ABSTRACT
Mediterranean pine nuts are recognized for their high nutritional value and healthy benefits. The aim of the study was to analyze the composition of pine nuts harvested from Pinus pinea L. across three different geographic macrozones in Chile. Proximate analysis, minerals, fatty acid composition, phytosterols, tocopherols, vitamin C, phenolic compounds, and antioxidant capacity were determined. The major component was fat (422.5±3.3–469.2±0.7 g/kg), followed by protein (320.0±1.4–352.5±1.0 g/kg). The predominant phytosterol was β-sitosterol (average 18,177.7±763.8 µg/kg oil), and the main vitamin E isomer was γ-tocopherol (average 1071.1±109.8 µg/kg oil). Phenolics reached an average of 0.34±0.05 mg gallic acid equivalents/g. Vitamin C and ORAC exhibited an average of 27.7±1.4 mg/kg and 8.54+0.03 µmol Trolox equivalents/g, respectively. Linoleic was the most abundant fatty acid, followed by oleic acid. The chemical composition of pine nuts harvested in different macrozones reveals good nutritional properties and potential health benefits.
RESUMEN
El piñón de pino mediterráneo es reconocido por su elevado valor nutricional y saludable. El objetivo del trabajo fue analizar la composición de piñones de Pinus pinea L. cultivados en tres macrozonas geográficas de Chile. Se realizó análisis proximal, minerales, composición de ácidos grasos, fitoesteroles, tocoferoles, vitamina C, compuestos fenólicos y capacidad antioxidante. El principal componente fue grasa (422.5±3.3 a 469.2±0.7 g/kg), seguida de proteína (320.9±1.4 a 352.5±1.0 g/kg). El fitoesterol predominante fue β-sitosterol (promedio 18,177.7±763.8 µg/kg aceite), y el principal isómero de vitamina E fue γ-tocoferol (promedio 1071.1±109.8 µg/kg aceite). Los polifenoles alcanzaron un promedio de 0.34±0.05 mg GAE/g. La vitamina C y ORAC fueron 27.7±1.4 mg/kg y 8.54±0.03 µmol TE/g, respectivamente. El ácido graso más abundante fue el linoleico, seguido del oleico. La composición química de piñones de pino cultivados en diferentes macrozonas chilenas muestra buenas propiedades nutricionales y potenciales beneficios saludables.
PALABRAS CLAVE:
1. Introduction
Pinus pinea L., a major tree nut species known as stone pine, is an evergreen conifer and the largest producer of commercial pine nuts. It is endemic to the Mediterranean Basin, where the seeds have been part of the Mediterranean diet for over 20 centuries. This diet is well recognized as a dietary pattern that reduces some metabolic syndrome risk factors (Estruch et al., Citation2006; Rees et al., Citation2014; Ros, Citation2015). In fact, in the last decade, the demand for pine nuts has risen due to the growing evidence of the association of tree nuts consumption to a wide range of health benefits (Estruch et al., Citation2013; Jenkins et al., Citation2011; Rees et al., Citation2014; Sabaté & Ang, Citation2009). Moreover, in multivariate analyses, Bao et al. (Citation2013) observed an inverse association between nut consumption with the risk of major causes of death (cancer, heart, and respiratory disease). Consequently, the global market for pine nut is increasing worldwide, especially since production is undergoing a significant reduction caused by a pest (Leptoglossus occidentalis), which has affected P. pinea forests across all the main producer countries (Bracalini et al., Citation2013).
The oil content of most nuts ranges from 440 to 740 g/kg (Kornsteiner-Krenn, Wagner, & Elmadfa, Citation2013; Ros & Mataix, Citation2006; Ryan, Galvin, O’Connor, Maguire, & O’Brien, Citation2006; Sabaté & Wien, Citation2010; USDA Database, Citation2015), and for pine nuts, it reaches around 500 g/kg (Evaristo, Batista, Correia, Correia, & Costa, Citation2010). Although tree nuts are high fat, energy-dense foods, it has been recognized that these seeds have a healthy fatty acid profile. Additionally, the intake of nuts has been associated with reduced body mass index and advocated in weight maintaining diets (Bes-Rastrollo et al., Citation2009; Flores-Mateo, Rojas-Rueda, Basora, Ros, & Salas-Salvadó, Citation2013; Ibarrola-Jurado et al., Citation2013; Lutz & Luna, Citation2016; Mattes & Dreher, Citation2010).
