ABSTRACT
The effects of thermal treatment on the ultrastructure and quality of Chilgoza pine nuts stored at near-freezing temperature were investigated. The moisture content of pine nuts was adjusted to 13.3%, compared to the initial 17.3% moisture content. Thermal treatment improved the storage stability. The hydrolysis of lipids was delayed in low-moisture pine nuts, resulting in a significant inhibition of free fatty acid accumulation in pine nuts. Low-moisture pine nuts also showed lower peroxide and thiobarbituric acid (TBA) values compared with the control. Ultrastructure characterization revealed that thermal treatment maintained the cell integrity, deferred the degradation of the plasmalemma and protected the internal lipid droplet. Thermal treatment better retained the antioxidant components, including total phenolics and vitamin E, and reduced the activities of lipase and lipoxygenase in pine nuts. These results indicated that thermal treatment could retard the senescence and deterioration of quality in pine nuts stored at a near-freezing temperature.
RESUMEN
Se investigaron los efectos del tratamiento térmico en la ultrastructura y la calidad del piñón de pino Chilgoza almacenado a una temperatura cercana a congelación. El contenido de humedad de los piñones de este pino fue de 13,3%, en comparación con el contenido de humedad inicial de 17,3%. El tratamiento térmico mejoró la estabilidad de almacenamiento. La hidrólisis de los lípidos se retardó en los piñones de pino de baja humedad, resultando en una inhibición significativa de la acumulación de ácidos grasos libres en los piñones. Los piñones de pino de baja humedad también mostraron unos índices bajos de peróxido y TBA en comparación con la muestra control. La caracterización de la ultrastructura reveló que el tratamiento térmico mantuvo la integridad de las células, atrasó la degradación de la plasmalema y protegió la gota lipídica interna. El tratamiento térmico retuvo de forma más favorable los componentes antioxidantes, como el total fenólico y la vitamina E, además redujo la actividad de la lipasa y la lipoxigenasa en los piñones. Estos resultados indicaron que el tratamiento térmico podría retardar la senescencia y la deterioración de la calidad de los piñones almacenados a una temperatura cercana a la congelación.
1. Introduction
The Chilgoza pine nut (Pinus gerardiana) is the edible seed of the Chilgoza pine, which belongs to the Pinaceae family, mainly distributed in the natural regions of Afghanistan, Pakistan, India and China (Hoon, Choo, Watawana, Jayawardena, & Waisundara, Citation2015). It is an important international trade commodity worldwide and is used as a food ingredient in desserts, sauces and salads. The Chilgoza pine nut is rich in unsaturated fatty acids (over 90% of total fatty acids), especially polyunsaturated fatty acids, which offer health benefits, particularly in relation to decreasing undesirable low-density cholesterol (Cai, Xiao, Liu, & Ying, Citation2013; Lee, Lee, Lee, Kim, & Rhee, Citation2004; Matthaus & Ozcan, Citation2013; Ozcan, Dagdelen, Kara, & Kanbur, Citation2013; Venkatachalam & Sathe, Citation2006). As pine nuts contain high amounts of fats and unsaturated fatty acids, they are prone to hydrolytic and oxidative rancidity, which leads to quality loss during storage (Kaijser, Dutta, & Savage, Citation2000; Xie, Miles, & Calder, Citation2016). The quality deterioration of pine nuts in storage is also caused by the metabolism of nuts and microorganisms, which depends on storage conditions including temperature, moisture content and gas composition.
Postharvest thermal treatment has proven beneficial to control respiration intensity (Dillahunty, Siebenmorgen, Buescher, Smith, & Mauromoustakos, Citation2000), inhibit microbial growth (Genkawaa, Uchino, Inoue, Tanaka, & Hamanaka, Citation2008; Rajarammanna, Jayas, & White, Citation2010) and retard oxidative rancidity (Bao, Zhu, Luo, & Shen, Citation2015). Near-freezing temperature storage is also regarded as an effective system that offers the possibility of storing grain for the long term without significant deterioration in quality (Fukai, Matsuzawa, & Ishitani, Citation2003; Rehman, Citation2006). In a previous study, pine nuts were stored at −3 and −1°C to compare the differences in quality changes, and we found that −3°C had a better effect on pine nut storage than −1°C (Cai, Liu, & Ying, Citation2013). However, no information is available on the ultrastructure and lipid quality in low-moisture pine nuts stored at near-freezing temperature. The objective of this study was to examine the changes in lipid content, free fatty acids (FFAs), peroxide value (PV), thiobarbituric acid (TBA) value, ultrastructure, total phenolics, vitamin E and activities of lipid metabolism-related enzymes, including lipase and lipoxygenase (LOX), of low-moisture pine nuts during near-freezing temperature storage. The outcomes of this study should offer a better understanding of how thermal treatment delays rancidity and preserves lipid quality in pine nuts during near-freezing temperature storage.
