ABSTRACT
This study investigated the antioxidant activity of peptides isolated from dry-cured pork loins inoculated with probiotic strains of lactic acid bacteria (LAB). The antioxidant activity of the isolated peptide fraction (an average of 13 kDa) was determined by radical-scavenging activities: ABTS and DPPH, reducing power and metal chelating assay (Fe2+ and Cu2+). The highest antioxidant activity was noted against ABTS and DPPH. The results suggest no influence of probiotic LAB on the relative quantity of peptides and the lack of relationship between this parameter and antioxidant activity measures. The differences in antioxidant activity among the tested batches may be associated with the influence of the LAB strains on the peptides’ composition and the amino acid position in them.
RESUMEN
Este estudio investigó la actividad antioxidante de péptidos aislados de lomo de cerdo curado en seco inoculado con cepas probióticas de bacteria ácido-láctica (LAB). Se determinó la actividad antioxidante de la fracción de péptidos aislados (un promedio de 13 kDa) mediante actividad de barrido de radicales: ABTS y DPPH, poder reductor férrico (RP) y ensayo quelante de metal (Fe2+ y Cu2+). La mayor actividad antioxidante se observó contra ABTS y DPPH. Los resultados sugieren que no existe ninguna influencia de LAB probióticas en la cantidad relativa de péptidos y la falta de relación entre este parámetro y las mediciones de actividad antioxidante. Las diferencias en la actividad antioxidante entre los grupos examinados podrían asociarse a la influencia de las cepas de LAB en la composición de los péptidos y su posición de los aminoácidos.
PALABRAS CLAVE:
1. Introduction
Dry-cured meats are gaining immense importance among products of animal origin. This has been confirmed by their steadily increasing number and assortment in stores. They are valued by consumers primarily due to their characteristic flavour and aroma, both of which result from distinct production technology (Dave & Ghaly, Citation2011). They are also of interest to scientists, largely due to the presence of active molecules in them. These include not only, for example, anserine, carnosine, linoleic acid and coenzyme Q10, but also biologically active peptides, that is, short sequences encoded in the structure of the protein (Arihara, Citation2006). Among the various activities attributed to peptides from meat products, the antioxidant properties of these compounds draw particular attention. The peptides, when ingested together with the product, may be helpful in the fight against reactive forms of oxygen, thus preventing diseases caused by oxidative stress, which is caused by the presence of a number of highly reactive oxygen species generally called ROS. An excess of ROS, both due to excess production or impairment of antioxidants, or both, is harmful, for example, changes to the oxidation of biological membrane lipids involving free radicals have a disruptive effect at the level of the cell and of the entire organism. As an example, proteins are a group of molecules affected by ROS (Sarmadi & Ismail, Citation2010). Cleavage of the peptide bond, amino acid modification and the formation of cross-linked peptide aggregates take place during protein oxidation by ROS, which leads to the formation of protein derivatives possessing highly reactive carbonyl groups (ketones and aldehydes) that are involved in complications in diabetes and in many age-related diseases (Chakrabarti, Jahandideh, & Wu, Citation2014; Stadtman & Levine, Citation2003). Living organisms are protected from the effects of oxidative stress by various defence systems. The ability of these protective systems gradually decreases with age, resulting in a disruption of the normal redox balance. Intake of antioxidant compounds along with food helps to maintain the state of equilibrium of pro-oxidants/antioxidants in the body (Chakrabarti et al., Citation2014).
Thus, there is growing interest in food proteins and in their constituent peptides as potential candidates for use as antioxidative components of functional products. These products could be used to maintain human health, especially for the prevention and treatment of chronic diseases which are either caused or promoted by oxidative stress (Sarmadi & Ismail, Citation2010). The most attractive characteristic of peptides, in comparison to their synthetic equivalents, is their ability to exert very few side effects in humans because of their natural origin. In this context, food proteins and the peptides they release can be potential functional food ingredients (Chakrabarti et al., Citation2014).
It has been reported that bioactive peptides acting as antioxidants can come from a variety of natural proteins, such as grains, legumes, milk, meat, eggs and fish, and from various marine organisms (Sarmadi & Ismail, Citation2010). Fermented food products are a good source of amino acids and peptides which possess antioxidant properties. They show significant antioxidant activity which may have beneficial effects in promoting human health and in food processing (Chakrabarti et al., Citation2014; Escudero, Mora, Fraser, Aristoy, & Toldrá, Citation2013; Faithong & Benjakul, Citation2014).
This study aimed at evaluating the antioxidative activity of peptides isolated from dry-cured pork loins inoculated with probiotic strains of lactic acid bacteria (LAB). Radical-scavenging activities: ABTS ([2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] and DPPH (2,2-diphenyl-1-picrylhydrazyl), ion-chelating activity (Fe2+ and Cu2+) and reducing power (RP) of the peptide isolates were determined.