Health scientific societies and legislation agencies such as the US Food and Drug Administration (Food and Drug Administration [FDA], Citation2003) and the Canadian Cardiovascular Society (Anderson et al., Citation2013) recommend the regular consumption of nuts to the general population, in the context of a healthy diet, to prevent the risk of cardiovascular diseases. The cardio-protective constituents of pine nuts include unsaturated fatty acids, phytosterols, various tocopherols (α-, γ-, and δ-tocopherols), and squalene (Maguire, O’Sullivan, Galvin, O’Connor, & O’Brien, Citation2004; Nasri et al., Citation2009; Wolff & Bayard, Citation1995). While most tree nuts oils show a high content of monounsaturated fatty acids, mainly oleic acid (18:1n-9), pine nut oil exhibits a fatty acid profile in which polyunsaturated (PUFA) are more abundant, especially linoleic acid (18:2n-6) (Evaristo et al., Citation2010; Kornsteiner-Krenn et al., Citation2013; Nasri, Khaldi, Hammami, & Triki, Citation2005; Nergiz & Dönmez, Citation2004; Ros, Citation2009; Ros & Mataix, Citation2006; Ryan et al., Citation2006; Venkatachalam & Sathe, Citation2006). Pine nut oil is also a good source of dietary phytosterols, components of plant cell membranes that have been reported to inhibit the intestinal absorption of cholesterol, thereby lowering total plasma cholesterol and LDL levels (De Jong, Plat, & Mensink, Citation2003; Ferguson, Stojanovski, MacDonald-Wicks, & Garg, Citation2016; Gylling et al., Citation2014). These compounds possess other beneficial properties, including anti-inflammatory, anti-atherogenic, and antioxidant activities, as well as anticancer effects (Rocha et al., Citation2016; Rudkowska, Citation2010).
The various bioactives contained in pine nuts also include phenolic compounds (Bolling, Chen, McKay, & Blumberg, Citation2011), although their content is low compared to other tree nuts. The USDA database (Citation2015) reports 68 mg gallic acid equivalents (GAE)/100 mg, while the Phenol-Explorer database (http://phenol-explorer.eu/contents/food/724) reports 58 mg GAE/100 mg in these nuts. The latter source also indicates a low content of lignans, reporting the presence of matairesinol and secoisolariciresinol (0.2 and 0.4 mg/kg, respectively).
P. pinea L. trees were brought into South America by Italian and Spanish immigrants mainly, who planted them for ornamental purposes, dune stabilization, and cattle shading. The Chilean Forestry Institute (INFOR) has developed a series of inventories and field assays to evaluate the potential for growing P. pinea in this country. However, there is no information available on the chemical composition of pine nuts harvested from P. pinea trees grown in Chile.
Taking into account the benefits of pine nut consumption and the agro-economic potential of growing this foreign species in the country, the aim of this study was to analyze and compare the chemical composition of pine tree nuts harvested from P. pinea L. plantations. For this purpose, three Chilean macrozones that exhibit diverse environmental conditions were selected to harvest the pine nuts, in order to establish if the environment affects their nutritive value.
2. Materials and methods
2.1. Materials
Pine nut seeds were collected from June to November 2013, in three selected Chilean geographical growth macrozones (Loewe, Balzarini, Álvarez-Contreras, Delard, & Navarro-Cerrillo, Citation2016). The samples were gathered from P. pinea L. plantations, windbreaks, groves, and isolated trees. The selected macrozones were previously defined as North (N): 31–33° latitude, n = 42 trees sampled; Dry Coast (DC): 34–35° latitude, n = 89 trees sampled; and South (S): 36–38° latitude, n = 36 trees sampled, between Coquimbo and Araucanía regions, covering a length of 1300 km. The number of trees was defined taking into account the variability of each macrozone, in order to have a representative sample of each. Cones were collected and pine nuts in shell kept in plastic nets tagged with their individual tree identification at room temperature until they were manually shelled.
Sample preparation: Shelled pine nuts were dried at 40°C for 40 min until humidity reached 40 g/kg. The seeds were ground with a kitchen processor (Moulinex®) and sieved to 0.5 mm, then they were frozen in sealed plastic bags at −20°C until analysis.
2.2. Methods
All reagents and solvents were analytical grade chemicals from Merck (Darmstadt, Germany) and the standards (gallic acid, cathechin, caffeic acid, Trolox, β-sitosterol, campesterol, stigmasterol, α-, γ-tocopherol, ascorbic acid, dehydroascorbic acid) were Sigma Chemical Co (St. Louis, MO, USA). For the fatty acid profile, a standard containing the following relative percentage of methyl esters (by wt) was used: palmitate (16:0) 6%, stearate (18:0) 3%, oleate (18:1n-9) 35%, linoleate (18:2n-6) 50%, linolenate (18:3n-3) 3%, arachidate (20:0) 3% (FAME GLC 15A, Walnut Standard, Nu Check Prep. Inc., MN, USA).
2.2.1. Proximate analysis
The following components were determined using the AOAC methods for nuts and nut products (Association of Official Analytical Chemists [AOAC], Citation2012). Moisture was determined using method 925.40; ash was determined using method 950.49. Protein content was determined by Kjeldahl assay (AOAC 950.48) using a nitrogen digestor DK6 (VELP®) and a nitrogen distiller UDK 129 (VELP®), applying a factor of 5.3 to convert nitrogen to proteins (Greenfield & Southgate, Citation1972). Fat was assessed using method 948.22; and dietary fiber (total, soluble, and insoluble) was measured using the enzymatic-gravimetric method 991.43.
2.2.2. Minerals
Ca, Cu, Fe, Zn, K, and Na were determined according to AOAC multielement method 986.15 (AOAC, Citation2012) by inductively coupled plasma atomic emission spectrometry (ICP) (Shimadzu, model ICPE-9000). For this purpose, the samples were ash dried at 450°C (Boeco® furnace). Working standard solutions of minerals were prepared by stepwise dilutions of a multielement ICP Certipur® multielement standard V solution Merck (Darmstadt, Germany).
