was calculated by the following equation:
DPPH radical scavenging activity (%) = [(Abs control × Abs sample)]/(Abs control)] × 100 where Abs control is the absorbance of DPPH radical + ethanol; Abs sample is the absorbance of DPPH radical + sample extract /standard. The DPPH radical scavenging activity (%) of the extracts was analyzed in triplicate.
ABTS radical scavenging activities
ABTS radical cation decolourization assay to determine the free radical scavenging activity of plant extracts was carried out as described by Re et al. (Citation1999). Stock solutions (ABTS•+) were prepared by reacting a 7 mM ABTS solution with 2.4 mM potassium persulphate solution in equal quantities and the mixture was allowed to stand in the dark at room temperature for 16–18 h before use. The stock solution was then diluted by mixing 1 mL ABTS solution with 53 ml of ethanol to obtain an absorbance of 0.705 units at 734 nm. One millimeter of diluted ABTS working standard solution was mixed with 1 ml of plant extract/standard and the absorbance was measured after 7 min at 734 nm using the spectrophotometer. The ABTS scavenging capacity of the extracts was compared with that of rutin and BHT as reference standards. The percentage inhibition was calculated as ABTS radical scavenging activity (%) = [(Abs control – Abs sample)]/(Abs control)] × 100, where Abs control is the absorbance of ABTS radical + ethanol; Abs sample is the absorbance of ABTS radical + sample extract /standard. The ABTS scavenging capacity of the extracts were analyzed in triplicate
Preparation of beef samples
Fresh beef samples (Muscularis longissimus thoracis et lumborum) were obtained from 40 nondescript cattle at high throughput commercial abattoir and processed after 48 h postmortem. Beef samples were cut into small cubes after removal of visible fat and connective tissues and minced in a sterile meat grinder (CombinMax600, China). A portion (1200 g) of the ground beef were randomly assigned to one of the following treatments: (1) NC (negative control, meat without additives); (2) BP (meat with 1 g/kg B. pilosa extract); (3) ML (meat with 1 g/kg M. oleifera extract), (4) BPML (meat with 1 g/kg B. pilosa and M. oleifera extract) and (5) PC (positive control, meat with 0.2 g/kg BHT). Immediately after adding the extracts and BHT, the ground beef samples were aerobically packed in polyethylene bags (O2 permeability = 6000–8000 cm3/(24 h × m2 × atm), water vapor transmission = 83 g/(24 h × m2) and 50% relative humidity) and stored at 4 ± 1°C and analyzed on 0, 3 and 6 days of storage for pH and thiobarbituric acid-reactive substances (TBARS).
pH determination
The pH of the fresh ground beef sample was determined as described by Muthukumar et al. (Citation2014) with slight modifications. A 5 g portion of the sample was blended in 25 ml of deionized distilled water for 60 s using homogenizer (Model Polytron® PT 2500 E Stand Dispersion Device, Kinematica AG, Switzerland). The pH values were measured using a standardized electrode attached to a digital pH meter (CRISON Instruments S.A., Alella, Spain). The pH analysis was carried out in eight replicates per each treatment and storage day
Determination of lipid oxidation
The lipid oxidation of the fresh ground beef was determined by quantifying the TBARS in 5 g of sample using the aqueous acid extraction method of Raharjo, Sofos, and Schmidt (Citation1992). The values of TBARS obtained were multiplied by 10 and expressed as micrograms of malonaldehyde (MDA) per gram of meat. All TBARS analysis was carried out in four replicates per each treatment and storage day.
Statistical analysis
Data obtained on antioxidant contents of the plant extracts were analyzed using PROC ANOVA procedures of the Statistical Analysis System (SAS, version 9.1.3 of 2007). The pH and lipid oxidation values were analyzed using PROC GLM procedures of SAS (version 9.1.3 of 2007). Differences in mean values were computed using Tukey’s Studentized Range procedures for multiple comparisons.
