?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The evidence shows that probiotics can reduce cardiovascular diseases associated to high cholesterol levels, but it occurs just when they survive gastrointestinal conditions. The aim of this study was to evaluate the ability of reference strains (RS) and food isolates strains (FIS) to survive acid and bile and to determine in-vitro cholesterol assimilation. FIS were more tolerant to acid than RS, showing significantly different growth response. FIS were more resistant to the presence of bile, in order descendent to oxgall, taurocholic acid, and cholic acid. The survival percentages ranged from 0% to 100%, presenting strain dependence. The most tolerant strains were tested for cholesterol test, showing RS lower percentages than FIS. In FIS, the percentage was negatively affected when the concentration of bile salt was increased. Therefore, to study the viability of different strains in gastrointestinal conditions is crucial because of the strain-dependence nature.
RESUMEN
La evidencia científica muestra que los probióticos pueden reducer enfermedades cardiovasculares asociadas a altos niveles de colesterol pero sólo cuando sobreviven a condiciones gastrointestinales. El objetivo del estudio fue evaluar la supervivencia a ácido y bilis de cepas aisladas de alimentos (FIS) y de referencia (RS) y determinar in vitro, su asimilación de colesterol. FIS mostraron mayor tolerancia al ácido que RS, mostrando diferencias significativas en el crecimiento. FIS mostró mayor resistencia a la bilis; en orden descendente: oxgall, ácido taurocólico y ácido cólico. El porcentaje de supervivencia fue 0–100%, siendo cepa dependiente. Las cepas más tolerantes fueron evaluadas para su asimilación de colesterol, mostrando RS menores porcentajes que FIS. En éstas, el porcentaje fue afectado negativamente al incrementar la concentración de la sal biliar. Por lo tanto, es importante evaluar la viabilidad de las cepas en condiciones intestianles por su caracter cepa-dependiente.
Introduction
Current scientific evidence links high levels of plasma cholesterol with the development of diseases and recognizes plasma cholesterol as one of the principal factors for developing cardiovascular disease. There is strong evidence linking lower levels of cholesterol to a reduced risk of cardiovascular disease. When cholesterol is reduced by 1%, the risk of developing these diseases is reduced by between 2% and 3% (Manson et al., Citation1992; Xie et al., Citation2011). Currently, treatment for hypercholesterolemia entails a combination of diet and drug therapy. However, the long-term use of drugs may lead to adverse side effects for the patient. In addition, there is a great economic investment required to maintain treatment for these chronic conditions (Ooi & Liong, Citation2010; Ledesma Velazco, Citation2011). For this reason, alternative treatments are continuously being sought that may help patients with these conditions improve their health, and to offer more options to counteract symptoms, lower the risks associated with hypercholesterolemia, and improve their quality of life.
Probiotic bacteria are microorganisms that have been scientifically proven to be beneficial to physical health. One of these benefits is the ability to lower serum cholesterol levels. Since Eli Metchnikoff times, the beneficial effects from ‘bulgars’ (later known as probiotics) have been under study. One of the first to link the intake of probiotics with the cholesterol-lowering effects was Mann and Spoerry (Citation1974) in a Massai population. This cholesterol-lowering effect occurs at the intestinal level, making it important for probiotics to reach the colon alive, by surviving the high concentrations of acid and bile salt in the intestinal tract (Kumar et al., Citation2012).
The aim of this study was to investigate, via the use of in-vitro techniques, the ability of different genera of probiotic bacteria (Lactobacillus, Lactococcus, and Bifidobacterium) to survive in the presence of acid and bile and to study their cholesterol assimilation properties.
Material and methods
Biological materials
For the study, RS were achieved by ATCC and DSMZ () and food isolates strains (FIS) from different foods such as yogurt with health claims (4), fermented milk (1), and infant formula (2), all of which were labelled as using these microorganisms in their formulations. The isolation of the strains was performed following the methods by González Martínez (Citation2005): Lactobacillus and Bifidobacterium were identified by API system (bioMériux I’Etoile France); API®CH50 and API 20®A, PCR method, and fructose-6-phosphate phospoketolase test, respectively. The following strains were isolated from these products: Lactococcus lactis lactis, Lactobacillus casei rhamnosus, Lactobacillus pentosus, Lactobacillus paracasei paracasei, Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus casei Shirota, Bifidobacterium spp., and Bifidobacterium lactis. Strains were cryopreserved in glycerol and stored at −20°C or lyophilized and preserved at 4°C. The strains were reactivated in MRS broth (de Man, Rogosa, and Sharpe) (BD®) and incubated at 37°C for 24–48 h under anaerobic conditions.
Table 1. Strains analysed: 1–9 are food (FIS) isolates and 10–17 are reference (RS).