2.2.3. Phytosterols
Phytosterols were analyzed adapting the method of Maguire et al. (Citation2004) in pine nut oil, after saponification with 3% methanolic NaOH. Phytosterols were extracted further with hexane for their identification and quantification by HPLC. A liquid chromatograph with an ultraviolet-diode array detector (UV-DAD) at a wavelength of 210 nm (Merck Hitachi Lachrom L-7455) equipped with a RP-18 column was used, applying an isocratic mobile phase containing acetonitrile:methanol (70:30 v/v) at 1 mL/min, at 30°C, according to Shah, Kekare, and Vaidya (Citation2010). The volume injected was 20 µL. Retention times: stigmasterol 26.29 min, β-sitosterol 29.44 min. Results were expressed as micrograms per kilogram oil (µg/kg oil).
2.2.4. Tocopherols
Tocopherols were measured by the IUPAC standard method 2432 (International Union of Pure and Applied Chemistry [IUPAC], Citation1992). Both α- and γ-tocopherols in pine nut oil were determined after saponification with 50% KOH and 1% ethanolic pyrogallol. Both species were identified and quantified by HPLC using an UV-DAD at a wavelength of 292 nm (Merck Hitachi Lachrom L-7455) equipped with a RP-18 column, at 25°C, using an isocratic mobile phase containing methanol:water (ultrapure) (99:1, v/v), as described by Maguire et al. (Citation2004). The volume injected was 20 µL. Retention time: α-tocopherol 8.01 min, γ-tocopherol 7.09 min. Results were expressed as milligrams per kilogram oil (µg/kg oil).
2.2.5. Total phenolics
Total phenolics content (TPC) was assayed using the method of Singleton and Rossi (Citation1965), adjusted to a microplate reader (Synergy Multidetection HT, Biotek®). A gallic acid standard solution series was prepared ranging from 235 to 1180 µmol/L. Extracts of the samples were obtained using acetone:water (7:3 v/v). From each standard solution and sample, 50 µL was mixed with 2.5 mL Folin–Ciocalteu reagent and 2.0 mL sodium carbonate solution. The samples were mixed and incubated at 20°C for 30 min and absorbance was read at 760 nm. The results were expressed as milligrams of GAE/gram (mg GAE/g).
2.2.6. Antioxidant capacity
ORAC (Oxygen Radical Absorbance Capacity) was determined as described by Ou et al. (Citation2002) and Huang, Ou, and Prior (Citation2005), adjusted for the microplate reader used (Synergy Multidetection HT, Biotek®). The fluorescence of the probe (fluorescein) decreases in time, and the decay kinetics is followed by reading every min, for 90 min, at 485 nm (excitation) and 528 nm (emission). Results were expressed as micromoles of Trolox equivalents (TE)/gram (μmol TE/g).
2.2.7. Vitamin C
Vitamin C content (ascorbic and dehydroascorbic acids) was measured by the method of Gökmen, Kahraman, Demir, and Acar (Citation2000). For this, 0.6 g pine nuts were added 50 mL 5% orthophosphoric acid containing 5 mM dithiothreitol (DTT), homogenized in an ultraturrax for 2 min, and stirred for 30 min in an orbital shaker in complete darkness. Then, it was centrifuged for 10 min at 1792 × g at 25°C and filtered to be analyzed by HPLC using an UV-DAD at a wavelength of 240 nm (Merck Hitachi Lachrom L-7455) equipped with a RP-18 column. The isocratic mobile phase was 25 mM KH2PO4, adjusted with H3PO4, pH 3.0; at 0.8 mL/min at 35°C. The volume injected was 20 µL. Retention time of ascorbic acid: 4.23 min.
2.2.8. Fatty acids profile
Lipids were extracted using the methods of Bligh and Dyer (Citation1959) and Folch, Lees, and Sloane-Stanley (Citation1957). In this fraction, the relative fatty acid profile was determined by the Official Method ISO 12966:2011 for fatty acids in oils, animal, and vegetable fats (International Organization for Standardization [ISO], Citation2000). Analysis of fatty acid methyl esters was carried out using an HP 5890 gas chromatograph (Hewlett Packard HP series II 5890, Palo Alto, CA, USA) with flame ionization detector (FID) and integrated software Young Lin Autochro-3000®, version 2.0 (YL Instruments, Korea), using a HP-FFAP column (25 m × 0.2 µm diameter). The temperatures used were injector 210°C; detector 240°C; oven: initial 150°C × 2 min, ramp 10°C/min until 230°C, ramp 6°C/min until 215°C, ramp 30°C/min, total time: 45 min. Helium (carrier): 1 mL/min, hydrogen (FID): 30 mL/min (15 psi), air (FID): 300 mL/min (35 psi). Injection volume: 1 µL. The relative composition of fatty acids was expressed as percentage of each identified fatty acid in the total of fatty acids measured in the pine nut oil.