Results and discussion
Identification and quantification of phytochemicals of moringa oleifera and biden pilosa leaf extracts
The phytochemical composition of the extracts as revealed by GC-MS analysis is presented on . The extract of B. pilosa exhibited more volatile compounds (20 compounds) than M. oleifera (13 compounds) during maximum run time of 18.23 min ( and ). The number of compounds identified in this study was relatively higher than those reported by Al-Owaisi et al. (Citation2014) for M. oleifera extract and Chien et al. (Citation2009) for B. pilosa extract. This difference could be due to variation in extraction solvents and method of analysis. The use of different solvents has been reported to cause noticeable effect and greater differences in chemical composition of extracts (Mohamed, Ali, EL-Baz, Hegazy, & Kord, Citation2014). Among the phytochemicals identified in the extracts (), the most prevailing compounds which have been reported for strong antioxidants activities were tetradecanoic acid (Mujeeb, Bajpai, & Pathak, Citation2014), n-hexadecanoic acid, and hexadecanoic acid ethyl ester (Rajeswari, Murugan, & Mohan, Citation2012), phytol (de Moraes et al., Citation2014), DL-alpha-tocopherol (Di Mambroa, Azzolinib, Valimb, & Fonseca, Citation2003) and phenol, 2,2ʹ-methylenebis [6-(1,1-dimethylethyl)]-4-methyl (Mujeeb et al., Citation2014). Antioxidant compounds are the major constituents of medicinal plant and they possess redox properties that can adsorb and neutralize free radicals, quench singlet and triplet oxygen or decompose peroxide in cell and muscle food (Adedapo et al., Citation2011: Moyo, Oyedemi, Masika, & Muchenje, Citation2012). Other biological activities which have been reported for the identified compounds include antimicrobial, anticancer, anti-inflammatory, antidiabetic, hypocholesterolemic and cell death prevention (Cisneros, Paredes, Arana, & Cisneros-Zevallos, Citation2014; Peng et al., Citation2015; Yin et al., Citation2014).
Table 1. Chemical composition of leaf extract of Moringa oleifera and Bidens pilosa.
Tabla 1. Composición química del extracto de hoja de Moringa oleifera y Bidens pilosa.
Table 2. Bioactivity of phytocomponents identified in the leaf extracts of Moringa oleifera (M. oleifera) and Bidens pilosa (B. pilosa) by GC-MS.
Tabla 2. Bioactividad de los fitocomponentes identificados en los extractos de hoja de Moringa oleifera (M. oleifera) y Bidens pilosa (B. pilosa) mediante GC-MS.
Antioxidant activities of the plant extracts
The antioxidant activities of plant extracts are presented in . The extracts of M. oleifera revealed higher amount of antioxidant activities than the extract of B. pilosa. The percentage inhibition of DPPH radicals (p < 0.05) for M. oleifera extracts, B. pilosa extract, rutin, and BHT were 75.9, 77.1, 73.8 and 70.6% while that of ABTS radicals were 82.8, 83.24, 79.3 and 85.0%, respectively (). The DPPH radical scavenging activities of B. pilosa and M. oleifera extract demonstrated significant strong antioxidant activity, and compared favourably with the standard rutin and BHT which are derivatives of phenolic compounds. In overall, the ABTS radical scavenging activities of extract showed greater antioxidant activity than DPPH radical. This could be attributed to differences in mechanism of action and reaction of DPPH and ABST radical. The ABTS has been reported to be soluble in aqueous and organic solvents, and can therefore determine both hydrophilic and lipophilic antioxidant capacities (Abegg, Alabarse, Schuller, & Benfato, Citation2012). However, our observations agreed with the finding of Moyo et al. (Citation2012) who found that scavenging ability of M. oleifera extract against ABTS was greater than DPPH radicals. The radical scavenging activities of M. oleifera extract observed in this study were comparable with those reported by Sultana, Anwar, and Ashraf (Citation2009) while that of B. pilosa extract were slightly lower than those reported by Adedapo et al. (Citation2011). Also, the presence of chlorophyll and carotenoid has been reported to contribute significantly to antioxidant activity of plant species through their ability to scavenging reactive oxygen species, singlet molecular oxygen and peroxyl radicals (Bunea et al., Citation2012). The chlorophyll a (2.62 ± 0.05 mg/g DW) and b (0.98 ± 0.01 mg/g DW) contents in B. pilosa were significantly higher (p < 0.05) than those of M. oleifera leaves (). The total chlorophyll values were 3.60 ± 0.04 and 1.46 ± 0.01 mg/g DW for B. pilosa and M. oleifera respectively (p < 0.05). The total carotenoid content was higher for B. pilosa (0.73 ± 0.00 mg/g DW) and lower for M. oleifera (0.39 ± 0.00 mg/g DW) (). The presence of total chlorophyll and carotenoid together with synergistic effect of phytochemicals could be responsible for stronger free radical scavenging activities displayed by B. pilosa extract in this study.