2. Materials and methods
2.1. Materials and chemicals
Samples of freshly harvested Chilgoza pine nuts (P. gerardiana) were obtained from northern Pakistan (latitude 34–35°N and longitude 73–75°E, altitude 2000 m or higher) with an initial moisture content of approximately 17.3% (wet basis). Folin–Ciocalteu reagent, linoleic acid, 1,1,3,3-tetrathoxypropane and α-tocopherol were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All chemicals used in the experiments were of analytical grade.
2.2. Sample treatment
Low-moisture pine nuts were obtained by drying at 35°C in a hot air oven for 7 h to a moisture content of 13.3%. Since many researchers have reported the superiority of low moisture content of cereals and oil products in storage, a moisture range of 13–17% was selected in the previous study. The initial moisture content of pine nuts is 17.3%, and the pine nuts were conditioned in a hot air oven at 35°C and 20% relative humidity for 3 and 7 h to bring the moisture contents to 15.1% and 13.3%, respectively. Pine nuts with 17.3%, 15.1%, and 13.3% moisture contents were stored at −3 and −1°C for 12 months. The moisture content of 13.3% was found to be most beneficial in reducing the respiration intensity and inhibiting the biological activities, and 13.3% was accordingly chosen in this study. The freezing temperature of the low-moisture pine nuts was determined by differential scanning calorimetry to be −4.9°C, while the freezing temperature of the normal moisture pine nut was −4.0°C. A storage temperature of −3°C was chosen with the original 17.3% moisture nuts as a control. For storage, pine nuts (200 g) were sealed in polyethylene pouches (90 µm thickness, 18 cm × 20 cm) with oxygen permeability of 5 cm3/m2/24 h at 20°C and 70% RH. The initial O2 and CO2 contents in the pouches with air were 21% and 0.03%, respectively. Both treated and control nuts were stored at −3°C in the dark for 6 months. Twenty-one bags of pine nuts were included in each treatment, and every month, three replicates from each treatment group were randomly selected and analyzed.
2.3. Gas analysis of headspace
The concentration of O2 and CO2 in the pouches was determined using a SCY-2A O2 and CO2 Analyzer (Xinrui Instrument Co., China). Gas samples were taken from the pouches with a 20-mL syringe.
2.4. Lipid content
Lipid content was determined by the method of AOCS (Citation1998). Pine nut oil was extracted from a 5-g nut sample by a Soxhlet extraction apparatus using ether as a solvent for 24 h. Lipid content was expressed on a dry basis.
2.5. Determination of FFAs, PV and thiobarbituric acid reactive substances value
Pine nut oil was extracted from a 5-g nut sample by a Soxhlet extraction apparatus using ether as a solvent for 8 h. PV was then determined following the AOCS method (Citation1998). FFAs were determined by the method of Zhao, Xiong, Qiu and Xu (Citation2007) with some modification. The oil samples (2 g) were dissolved in a 50-mL mixture of ether:ethanol (2:1, v/v) with heating. After cooling to room temperature, the mixture was titrated with 0.01 mol/L potassium hydroxide using phenolphthalein solution as the indicator. The milligram amount of KOH exhausted per gram of oil sample was used to determine the FFA.
Thiobarbituric acid reactive substances (TBARS) values were determined according to the official method of AOCS (Citation1998). An oil sample (0.2 g) was weighed into a 25-mL flask and brought up to the mark with 1-butanol. Five milliliters of this solution was mixed with 5 mL of 2 mg/mL 2-TBA reagent (in 1-butanol). The solution was thoroughly mixed and heated in a water bath at 95°C for 2 h. Then, the samples were removed from the water bath and cooled in an ice bath. The absorbance was detected at 532 nm. TBARS values were calculated from a standard curve of malondialdehyde (MDA) prepared using 1,1,3,3-tetrathoxypropane and expressed as mg of MDA per kg oil.