2. Materials and methods
2.1. Preparation of dry-cured meat products
The study was carried out on pork meat cuts (n = 12) of the Polish White Large breed. Loins (m. longissimus thoracis) were excised at 24 h postmortem from half-carcasses chilled at 4°C at a local slaughterhouse (Lublin, Poland). At 48 h postmortem, all loins underwent curing using a surface massage with a curing mixture (18 g of sea salt, 9.7 g of curing salt and 0.3 g of NaNO3 per kg of loin). All batches were then kept at 4°C for 24 h to allow the curing salt to diffuse. After the salting stage, three loins were regarded as the control sample (C). The remaining loins were randomly divided in three experimental batches with three loins each and inoculated with 0.2% (v/w) of Lactobacillus rhamnosus LOCK900 (LOCK), Lactobacillus acidophilus Bauer Ł0938 (BAUER) and Bifidobacterium animalis ssp. lactis BB-12 (BB12), respectively, to achieve an initial level of 106–107 CFU/g of meat. The LOCK and BAUER strains were derived from the Collection of Pure Cultures of Industrial Microorganisms, Institute of Fermentation Technology and Microbiology at the Technical University of Łodź. BB-12 was obtained from Christian Hansen’s collection of dairy cultures (strain deposit number: DSM15954). These LAB strains were chosen on the basis of publications describing attempts to apply them for meat fermentation (Libera, Karwowska, Stasiak, & Dolatowski, Citation2015; Trząskowska, Kołożyn-Krajewska, Wójciak, & Dolatowski, Citation2014). Subsequently, the meat portions were hung at 16 ± 1°C in a laboratory ageing chamber with a relative humidity of 75 ± 5% for 21 days. Then, whole pieces of loins were vacuum-packed and aged at 4 ± 1°C for 9 months. Three independent experimental trials were conducted.
2.2. Determination of physicochemical parameters
Active acidity (pH) was measured in a slurry made by mixing the minced sample (10 g) with distilled water (100 mL) for 1 min using a homogeniser (Ultra-Turrax T25 Basic, IKA, Staufen, Germany). The pH value was determined with a digital pH meter (CPC-501, Elmetron, Zabrze, Poland) equipped with a pH electrode (ERH-111; Hydromet, Gliwice, Poland). The pH-meter was standardised with buffer solutions at pH 4.0, 7.0 and 9.0, prior to determination. The oxidation–reduction potential (ORP) was measured with a platinum electrode (ERPt-13; Hydromet, Gliwice, Poland) in a slurry previously obtained for pH measurement. The water activity (aw) measurements were carried out at 20 ± 1°C, using a LabMaster-aw instrument (Novasina AG, Lachen, Switzerland) with a temperature-controlled measuring chamber. Novasina humidity standards based on saturated salt solutions were used for calibration.
2.3. Microbiological analysis
Total viable counts (TVCs) were determined using plate count agar after incubation at 30°C for 72 h (ISO 4833-2:Citation2013). The population of LAB (including probiotic bacteria) was determined according to ISO 15214, Citation1998. Samples for the microbiological analyses were taken as a cross section from the middle of the loins being tested. Microbiological analyses were carried out at the Eurocontrol Laboratory (Dęblin, Poland). The analyses were made in triplicate and the counts were expressed as colony forming units (log CFU/g).
2.4. Isolation of peptides and sodium dodecyl sulphate polyacrylamide gel electrophoresis
The extraction was performed according to the method of Mora, Sentandreu and Toldrá (Citation2010). Peptides isolated from dry-cured loins were analysed using sodium dodecyl sulphate polyacrylamide gel electrophoresis according to the method of Laemmli (Citation1970), using 14% separating gel and 5% stacking gel. A volume of 20 μl of peptides was loaded onto the gel. Electrophoresis was conducted at a constant current of 50 V for the stacking gel and 100 V for the separating gel by using Mini-PROTEAN® Tetra Cell (Bio-Rad Laboratories, Hercules, CA, USA). Gels were stained using 0.05% Coomassie Brilliant Blue R-250 dissolved in 15% (v/v) methanol and 5% (v/v) acetic acid and de-stained with 30% (v/v) methanol and 10% (v/v) acetic acid.
2.5. Radical-scavenging activity
Scavenging activity of the peptide isolates against the DPPH free radical was assessed according to the method of Wu, Chen and Shiau (Citation2003) and against the ABTS+ radical cation according to Re et al. (Citation1999).
2.6. Determination of ion chelating activity
The method of Decker and Welch (Citation1990) was used to investigate the ferrous ion-chelating ability of peptides. Copper-chelating activity was measured according to the Torres-Fuentes, Alaiz and Vioque (Citation2011) method.
2.7. Determination of RP
The RP of the samples was determined by the method described by Mora, Escudero, Fraser, Aristoy and Toldrá (Citation2014).
2.8. Statistical analysis
All assays were performed in five replicates and the results are presented as mean ± standard deviation. The data were analysed by one-way ANOVA using SAS statistical software. The significance of the differences between the mean values was calculated at a significance level of p < 0.05 using T-Tukey’s range test. For hierarchical cluster analysis (HCA), the Ward’s method was applied.
