ABSTRACT
This contribution looks at the possibility of replacing acid-thinned corn starch by chicory inulin as gelling coagents in gummy jellies made with gelatin. A creamy gel could be formed after stirring (70°C/10 min) an inulin water solution (240 g kg−1) without previous cooking. Starch replacement by inulin (90 g kg−1 raw mass) in jellies provided a slightly softer, springier and stickier texture, enhanced strawberry, sweet and sour flavors, and hardly affected the red color. Inulin/Oligosaccharides remained without be degraded to free sugars in jellies after processing (mixing at 80°C and pH 3.2 for 5 min and further drying at 30% relative humidity and 25°C for 24 h). Therefore, chicory inulin acted as stable and neutral flavoring ingredient and can be used as gelling coagent to develop gummy jellies enriched in dietary fiber with potential prebiotic activity.
RESUMEN
Este estudio analiza la posibilidad de sustituir almidón de maíz por inulina de achicoria como agente co-gelificante en caramelos de goma elaborados con gelatina. A partir de una solución acuosa de inulina (240 g kg−1) se pudo formar un gel cremoso mediante agitación (70°C /10 min) sin necesidad de cocción previa. La sustitución de almidón por inulina (90 g kg−1 masa cruda) en los caramelos proporcionó una textura ligeramente más blanda, elástica y adhesiva, potenció los sabores a fresa, dulce y ácido, y apenas afectó el color rojo. La inulina añadida no se degradó a azúcares simples tras el procesado (mezclado a 80°C y pH 3,2 durante 5 min y posterior secado a 30% H.R. y 25°C durante 24 h). Por tanto, la inulina ha resultado ser un ingrediente estable y de sabor neutro que puede ser empleado para desarrollar caramelos de goma enriquecidos en fibra dietética con potencial actividad prebiótica.
Introduction
In recent years, the enrichment of candies with dietary fiber has come to be seen as a suitable strategy to increase their nutritional quality, including gummy jellies (Koh, Jiang, Kasapis, & Foo, Citation2011). Gummy jellies, which are very popular products, widely consumed by children and teenagers, are elaborated with concentrated sugar solutions, gelatin (combined or not with other gelling agents) and other minor ingredients (coloring, acid and flavoring agents). The use of gels based on gelatin and modified starch is extensive in the industry and provides products characterized by a firm structure, while being soft and chewy (Burey, Bhandari, Rutgers, Halley, & Torley, Citation2009). Both gelling agents act synergistically: gelatin provides springy and transparent gels, while starch increases the hardness and opacity of gelatin candies without affecting their fracturability or springiness (Marfil, Anhê, & Telis, Citation2012). Starch is a cheap ingredient with good gelling properties but is also a source of digestible sugars, and, for this reason, the industry is looking at the use of non-starchy gelling polysaccharides to meet the new demand for less caloric candies containing dietary fiber (Goncalves & Rohr, Citation2009; Koh et al., Citation2011).
Inulin is a nondigestible polysaccharide belonging to the fructan group. It is formed of fructose chains (linked by β-(2-1)-d-fructosyl-fructose bonds) (Ritsema & Smeekens, Citation2003) and is obtained industrially from chicory root (Cichorium intybus). Inulin has been used to improve the marketability of foodstuffs as health-promoting food products due to their beneficial physiological features and prebiotic effects (Flamm, Glinsman, Kritchevsky, Prosky, & Roberfroid, Citation2001; Tungland & Meyer, Citation2002). Moreover, inulin has a gel-formation capability and so can be used as gelling agent in food products (Bot, Erle, Vreeker, & Agterof, Citation2004; Chiavaro, Vittadini, & Corradini, Citation2007; Evageliou, Tseliou, Mandala, & Komaitis, Citation2010; Harrington & Morris, Citation2009; Hébette et al., Citation1998; Paradiso et al., Citation2015). Inulin gel can be obtained by shearing or heating–cooling an inulin suspension (Kim, Faqih, & Wang, Citation2001) as being composed of a tri-dimensional network of insoluble submicron crystalline particles containing large amounts of immobilized water, which ensures its physical stability. A gel-like structure with a white creamy appearance and a spreadable texture is formed when inulin is thoroughly mixed with water or other aqueous liquids (André et al., Citation1996; Franck, Citation2002).
Little information is available concerning the potential of inulin as a functional ingredient in candy products, and, in particular, for use as gelling agent in soft candies. Inulin has a synergistic effect with gelatin (Roberfroid, Citation2005) and therefore offers good possibilities for use as co-gelling agent in gummy jellies, a product where gel strength mainly depends on gelatin. The addition of inulin in low proportions should have little impact on the rheological properties and sensory quality of products due to its neutral or slightly sweet taste and the limited effect on viscosity (Franck, Citation2002; Kalyani Nair, Kharb, & Thompkison, Citation2010), while its use at relatively high levels might even enhance the taste, mouthfeel and shelf life without significantly altering the specific application characteristics (Tungland & Meyer, Citation2002). In the case of gummy jellies, an acid product with a potential risk of inulin acid hydrolysis, the impact of using inulin gels on the resulting flavor, color and texture has not been assessed.
The objective of the present study was to determine the effect of replacing starch by inulin on the texture, color and flavor of gummy jellies. Acid-thinned starch was completely replaced by chicory inulin in a commercial formulation for jellies and the degradation of inulin to free sugars was assessed in the final product.