2.6. Electron microscopy
Specimens were obtained from pine nut kernels for transmission electron microscopy (TEM). First, the samples were fixed with 25 mL/L glutaraldehyde in phosphate buffer (pH 7.0) for 6 h, washed three times in phosphate buffer (pH 7.0) for 15 min and then post-fixed with 10 mL/L OsO4 in phosphate buffer (pH 7.0) for 1 h and again washed three times in phosphate buffer (pH 7.0). Each sample was dehydrated using a gradient of ethanol (500, 700, 800, 900, 950 and 1000 mL/L) for 20 min at each step and then mixed with absolute acetone for 20 min. The samples were added to the absolute acetone and Spurr resin mixture (1:1, v/v) for 1 h and then added to an absolute acetone and Spurr resin mixture (1:3, v/v) for 3 h and finally to 100% Spurr resin overnight. The samples were observed by a TEM Model JEM-1230 (JEOL, Tokyo, Japan).
2.7. Determination of lipase and LOX activity
Lipase (E.C. 3.1.1.3) activity was determined by alkali titration, as described by Molteberg, Vogt, Nilsson and Frolich (Citation1995), with some modifications. Olive oil (AP) was used as the substrate and 0.01 mol/L KOH solution for titration. The enzyme extraction of pine nut samples was performed using cold acetone at 4°C. To 1 g of crude enzyme extract, 5 g of olive oil previously solubilized into 2.5 mL of hexane was added. The mixture was incubated at 30°C for 30 min. At the end of the incubation, 25 mL of acetone/ethanol (1:1, v/v) was added to stop the reaction. The FFAs were titrated with 0.01 mol/L KOH using phenolphthalein as the indicator. The control consisted essentially of the same components mentioned above, except for the heat-inactivated enzyme extract. Lipase activity was expressed as mmol FFA/g enzyme/min after 30 min incubation.
LOX (E.C. 1.13.1.13) activity was assayed according to the method of Surrey (Citation1964) based on the absorption (234 nm) of conjugated dienes. The substrate was prepared by using Tween 20 to solubilize linoleic acid. The pine nut samples (2 g) were homogenized with 10 mL of 0.05 mol/L phosphate buffer (pH 7.0), followed by centrifugation at 12,000 rpm for 15 min at 4°C. The supernatant was obtained as the crude enzyme solution. The assays were performed at 30°C. The reaction mixture contained 2 mL 0.1 mol/L phosphate buffer (pH 7.0), 0.8 mL substrate and 0.2 mL enzyme extract in a final assay volume of 3 mL. The increase in absorbance at 234 nm versus the blank was recorded. The blank contained 0.2 mL distilled water instead of enzyme extract. One unit of LOX activity was defined as a change in absorbance of 0.001/min in a 3-mL volume.
2.8. Determination of antioxidant components
The Vitamin E (α-tocopherol) content of pine nut was determined by the method of Karabulut, Topcu, Yorulmaz, Tekin and Ozay (Citation2005). The content of α-tocopherol was determined using a normal-phase HPLC (Waters Co. Ltd., Milford, MA, USA) with a Phenomenex Luna Silica column (5 µm, 250 × 4.6 mm). Isocratic elution using n-hexane/isopropanol (99:1, v/v) was maintained at a column temperature of 30°C with a flow rate of 1 mL/min. The injection volume of the samples was 20 µL, and the eluate was monitored at 292 nm. An external standard method with the authentic compound was used to quantify the identified compound.
Total phenolics content was determined by the Folin–Ciocalteu assay described by Singleton, Orthofer and Lamuela-Raventos (Citation1999) with some modifications. Briefly, the defatted samples were extracted twice with 95% ethanol, and the extracts were combined. The extracts (0.5 mL) were mixed with Folin–Ciocalteu reagent (0.5 mL) and distilled water (1 mL). The mixture was shaken and let stand for 4 min prior to the addition of saturated sodium carbonate solution (0.5 mL). The total volume was adjusted to 10 mL using distilled water, and the solution was thoroughly mixed. The mixture was kept at 37°C in a water bath for 30 min and centrifuged at 5000 rpm for 10 min. The absorbance was read at 725 nm on a UV/vis spectrophotometer using 95% ethanol as a blank. Total phenolics content was determined using a standard curve prepared for gallic acid and expressed as mg gallic acid equivalents/100 g nuts (dry basis).
2.9. Statistical analysis
All the experiments were repeated three times. The differences among different groups were determined using ANOVA and Duncan’s multiple range test (p < 0.05), using SAS software (version 9.2, Cary, NC, USA).