Table 3. Antioxidant activities of the plant extracts.
Tabla 3. Actividad antioxidante de los extractos de plantas.
Table 4. Carotenoid and chlorophyll contents of Moring oleifera and Bidens pilosa leaf extracts.
Tabla 4. Contenido de carotenoides y clorofila de los extractos de hoja de Moring oleifera y Bidens pilosa.
Effect of moringa oleifera and bidens pilosa leaf extracts on pH
The pH value on fresh ground beef during storage at 4°C is shown in . There was no significant difference (p > 0.05) in the pH values of the fresh ground beef across the treatment, indicating that the pH of the extracts did not affect the pH of the meat sample. However, all the beef samples treated with plant extracts had lower pH values compared with control and BHT treatments during the storage period. Similar results have been reported by Aytul, Korel, Arserim-Uçar, Uysal, and Bayraktar (Citation2008) in raw beef meat treated with olive leaf extract and Muthukumar et al. (Citation2014) in raw pork patties treated with Moringa oleifera extracts. This result, however, is in contrast to the findings of Shah et al. (Citation2015) who observed significant change in the pH of modified atmosphere packaged raw beef treated with Moringa oleifera leaf extracts.
Table 5. Effect of Moringa oleifera (ML) and Bidens pilosa (BP) leaf extracts on pH and TBARS values during refrigeration storage (4°C).
Tabla 5. Efecto de los extractos de hoja de Moringa oleifera (ML) y Bidens pilosa (BP) en los valores pH y TBARS durante el almacenamiento en frío (4° C).
Effect of moringa oleifera and biden pilosa leaf extracts on TBARS
Oxidation is the main non-microbial cause of quality deterioration in processed meat products during storage (Falowo et al., Citation2014). The results of TBARS analysis showed that application of M. oleifera and B. pilosa extracts can protects ground beef against lipid oxidation during the storage period (.). Although, the effect of the leaf extracts on TBARS values in ground beef were not statistically different (p > 0.05) compared with control during the storage periods. However, at day 6, the overall TBARS values of the beef samples containing extracts were lowered (ranging from 0.85 ugMDA/g to 0.94 ugMDA/g) compared with the control and BHT treatments at 1.14 ugMDA/g and 0.98 ugMDA/g, respectively. The inhibitory effects of these extracts against TBARS formation could be attributed to the inherent phyto-constituents and antioxidant activity as mentioned above. Other study has reported a positive correlation between phytochemical content or antioxidant activity of plant extracts and reduction in lipid oxidation in meat products (Jayathilakan, Sharma, Radhakrishna, & Bawa, Citation2007). However, the addition of B. pilosa extracts exhibited higher antioxidant activity at 0.5 and 1 g/kg than the M. oleifera extracts. Possible reasons for higher antioxidant activity of B. pilosa extract on TBARS values could be linked to higher inherent phytochemicals and the presence of antioxidant compound such as Phenol, 2, 2ʹ-methylenebis [6- (1, 1-dimethylethyl)]-4-methyl (BHT) which is absent in M. oleifera extracts. Our results is in line with the findings of Muthukumar et al. (Citation2014) and Shah et al. (Citation2015) who reported a lower TBARS values in raw pork and beef patties treated with Moringa plant extract.
Conclusion
This study has revealed that both M. oleifera and B. pilosa leaf extracts have substantial amounts of phytochemicals with significant free radical scavenging activity. The application of the M. oleifera and B. pilosa leaf extracts at 0.5 and 1 g/kg concentration can delay the formation of lipid oxidation in meat products during refrigerated storage. It also showed that the antioxidant potential of B. pilosa is much greater than M. oleifera. Moreso, both M. oleifera and B. pilosa leaf extracts could be used as a potential source of antioxidants to replace synthetic antioxidant in meat industry
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abegg, M.A., Alabarse, P.V.G., Schuller, A.K., & Benfato, M.S. (2012). Glutathione levels in and total antioxidant capacity of Candida sp. cells exposed to oxidative stress caused by hydrogen peroxide. Revista Da Sociedade Brasileira De Medicina Tropical, 45, 5. doi:10.1590/S0037-86822012000500015
- Adedapo, A., Jimoh, F., & Afolayan, A. (2011). Comparison of the nutritive value and biological activities of the acetone, methanol and water extracts of the leaves of Bidens pilosa andChenopodium album. Acta Poloniae Pharmaceutica Drug Research, 68(1), 83–92.