Materials and methods
Gelling agents
Three gelling agents were used to manufacture the jelly: (1) chicory inulin (Orafti® GR, Beneo, Tienen, Belgium), (2) acid-thinned corn starch (Cleargum® Mb 76, Roquette Laisa, Valencia, Spain); and (3) type A pig skin gelatin (Juncá Gelatines, Girona, Spain). The chicory inulin consisted of a white odorless powder containing 899 g inulin, 11 g sucrose, 37 g glucose, 33 g fructose and 20 g water kg−1 product. The average degree of polymerization (DP) of inulin was ≥10. This medium-chain inulin was chosen because it seemed to have a good balance between its solubility in water, chemical stability and gelling properties. The maximum solubility of inulin powder in water at 90°C was 350 g L−1. The inulin water solution (240 g kg−1) was prepared by stirring (≈800 rpm) at 70°C for 10 min (at pH 6). The caloric value of inulin powder declared by the supplier was 8.1 kJ g−1. The acid-thinned corn starch was in the form of a white–yellow odorless powder of high purity (89 g starch and 11 g water per 100 g product). A starch water dispersion (410 g kg−1) was prepared by stirring at 25°C for 3 min (at pH 6). The caloric value of starch powder declared by the supplier was 14.8 kJ g−1. The pig skin gelatin used consisted of a granulated (4 mm average diameter) powder with a pale yellow color, a neutral odor and a neutral flavor. Gelatin strength was 250 °Bloom (measured at 66.7 g gelatin kg−1 water and 10°C). The gelatin water solution was prepared by stirring (≈800 rpm) for 20 min (at pH 5 and 80°C) until complete dissolution.
Jelly manufacturing
Two jelly formulations denominated starch (ST) and inulin (IN) were compared: (1) ST (jellies containing 90 g starch kg−1) and (2) IN (jellies containing 90 g inulin kg−1). The proportions of the ingredients in the raw jellies are described in . The amounts of sugars and/or moisture of ingredients (inulin powder, starch powder and/or sucrose) are included in the respective sugar and/or water total percentages. The jellies were manufactured in a pilot plant following a procedure provided by a local company. The main raw ingredients (sucrose, corn glucose syrup and starch water dispersion) were homogenized by stirring and heating at 120°C for 5 min. After cooking, the temperature of the product (liquor) was reduced to 80°C and then the gelatin water solution was added and homogenized. Next, the respective water solutions (previously stirred for 10 min at 25°C) containing the acidifying, flavoring and coloring agents were transferred and then the liquor was homogenized for 5 min at 80°C. Finally, it was checked that the final concentration of soluble solids reached 78 ± 0.01 °Brix, as measured with a hand refractometer (Atago Co. Ltd. Minato-ku, Tokyo, Japan). The hot liquor was then poured into the starch powder molds (printed in trays), which were previously conditioned at 30°C and 10% relative humidity (RH) for 24 h. The trays containing hot jellies were kept in a cooling–drying chamber with circulating air at 21°C and 35% RH for 24 h. After drying, the trays were inverted to remove the jellies from the molds and were gently brushed to eliminate the rest of the starch powder. Finally, the jellies were polished with carnauba wax to avoid an excessively sticky surface for further handling. The IN-jellies were elaborated following the same procedure as described above (without starch) and inulin solution was added after cooking at 80°C and stirred until completely homogenized. The average dimensions of the jelly pieces (truncated flat cones) were 28 mm (minor diameter) × 30 mm (major diameter) × 8 mm (height). The average final weight of the jelly pieces was 7 ± 0.01 g. The jellies were packed in a polystyrene tray Aerpack B5-37 placed in a Cryovac BB3050 bag (7 g/24 h/m2 water vapor transmission rate at 38°C and 90% RH) (Sealed Air Corporation, Ozarów Maz, Poland) and kept at 25°C for a week before analysis. Three different batches (replicas) were made.
Table 1. Gummy jelly formulation.
Tabla 1. Formulación de los caramelos de goma.
Moisture, soluble solids and pH
Some physicochemical characteristics were determined in jellies for their potential effect on the color, flavor and texture. The moisture content (g kg−1) was determined by Karl Fischer titration (0.1 g kg−1 accuracy) using an automatic Titrino 702 SM instrument equipped with a No. 6.0338.100 double Pt-wire electrode (Methrom Schweiz, Zofingen, Switzerland). A 0.1-g sample was dissolved in a medium composed of 20 ml Hidranal® dry methanol and 20 ml formamide (Sigma-Aldrich, St. Louis, Missouri, USA). The sample solution was heated at 50°C until dissolution and was then titrated to dryness with Hidranal® Composite (Sigma-Aldrich). Total soluble solids (expressed as g kg−1) were determined using a hand-held Atago refractometer, placing a 2-mm-thick sliced sample in the visor for the measurements. The pH was measured using a micropH 2001 m (Crison, Barcelona, Spain) equipped with a glass combined electrode, Cat. No. 52-22 (Ingold Electrodes, Wilmington, USA). The samples were cut into thin slices, mixed with hot water (1:3, w/w) at 25°C and constantly stirred until complete dissolution.
Texture profile analysis
A Texture Profile Analysis (TPA) was performed using a QTS-25 Texture Analyzer (Brookfield Engineering, Harlow, Essex, England) equipped with the Texture-Pro program v. 2.1. Testing conditions were chosen on the basis of previous texture studies performed in inulin gels (Chiavaro et al., Citation2007; Glibowski & Wasko, Citation2008; Kim et al., Citation2001) and the information provided by Pons and Fiszman (Citation1996). The samples were compressed twice with a flat cylindrical probe (20 mm in diameter), which allowed the sample to be deformed without being penetrated. The testing conditions were 24°C room temperature; two consecutive cycles of 50% compression; cross-head moved at a constant speed of 0.5 mm s−1 and a trigger point of 0.05 N. Both the crosshead speed and compression percentage were those recommended to obtain values that are closely correlated with sensory responses in gelatin and polysaccharide-based gels (Muñoz, Pangborn, & Noble, Citation1986). The TPA force was obtained as described by Bourne (Citation1968) and Mochizuki (Citation2001). The texture variables analyzed were (1) hardness (N): force required to compress the material by a given amount; (2) cohesiveness (no units): strength of the internal bonds in the sample, the value being the ratio of the areas (force × time) resulting from the second and first bites; (3) springiness (mm): elastic recovery that occurs when the compressive force is removed, calculated as sample height recovered during the time elapsed from the end of the first bite to the beginning of the second; (4): gumminess (N): the energy required to break down a semi-solid food ready for swallowing, the values being the result of multiplying hardness × cohesiveness; and (5) chewiness (N mm): energy required to chew a solid food into a state ready for swallowing and its value is the result of multiplying hardness × cohesiveness × springiness. Adhesiveness was not included in the TPA because the jellies were covered with carnauba wax, a lubricant material. To minimize possible deviations in texture values, the dimensions of all the jelly pieces were previously checked using a calibrator of 0.1 mm accuracy.