3. Results and discussion
3.1. Physicochemical parameters
LAB are the major producers of lactic acid, which is responsible for a decrease in the pH value during the preparation of dry-cured meat products; thus, it contributes to limiting the growth of unfavourable microflora and to improving the safety and quality of dry-cured meat products. Low acidity affects the activity of enzymes involved in proteolysis. Flores, Marcus, Nieto, Navarro and Lorenzo (Citation1997) pointed out that protein hydrolysis is greater in highly acidic environments. The statistically significant differences in the pH values (p < 0.05) between the samples () were noted. Pork loin batches inoculated with the probiotic strain showed lower pH than samples that had undergone spontaneous fermentation. The significant differences (p < 0.05) possibly resulted from differences in lactic acid production by the inoculated strains, with each one dominating in their respective sample; this confirms the effectiveness of LAB to acidify the pork loins. The lowest pH value (p < 0.05) was observed in the sample with L. rhamnosus LOCK900 (5.52), which also had a high number of TVC and LAB (6.97 and 6.89 log CFU/g, respectively). A similar relationship was also confirmed in the BB12, thus suggesting that higher LAB counts corresponded to lower pH values in the samples. These results are consistent with other studies on dry-cured meat products subjected to fermentation using Lactobacillus (Skwarek, Dolatowski, & Kołożyn-Krajewska, Citation2014) or Bifidobacterium (Libera et al., Citation2015) as the starter cultures. Other authors found similar pH values: 5.04 in probiotic loins stored for 6 months (Neffe-Skocińska, Jaworska, Kolożyn-Krajewska, Dolatowski, & Jachacz-Jówko, Citation2015), 5.77 in probiotic neck stored for 6 months (Libera et al., Citation2015), 5.56 in probiotic hams stored for 5 months (Skwarek et al., Citation2014) and 5.42 in probiotic loins stored for 4 months (Okoń & Dolatowski, Citation2014).
Table 1. Physicochemical parameters of dry-cured loins with probiotic strains of LAB (mean ± standard deviation).
Tabla 1. Parámetros fisicoquímicos de lomo curado en seco con cepas probióticas de LAB (promedio±desviación estándar).
The oxidation–reduction (redox) value is defined as a biochemical system’s ability to accept or donate electrons. A lower redox potential suggests greater ability to donate electrons and to eliminate free radicals. Thus, the ORP may be an indicator of antioxidant ingredients in the product. The highest ORP value (p < 0.05) recorded in sample C (292.967 mV) showing that an increase in the system’s ability to retrieve electrons (oxidation) indicates lower content of reducing substances in the sample. The ORP values of dry-cured loins inoculated with probiotics were significantly lower (p < 0.05) than those obtained for C, averaged 285.633 mV (). However, there was no significant difference (p > 0.05) between type strains in achieved ORP levels. These results coincide with those of other authors, who indicated higher ORP values for samples subjected to spontaneous fermentation as compared to dry-cured meat products inoculated with a probiotic starter culture (Okoń & Dolatowski, Citation2014; Skwarek et al., Citation2014). The probable cause of the reduction in ORP could have been inoculation with LAB, which contributed to acidity reduction of the products and resulted in activating the protease and led to accelerated proteolytic changes inducing the formation of peptides with antioxidant properties in the dry-cured meat products (Mora et al., Citation2014; Faithong & Benjakul, Citation2014; Okoń & Dolatowski, Citation2014).
The availability of water is one of the most important factors not only affecting both the growth and activity of microorganisms, but also enzymes involved in muscle proteolysis in dry-cured meat products. Water activity (aw) may affect biochemical reactions, such as the Maillard reaction, degradation of vitamins, denaturation of proteins and oxidation of lipids (Maltini, Torreggiani, Venir, & Bertolo, Citation2003). The microbiological safety of dry-cured meat products is directly influenced by the water activity. Microorganisms generally grow best between aw values of 0.980 and 0.995; growth of pathogens is prevented at aw of 0.85 (Dave & Ghaly, Citation2011). All batches in the study reached an aw of more than or equal to 0.900, which indicates relatively good conditions for further development of LAB (). Maximum levels were achieved in samples C and BB12 (0.912 and 0.913, respectively); significantly lower results (p < 0.05) were achieved for LOCK and BAUER (0.901 and 0.900, respectively).
3.2. Microbiological analysis
Dry-cured meats are generally considered as safe products with an extended shelf life. Their quality results from biochemical, microbiological, physical and sensory changes occurring during meat fermentation and ageing under certain technological conditions. This is due to, among others, the presence of natural and/or inoculated microflora resulting in a pH and water activity decrease, thereby limiting the conditions for growth of undesirable pathogenic or spoilage microorganisms. Fermented food products (based on meat, fish, vegetables and dairy products) are produced by using LAB, including probiotics.
A TVC value above 106 CFU/g indicates the presence of the dominant microorganisms in the product, but the limits are not applicable if LAB were used as a processing aid (HPA, Citation2009). In the study, the total number of live bacteria in dry-cured loins was determined on the similar level; however, it had significantly lower (p < 0.05) values for BAUER ().