- Al-Owaisi, M., Al-Hadiwi, N., & Khan, S.A. (2014). GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pacific Journal of Tropical Biomedicine, 4(12), 964–970. doi:10.12980/APJTB.4.201414B295
- Aytul, K.K., Korel, F., Arserim-Uçar, D.K., Uysal, I., & Bayraktar, O. (2008). Efficacy of olive leaf extract for enhancing quality of beef cubes. Download fromhttp:// www.icomst.helsinki.fi/ICoMST2008/CD%20Papers/General%20speak ersþposters-3p%20papers/Session2/2A/2A.9.Korel.pdf
- Bartolome, A.P., Villaseñor, I.M., & Yang, W. (2013). Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evidence-Based Complementary and Alternative Medicine, 2013, 1–51. doi:10.1155/2013/340215
- Bunea, C.I., Pop, N., Babeş, A.C., Matea, C., Dulf, F.V., & Bunea, A. (2012). Carotenoids, total polyphenols and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chemistry Central Journal, 6(1), 1–66. doi:10.1186/1752-153X-6-66
- Chien, S.C., Young, P.H., Hsu, Y., Chen, C., Tien, Y., Shiu, S.Y. … Yang, W. (2009). Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry, 70, 1246–1254. doi:10.1016/j.phytochem.2009.07.011
- Cisneros, F.H., Paredes, D., Arana, A., & Cisneros-Zevallos, L. (2014). Chemical composition, oxidative stability and antioxidant capacity of oil extracted from roasted seeds of Sacha-Inchi (Plukenetia volubilis L.). Journal of Agriculture and Food Chemistry, 62, 5191–5197. doi:10.1021/jf500936j
- Dai, J., & Mumper, R.J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules, 15, 7313–7352. doi:10.3390/molecules15107313
- De Moraes, J., De Oliveira, R.N., Costa, J.P., Junior, A.L.G., De Sousa, D.P., Freitas, R.M. … Pinto, P.L.S. (2014). Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis Mansoni. PLOS Neglected Tropical Diseases, 8(1), 2617. doi:10.1371/journal.pntd.0002617
- De Oliveiraa, T.L.C., De Carvalhob, S.M., Soaresa, R.D., Andradeb, M.A., Cardosob, M.D., Ramos, E.M., & Piccolia, R.H. (2012). Antioxidant effects of Satureja montana L. essential oil on TBARS and colorof mortadella-type sausages formulated with different levels of sodium nitrite. LWT - Food Science and Technology, 45, 204–212. doi:10.1016/j.lwt.2011.09.006
- Di Mambroa, V.M., Azzolinib, A.E.C.S., Valimb, Y.M.L., & Fonseca, M.J.V. (2003). Comparison of antioxidant activities of tocopherols alone and in pharmaceutical formulations. International Journal of Pharmaceutics, 262, 93–99. doi:10.1016/S0378-5173(03)00333-8
- Falowo, A.B., Fayemi, P.O., & Muchenje, V. (2014). Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Research International, 64, 171–181. doi:10.1016/j.foodres.2014.06.022
- Falowo, A.B., Muchenje, V., Hugo, C.J., & Charimba, G. (2016). In vitroantimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects on ground beef quality during cold storage. Cyta - Journal of Food, 1–6. doi:10.1080/19476337.2016.1162847
- Hazra, S., Biswas, S., Bhattacharyya, D., Das, S.K., & Khan, A. (2012). Quality of cooked ground buffalo meat treated with the crude extracts of Moringa oleifera (Lam.) leaves. Journal of Food Science and Technology, 49(2), 240–245. doi:10.1007/s13197-011-0383-3
- Jayathilakan, K., Sharma, G.K., Radhakrishna, K., & Bawa, A.S. (2007). Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chemistry, 105, 908–916. doi:10.1016/j.foodchem.2007.04.068
- Kim, S., Cho, A.R., & Han, J. (2013). Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control, 29, 112–120. doi:10.1016/j.foodcont.2012.05.060
- Lichtenthaler, H.K. (1987). Chlorophylls and caroteniods: Pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