Tabla 1. Cepas analizadas: 1 – 9 son aisladas de alimentos (FIS) y 10 – 17, cepas de referencia (RS).
Cultures were maintained at −4°C and subcultured weekly with 1% v/v of the inoculum in 3 mL of MRS broth and were incubated at 37°C for 48 h under anaerobic conditions.
Acid tolerance
Tolerance to acid was evaluated in the FIS and RS strains, following methods proposed by Pereira and Gibson (Citation2002) with the following modifications: a fresh culture of the strain of interest was centrifuged at 1087.59g for 10 min at 24°C. The pellet was resuspended in MRS broth and adjusted to pH 2 using HCl, then incubated for 2 h under anaerobic conditions with an overlay of sterile mineral oil in the test tube.
Bile tolerance
For this study, the methods described by Liong and Shah (Citation2005) were followed with the following modifications: three MRS broths were prepared that contained oxgall, cholic acid, or taurocholic acid at concentrations of 0.30% w/v. These broths were inoculated with the strain of interest and incubated at 37°C for 8 h under anaerobic conditions, using the Gas Pak system and envelopes (BD®); MRS broth without bile salt was used as a control. Subsequently, quantification of viable organisms was performed by pour plate counts on MRS agar at the beginning and end of the test to determine the survival of the strains tested in the various salt sources. The plates were incubated at 37°C for 48 h under anaerobic conditions using Gas Pak envelopes and the Gas Pak system.
Cholesterol assimilation assay
To test the ability of each of the selected strains to assimilate cholesterol, based on their tolerance to acid and bile, the methods by Pereira and Gibson (Citation2002) were used with the following modifications: strains of interest were inoculated into tubes containing 10 mL MRS broth, 0.2% or 0.4% w/v oxgall, and an acid solution of cholesterol to reach a 100–mg/L final concentration. Cultures were incubated at 37°C for 12 h under anaerobic conditions (with sterile mineral oil). Throughout the incubation period, each hour an aliquot was taken to measure the optical density at 650 nm (un-inoculated sterile broth was used as a control).
After 12 h, cholesterol assimilation was determined as follows. Bacterial cells were removed by centrifugation (3000g for 10 min at 4°C), the supernatant was separated, and the amount of liquid removed was measured. The cells were dried to a constant weight in an 80°C oven and subsequently dissolved in sterile milli-Q water in an equal volume that was initially removed.
Subsequently, the cholesterol assay was performed following the methods described by Gilliland, Nelson, and Maxwell (Citation1985). The percentage of cholesterol assimilation was determined by the equation established by Al-Saleh, Metwalli, and Abu-Tarboush (Citation2006):
where A is the cholesterol remained with the pellet (as percentage), B is the absorbance of the sample containing the cells, and C is the absorbance of the sample without cells. It was observed that sample containing no cells has no pellet and cholesterol was determined in the whole system.
Statistical analysis
The results were statistically analysed using the SPSS 15.0 software. Descriptive statistics were used in all of the results obtained for the analysis of tolerance to acid and bile (n = 2) and of cholesterol assimilation (n = 3). The results for cholesterol assimilation were submitted for analysis of variance, as well as Kruskal–Wallis testing to determine the difference between groups, followed by the post-hoc Duncan test.
Results and discussion
Acid tolerance
The results of strain survival at acidic pH (pH = 2) are presented in . The pH of the stomach is 1.5 (Giannella, Broitman, & Zamcheck, Citation1972) and according to Berrada, Lemeland, Laroche, Thouvenot, and Piaia (Citation1991), the average residence time of food at this pH is 90 min. Thus, according to Chou and Weimer (Citation1999), the in-vitro tolerance tests used to determine if microorganisms have the potential to be selected, as probiotics must demonstrate that they are able to withstand this amount of time and pH without losing viability. In this study, strains isolated from food were more tolerant to the presence of acid compared to reference strains (RS).
Figure 1. Acid survival of FIS (1–9) and RS (11–17) strains at pH = 2. Results are expressed in log10 CFU/time of exposure (n = 2).
Figura 1. Supervivencia de cepas FIS (1–9) y RS (11–17) a pH = 2. Resultados son expresados en log10 de UFC/tiempo de exposición (n = 2).