3. Results and discussion
3.1. Gas analysis of headspace
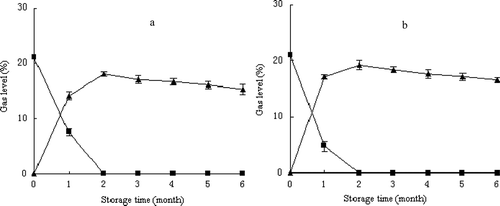
The gas analysis of the headspace of pine nut pouches showed a decrease in O2 and an increase in CO2 levels in both treated and controlled nuts during storage ()). After the first month of storage, the concentration of O2 declined from 21% to 7.5% in treated nuts and to 4.8% in the control. At the same time, the concentration of CO2 increased from 0.03% to 14.1% in the low-moisture nuts and 17.1% in the control nuts. O2 declined to near 0% in the pouches after 2 months of storage. The changes of the gas composition in the headspace of the pouches could be attributed mainly to the respiratory intensity, indicating that low moisture effectively reduced the metabolism of the nuts.
Figure 1. Changes in O2 (■) and CO2 (▲) content of pine nuts treated with low-moisture (a) versus the control (b) stored at −3°C. Vertical bars represent standard deviations from triplicate measurements.
Figura 1. Cambios en el contenido de O2 (■) y CO2 (▲) de los piñones de pino tratados con baja humedad (A) frente a la muestra control (B), almacenados a −3°C. Las barras verticales representan las desviaciones estándar de mediciones triplicadas.
3.2. Lipid content
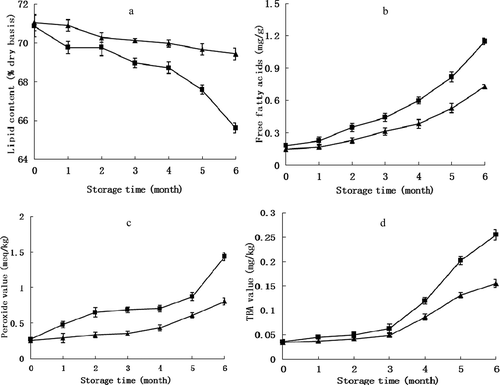
Lipids were the main constituent of the edible nut kernel. Nuts contain essential fatty acids that are beneficial for the human diet, and the oil obtained from nuts has a more equilibrated proportion of fatty acids than animal fat (Venkatachalam & Sathe, Citation2006). A significant reduction in the lipid content of nuts could be observed as lipid quality degraded; so, the lipid content of pine nuts is the most obvious parameter reflecting nut quality attributes. In this study, the lipids in untreated pine nuts gradually broke down during storage, resulting in a significant decrease (p < 0.05) in lipid content ()). Treated nuts maintained a higher level of lipid content during 6 months of storage. Similar results have also been found by Kaijser et al. (Citation2000) in macadamia nuts.
Figure 2. Changes in lipid content (a), free fatty acids (b), peroxide value (c) and TBA value (d) of pine nuts treated with low-moisture (▲) versus the control (■) stored at −3°C. Vertical bars represent standard deviations from triplicate measurements.
Figura 2. Cambios en el contenido lipídico (A), ácidos grasos libres (B), índice de peróxido (C) y valor TBA (D) de los piñones de pino tratados con baja humedad (▲) frente a la muestra control (■), almacenados a −3°C. Las barras verticales representan las desviaciones estándar de mediciones triplicadas.
3.3. FFAs, PV and TBA values
There are at least two processes that affect the degradation of lipids in nuts during storage. One is the hydrolysis of lipids to produce FFAs; the other involves the oxidation of lipids (involving FFAs) to produce hydroperoxides as primary oxidation products and MDA as a secondary oxidation product (Atungulu, Miura, Atungulu, Satou, & Suzuki, Citation2007). Currently, there are many methods to determine lipid quality. Clearly, the quality of lipids cannot be evaluated using only a single index. Therefore, three assay methods were used in this study to investigate the changes in lipid quality of pine nuts during storage. FFA was determined as an index of hydrolysis of lipid. The lipid oxidation of pine nuts was evaluated for primary oxidation products and TBARS values for secondary oxidation products.
The lipid quality of pine nuts as evaluated by FFA, PV and TBA values is summarized in ), respectively. In this study, the treated and control nuts all showed an increasing trend in FFA. Compared to control nuts, the increase of low-moisture nuts in FFA was minimal throughout the storage period, and the difference was significant (p < 0.05). This trend is in agreement with observations by Genkawaa et al. (Citation2008), who found that rice with 11% moisture showed the lowest degree of lipid hydrolysis. However, pine nuts stored at −3°C all maintained good quality throughout the whole storage period, with FFA lower than 1.4 mg/g, which is considered a critical threshold value in the nut processing industry.