Table 2. Number of total viable counts (TVC) and lactic acid bacteria (LAB; log CFU/g) in dry-cured loins with probiotic strains of LAB (mean ± standard deviation).
Tabla 2. Número total de recuentos viables (TVC) y bacteria ácido-láctica (LAB; log CFU/g) en lomo curado en seco con cepas probióticas de LAB (promedio±desviación estándar).
It was determined in the study that LAB were the predominant microflora after 9 months of storage. In any case, the level of LAB reached more than 106 CFU/g. The minimum population of LAB was found for BAUER (p < 0.05). These results are consistent with other studies on dry-cured meat products subjected to fermentation by using a probiotic bacteria strain. The level of B. animalis ssp. lactis BB12 was from 7.41 after 6 months to 6.15 log CFU/g after 12 months of ageing (Libera et al., Citation2015), and L. acidophilus Bauer reached a level of 6.82 log CFU/g after 6 months of storage (Jaworska, Neffe, Kołożyn‐Krajewska, & Dolatowski, Citation2011). It is not surprising that loins without the addition of starter cultures presented a relatively high LAB, as LAB are common in the natural microflora of fermented meats (Jaworska et al., Citation2011).
3.3. Peptide profile
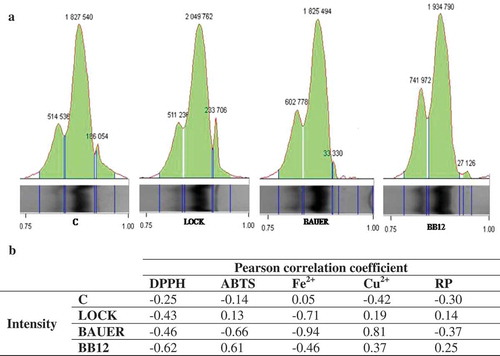
In the present study, lactic acid fermentation with probiotic strains was conducted for a period of 9 months and peptide isolates were obtained. The Coomassie-stained protein patterns of all samples do not show qualitative differences (). The obtained fractions had an average molecular weight of 13 kDa and did not show the presence of larger proteins, which excluded their participation in shaping the antioxidant properties. The gels clearly showed peptide bands in samples obtained after fermentation using the Coomassie stains. The quantitative differences were determined by densitometric analysis () which compares the relative quantity of peptides in each sample with the control sample. On the basis of the intensity (Int) and the correlation analysis, the results suggest no influence of probiotic LAB on the relative quantity of peptides and the lack of relationship between this parameter and antioxidant activity measures.
Figure 1. SDS-PAGE electrophoresis results.
MW: Molecular weight standard (kDa); C: sample without probiotic starter culture; LOCK: sample with probiotic starter culture Lactobacillus rhamnosus LOCK900; BAUER: sample with probiotic starter culture Lactobacillus acidophilus Bauer Ł0938; BB12: sample with probiotic starter culture Bifidobacterium animalis ssp. lactis BB-12.
Figura 1. Resultados electroforéticos de SDS-PAGE.
MW: peso molecular estándar (kDa); C: muestra sin cultivo iniciador probiótico; LOCK: muestra con cultivo iniciador probiótico Lactobacillus muestra con cultivo iniciador probiótico Lactobacillus acidophilus Bauer Ł0938; BB12: muestra con cultivo iniciador probiótico Bifidobacterium animalis ssp. lactis BB-12.
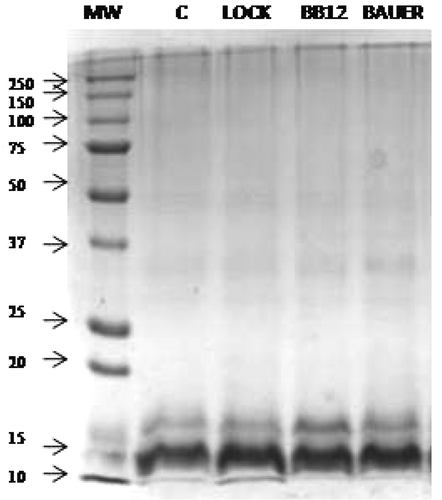
Figure 2. Representative densitogram of peptide isolates after fermentation (a). The correlation (Pearson’s correlation coefficient) between relative intensity and antioxidant activity measures of the tested samples (b).
Relative front in the range 0.75–1.00 was shown. Blackouts expressed numerically as intensity (Int).C: Sample without probiotic starter culture; LOCK: sample with probiotic starter culture Lactobacillus rhamnosus LOCK900; BAUER: sample with probiotic starter culture Lactobacillus acidophilus Bauer Ł0938; BB12: sample with probiotic starter culture Bifidobacterium animalis ssp. lactis BB-12.
Figura 2. Densitograma representativo de aislados de péptidos después de la fermentación (a). La correlación (coeficiente de correlación de Pearson) entre la intensidad relativa y las mediciones de actividad antioxidante de las muestras examinadas (b).