2.3. Statistical analysis
The chemical analyses were performed in duplicate or triplicate (as indicated in tables); each replicate was quantified in duplicate, unless stated otherwise. All data given represent mean values ± standard error. Statistical analysis was achieved using Kruskal–Wallis test and the Conover post hoc multiple comparison between macrozones (Conover, Citation1999). A p < 0.05 was considered significant. A principal component analysis was also done, generating a biplot for chemical composition of pine nuts and climate variables of the three macrozones. Analyses were executed using the software Infostat® (Di Rienzo et al., Citation2013).
3. Results and discussion
The Chilean Forestry Institute (INFOR) developed a series of field assays to evaluate the potential for growing P. pinea in this country, and three geographic macrozones (N, DC, and S) were chosen to compare the chemical composition of the harvested pine nuts.
3.1. Proximate composition and mineral content
The proximate composition of stone pine nuts is shown in (moisture, ashes, crude protein, fat, and total dietary fiber). The most abundant component was fat, ranging from 422.5 ± 3.3 to 469.2 ± 0.7 g/kg, followed by protein, ranging from 320.9 ± 1.4 to 352.5 ± 1.0 g/kg. The contents of fats and proteins exhibited differences between the growing macrozones (p < 0.05), although the values are similar in terms of the nutritive value of these tree nuts, being a good source of both macronutrients (Evaristo et al., Citation2010; Nergiz & Dönmez, Citation2004; Ruggeri, Cappelloni, Gambelli, Nicoli, & Carnovale, Citation1998). The total dietary fiber content was similar in the seeds collected in the three tested macrozones (average 116.8 ± 1.0 g/kg), with a predominance of the insoluble fraction (average 96.1 ± 1.3 g/kg). These results indicate that pine nuts grown in Chile possess higher contents of proteins, lipids, and dietary fiber than walnuts, almonds, and hazelnuts, which are the nuts most frequently consumed in the country.
Table 1. Chemical composition of Chilean pine nuts from different macrozones (g/kg).
Tabla 1. Composición química de piñón de pino cultivado en diferentes macrozonas de Chile (g/kg).
Among the minerals contained in pine nuts, K and P are the most abundant, followed by Mg (Evaristo et al., Citation2010; Nergiz & Dönmez, Citation2004). The main mineral measured in Chilean pine nuts was K, ranging from nearly 4 to 9 g/kg, exhibiting a low content of Na (). The seeds also contain some trace minerals with nutritional interest such as Cu, Zn, and Fe. In this study, K was the most abundant mineral, which is well recognized as a healthy mineral as opposed to Na, which relates to cardiovascular and other noncommunicable diseases (Campbell, Correa-Rotter, Neal, & Cappuccio, Citation2011). Since the analysis was performed on a single pooled sample of each macrozone, it is not possible to apply a statistics to conclude if the geographical zones affect the mineral content, although some mineral levels observed (Cu, Fe, K, Na) were around 50% lower in the DC zone, an aspect that should be further investigated.
Table 2. Mineral content of Chilean pine nuts from different macrozones (mg/kg).
Tabla 2. Contenido de minerales de piñón de pino cultivado en diferentes macrozonas de Chile (mg/kg).
3.2. Tocopherols and sterols
Vitamin E average contents were 59.0 ± 12.6 and 1071.1 ± 109.8 µg/kg oil for α- and γ-tocopherols, respectively (). In fact, for all nut oils, the antioxidant capacity is due mainly to their content of tocopherols (Espín, Soler-Rivas, & Wichers, Citation2000), and tree nuts are recognized among the richest sources of vitamin E, the principal lipid-soluble vitamin, along with seeds and vegetable oils. After analyzing 10 different tree nut types, Esche, Müller, and Engel (Citation2013) observed that the levels of tocopherols were highest in pine nuts (0.33 mg/g nut).
Table 3. Main phytosterols and tocopherols of Chilean pine nut oil from different macrozones (µg/kg oil).
Tabla 3. Principales fitoesteroles y tocoferoles en aceite de piñón de pino cultivado en diferentes macrozonas de Chile (µg/kg aceite).
Nasri, Fady, and Triki (Citation2007) analyzed seven populations of pine nuts from different European Mediterranean countries and reported very high levels of sterols (4376 mg/kg oil). The authors observed that β-sitosterol is the most abundant species (74%) in all P. pinea populations (3208 ± 253 mg/kg oil), followed by campesterol (661 ± 32 mg/kg TL), ∆5-avenasterol (296 ± 67 mg/kg oil), and ∆5,24-stigmastadienol (21 ± 5 mg/kg oil). The β-sitosterol content observed in our study () was in average 18.18 ± 0.76 mg/kg oil, that is, over 60% higher than stigmasterol in the pine nuts grown in the three Chilean macrozones studied. The predominance of β-sitosterol has been consistently observed in pine nuts, accounting for over 60% of total free sterols/stanols in other edible nuts such as almonds, hazelnuts, and walnuts (Esche et al., Citation2013; Segura, Javierre, Lizarraga, & Ros, Citation2006).