CIELAB color
Instrumental color was measured using a CR-200/08 Chroma Meter II (Minolta Ltd., Milton Keynes, UK) with a D65 illumination standard, 2° observer angle, and aperture size of 50 mm and calibrated against a standard white tile. Reflectance was measured on jelly surface. The results were expressed as CIELAB values: lightness (L*), redness (a*) and yellowness (b*). The chroma (C*) and hue angle (h) (expressed as sexagesimal degrees) values were calculated as follows: C* = √(a*2 + b*2); h = tan−1(b*/a*).
Sensory analysis
A sensory descriptive analysis (ISO 4121, Citation2003) was performed to assess jelly color, flavor and texture. Eleven panelists selected from university personnel were specifically trained in four sessions according to ISO 8586 (Citation2012). The first two training sessions were concerned with identifying, selecting and quantifying the sensory attributes to be used for treatment evaluation. After consensus, nine sensory descriptors were chosen: hardness, springiness, gumminess, chewiness, adhesiveness, red tonality, strawberry flavor, sourness and sweetness. The descriptors were quantified by using intensity scales graduated in 1-point intervals: 1: absent; 2: slight; 3: moderate; 4: intense; and 5: very intense (see ). The results were expressed as Sensory Scores. Testing was conducted in the sensory evaluation laboratory kept at 25°C. In each session, panelists were given reference samples, experimental gummy jellies, breadsticks and water. Samples from different treatments were analyzed individually. Two randomly coded samples were assessed by each panelist in each session.
Table 2. Sensory descriptors and reference scales used for the quantitative descriptive analysis of gummy jellies.
Tabla 2. Descriptores sensoriales y escalas de referencia usadas para el análisis descriptivo de los caramelos de goma.
Inulin
Inulin (including inulin molecules with different DP, fructooligosaccharides and inulooligosaccharides) was analyzed by High Performance Size Exclusion Chromatography (HPSEC). The HPSEC system was equipped with a L-6200 pump (Merck-Hitachi, Darmstadt, Germany), a 2050 Plus autosampler (Jasco Inc., Easton, UK), a L-7490 Lachrome refractive index detector (Merck-Hitachi) and Waters 500, 250 and 120 Ultrahydrogel columns in line with an Ultrahydrogel guard column. A water extract sample was filtered using a 0.20-µm filter to remove proteins prior to analysis. For HPSEC analysis, the column was maintained at 30°C with an isocratic elution flow rate of 0.8 ml min−1. The eluent consisted of 0.4 M sodium acetate pH 3.0, which was used for the preparation of the solutions and the mobile phase. Injection volumes were 20 µl with run time 50 min. Inulin was quantified using a standard (Chicory Inulin, No. 12255, Sigma, St. Louis, Missouri, USA) to perform the calibration curve at a concentration ranging from 9.9 to 15.7 g inulin/oligosaccharides 100 g−1 sample.
Statistic
A randomized statistical design was followed for the experiment. Twenty-four jelly units were sampled (4 jelly pieces × 3 manufacturing batches × 2 co-gelling agents). Average values of the dependent variables for individuals were analyzed using the Statistix 8.0 for Windows software (Analytical Software, Tallahassee, FL, USA). The effects of treatment (ST or IN) on the dependent variables were determined by one-way analysis of variance. The Tukey test was used to compare the least square means, which were considered to be statistically different when P < 0.05.
Results and discussion
Effects on moisture, soluble solids and pH
The gummy jellies elaborated with hot liquor at 78.0°Brix had final total soluble solids values of above 80°Brix. The total soluble solids were slightly higher in the IN-jellies than in the ST-jellies, while the moisture content was similar in the jellies elaborated with inulin and starch (see ). This may be explained by the different sorption properties of gelatinized starch and inulin. Zimeri and Kokini (Citation2003) compared moisture sorption isotherms and found that gelatinized corn starch had a lower water activity than pre-solubilized inulin, indicating differences in their respective water-binding capacities, which may explain why the IN-jellies had higher soluble solid values than ST-jellies after molding, drying and storage. The jellies had an acidic pH due to the incorporation of citric and lactic acids to reproduce the natural acidity of strawberry. The pH was slightly lower in the IN-jellies than in the ST-jellies. The fact that the pH values of the starch and inulin dispersions were similar (6), which suggests that there were some differences in the quantity and/or the type of degradation compounds formed from inulin and starch. Part of the acids added might had reacted with the glucose chains released from gelatinized starch, while inulin was less exposed to thermal degradation. The relevance of pH for the chemical stability of gelling polysaccharides is well known. The glycosidic bonds can be broken in an acidic medium, resulting in the formation of low molecular fractions from the branched and linear chains of polysaccharides (Wang, Truong, & Wang, Citation2003). Despite being quite acid-resistant, inulin can be hydrolyzed to oligosaccharides and sugars at a pH of around 3.5. For this reason, a reduction in pH from 5 to 3 decreased the DP of gelled inulin (prepared at 200 g kg−1 and 80°C for 30 min and then kept at 5°C for 21 h) from 165 g kg−1 (2787 g mol−1 molecular weight) to 45 g kg−1 (731 g mol−1), producing soft creamier gels (Glibowski & Wasko, Citation2008). Other authors reported similar findings for other inulin gels (Franck, Citation2002; Kim et al., Citation2001), although there is no specific information on gelled candies made with inulin. Whatever the case, inulin hydrolysis would be more intense in aqueous media with a higher water activity than in media with a high concentration of sugars, such as jellies. On the other hand, gel strength increases at pH values lower than the isoelectric point of pig skin type A gelatin (pH from 6.0 to 9.5), which makes this gelatin particularly suitable for acid jellies (Edwards, Citation2000). In our study, the differences in the pH or moisture/solids content observed between treatments can be considered as minor or irrelevant, and any possible difference in texture, flavor or color observed between both types of jelly can probably be attributed to the different properties provided by chicory inulin and cooked corn starch.