Se mostró un principio relativo de rango 0,75–1,00. Censuras expresadas de forma numérica como la intensidad (Int)C: muestra sin cultivo iniciador probiótico; LOCK: muestra con cultivo iniciador probiótico Lactobacillus rhamnosus LOCK900; BAUER: muestra con cultivo iniciador probiótico Lactobacillus acidophilus Bauer Ł0938; BB12: muestra con cultivo iniciador probiótico Bifidobacterium animalis ssp. lactis BB-12.
3.4. Determination of antioxidant activity
Free radicals and reactive oxygen species have a twofold importance in the cells of living organisms. They are important intermediaries in the transmission of signals and play an important role in the production of biologically active and essential nutrients. They can also be toxic and play a causal role in various diseases or spoilage. Therefore, the potential of peptide isolates for protection against reactive oxygen species was determined. Antioxidant compounds from dry-cured loins after 9 months of ageing showed activity against radicals, on average 76.48% in the DPPH test and 89.36% in the ABTS test (). Considering the ability to capture free radicals, the LAB-inoculated attempts (except for BB12) had significantly higher activity (p < 0.05) than the C. Isolated peptides have scavenging ability against the ABTS radical at a higher level as compared to the DPPH radical, which was confirmed by the results of other studies (Faithong & Benjakul, Citation2014; Sachindra & Bhaskar, Citation2008). By using DPPH, only hydrophobic compounds can be determined as opposed to the method using the ABTS radical, which can determine both the hydrophobic and hydrophilic antioxidants in the same sample (Sun & Tanumihardjo, Citation2007). Transition metal ions (especially copper and iron) are actively involved in oxidative processes leading to the formation of hydroxyl and peroxyl radicals (Fenton reaction and Haber–Weiss reaction). Chelating agents have the effect of masking for metal ions and thus, they contribute to the fight against oxidative stress (Lee, Hwang, Heo, Lee, & Park, Citation2005). The results of the chelating ability of peptides from dry-cured loins for Cu2+ and Fe2+ are presented in . All samples showed the ability to chelate copper ions from 52.14% for C to 65.30% for BAUER. All samples with the probiotic strain had higher (p < 0.05) ability to chelate copper ions than C, and this was also observed by other authors (Liu, Kong, Xiong, & Xia, Citation2010). The lowest level of ability to chelate Fe2+ was determined for samples C and BAUER. Higher values (p < 0.05) were obtained for LOCK and BB12, and the differences between them proved to be statistically insignificant (p > 0.05). Generally, the dry-cured loins had better ability to chelate copper ions than iron ions. These results correspond with other studies (Chen, Muramoto, Yamauchi, Fujimoto, & Nokihara, Citation1998; Kong & Xiong, Citation2006; Lee et al., Citation2005; Liu et al., Citation2010).
Table 3. Antioxidant capacity of dry-cured loins with probiotic strains of LAB (mean ± standard deviation).
Tabla 3. Capacidad antioxidante de lomo curado en seco con cepas probióticas de LAB (promedio±desviación estándar).
The RP was determined by measuring the ability to reduce ferric iron to ferrous iron. It is expressed as absorbance values measured at 700 nm and ranging from 1.38 to 2.88, which corresponded with the results of another study on dry-cured meat products (Mora et al., Citation2014). Higher absorbance values indicated higher RP. The dry-cured meat peptide isolates from C, LOCK and BB12 showed stronger RP (p < 0.05) than BAUER.
All tested batches showed antioxidant properties as a result of the presence of compounds capable of reacting with ROS and therefore of creating more stable compounds. In the available literature, several assessment tests were made in order to clarify the specific properties of the proteins, that is, in vitro research or a study structure versus activity relationships of peptides (QSAR) (Zou, He, Li, Tang, & Xia, Citation2016). The properties of proteins and peptides are highly dependent characteristics of the compound’s chemical structure. Thus, the differences in antioxidant activity among the tested batches may be associated with the influence of the LAB strains on the peptides’ composition and the amino acid position in them. The high capacity to react with the DPPH is associated with the presence of hydrophobic amino acids. Peptides containing the amino acid residues Val, Leu, Ile, Ala, Phe, Lys or Cys at the N-terminal have been reported to act as a radical-scavenging factor (Ren et al., Citation2008; Suetsuna, Ukeda, & Ochi, Citation2000). In addition, residues of Tyr, especially when at the C-terminal, are very important for the activity of the radical scavenging of peptides because of their special design features (presence of indole and imidazole groups) (Chen et al., Citation1998; Escudero et al., Citation2013; Saiga, Tanabe, & Nishimura, Citation2003). Acidic and/or basic amino acids play an important role in chelating Fe2+ and Cu2+ by peptides (Saiga et al., Citation2003). Cys, His, Asp and Glu are the most frequently reported amino acids contributing to metal chelating activity. On the other hand, some carboxyl groups (Glu, Asp) and amino groups (Lys, Arg) in the side chains can also act as chelating agents (Guo et al., Citation2014; Liu et al., Citation2010; Suetsuna et al., Citation2000). In addition, the position of the amino acid plays an important role in the chelating activity. The imidazole ring may correspond with the metal ion, showing higher affinity when it is at the N-terminus and not at the C-terminus (Chen et al., Citation1998). Therefore, the observed differences in the chelating activity may be due to the position of the amino acids making up the peptides. Antioxidant activity can also be determined by the species of microorganisms present in the fermented food, as demonstrated in the study. The effect of LAB antioxidant activity can be explained by the release of compounds with antioxidant activity in fermentation. These microorganisms activate the enzymatic (e.g. proteolysis, lipolysis) and non-enzymatic (e.g. exopolysaccharides, glutathione) mechanism to protect against oxidative damage (Kim et al., Citation2005; Lee et al., Citation2005; Spyropoulos, Misiakos, Fotiadis, & Stoidis, Citation2011). Numerous studies confirm that probiotic bacteria support the production of antioxidant biomolecules. Wang, Wu and Shyu (Citation2014) report that the Lactobacillus strains had significant antioxidant activity by reaction with DPPH and Fe2+. Lactobacillus casei strains exhibited the ability to chelate Fe2+ and Cu2+ in a range of 1.1–10.6 ppm and from 1.35 to 21.8 ppm, respectively (Lee et al., Citation2005). Lactobacillus bulgaricus LB207 showed high antioxidant activity in the inhibition of lipid peroxidation (Kim et al., Citation2005).