3.3. TPC, ORAC, and vitamin C
Shelled pine nuts do not contain phenolic compounds in amounts comparable with other tree nuts, which are consumed with the thin tan-brown skin that lines the nut, such as walnuts (Pellegrini et al., Citation2006). The TPC observed () was lower than the content reported for pine nuts by Alasalvar and Shahidi (Citation2008) and Alasalvar and Bolling (Citation2015). In terms of antioxidant capacity, the ORAC values observed in Chilean seeds are similar to those reported by these authors (7.19 µmol TE/g) and others (Kornsteiner, Wagner, & Elmadfa, Citation2006; Wu et al., Citation2004), while the vitamin C content observed in Chilean pine nuts is not outstanding, reaching an average of 27.7 ± 1.4 mg/kg.
Table 4. Phenolic compounds, antioxidant capacity, and vitamin C content of Chilean pine nuts from different macrozones.
Tabla 4. Compuestos fenólicos, capacidad antioxidante y contenido de vitamina C de piñón de pino cultivado en diferentes macrozonas de Chile.
3.4. Relative fatty acid composition
shows the relative fatty acid profile of pine tree nuts, expressed as percentage of each species in the whole oil extracted. The predominant fatty acids were polyunsaturated, reaching averages from 48.01 ± 0.09% (N) to 51.08 ± 0.04% (DC), represented mainly by linoleic acid, followed by monounsaturated (mainly oleic acid), with average amounts from 37.13 ± 0.03% (DC) to 40.92 ± 0.05% (N). The most abundant fatty acids observed in Chilean pine nut oils were linoleic and oleic acids. This result is consistent with those reporting that pine nut oil contains predominantly linoleic acid (around 46%) and oleic acid (38%), while Maritime pine nut (Pinus pinaster) oil also contains two fatty acids that are unique among tree nut oils: pinoleic and sciadonic acids (Shahidi & Miraliakbari, Citation2005). Pinolenic acid (5,9,12–18:3) and other Δ5 polymethylene-interrupted PUFA have been identified in pine nuts (Wolff, Pédrono, Pasquier, & Marpeau, Citation2000). Pinolenic acid has been reported in levels ranging from 0.0 to 3.70 g/100 g oil in pine nuts from other pine species (Bagci & Karaagacli, Citation2004; Nasri, Khaldi, Fady, & Triki, Citation2005; Wolff & Bayard, Citation1995) especially Pinus sibirica. As shown in , this fatty acid was not identified in the Chilean samples analyzed.
Table 5. Relative percentage of fatty acids of Chilean pine nut oil from different macrozones.
Tabla 5. Porcentaje relativo de ácidos grasos en aceite de piñón de pino cultivado en diferentes macrozonas de Chile.
The composition of pine nuts varies among species, depending on geographical and climatic conditions (Sagrero-Nieves, Citation1992; Savage, Citation2001; USDA, Citation2015). In the particular macrozones studied, S is the coolest, with an annual average temperature of 13.2°C, annual max average temperature of 19.8°C, and thermal oscillation of 12.3°C and it is also the most humid (annual rainfall of 1047 mm). Pine nuts collected there presented the lowest protein content. On the contrary, macrozone N is characterized by the most challenging climate variables, with an annual average temperature of 14.1°C, annual maximum average temperature of 21.9°C, thermal oscillation of 14.3°C, and annual rainfall of 383.7 mm, and pine nuts collected there showed the highest ashes and α-tocopherol contents. Pine nuts collected in the transition macrozone DC, characterized by intermediate climate variables (annual average temperature 13.6°C, annual maximum average temperature 21.0°C; thermal oscillation 14°C; annual rainfall 648.3 mm), showed the highest values for moisture, protein, and lipids.
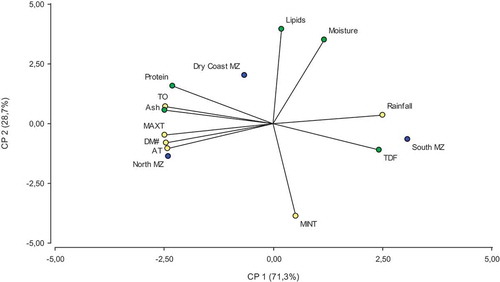
The biplot of the two principal components () explains 100% of the variability. Differences were observed between some components of pine nuts grown in the three Chilean macrozones studied. Pine nuts from S, an environment with higher rainfall, contained more dietary fiber, while those harvested in N, a macrozone characterized by high average temperature, maximum average temperature, thermal oscillation, and quantity of dry months, showed a high content of ashes. shows that pine nuts collected in DC macrozone showed high contents of moisture, proteins, and lipids. Finally, lipids seem to increase with a decrease in the minimum average temperature.
Figure 1. Biplot for the chemical composition of pine nuts and climate variables by macrozone. MAXT: maximum average temperature, AT: average temperature, MINT: minimum average temperature, DM#: dry month number, TO: thermal oscillation, TDF: total dietary fiber.
Figura 1. Biplot de la composición química de piñones de pino y variables climáticas por macrozona. MAXT: temperatura máxima promedio, AT: temperatura promedio, MINT: temperatura mínima promedio, DM#: número de meses secos, TO: oscilación térmica, TDF: fibra dietética total.
The results indicate that even though the environmental conditions may affect the composition of pine nuts, all the seeds grown in Chile present a chemical composition largely similar to those produced in the Mediterranean zone, in most of their components. Consequently, Chile represents an adequate terrain to produce this highly appreciated tree nut, augmenting the diversity of the local agriculture.