Table 3. Effect of replacing starch by inulin on moisture, total soluble solids and pH of gummy jellies.
Tabla 3. Efecto de la sustitución de almidón por inulina sobre el contenido en humedad, sólidos solubles y el pH de los caramelos de goma.
Effects on texture
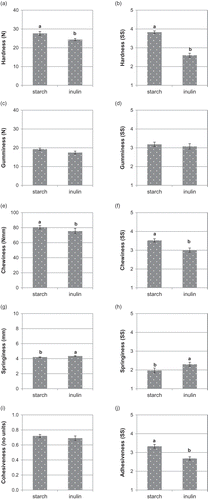
According to the TPA test (), hardness and chewiness were lower in the IN-jellies (24.5 N and 75.4 N mm, respectively) than in the ST-jellies (27.7 N and 80.6 N mm); in contrast, springiness was higher in the IN-jellies (4.35 mm) than in the ST-jellies (4.21 mm), while there were no differences between treatments for gumminess (IN-jellies: 17.4 N; ST-jellies: 19.3 N) and cohesiveness (IN-jellies: 0.7 no units; ST-jellies: 0.7 no units). According to this, the use of inulin decreased gel strength, resulting in weaker and more easily deformable jellies; however, the IN-jellies were more elastic and recovered their original dimensions better than the ST-jellies. As expected, the chewiness value was more useful than gumminess value for discriminating elasticity in jellies, a solid matrix. In general, sensory texture scoring () reproduced the results obtained by TPA, as has also been reported in isomaltulose-based gummy confections (Periche, Heredia, Escriche, Andrés, & Castelló, Citation2014). Hardness (resistance to the first bite using incisor teeth) and chewiness (number of chews required for swallowing) also scored lower in the IN-jellies (2.6 and 3.0, respectively) than in the ST-jellies (3.7 and 3.2, respectively). By contrast, springiness (inversely, the time required to recover the original dimensions after compression using the ring finger) scored slightly higher in the IN-jellies (2.3) than in the ST-jellies (1.9), while there were no differences in gumminess (elasticity during mastication using molar teeth) between the IN-jellies (3.1) and the ST-jellies (3.2). Moreover, adhesiveness (stickiness after the first bite using incisor teeth) scored lower in the IN-jellies (2.7) than in the ST-jellies (3.3). Thus, both instrumental and sensory analyses confirmed that the replacement of starch by inulin resulted in slightly softer, springier and stickier jellies.
Figure 1. Effect of replacing starch by inulin on the instrumental (a, c, e, g and i) and sensory (b, d, f, h and j) texture of gummy jellies.Note: Means with different superscripts are different at P ≤ 0.05 (standard error of the mean in bars). SS: Sensory scores.
Figura 1. Efecto de la sustitución de almidón por inulina sobre la textura instrumental (a, c, e, g y i) y sensorial (b, d, f, h y j) de los caramelos de goma.Nota: Medias con diferentes superíndices son diferentes para P ≤ 0,05 (error estándar de la media en barras). SS: puntuaciones sensoriales.
The differences in texture observed between treatments could be explained by the different rheological properties of starch and inulin gels as well as their possible synergies with gelatin. Inulin water solutions/dispersions ranging from 250 to 500 g kg−1 are able to form thermoreversible gels at low temperatures (Flamm et al., Citation2001). Gel strength will depend on the mechanical treatment and the temperature applied during gel preparation. Gel formation from inulin solution by shearing at high temperature (80°C) favors the dispersion of small insoluble inulin particles, inducing a gel-like texture. This sol–gel transition is the result of association of polymer molecules in the polymer solution. Therefore, inulin gels formed under heating (while stirring) are stronger, smoother and more uniform than those obtained without heating (Kim et al., Citation2001). In our study, the creamy inulin gel prepared by stirring at 70°C had a suitable strength for use in jellies. Moreover, the synergy between gelatin and inulin has been documented. Inulin can be added at 25–100 g kg−1 to gelatin gel (type B at 50 g kg−1) without affecting the texture, while it was necessary to add high quantities of inulin (200 g kg−1) to decrease gel elasticity (Harrington & Morris, Citation2009), which is much higher quantity than those used in our study. On the other hand, acid-thinned corn starch granules suffer complete gelatinization at cooking temperatures (>70°C) (Yousif, Gadallah, & Sorour, Citation2012). Further transformations of gelatinized starch during jelly processing include gelation and retrogradation. Irreversible gelation occurs as the result of cooking in the presence of excessive water. The granules of starch swell, increasing their size and viscosity until they break. When this occurs, a mixture of fragments of swollen amylopectin-rich granules, dissolved and hydrated granules, and dissolved molecules of amylose is formed (Langton & Hermansson, Citation1989). After gelation, starch gels suffer retrogradation due to the reorganization and crystallization of gelled amylose and amylopectin molecules, which leads to retraction and hardening in starch-based gelled candies (Díaz, Ros, & Bañón, Citation2010), a defect that can be partially corrected using acid-treated starch (Sandhu, Singh, & Lim, Citation2007). The retrogradation rate of acid-thinned starch gels increased as hydrolysis proceeded (Kang, Kim, Lee, & Kim, Citation1997).