4. HCA results
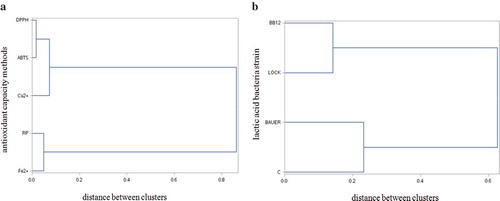
Numerous antioxidant capacity assays are commonly applied in relation to meat products. However, it is not clear which assay is all-purpose. Measuring the antioxidant activity of peptides is currently popular due to their biologically active properties. However, they give different results because the investigations use different model compounds with many side reactions and other disturbing elements (Rácz, Papp, Balogh, Fodor, & Héberger, Citation2015) The aim of the study was to compare the antioxidant capacity assays by using statistical methods (HCA) and to select the most representative ones. The hierarchical clustering procedure results can be displayed graphically by using a tree diagram, also known as a dendrogram, in which all of the steps in the hierarchical procedure are shown. Grouping and detection of similarities and differences among the methods is also revealed. Biglari, AlKarkhi and Easa (Citation2009) and Patras et al. (Citation2011) studied the effect of long-term cold storage on antioxidant compounds in dates using cluster analysis and found it to be quite a useful technique for classification. It should be noted that cluster analysis can report similarities but is not able to rank the different methods, only to assume the connections (Rácz et al., Citation2015).
The statistical methods presented in this study are relevant for a comparison of other antioxidant capacity techniques. The comparison takes into account the impact of induced fermentation to the antioxidant properties by using probiotic or potential probiotic LAB with spontaneous fermentation in dry-cured loin. ) presents the different clusters and connections between the antioxidant capacity methods in the analysed samples. It compares and clusters five antioxidant capacity methods. Two clusters were observed in this case. Two groups were clearly separated, one contained the ABTS, DPPH and Cu2+ techniques and the other contained the RP and Fe2+ methods. ) presents the grouping pattern for the LAB strain data set. The LOCK and BB12 strains clearly formed a distinct group and the antioxidant tests for them were more closely connected to one another. The second cluster formed C and BAUER. This means that the results of these techniques are more similar (related more closely) to one another in the two separate groups. This indicates that the first cluster (LOCK and BB12) and second cluster (C and BAUER) show different behaviour according to the type of antioxidant test.
Figure 3. Dendrogram resulting from the Ward’s method of hierarchical cluster analysis of antioxidant capacity methods (a) and lactic acid bacteria strain (b).
C: Sample without probiotic starter culture; LOCK: sample with probiotic starter culture Lactobacillus rhamnosus LOCK900; BAUER: sample with probiotic starter culture Lactobacillus acidophilus Bauer Ł0938; BB12: sample with probiotic starter culture Bifidobacterium animalis ssp. lactis BB-12.
Figura 3. Resultado del dendrograma del método de análisis de agrupaciones jerárquicas de Ward acerca de los métodos de capacidad antioxidante (a) y cepa de bacteria ácido-láctica (b).
C: muestra sin cultivo iniciador probiótico; LOCK: muestra con cultivo iniciador probiótico Lactobacillus rhamnosus LOCK900; BAUER: muestra con cultivo iniciador probiótico Lactobacillus acidophilus Bauer Ł0938; BB12: muestra con cultivo iniciador probiótico Bifidobacterium animalis ssp. lactis BB-12.
5. Conclusions
The effect of free radical neutralisation in dry-cured loin can be obtained by fermentation of LAB. These data show that dry-cured meat products exhibit antioxidant activity that can be attributed to the ability of a proton donor, as evidenced by the results of DPPH radical scavenging and ABTS. Furthermore, the products showed a chelating effect and RP and could protect against oxidative damage. Dry-cured meat products may also be regarded as electron donors which can react with free radicals and convert them into more stable products, thus ending the terminal radical chain reactions. The lack of relationship between relative quantity of peptides and antioxidant activity measures suggests that the differences in antioxidant activity among the tested batches may result from the influence of the LAB strains on the composition of the peptides and position of amino acid in them, which needs further investigation.