The changes in the PV of pine nuts during storage are shown in Figure 2c. Similar to FFA, nuts with different moisture content stored at −3°C all showed increasing trends in PV. The initial PV of pine nuts was very low (0.25 and 0.27 meq/kg oil, respectively), indicative of good initial lipid quality (Evranuz, Citation1993). In the first 2 months and the last 2 months, the PV of nuts with 17.3% moisture content increased rapidly. Nuts with 13.3% moisture content in PV remained relatively steady throughout storage, and the difference was significant (p < 0.05). By the end of storage, nuts with low moisture content had lower PV (0.82 meq/kg oil), which was only approximately 57% of the control nuts.
In this study, the TBA values of pine nuts remained steady under both moisture conditions during the first 3 months, and then both increased sharply from the beginning of the 4th month ()). At the end of storage, the TBA value of treated nuts was significantly lower (p < 0.05) than in the control. All the above results clearly showed that low-moisture storage could retard lipid oxidation and alleviate the degree of hydrolysis of lipids to preserve the quality of pine nuts.
3.4. Ultrastructure characteristics
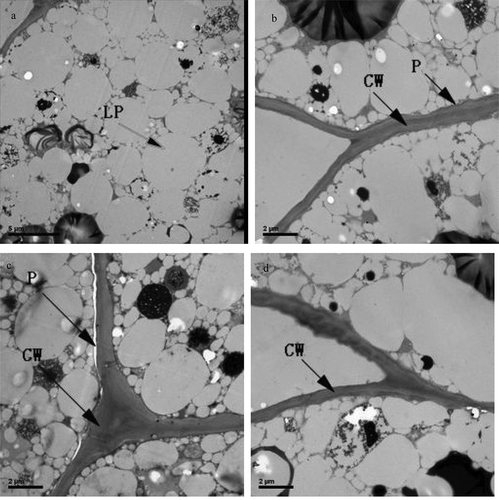
TEM analysis of pine nut kernels revealed that a cross-sectional view of a single cell may contain over 300 oil droplets, which in total comprise more than 70% of the section area. Oil droplets were spherical in shape, ranging in size from 0.3 to 3.0 µm ()). During the initial period of storage, the integrity of the cell wall and plasmalemma was recorded ()). After 6 months of storage, the plasmalemma of the cells of the control pine nut kernel was broken ()), which indicated the beginning of degradation of the cell wall (Katavic, Agrawal, Hajduch, Harris, & Thelen, Citation2006). The loss of membrane integrity could be caused by tissue injury, lipid oxidation and seed senescence (Murphy, Citation2001). On the other hand, low-moisture nuts showed intact cell wall and plasmalemma structures ()). These results indicate that the deterioration of lipid quality would be aggravated when accompanied by the degradation of the plasmalemma in the control samples, and thermal treatment can offer better protection for lipid quality through maintenance of the subcellular structure.
Figure 3. Transmission electron micrographs of pine nuts parenchyma cell wall (CW), plasmalemma (P) and lipid droplet (LP) stored at −3°C for 6 months; (a, b) pine nut before storage; (c) control treated pine nut after 6 months of storage; (d) low-moisture treated pine nut after 6 months of storage. Micrographs for B, C and D were taken at ×10,000 magnification (bar = 2 µm). Micrographs for A was taken at ×6000 magnification (bar = 5 µm).
Figura 3. Micrografía electrónica de transmisión de la pared celular parenquimática de piñones de pino (CW), plasmalema (P) y gota lipídica (LP), almacenados a −3°C durante 6 meses. (A, B) piñones antes del almacenamiento; (C) muestra control de piñones tratada después de 6 meses de almacenamiento; (D) piñones de pino de baja humedad tratados después de 6 meses de almacenamiento. Las micrografías de B, C y D se realizaron a × 10 000 de magnificación (bar = 2 µm). La micrografía de A se realizó a × 6 000 de magnificación (bar = 5 µm).
3.5. Lipase and LOX activity
The occurrence of such enzymes as lipase and LOX in the pine nut contributes to the worsening of lipid quality, organoleptic features and losses of nutrients (Ramezanzadeh et al., Citation1999). In this study, statistically significant changes (p < 0.05) were observed in the activity of lipase. The thermal treatment significantly reduced the activity of lipase during storage at low temperature ()), which might be attributable to the weakened water–oil interface after low-moisture treatment. In some tomato cultivars, Lisiewska and Kmiecik (Citation2000) observed a significant decrease in lipase activity during a period of 6 months of frozen storage. The activity of lipase in pine nut, especially in nuts with 17.3% moisture, would be to a certain extent responsible for the deterioration of lipid quality during storage. This finding is supported by the results reported by Molteberg et al. (Citation1995), who found that appropriate storage conditions for the inactivation of lipolytic enzymes are essential in achieving stable oat products.