- Liyana-Pathiranan, C.M., Shahidi, F., & Alasalvar, C. (2006). Antioxidant activity of Cherry laurel fruit (Laurocerasus officinalis Roemi.) and its concentrated juice. Food Chemistry, 99, 121–128. doi:10.1016/j.foodchem.2005.06.046
- Mohamed, A.A., Ali, S.I., EL-Baz, F.K., Hegazy, A.K., & Kord, M.A. (2014). Chemical composition of essential oil and in vitro antioxidant and antimicrobial activities of crude extracts of Commiphora myrrha resin.. Industrial Crops and Products, 57, 10–16. doi:10.1016/j.indcrop.2014.03.017
- Moyo, B., Masika, P.J., Hugo, A., & Muchenje, V. (2011). Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology, 10(60), 12925–12933. doi:10.5897/AJB10.1599
- Moyo, B., Oyedemi, S., Masika, P.J., & Muchenje, V. (2012). Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Science, 91, 441–447. doi:10.1016/j.meatsci.2012.02.029
- Mujeeb, F., Bajpai, P., & Pathak, N. (2014). Phytochemical Evaluation, Antimicrobial activity, and determination of bioactive components from leaves ofAegle marmelos. Biomedicine Research International, 2014, 11. doi:10.1155/2014/497606
- Muthukumar, M., Naveena, B.M., Vaithiyanathan, S., Sen, A.R., & Sureshkumar, K. (2014). Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. Journal of Food Science and Technology, 51(11), 3172–3180. doi:10.1007/s13197-012-0831-8
- Peng, W., Li, D., Zhang, M., Ge, S., Mo, B., Li, S., & Ohkoshi, M. (2015). Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi Journal of Biological Sciences, 1319–1562. doi:10.1016/j.sjbs.2015.10.026
- Radha Krishnan, K.R., Babuskina, S., AzhaguSaravanaBabu, P., Sasikala, M., Sabina, K., Archana, G. … Sukumar, M. (2014). Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. International Journal of Food Microbiology, 171, 32–40. doi:10.1016/j.ijfoodmicro.2013.11.011
- Raharjo, S., Sofos, J.N.S., & Schmidt, G.R. (1992). Improved speed specificity, and limit of determination of an aqueous acid extraction thiobarbituric acid-C18 method for measuring lipid peroxidation in beef. Journal of Agriculture and Food Chemistry, 40, 2182–2185. doi:10.1021/jf00023a027
- Rajeswari, G., Murugan, M., & Mohan, V.R. (2012). GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Research Journal of Pharmaceutical, Biological and Chemical Sciences, 3, 301.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C.A. (1999). Antioxidant activity applying an improved ABTS radical cation decolourising assay. Free Radical Biology and Medicine, 26, 1231–1237. doi:10.1016/S0891-5849(98)00315-3
- Shah, M.A., Don Bosco, S.J., & Mir, S.A. (2015). Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packaging and Shelf Life, 3, 31–38. doi:10.1016/j.fpsl.2014.10.001
- Soladoye, O.P., Juárez, M.L., Aalhus, J.L., Shand, P., & Estévez, M. (2015). Protein oxidation in processed meat: Mechanisms and potential implications on human health. Comprehensive Reviews in Food Science and Food Safety, 14(2), 106–122. doi:10.1111/crf3.2015.14.issue-2
- Sultana, B., Anwar, F., & Ashraf, M. (2009). Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules, 14, 2167–2180. doi:10.3390/molecules14062167
- Velasco, V., & Williams, P. (2011). Improving meat quality through natural antioxidants. Chilean Journal of Agricultural Research, 71, 2. doi:10.4067/S0718-58392011000200017
- Yin, Y., He, Y., Liu, W., Gan, L., Fu, C., Jia, H., & Li, M. (2014). The durative use of suspension cells and callus for volatile oil by comparative with seeds and fruits in Capparis spinosa L. Plos One, 2014(9), 11. doi:10.1371/journal.pone.0113668