4. Conclusion
The results obtained demonstrate that P. pinea L. seeds harvested from trees grown in three Chilean geographical macrozones, differing in climatic and growth conditions, exhibit good nutritional and healthy properties. All pine nuts harvested constitute an adequate source of nutrients and bioactives that clearly justify their inclusion as part of a healthy diet, in which they contribute to enhance the beneficial effects on health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alasalvar, C., & Bolling, B.W. (2015). Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. British Journal of Nutrition, 113(Suppl S2), S68–S78. doi:10.1017/S0007114514003729
- Alasalvar, C., & Shahidi, F. (2008). Tree nuts: Composition, phytochemicals, and health effects. Nutraceutical science and technology series. Boca Raton, FL: CRC Press.
- Anderson, T.J., Grégoire, J., Hegele, R.A., Couture, P., Mancini, G.B., McPherson, R., … Ur, E. (2013). 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Canadian Journal of Cardiology, 29, 151–167. doi:10.1016/j.cjca.2012.11.032
- Association of Official Analytical Chemists (AOAC). (2012). Official methods of analysis of AOAC International (19th ed.). G.W. Latimer (Ed.).Gaythersburg, MD: AOAC International. ISBN:0-935584-83-8.
- Bagci, E., & Karaagacli, Y. (2004). Fatty acid and tocochromanol patterns of Turkish pines. Acta Biologica Cracoviensia Series Botanica, 46, 95–100.
- Bao, Y., Han, J., Hu, F.B., Giovannucci, E.L., Stampfer, M.J., Willett, W.C., & Fuchs, C.S. (2013). Association of nut consumption with total and cause-specific mortality. New England Journal of Medicine, 369, 2001–2011. doi:10.1056/NEJMoa1307352
- Bes-Rastrollo, M., Wedick, N.M., Martínez-González, M.A., Li, T.Y., Sampson, L., & Hu, F.B. (2009). Prospective study of nut consumption, long-term weight change, and obesity risk in women. American Journal of Clinical Nutrition, 89, 1913–1919. doi:10.3945/ajcn.2008.27276
- Bligh, E., & Dyer, W. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917. doi:10.1139/o59-099
- Bolling, B.W., Chen, C.-Y.O., McKay, D.L., & Blumberg, J.B. (2011). Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutrition Research Reviews, 24, 244–275. doi:10.1017/S095442241100014X
- Bracalini, M., Benedettelli, S., Croci, F., Terreni, P., Tiberi, R., & Panzavolta, T. (2013). Cone and seed pests of Pinus pinea: Assessment and characterization of damage. Journal of Economic Entomology, 106, 229–234. doi:10.1603/EC12293
- Campbell, N., Correa-Rotter, R., Neal, B., & Cappuccio, F.P. (2011). New evidence relating to the health impact of reducing salt intake. Nutrition, Metabolism & Cardiovascular Diseases, 21, 617–619. doi:10.1016/j.numecd.2011.08.001
- Conover, W.J. (1999). Practical nonparametric statistics. New York, NY: John Wiley & Sons. ISBN:978-0471160687.
- De Jong, A., Plat, J., & Mensink, R.P. (2003). Metabolic effects of plant sterols and stanols (review). The Journal of Nutritional Biochemistry, 14, 362–369. doi:10.1016/S0955-2863(03)00002-0
- Di Rienzo, J.A., Casanoves, F., Balzarini, M.G., Gonzalez, L., Tablada, M., & Robledo, C.W. (2013). InfoStat version 2014. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Retrieved September 15, 2015, from http://www.infostat.com.ar
- Esche, R., Müller, L., & Engel, K.-H. (2013). Online LC-GC-based analysis of minor lipids in various tree nuts and peanuts. Journal of Agricultural and Food Chemistry, 61, 11636–11644. doi:10.1021/jf403900q
- Espín, J.C., Soler-Rivas, C., & Wichers, H.J. (2000). Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picryl-hydrazyl radical. Journal of Agricultural and Food Chemistry, 48, 648–656. doi:10.1021/jf9908188
- Estruch, R., Martínez-González, M.A., Corella, D., Salas-Salvadó, J., Ruíz-Gutiérrez, V., Covas, M.I., … Ros, E. (2006). Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Annals of Internal Medicine, 145, 1–11. doi:10.7326/0003-4819-145-1-200607040-00004
- Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M.-I., Corella, D., Arós, F., … Martínez-González, M.A. (2013). Primary prevention of cardiovascular disease with a Mediterranean diet. New England Journal of Medicine, 368, 1279–1290. doi:10.1056/NEJMoa1200303
- Evaristo, I., Batista, D., Correia, I., Correia, P., & Costa, R. (2010). Chemical profiling of Portuguese Pinus pinea L. nuts. Journal of the Science of Food and Agriculture, 90, 1041–1049. doi:10.1002/jsfa.3914
- Ferguson, J.J.A., Stojanovski, E., MacDonald-Wicks, L., & Garg, M.L. (2016). Fat type in phytosterol products influence their cholesterol-lowering potential: A systematic review and meta-analysis of RCTs. Progress in Lipid Research, 64, 16–29. doi:10.1016/j.plipres.2016.08.002
- Flores-Mateo, G., Rojas-Rueda, D., Basora, J., Ros, E., & Salas-Salvadó, J. (2013). Nut intake and adiposity: Meta-analysis of clinical trials. American Journal of Clinical Nutrition, 97, 1346–1355. doi:10.3945/ajcn.111.031484
- Folch, J., Lees, M., & Sloane-Stanley, G.M. (1957). A simple method of isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
- Food and Drug Administration. (2003). Qualified health claims: Letter of enforcement discretion – Nuts and coronary heart disease. Rockville, MD: US Food and Drug Administration.