Figure 4. Changes in lipase activity (a) and LOX activity (b) of pine nuts treated with low moisture (▲) versus the control (■) stored at −3°C. Vertical bars represent standard deviations from triplicate measurements.
Figura 4. Cambios en la actividad de la lipasa (A) y la actividad de LOX (B) de los piñones de pino tratados con baja humedad (▲) frente a la muestra control (■), almacenados a −3°C. Las barras verticales representan las desviaciones estándar de mediciones triplicadas.
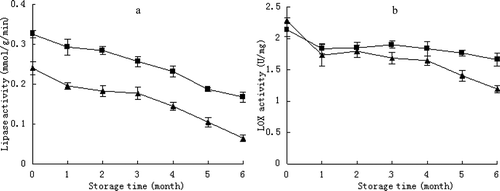
LOX is an iron-containing dioxygenase that specifically catalyzes the oxidation of polyunsaturated fatty acids to produce conjugated unsaturated fatty acid hydroperoxides, the deterioration of which causes off-flavors in food (Gökmen, Savaş Bahçeci, Serpen, & Acar, Citation2005). Both the low-moisture and control nuts showed a slight decrease in LOX activity when stored at −3°C ()). A similar trend was observed in rice bran, as described by Ramezanzadeh et al. (Citation1999), who reported that rice bran stored at low temperature showed little change in LOX activity. However, from month 3, significantly lower LOX activity was observed in the low-moisture nuts (p < 0.05) than in the control.
3.6. Total phenolics and vitamin E content
Antioxidative and free radical-scavenging properties of the polyphenolic compounds in several plant extracts have been reported, suggesting possible protective roles of polyphenolic compounds in reducing the risk of cardiovascular diseases in humans (Velioglu, Mazza, Gao, & Oomah, Citation1998). The total phenolics contents in control and treated nuts are shown in . Both control and treated nuts showed a decreasing trend in total phenolics content during storage. Both low-moisture and control nuts begin to show significantly lower total phenolics contents from the 5th month and the 3rd month, respectively (p < 0.05). At the end of storage, low-moisture nuts retained approximately 84.6% of the initial total phenolics, which is significantly higher than 66.6% in the control (p < 0.05). The results showed that low-moisture pine nuts stored at −3°C may have better inhibition than nuts with 17.3% moisture content due to the degradation of phenolics compounds.
Table 1. Changes in total phenolics and vitamin E content of pine nuts treated with low moisture versus the control stored at −3°C.a
Tabla 1. Cambios en el contenido total fenólico y de Vitamina E en piñones de pino tratados con baja humedad frente a la muestra control, almacenados a −3°C.a
Vitamin E, which acts in plants as an antioxidant, antimicrobial, photoreceptor and visual attractor, is a fat-soluble antioxidant that functions as a scavenger of peroxyl radicals (Taipina, Lamardo, Rodas, & Del Mastro, Citation2009). The changes in the contents of Vitamin E in treated and control pine nuts during storage are shown in . The Vitamin E (α-tocopherol) content (117.45 mg/kg oil) observed in this study is higher than reported by Kornsteiner, Wagner and Elmadfa (Citation2006), who reported a α-tocopherol content of 41 mg/kg oil in pine nuts grown in Greece. All samples showed a decreasing trend in vitamin E content throughout the storage period. The content of Vitamin E in the low-moisture nuts and control began to be significantly lower than the initial content after the 4th month and 1st month, respectively. By the end of the last month, the treated nuts were found to contain significantly more (p < 0.05) vitamin E than the control. Our results are consistent with the findings of Fourie and Basson (Citation1989), who showed that the Vitamin E content in various nuts decreased during storage as a result of its exhaustion during auto-oxidation. Naturally occurring Vitamin E represents an essential antioxidant component in human nutrition, required for the preservation of lipids in stable form in biological systems as well as in foods (Chun, Lee, & Eitenmiller, Citation2005).