The use of starch increases gel strength and hardness in gelatin-based gummy confections (Marfil et al., Citation2012); however, inulin has relatively few applications in candies and there is little information available on the gelatin–inulin interaction in such products. Inulin has been successfully used (at 4–13 g kg−1) as a texturizing agent to increase firmness in soft jellies made without other gelling agents (Goncalves & Rohr, Citation2009). Inulin has also been tested with good results to increase the fiber content in durum wheat spaghetti. A high quantity (150 g kg−1) of higher molecular weight inulin was incorporated in pasta without any effect on firmness and cooking loss (Aravind, Sissons, Fellows, Blazek, & Gilbert, Citation2012). In a similar study, the addition of inulin (up to 100 g kg−1) decreased the swelling index and firmness but did not affect elasticity and adhesiveness in cooked pasta (Brennan, Kuri, & Tudorica, Citation2004). Another rheological study confirmed that inulin can be incorporated as a fiber in low-caloric milk chocolate within of a wide technological margin (670–860 g kg−1) (Abassi & Farzanmehr, Citation2009). Inulin has also been compared with other gelling polysaccharides used as functional ingredients in food products. For example, an inulin gel had lower strength and firmness and similar elasticity to a gel containing acyl gellan gel (Evageliou et al., Citation2010).
Effects on color and flavor
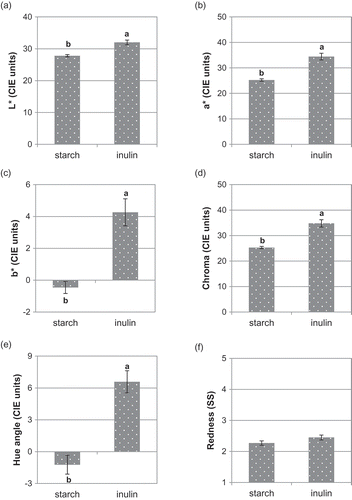
As regards color (), the IN-jellies had higher values of lightness (L*), redness (a*) and yellowness (b*) (32.0, 34.4 and 4.3 CIE units, respectively) than the ST-jellies (27.8, 25.3 and −0.5 CIE units, respectively). The values of chroma (C*) and hue angle (h*) were also higher in the IN-jellies (34.8 and 6.6 CIE units, respectively) than in the ST-jellies (25.3 and −1.2 CIE units). According to these reflectance values, the IN-jellies were lighter and had a more reddish tonality than the ST-jellies. This may be explained by the inherent optical properties of each gelling agent. Inulin water solution is a colorless liquid that can increase jelly lightness, enhancing the reddish tonality, while starch gel is slightly opaque. In a similar study (Marfil et al., Citation2012), the incorporation of acid modified corn starch increased opacity in gelatin gels. In contrast, the panellists did not score differences (P > 0.05) in red tonality (dark to bright) between IN-jellies (2.4) and ST-jellies (2.3) so that, from a sensory point of view, the use of starch or inulin was considered irrelevant for redness in ‘strawberry-colored’ jellies. A similar finding was reported in another study on soft candies containing inulin (at 4–13 g kg−1) and red coloring (Goncalves & Rohr, Citation2009).
Figure 2. Effect of replacing starch by inulin on the CIELAB color (a, b, c, d and e) and the redness tonality (f) of gummy jellies.Note: Means with different superscripts are different at P ≤ 0.05 (standard error of the mean in bars). SS: Sensory scores.
Figura 2. Efecto de la sustitución de almidón por inulina sobre el color CIELab (a, b, c, d y e) y la tonalidad roja (f) de los caramelos de goma.Nota: Medias con diferente superíndice son diferentes para P ≤ 0,05 (error estándar de la media en barras). SS: puntuaciones sensoriales.
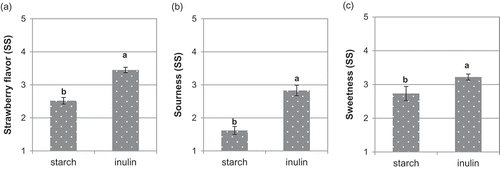
Strawberry flavor clearly scored higher in the IN-jellies (3.4) than in the ST-jellies (2.5) (). Although there is no specific information on candies, it is generally accepted that inulin, a polysaccharide with a bland neutral taste without any off-flavor or aftertaste, can be combined with other food ingredients without modifying delicate flavors, even enhancing the natural flavor of some products, such as desserts (Tárrega & Costell, Citation2006), bakery products, breakfast cereals or low-fat dairy products (Franck, Citation2002). Sourness and sweetness are also typical flavor attributes of strawberry jellies. Sourness scored higher in the IN-jellies (2.9) than in the ST-jellies (1.6), which was coherent with the lower pH observed in the IN-jellies. The relationship between sourness and pH is well known in food products. Amerine, Roessler, and Ough (Citation1965) reported that differences of 0.05 pH units were easily detected by a sensory panel trained for assessing sourness in citric or lactic acid solutions (at pH around 3.3), although this was not confirmed in further studies (CoSeteng, McLellan, & Downing, Citation1989). Sourness mainly depends on the concentration of citric and lactic acids and is the first dominant sensation in the mouth during tasting of this type of soft candy, while strawberry perception predominates at the end (Saint-Eve et al., Citation2011). Moreover, inulin, with its more neutral flavor than cooked starch, could also have enhanced the perception of sourness, as it did with the strawberry flavor. Similarly, sweetness also scored higher in the jellies made with inulin (3.2) compared with those made with starch (2.7). Inulin has no sweet flavor, although the chicory inulin powder used in our study was slightly sweet (10% relative sweetening power with respect to sucrose) because it contains oligosaccharides and hydrolyzed sugars (Franck, Citation2002). Ten DP inulins contain one molecule of glucose for every nine molecules of fructose, since fructose has a higher sweetening power (173%) than glucose (74%) (Bellisle & Drewnowski, Citation2007); jellies made with inulin contained a higher proportion of fructose-based molecules (oligosaccharides and sugars) than those made with starch, which may explain the differences in sweetness observed between both jelly types. Unlike our findings, the addition of inulin (at 4–13 g kg−1) did not improve the flavor in soft candies containing strawberry and milk flavorings (Goncalves & Rohr, Citation2009). In another study on cooked wheat pasta, inulin had little impact on the flavor and could be used within of a wide sensory margin (Aravind et al., Citation2012). Our sensory assessment showed that chicory inulin clearly enhanced jelly flavor compared with cooked (acid-thinned) corn starch. Moreover, this flavor enhancement could also have been influenced by the changes observed in jelly texture. In general, overall flavor is perceived stronger in softer gels with a rapid breakdown rate. Therefore, the compounds responsible for strawberry, sweet and sour flavors may be released more quickly in the mouth from weak and fragile gels, as occurred in IN-jellies, while the same flavoring compounds would be more tightly retained in strong and cohesive gels (Boland, Delahunty, & Van Ruth, Citation2006; Kälviäinen, Roininen, & Tuorila, Citation2000).