- Gökmen, V., Kahraman, N., Demir, N., & Acar, J. (2000). Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. Journal of Chromatography A, 881, 309–316. doi:10.1016/S0021-9673(00)00080-7
- Greenfield, H., & Southgate, D.A.T. (1972). Food composition data production, management and use. London: Elsevier Applied Science.
- Gylling, H., Plat, J., Turley, S., Ginsberg, H.N., Ellegård, L., Jessup, W., … Chapman, M.J. (2014). Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis, 232, 346–360. doi:10.1016/j.atherosclerosis.2013.11.043
- Huang, D., Ou, B., & Prior, R.L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53, 1841–1856. doi:10.1021/jf030723c
- Ibarrola-Jurado, N., Bulló, M., Guasch-Ferré, M., Ros, E., Martínez-González, M.A., Corella, D., … Calbet, J.A.L. (2013). Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: The PREDIMED study. Plos One, 8, e57367. doi:10.1371/journal.pone.0057367
- International Organization for Standardization (ISO). (2011). Animal and vegetable fats and oils - Preparation of methyl esters of fatty acids. Reference 12966-2:2011. Retrieved April 18, 2016, from http://www.iso.org/iso.
- International Union of Pure and Applied Chemistry. (1992). Method No 2432: Determination of tocopherols and tocotrienols in vegetable oils and fats by HPLC. Standard methods for the analysis of oils fats and derivatives (7th ed.). Oxford: Blackwell.
- Jenkins, D.J.A., Kendall, C.W.C., Banach, M.S., Srichaikul, K., Vidgen, E., Mitchell, S., … Josse, R.G. (2011). Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care, 34, 1706–1711. doi:10.2337/dc11-0338
- Kornsteiner, M., Wagner, K.-H., & Elmadfa, I. (2006). Tocopherols and total phenolics in 10 different nut types. Food Chemistry, 98, 381–387. doi:10.1016/j.foodchem.2005.07.033
- Kornsteiner-Krenn, M., Wagner, K.-H., & Elmadfa, I. (2013). Phytosterol content and fatty acid pattern of ten different nut types. International Journal for Vitamin and Nutrition Research, 83, 263–270. doi:10.1024/0300-9831/a000168
- Loewe, M.V., Balzarini, M., Álvarez-Contreras, C.A., Delard, R.C., & Navarro-Cerrillo, R.M. (2016). Fruit productivity of Stone pine (Pinus pinea L.) along a climatic gradient in Chile. Agricultural and Forest Meteorology, 223, 203–216. doi:10.1016/j.agrformet.2016.04.011
- Lutz, M., & Luna, L. (2016). Nuts and body weight: An overview. Journal of Nutrition and Health Sciences, 3, 104. doi:10.15744/2393-9060.3.104
- Maguire, L.S., O’Sullivan, S.M., Galvin, K., O’Connor, T.P., & O’Brien, N.M. (2004). Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. International Journal of Food Sciences and Nutrition, 55, 171–178. doi:10.1080/09637480410001725175
- Mattes, R.D., & Dreher, M.L. (2010). Nuts and healthy body weight maintenance mechanisms. Asia Pacific Journal of Clinical Nutrition, 19, 137–141.