4. Conclusions
The moisture content of pine nuts was adjusted to 13.3%, with the initial moisture content of 17.3% as a control. Thermal treatment improved the storage stability. The hydrolysis of lipids was delayed in low-moisture pine nuts, resulting in a significant inhibition of FFA accumulation in pine nuts. Low-moisture pine nuts also showed lower peroxide and TBA values compared with control pine nuts. Ultrastructure investigation revealed that thermal treatment maintained the integrity of the cell, deferring the degradation of the plasmalemma and protecting the internal lipid droplet. Thermal treatment better preserved the antioxidant components, including total phenolics and vitamin E, and reduced the activities of lipase and LOX in pine nuts. This study demonstrated that combining thermal treatment with near-freezing temperature storage could be a potentially highly effective approach for the long-term storage of pine nuts.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31401478), the National Postdoctoral Science Foundation of China (2015M570760), China Scholarship Council (201508210023), the Postdoctoral Special Funding of Chongqing City (Xm2015021), the Research Project from Science & Technology Department of Liaoning Province of China (number 2015103020).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOCS. (1998). Official methods and recommended practices of the American oil chemists’ society (5th ed.). Champaign, IL: Author.
- Atungulu, G., Miura, M., Atungulu, E., Satou, Y., & Suzuki, K. (2007). Activity of gaseous phase steam distilled propolis extracts on peroxidation and hydrolysis of rice lipids. Journal of Food Engineering, 80, 850–858. doi:10.1016/j.jfoodeng.2006.08.007
- Bao, Y.L., Zhu, S.C., Luo, Y.K., & Shen, H.X. (2015). Comparison of postmortem changes in Blunt-Snout Bream (Megalobrama amblycephala) during short-term storage at chilled and partial freezing temperatures. Journal of Aquatic Food Product Technology, 24, 752–761. doi:10.1080/10498850.2013.809832
- Cai, L.Y., Liu, C.H., & Ying, T.J. (2013). Changes in quality of low-moisture conditioned pine nut (Pinus gerardiana) under near freezing temperature storage. CyTA-Journal of Food, 11, 216–222. doi:10.1080/19476337.2012.727032
- Cai, L.Y., Xiao, L., Liu, C.H., & Ying, T.J. (2013). Functional properties and bioactivities of pine nut (Pinus gerardiana) protein isolates and its enzymatic hydrolysates. Food and Bioprocess Technology, 6, 2109–2117. doi:10.1007/s11947-012-0885-7
- Chun, J., Lee, J., & Eitenmiller, R.R. (2005). Vitamin E and oxidative stability during storage of raw and dry roasted peanuts packaged under air and vacuum. Journal of Food Science, 70, C292–C297. doi:10.1111/j.1365-2621.2005.tb07176.x
- Dillahunty, A.L., Siebenmorgen, T.J., Buescher, R.W., Smith, D.E., & Mauromoustakos, A. (2000). Effect of moisture content and temperature on respiration rate of rice. Cereal Chemistry, 77, 541–543. doi:10.1094/CCHEM.2000.77.5.541
- Evranuz, E.Ö. (1993). The effects of temperature and moisture content on lipid peroxidation during storage of unblanched salted roasted peanuts: Shelf life studies for unblanched salted roasted peanuts. International Journal of Food Science & Technology, 28, 193–199. doi:10.1111/j.1365-2621.1993.tb01264.x
- Fourie, P.C., & Basson, D.S. (1989). Changes in the tocopherol content of almond, pecan and macadamia kernels during storage. Journal of the American Oil Chemists’ Society, 66, 1113–1115. doi:10.1007/BF02670095
- Fukai, Y., Matsuzawa, T., & Ishitani, T. (2003). Effects of moisture content and storage temperature of husked rice on taste and changes in physicochemical properties. Journal of the Japanese Society for Food Science and Technology-Nippon Shokuhin Kagaku Kogaku Kaishi, 50, 243–253. doi:10.3136/nskkk.50.243
- Genkawaa, T., Uchino, T., Inoue, A., Tanaka, F., & Hamanaka, D. (2008). Development of a low-moisture-content storage system for brown rice: Storability at decreased moisture contents. Biosystems Engineering, 99, 515–522. doi:10.1016/j.biosystemseng.2007.12.011
- Gökmen, V., Savaş Bahçeci, K.S., Serpen, A., & Acar, J. (2005). Study of lipoxygenase and peroxidase as blanching indicator enzymes in peas: Change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. LWT-Food Science and Technology, 38, 903–908. doi:10.1016/j.lwt.2004.06.018