Figure 3. Effect of replacing starch by inulin on the strawberry (a), acid (b) and sweet flavor (c) of gummy jellies.Note: Means with different superscripts are different at P ≤ 0.05 (standard error of the mean in bars). SS: Sensory scores.
Figura 3. Efecto de la sustitución de almidón por inulina sobre el sabor a fresa (a), sabor ácido (b) y sabor dulce (c) de los caramelos de goma.Nota: Medias con diferente superíndice son diferentes para P ≤ 0,05 (error estándar de la media en barras). SS: puntuaciones sensoriales.
Inulin stability
The IN-jellies contained 121 ± 4 g inulin kg−1, which was similar to the maximum value expected for the final product (120 g kg−1). Therefore, sugar release, a possible limiting factor when using inulin in acidic jellies, was considered to be irrelevant. As already mentioned, inulin, as other polysaccharides, is susceptible to hydrolysis depending on the temperature and duration of exposure to the acidic environment. When hydrolysis occurs, the long-chain oligosaccharide is broken down into shorter chains and simple sugars. There is no available information on inulin stability in candies, although it has been reported that inulin gels are able to release reducing sugars at pH values around 3.5 (Glibowski & Wasko, Citation2008; Kim et al., Citation2001). On the other hand, oligofructose is quite stable in environments with a low pH at hot temperature. Oligofructose hydrolysis is insignificant at 60°C under acidic conditions (pH 3.3), although it could be considerable at 60–80°C (Matusek, Merész, Diem Le, & Örsi, Citation2009). Whatever the case, the intensity of hydrolyzing reactions in inulin gels cannot be fully extrapolated to gummy jellies, a product with a high concentration of sugars and with a low moisture content. In our study, inulin did not appear to be degraded in jellies despite that the conditions suitable for hydrolyzing inulin began when the hot liquor was acidified. This fact may be explained by the high chemical stability of the commercial chicory inulin used in our study. For example, it was obtained a 140 g kg−1 released sugars when a water solution of the same type of chicory inulin (125 g L−1) was hydrolyzed in a chemical reactor at pH 3.5 and 150°C for 5 min (León, Santos, López, & Antolín, Citation2005).
Regardless of other quality aspects, the replacement of starch by dietary fiber in food products aims to reduce their caloric value. Inulin suffers a low degree of acid hydrolysis in the stomach, although it can be degraded by bacteria that colonize the colon (Rossi et al., Citation2005); so, its caloric value is low compared with starch. Chicory inulin and oligofructose, like all the other carbohydrates that are more or less completely fermented in the human colon, have been given a caloric value of 6.3 kJ g−1, while dietary carbohydrates, which are absorbed as hexose (glucose, fructose), have a caloric value of 16.3 kJ g−1 (Roberfroid, Citation1993). According to these estimations, the replacement of 120 g kg−1 acid-thinned corn starch by chicory inulin would decrease the caloric value of gummy jellies by 120 kJ 100 g−1. This seems a modest reduction in the caloric value for a sugar product, but the use of inulin could be combined with other strategies, such as the use of sugar replacers, to elaborate dietary candies.
Conclusions
Acid-thinned corn starch can be replaced by chicory inulin as gelling coagent to develop gummy jellies enriched in dietary fiber. Unlike starch, inulin gel can be formed by shearing at hot temperature without previous cooking, which simplifies the manufacturing procedure and reduces the thermal treatment required. The replacement of starch by inulin modified the texture flavor and, to lesser extent, color in strawberry-type jellies. The jellies made with inulin were slightly softer, springier and stickier than those made with cooked starch. Moreover, the use of inulin enhanced strawberry, sweet and sour flavors, which suggests that inulin powder, with its pool of fructose-based molecules, is a neutral flavoring ingredient with a higher sweetening power than cooked starch. Inulin was not degraded in jellies despite the potential risk of hydrolysis due to the thermal and acidifying treatments applied in the manufacturing process. Therefore, chicory inulin can be used as gelling coagent to develop less caloric gelatin jellies enriched in dietary fiber with potential prebiotic activity. However, future studies should be carried out to optimize the ingredients (degree of inulin polymerization, inulin/gelatin ratio, replacement of sugars by low-caloric sweeteners and addition of acids) and processing conditions (pH, heating and cooling temperatures) in order to obtain new products with improved sensory and nutritional properties. Additionally, studies with potential consumers should be conducted to determine the acceptance of gummy jellies made with inulin.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abassi, S., & Farzanmehr, H. (2009). Optimization of the formulation of prebiotic milk chocolate based on rheological properties. Food Technology and Biotechnology, 47(4), 396–403.