Disclosure statement
The authors declare no conflict of interest.
References
- Arihara, K. (2006). Strategies for designing novel functional meat products. Meat Science, 74, 219–229. doi:10.1016/j.meatsci.2006.04.028
- Biglari, F., AlKarkhi, A.F., & Easa, A.M. (2009). Cluster analysis of antioxidant compounds in dates (Phoenix dactylifera): Effect of long-term cold storage. Food Chemistry, 112, 998–1001. doi:10.1016/j.foodchem.2008.06.063
- Chakrabarti, S., Jahandideh, F., & Wu, J. (2014). Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Research International, Article ID 608979, 11 pages. doi:10.1155/2014/608979
- Chen, H.M., Muramoto, K., Yamauchi, F., Fujimoto, K., & Nokihara, K. (1998). Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. Journal of Agricultural and Food Chemistry, 46, 49–53. doi:10.1021/jf970649w
- Dave, D., & Ghaly, A.E. (2011). Meat spoilage mechanisms and preservation techniques: A critical review. American Journal of Agricultural and Biological Sciences, 6, 486–510. doi:10.3844/ajabssp.2011.486.510
- Decker, E.A., & Welch, B. (1990). Role of ferritin as a lipid oxidation catalyst in muscle food. Journal of Agricultural and Food Chemistry, 38, 674–677. doi:10.1021/jf00093a019
- Escudero, E., Mora, L., Fraser, P.D., Aristoy, M.C., & Toldrá, F. (2013). Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chemistry, 138, 1282–1288. doi:10.1016/j.foodchem.2012.10.133
- Faithong, N., & Benjakul, S. (2014). Changes in antioxidant activities and physicochemical properties of Kapi, a fermented shrimp paste, during fermentation. Journal of Food Science and Technology, 51, 2463–2471. doi:10.1007/s13197-012-0762-4
- Flores, J., Marcus, J.R., Nieto, P., Navarro, J.L., & Lorenzo, P. (1997). Effect of processing conditions on proteolysis and taste of dry-cured sausages. Zeitschrift Für Lebensmitteluntersuchung Und -Forschung A., 204, 168–172. doi:10.1007/s002170050056
- Guo, L., Harnedy, P.A., Li, B., Hou, H., Zhang, Z., Zhao, X., & FitzGerald, R.J. (2014). Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends in Food Science & Technology, 37, 92–105. doi:10.1016/j.tifs.2014.02.007
- HPA. (2009). Guidelines for assessing the microbiological safety of ready-to-eat foods placed on the market. London: Health Protection Agency.
- ISO 15214:1998. Microbiology of food and animal feeding stuffs - horizontal method for the enumeration of mesophilic lactic acid bacteria - colony-count technique at 30 °C.
- ISO 4833-2:2013. Microbiology of the food chain - horizontal method for the enumeration of microorganisms - Part 2: Colony count at 30 °C by the surface plating technique.
- Jaworska, D., Neffe, K., Kołożyn‐Krajewska, D., & Dolatowski, Z. (2011). Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3′/IV in dry fermented pork loins. International Journal of Food Science & Technology, 46, 2491–2497. doi:10.1111/j.1365-2621.2011.02772.x
- Kim, H.S., Chae, H.S., Jeong, S.G., Ham, J.S., Im, S.K., Ahn, C.N., & Lee, J.M. (2005). Antioxidant activity of some yogurt starter cultures. Asian-Australasian Journal of Animal Sciences, 18, 255–258. doi:10.5713/ajas.2005.255
- Kong, B., & Xiong, Y.L. (2006). Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. Journal of Agricultural and Food Chemistry, 54, 6059–6068. doi:10.1021/jf060632q
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. doi:10.1038/227680a0
- Lee, J., Hwang, K.T., Heo, M.S., Lee, J.H., & Park, K.Y. (2005). Resistance of Lactobacillus plantarum KCTC 3099 from Kimchi to oxidative stress. Journal of Medicinal Food, 8, 299–304. doi:10.1089/jmf.2005.8.299
- Libera, J., Karwowska, M., Stasiak, D.M., & Dolatowski, Z.J. (2015). Microbiological and physicochemical properties of dry‐cured neck inoculated with probiotic of Bifidobacterium animali sssp. lactis BB‐12. International Journal of Food Science & Technology, 50, 1560–1566. doi:10.1111/ijfs.12806
- Liu, Q., Kong, B., Xiong, Y.L., & Xia, X. (2010). Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chemistry, 118, 403–410. doi:10.1016/j.foodchem.2009.05.013
- Maltini, E., Torreggiani, D., Venir, E., & Bertolo, G. (2003). Water activity and the preservation of plant foods. Food Chemistry, 82, 79–86. doi:10.1016/S0308-8146(02)00581-2