- Hoon, L.Y., Choo, C., Watawana, M.I., Jayawardena, N., & Waisundara, V.Y. (2015). Evaluation of the total antioxidant capacity and antioxidant compounds of different solvent extracts of Chilgoza pine nuts (Pinus gerardiana). Journal of Functional Foods, 18, 1014–1021. doi:10.1016/j.jff.2014.07.009
- Kaijser, A., Dutta, P., & Savage, G. (2000). Oxidative stability and lipid composition of macadamia nuts grown in New Zealand. Food Chemistry, 71, 67–70. doi:10.1016/S0308-8146(00)00132-1
- Karabulut, I., Topcu, A., Yorulmaz, A., Tekin, A., & Ozay, D.S. (2005). Effects of the industrial refining process on some properties of hazelnut oil. European Journal of Lipid Science and Technology, 107, 476–480. doi:10.1002/ejlt.200501147
- Katavic, V., Agrawal, G.K., Hajduch, M., Harris, S.L., & Thelen, J.J. (2006). Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics, 6, 4586–4598. doi:10.1002/pmic.200600020
- Kornsteiner, M., Wagner, K.-H., & Elmadfa, I. (2006). Tocopherols and total phenolics in 10 different nut types. Food Chemistry, 98, 381–387. doi:10.1016/j.foodchem.2005.07.033
- Lee, J.-W., Lee, K.-W., Lee, S.-W., Kim, I.-H., & Rhee, C. (2004). Selective increase in pinolenic acid (all-cis-5,9,12-18:3) in Korean pine nut oil by crystallization and its effect on LDL-receptor activity. Lipids, 39, 383–387. doi:10.1007/s11745-004-1242-2
- Lisiewska, Z., & Kmiecik, W. (2000). Effect of storage period and temperature on the chemical composition and organoleptic quality of frozen tomato cubes. Food Chemistry, 70, 167–173. doi:10.1016/S0956-7135(99)00110-3
- Matthaus, B., & Ozcan, M.M. (2013). Fatty acid, tocopherol and sterol contents of forest pine seed oil. Asian Journal of Chemistry, 25, 9845–9847. doi:10.14233/ajchem.2013.15503
- Molteberg, E.L., Vogt, G., Nilsson, A., & Frolich, W. (1995). Effects of storage and heat processing on the content and composition of free fatty-acids in oats. Cereal Chemistry, 72, 88–93.
- Murphy, D.J. (2001). The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progress in Lipid Research, 40, 325–438. doi:10.1016/S0163-7827(01)00013-3
- Ozcan, M.M., Dagdelen, A., Kara, H.H., & Kanbur, G. (2013). The effect of microwave and roasted processing on fatty acid composition of oils extracted from stone pine (Pinus pinea) nuts. La Rivista Italiana delle Sostanze Grasse, XC, 265–269.
- Rajarammanna, R., Jayas, D.S., & White, N.D.G. (2010). Comparison of deterioration of rye under two different storage regimes. Journal of Stored Products Research, 46, 87–92. doi:10.1016/j.jspr.2009.10.005
- Ramezanzadeh, F.M., Rao, R.M., Windhauser, M., Prinyawiwatkul, W., Tulley, R., & Marshall, W.E. (1999). Prevention of hydrolytic rancidity in rice bran during storage. Journal of Agricultural and Food Chemistry, 47, 3050–3052. doi:10.1021/jf981335r
- Rehman, Z.U. (2006). Storage effects on nutritional quality of commonly consumed cereals. Food Chemistry, 95, 53–57. doi:10.1016/j.foodchem.2004.12.017
- Singleton, V.L., Orthofer, R., & Lamuela-Raventos, R.M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
- Surrey, K. (1964). Spectrophotometric method for determination of lipoxidase activity. Plant Physiology, 39, 65–70. doi:10.1104/pp.39.1.65
- Taipina, M.S., Lamardo, L.C.A., Rodas, M.A.B., & Del Mastro, N.L. (2009). The effects of gamma irradiation on the vitamin E content and sensory qualities of pecan nuts (Carya illinoensis). Radiation Physics and Chemistry, 78, 611–613. doi:10.1016/j.radphyschem.2009.03.019
- Velioglu, Y.S., Mazza, G., Gao, L., & Oomah, B.D. (1998). Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. Journal of Agricultural and Food Chemistry, 46, 4113–4117. doi:10.1021/jf9801973
- Venkatachalam, M., & Sathe, S.K. (2006). Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry, 54, 4705–4714. doi:10.1021/jf0606959
- Xie, K.Y., Miles, E.A., & Calder, P.C. (2016). A review of the potential health benefits of pine nut oil and its characteristic fatty acid pinolenic acid. Journal of Functional Foods, 23, 464–473. doi:10.1016/j.jff.2016.03.003
- Zhao, S.M., Xiong, S.B., Qiu, C.G., & Xu, Y.L. (2007). Effect of microwaves on rice quality. Journal of Stored Products Research, 43, 496–502. doi:10.1016/j.jspr.2007.02.002