- Amerine, M.A., Roessler E.B., & Ough C.S. (1965). Acids and the acid taste. I. The effect of pH and titratable acidity. American Journal of Enology and Viticulture, 16, 29–37.
- André, I., Putaux, J.L., Chanzy, H., Taravel, F.R., Timmermans, J.W., & DeWit, D. (1996). Single crystals of inulin. International Journal of Biological Macromolecules, 18, 195–204. doi:10.1016/0141-8130(95)01075-0
- Aravind, N., Sissons, M.J., Fellows, C.M., Blazek, J., & Gilbert, E.P. (2012). Effect of inulin soluble dietary fiber addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chemistry, 132, 993–1002. doi:10.1016/j.foodchem.2011.11.085
- Bellisle, F., & Drewnowski, A. (2007). Intense sweeteners, energy intake and the control of body weight. European Journal of Clinical Nutrition, 61, 691–700. doi:10.1038/sj.ejcn.1602649
- Boland, A., Delahunty, C., & Van Ruth, S. (2006). Influence of the texture of gelatin gels and pectin gels on strawberry flavour release and perception. Food Chemistry, 96, 452–460. doi:10.1016/j.foodchem.2005.02.027
- Bot, A., Erle, U., Vreeker, R., & Agterof, W.G.M. (2004). Influence of crystallisation on the large deformation rheology of inulin gels. Food Hydrocolloids, 18, 547–556. doi:10.1016/j.foodhyd.2003.09.003
- Bourne, M.C. (1968). Texture profile of ripening pears. Journal of Food Science, 33, 223–226. doi:10.1111/j.1365-2621.1968.tb01354.x
- Brennan, C.S., Kuri, V., & Tudorica, C.M. (2004). Inulin-enriched pasta: Effects on textural properties and starch degradation. Food Chemistry, 86, 189–193. doi:10.1016/j.foodchem.2003.08.034
- Burey, P., Bhandari, B., Rutgers, R., Halley, P., & Torley, P. (2009). Confectionery gels: A review on formulation, rheological and structural aspects. International Journal of Food Properties, 12(1), 176–210. doi:10.1080/10942910802223404
- Chiavaro, E., Vittadini, E., & Corradini, C. (2007). Physicochemical characterization and stability of inulin gels. European Food Research and Technology, 225, 85–94. doi:10.1007/s00217-006-0385-y
- CoSeteng, M.Y., McLellan, M.R., & Downing, D.L. (1989). Influence of titratable acidity and pH on intensity of sourness of citric, malic, tartaric, lactic and acetic acids solutions and on the overall acceptability of imitation apple juice. Canadian Institute of Food Science and Technology Journal, 22, 46–51. doi:10.1016/S0315-5463(89)70300-x
- Díaz, P., Ros, J.M., & Bañón, S. (2010). Monitoring retrogradation in liquorice-type sweets of different size and hardness. Starke, 62(11), 558–565. doi:10.1002/star.200900205
- Edwards, W.P. (2000). Gums, gelled products and liquorice. In W.P. Edwards (Ed.), The science of sugar confectionery (pp. 121–144). Cambridge: The Royal Society of Chemistry Publishing.
- Evageliou, V., Tseliou, G., Mandala, I., & Komaitis, M. (2010). Effect of inulin on texture and clarity of gellan gels. Journal of Food Engineering, 101, 381–385. doi:10.1016/j.jfoodeng.2010.07.023
- Flamm, G., Glinsman, W., Kritchevsky, D., Prosky, L., & Roberfroid, M. (2001). Inulin and Oligrofructose as a dietary fiber: A review of the evidence. Critical Reviews in Food Science and Nutrition, 41(5), 353–362. doi:10.1080/20014091091841
- Franck, A. (2002). Technological functionality of inulin and oligofructose. British Journal of Nutrition, 87(2), S287–S291. doi:10.1079/BJN/2002550
- Glibowski, P., & Wasko, A. (2008). Effect of thermochemical treatment on the structure of inulin and its gelling properties. International Journal of Food Science and Technology, 43, 2075–2082. doi:10.1111/j.1365-2621.2008.01825.x
- Goncalves, A.A., & Rohr, M. (2009). Development of soft candies with addition of inulin. Alimentación Y Nutrición, 20(3), 471–478.
- Harrington, J.C., & Morris, E.R. (2009). Conformational ordering and gelation of gelatin in mixtures with soluble polysaccharides. Food Hydrocolloids, 23, 327–336. doi:10.1016/j.foodhyd.2008.03.003
- Hébette, C.L.M., Delcour, J.A., Koch, M.H.J., Booten, K., Kleppinger, R., & Mischenkod, N. (1998). Complex melting of semi-crystalline chicory (Chicorium intybus L.) root inulin. Carbohydrate Research, 310, 65–75. doi:10.1016/S0008-6215(98)00154-2
- ISO 4121. (2003). International organization for standardization publications. Sensory analysis methodology. Evaluation of food products by methods using scales. In sensory analysis methodology. Geneva: International Organization for Standardization. Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:4121:ed-2:v1:en
- ISO 8586. (2012). General guidance for the selection and training and monitoring of assessors. Part 1. Selected assessors. In Sensory analysis methodology. Geneva: International Organization for Standardization. Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:8586:ed-1:v2:en
- Kälviäinen, N., Roininen, K., & Tuorila, H. (2000). Sensory characterization of texture and flavor of high viscosity gels made with different thickeners. Journal of Texture Studies, 31, 407–420. doi:10.1111/j.1745-4603.2000.tb00299.x
- Kalyani Nair, K., Kharb, S., & Thompkison, D.K. (2010). Inulin dietary fiber with functional and health attributes – A review. Food Reviews International, 26(2), 189–203. doi:10.1080/87559121003590664
- Kang, K.J., Kim, K., Lee, S.K., & Kim, S.K. (1997). Relationship between molecular structure of acid-hydrolyzed rich starch and retrogradation. Korean Journal of Food Science and Technology, 29(5), 876–881.