- Mora, L., Escudero, E., Fraser, P.D., Aristoy, M.C., & Toldrá, F. (2014). Proteomic identification of antioxidant peptides from 400 to 2500Da generated in Spanish dry-cured ham contained in a size-exclusion chromatography fraction. Food Research International, 56, 68–76. doi:10.1016/j.foodres.2013.12.001
- Mora, L., Sentandreu, M.A., & Toldrá, F. (2010). Identification of small troponin T peptides generated in dry-cured ham. Food Chemistry, 123, 691–697. doi:10.1016/j.foodchem.2010.05.035
- Neffe-Skocińska, K., Jaworska, D., Kolożyn-Krajewska, D., Dolatowski, Z., & Jachacz-Jówko, L. (2015). The effect of LAB as probiotic starter culture and green tea extract addition on dry fermented pork loins quality. Biomed Research International, Article ID 452757, 9 pages. doi:10.1155/2015/452757
- Okoń, A., & Dolatowski, Z.J. (2014). Effect of probiotic bacteria on free amino acid profile and sensory traits of raw-ripening pork sirloin during storage. Żywność Nauka Technologia Jakość, 3, 92–107. doi:10.15193/zntj/2014/94/092-107
- Patras, A., Brunton, N.P., Downey, G., Rawson, A., Warriner, K., & Gernigon, G. (2011). Application of principal component and hierarchical cluster analysis to classify fruits and vegetables commonly consumed in Ireland based on in vitro antioxidant activity. Journal of Food Composition and Analysis, 24, 250–256. doi:10.1016/j.jfca.2010.09.012
- Rácz, A., Papp, N., Balogh, E., Fodor, M., & Héberger, K. (2015). Comparison of antioxidant capacity assays with chemometric methods. Analytical Methods, 7, 4216–4224. doi:10.1039/c5ay00330j
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237. doi:10.1016/S0891-5849(98)00315-3
- Ren, J., Zhao, M., Shi, J., Wang, J., Jiang, Y., Cui, C., & Xue, S.J. (2008). Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry, 108, 727–736. doi:10.1016/j.foodchem.2007.11.010
- Sachindra, N.M., & Bhaskar, N. (2008). In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresource Technology, 99, 9013–9016. doi:10.1016/j.biortech.2008.04.036
- Saiga, A.I., Tanabe, S., & Nishimura, T. (2003). Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry, 51(12), 3661–3667. doi:10.1021/jf021156g
- Sarmadi, B., & Ismail, A. (2010). Antioxidative peptides from food proteins: A review. Peptides, 31, 1949–1956. doi:10.1016/j.peptides.2010.06.020
- Skwarek, M., Dolatowski, Z.J., & Kołożyn-Krajewska, D. (2014). Effect of green tea infusion and pulverized pepper on colour of raw ripening probiotic hams. Żywność Nauka Technologia Jakość, 3, 108–122. doi:10.15193/zntj/2014/94/108-122
- Spyropoulos, B.G., Misiakos, E.P., Fotiadis, C., & Stoidis, C.N. (2011). Antioxidant properties of probiotics and their protective effects in the pathogenesis of radiation-induced enteritis and colitis. Digestive Diseases and Sciences, 56, 285–294. doi:10.1007/s10620-010-1307-1
- Stadtman, E.R., & Levine, R.L. (2003). Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids, 25, 207–218. doi:10.1007/s00726-003-0011-2
- Suetsuna, K., Ukeda, H., & Ochi, H. (2000). Isolation and characterization of free radical scavenging activities peptides derived from casein. The Journal of Nutritional Biochemistry, 11, 128–131. doi:10.1016/S0955-2863(99)00083-2
- Sun, T., & Tanumihardjo, S.A. (2007). An integrated approach to evaluate food antioxidant capacity. Journal of Food Science, 72, 159–165. doi:10.1111/j.1750-3841.2007.00552.x
- Torres-Fuentes, C., Alaiz, M., & Vioque, J. (2011). Affinity purification and characterisation of chelating peptides from chickpea protein hydrolysates. Food Chemistry, 129, 485–490. doi:10.1016/j.foodchem.2011.04.103
- Trząskowska, M., Kołożyn-Krajewska, D., Wójciak, K., & Dolatowski, Z. (2014). Microbiological quality of raw-fermented sausages with Lactobacillus casei LOCK 0900 probiotic strain. Food Control, 35, 184–191. doi:10.1016/j.foodcont.2013.07.002
- Wang, C.Y., Wu, S.J., & Shyu, Y.T. (2014). Antioxidant properties of certain cereals as affected by food-grade bacteria fermentation. Journal of Bioscience and Bioengineering, 117, 449–456. doi:10.1016/j.jbiosc.2013.10.002
- Wu, H.C., Chen, H.M., & Shiau, C.Y. (2003). Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Research International, 36, 949–957. doi:10.1016/S0963-9969(03)00104-2
- Zou, T.B., He, T.P., Li, H.B., Tang, H.W., & Xia, E.Q. (2016). The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules, 21(1), 72. doi:10.3390/molecules21010072