- Nasri, N., Fady, B., & Triki, S. (2007). Quantification of sterols and aliphatic alcohols in Mediterranean stone pine (Pinus pinea L.) populations. Journal of Agricultural and Food Chemistry, 55, 2251–2255. doi:10.1021/jf062911j
- Nasri, N., Khaldi, A., Fady, B., & Triki, S. (2005). Fatty acids from seeds of Pinus pinea L.: Composition and population profiling. Phytochemistry, 66, 1729–1735. doi:10.1016/j.phytochem.2005.05.023
- Nasri, N., Khaldi, A., Hammami, M., & Triki, S. (2005). Fatty acid composition of two Tunisian pine seed oils. Biotechnology Progress, 21, 998−1001. doi:10.1021/bp049568s
- Nasri, N., Tlili, N., Ben Ammar, K., Khaldi, A., Fady, B., & Triki, S. (2009). High tocopherol and triacylglycerol contents in Pinus pinea L. seeds. International Journal of Food Sciences and Nutrition, 60, 161–169. doi:10.1080/09637480802577854
- Nergiz, C., & Dönmez, İ. (2004). Chemical composition and nutritive value of Pinus pinea L. seeds. Food Chemistry, 86, 365–368. doi:10.1016/j.foodchem.2003.09.009
- Ou, B., Hampsch-Woodill, M., Flanagan, J., Deemer, E.K., Prior, R.L., & Huang, D. (2002). Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. Journal of Agricultural and Food Chemistry, 50, 2772–2777. doi:10.1021/jf011480w
- Pellegrini, N., Serafini, M., Salvatore, S., Del Rio, D., Bianchi, M., & Brighenti, F. (2006). Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Molecular Nutrition & Food Research, 50, 1030–1038. doi:10.1002/mnfr.200600067
- Rees, K., Hartley, L., Flowers, N., Clarke, A., Hooper, L., Thorogood, M., & Stranges, S. (2014). ’Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease (review). Cochrane Database of Systematic Reviews, 2013(8), Art. No CD009825. John Wiley & Sons. doi:10.1002/14651858.CD009825.pub2
- Rocha, V.Z., Ras, R.T., Gagliardi, A.C., Mangili, L.C., Trautwein, E.A., & Santos, D. (2016). Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis, 248(76), 76–83. doi:10.1016/j.atherosclerosis.2016.01.035
- Ros, E. (2009). Nuts and novel biomarkers of cardiovascular disease. American Journal of Clinical Nutrition, 89(5), 1649S–1656S. doi:10.3945/ajcn.2009.26736R
- Ros, E. (2015). Nuts and CVD. British Journal of Nutrition, 113(Suppl S2), S111–S120. doi:10.1017/S0007114514003924
- Ros, E., & Mataix, J. (2006). Fatty acid composition of nuts. Implications for cardiovascular health. British Journal of Nutrition, 96, S29–S35. doi:10.1017/BJN20061861
- Rudkowska, I. (2010). Plant sterols and stanols for healthy ageing. Maturitas, 66, 158–162. doi:10.1016/j.maturitas.2009.12.015
- Ruggeri, S., Cappelloni, M., Gambelli, L., Nicoli, S., & Carnovale, E. (1998). Chemical composition and nutritive value of nuts grown in Italy. Italian Journal of Biochemistry, 10, 243–252.
- Ryan, E., Galvin, K., O’Connor, T.P., Maguire, A.R., & O’Brien, N.M. (2006). Fatty acid profile, tocopherol, squalene and phytosterol content of brazil, pecan, pine, pistachio and cashew nuts. International Journal of Food Sciences and Nutrition, 57, 219–228. doi:10.1080/09637480600768077
- Sabaté, J., & Ang, Y. (2009). Nuts and health outcomes: New epidemiologic evidence. American Journal of Clinical Nutrition, 89, 1643S–1648S. doi:10.3945/ajcn.2009.26736Q
- Sabaté, J., & Wien, M. (2010). Nuts, blood lipids and cardiovascular disease. Asia Pacific Journal of Clinical Nutrition, 19, 131–136.
- Sagrero-Nieves, L. (1992). Fatty acid composition of Mexican pine nut (Pinus cembroides) oil from three seed coat phenotypes. Journal of the Science of Food and Agriculture, 59, 413–414. doi:10.1002/jsfa.2740590320
- Savage, G.P. (2001). Chemical composition of walnuts (Juglans regia L.) grown in New Zealand. Plant Foods for Human Nutrition, 56, 75–82. doi:10.1023/A:1008175606698
- Segura, R., Javierre, C., Lizarraga, M.A., & Ros, E. (2006). Other relevant components of nuts: Phytosterols, folate and minerals. British Journal of Nutrition, 96(Suppl S2), 36S–44S. doi:10.1017/BJN20061862
- Shah, W., Kekare, M.B., & Vaidya, V. (2010). Development and validation of high performance liquid chromatographic method for the simultaneous determination of β-sitosterol and lupeol in Vernonia cinerea Linn. International Journal of Pharma and Bio Sciences, 1, 1–5.
- Shahidi, F., & Miraliakbari, H. (2005). Tree nut oils. In F. Shahidi (Ed.), Bailey’s industrial oil and fat products (6th ed.). Hoboken, NJ: John Wiley & Sons.
- Singleton, V.L., & Rossi, J.A. (1965). Colorimetry of total phenolics with phosphor-molybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
- USDA, US Department of Agriculture ARS. (2015). USDA national nutrient database for standard reference, release 28. Nutrient Data Laboratory Home Page. United States Department of Agriculture. Retrieved April 18, 2016, from http://www.ars.usda.gov
- Venkatachalam, M., & Sathe, S.K. (2006). Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry, 54, 4705–4714. doi:10.1021/jf0606959
- Wolff, R.L., & Bayard, C.C. (1995). Fatty acid composition of some pine seed oils. Journal of the American Oil Chemists’ Society, 72, 1043–1046. doi:10.1007/BF02660719
- Wolff, R.L., Pédrono, F., Pasquier, E., & Marpeau, A.M. (2000). General characteristics of Pinus spp. Seed fatty acid compositions, and importance of Δ5-olefinic acids in the taxonomy and phylogeny of the genus. Lipids, 35, 1–22. doi:10.1007/s11745-000-0489-y
- Wu, X., Beecher, G.R., Holden, J.M., Haytowitz, D.B., Gebhardt, S.E., & Prior, R.L. (2004). Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. Journal of Agricultural and Food Chemistry, 52, 4026–4037. doi:10.1021/jf049696w