- Kim, Y., Faqih, M.N., & Wang, S.S. (2001). Factors affecting gel formation of inulin. Carbohydrate Polymers, 46, 135–145. doi:10.1016/S0144-8617(00)00296-4
- Koh, L., Jiang, B., Kasapis, S., & Foo, C. (2011). Structure, sensory and nutritional aspects of soluble-fiber inclusion in processed food products. Food Hydrocolloids, 25(2), 159–164. doi:10.1016/j.foodhyd.2010.03.013
- Langton, M., & Hermansson, A.M. (1989). Microstructural changes in wheat starch dispersions during heating and cooling. Food Structure, 8(1), 29–39.
- León, O., Santos, M., López, R., & Antolín, G. (2005). Optimización de la hidrólisis ácida de la inulina. Obtención de jugo fermentable para la producción de bioetanol. Ingeniería Química, 423, 199–203.
- Marfil, P.H.M., Anhê, A.C.B.M., & Telis, V.R.N. (2012). Texture and microstructure of gelatin/corn starch-based gummy confections. Food Biophysics, 7, 236–243. doi:10.1007/s11483-012-9262-3
- Matusek, A., Merész, P., Diem Le, T.K., & Örsi, F. (2009). Effect of temperature and pH on the degradation of fructo-oligosaccharides. European Food Research and Technology, 228, 355–365. doi:10.1007/s00217-008-0941-8
- Mochizuki, Y. (2001). Texture profile analysis. In R.E. Wrolstad (Ed.), Current protocols in food analytical chemistry (pp. H2.3.1.-H2.3.7). New York: John Wiley and Sons Inc.
- Muñoz, A.M., Pangborn, R.M., & Noble, A.C. (1986). Sensory and mechanical attributes of gel texture. II. Gelatine, sodium alginate and k-carrageenan gels. Journal of Texture Studies, 17, 17–36. doi:10.1111/j.1745-4603.1986.tb00711.x
- Paradiso, V.M., Giarnetti, M., Summo, C., Pasqualone, A., Minervini, F., & Caponio, F. (2015). Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocolloids, 45, 30–40. doi:10.1016/j.foodhyd.2014.10.027
- Periche, A., Heredia, A., Escriche, I., Andrés, A., & Castelló, M.L. (2014). Optical, mechanical and sensory properties of based-isomaltulose gummy confections. Food Bioscience, 7, 37–44. doi:10.1016/j.fbio.2014.05.006
- Pons, M., & Fiszman, S.M. (1996). Instrumental texture profile analysis with particular reference to gelled systems. Journal of Texture Studies, 27, 597–624. doi:10.1111/j.1745-4603.1996.tb00996.x
- Ritsema, T., & Smeekens, S. (2003). Fructans: Beneficial for plants and humans. Current Opinion in Plant Biology, 6(3), 223–230. doi:10.1016/S1369-5266(03)00034-7
- Roberfroid, M. (1993). Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Critical Reviews in Food Science and Nutrition, 33(2), 103–148. doi:10.1080/10408399309527616
- Roberfroid, M.B. (2005). Introducing inulin-type fructans. British Journal of Nutrition, 93(1), S13–S25. doi:10.1079/BJN20041350
- Rossi, M., Corradini, C., Amaretti, A., Nicolini, M., Pompei, A., Zanoni, S., & Matteuzi, D. (2005). Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Applied and Environmental Microbiology, 71(10), 6150–6158. doi:10.1128/AEM.71.10.6150–6158.2005
- Saint-Eve, A., Déléris, I., Panouillé, M., Dakowski, F., Cordelle, S., Schlich, P., & Souchon, I. (2011). How texture influences aroma and taste perception over time in candies. Chemosensory Perception, 4, 32–41. doi:10.1007/s12078-011-9086-4
- Sandhu, K.S., Singh, N., & Lim, S.T. (2007). A comparison of native and acid thinned normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT-Food Science and Technology, 40, 1527–1536. doi:10.1016/j.lwt.2006.12.012
- Tárrega, A., & Costell, E. (2006). Effect of inulin addition on rheological and sensory properties of fat-free starch-based dairy desserts. International Dairy Journal, 16, 1104–1112. doi:10.1016/j.idairyj.2005.09.002
- Tungland, B.C., & Meyer, D. (2002). Nondigestible oligo- and polysaccharides (dietary fiber): Their physiology and role in human health and food. Comprenhensive Reviews in Food Science and Food Safety, 1(3), 90–109. doi:10.1111/j.1541-4337.2002.tb00009.x
- Wang, Y., Truong, V., & Wang, L. (2003). Structures and rheological properties of corn starch as affected by acid hydrolysis. Carbohydrate Polymers, 52(3), 327–333. doi:10.1016/S0144-8617(02)00323-5
- Yousif, E.I., Gadallah, M.G.E., & Sorour, A.M. (2012). Physico-chemical and rheological properties of modified corn starches and its effect on noodle quality. Annals of Agricultural Science, 57(1), 19–27. doi:10.1016/j.aoas.2012.03.008
- Zimeri, J.E., & Kokini, J.L. (2003). Phase transitions of inulin–waxy maize starch systems in limited moisture environments. Carbohydrate Polymers, 51, 183–190. doi:10.1016/S0144-8617(02)00127-3