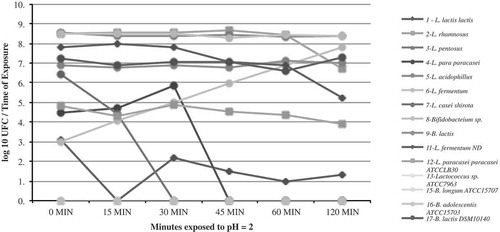
In addition, the acid presence had different effects on the growth of the various strains. Some strains were not tolerant to the presence of acid, as evidenced by the lack of growth in L. pentosus (FIS), and RS as Lactococcus sp. ATCC7963, Bifidobacterium longum ATCC15707, and Bifidobacterium adolescentis ATCC15703. In other cases, probiotic bacteria did not survive the acidic conditions after a 30–45-min interval, as seen in FIS: L. lactis lactis and L. casei. In other bacterial strains, such as Bifidobacterium sp., the growth rate is affected at the start of treatment but is able to recover towards the end. Finally, it is important to emphasize that the growth of FIS: L. fermentum, L. acidophilus, and B. lactis, as well as RS: B. lactis DSM10140, was not affected by the presence of acid.
Liong and Shah (Citation2005) demonstrated survival to acid (pH = 2) of reference probiotic strains; four strains of L. acidophilus, and seven strains of L. casei, and found that growth of L. acidophilus at a low pH decreased 3–5 log units after a 2-h exposure. In this study, FIS of same species proved to be acid tolerant, as its cell density remained the same throughout the treatment and there were observed as a reduction of 0.20 cycles after a 2-h exposure.
Al-Saleh et al. (Citation2006) evaluated the acid tolerance (pH = 2) of three strains of L. acidophilus, two of Bifidobacterium sp. (B. infantis and B. angulatum), and one of Streptococcus thermophilus, observing that the viability of the microorganisms was significantly affected after 1.5 h of incubation. The most tolerant strain was B. angulatum DSM 20098, making evident that acid tolerance is strain dependent. However, this effect varied among strains, in which a range of 10–30% survival was observed. In addition, Tokatl, Gülgör, Bagder Elmacj, Arslankoz Egleyen, and Özçelik (Citation2015) observed that survival of L. brevis strains to pH 2.5 by 4 h was 33–64%; showing strain-dependence variation. In this study was found that strains are not able to tolerate acidity (strains 3 FIS; 13, 15, and 16, RS); strains that partially tolerate acidity, such as strains 4 and 7 (FIS), which showed a total decrease in viability after 30 and 15 min of incubation, respectively; and strains that tolerated an acidic environment for 90 min (strains 8, 9 FIS; and 17 RS). The survival of the rest of the strains ranged between 42% and 98%.
Along with these findings, Al-Saleh et al. (Citation2006) found that adding 1% skim milk to the media increased the survival of the strains to 35%, suggesting that the matrix from which they were isolated, the types of stress to which they were subjected, and the ingredients in the media all influence the tolerance of the strains. It also suggests that the functional and technological properties of the same strain may vary in the presence of different food ingredients (Ranadheera, Baines, & Adams, Citation2010).
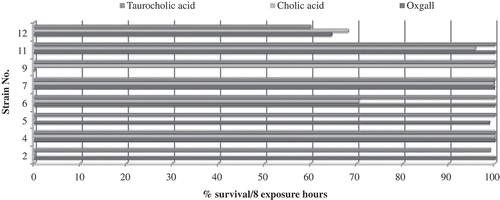
The bacterial survival differs upon treatment with the different bile salts analysed, and that these bile salts affect the survival of the strain. The majority of strains were more tolerant to the presence of oxgall, followed by taurocholic acid and finally cholic acid. In a group of RS of L. acidophilus and L. casei, Liong and Shah (Citation2005) observed strain-dependent tolerance responses when subjected to different bile salts (0.3% cholic acid, taurocholic acid, or oxgall) for a period of 12 h. They also found that these 11 lactococci strains had better tolerance to colic acid than to taurocholic acid, and that tolerance varied from strain to strain, suggesting that tolerance was also a strain-dependent feature.
The survival in each of the different types of bile salts varied between 0% and 100% and was unique to each strain, where 56% tolerated oxgall, 44% tolerated cholic acid, and 67% tolerated taurocholic acid. Importantly, strains such as L. rhamnosus and L. acidophilus can tolerate some bile salts (oxgall and taurocholic acid) but are completely inhibited by another bile salt (cholic acid). This is similar to B. lactis, which is able to survive cholic acid and taurocholic acid, but not oxgall.
Moreover, the RS showed less tolerance, as only 25% of the strains exposed to the different bile salts survived. Survival was between 90% and 100% for L. brevis and between 58% and 62% for L. paracasei paracasei. This variance in tolerance to bile salt was also demonstrated by Sahadeva et al. (Citation2011) in a study that investigated five types of fermented milk sold in Malaysia, which contained one or more of the following microorganisms: L. acidophilus, L. casei Shirota, S. thermophilus, Bifidobacterium sp., and L. casei. These were tested for bile tolerance at concentrations of 0%, 0.3%, and 2.0%. As the concentration of bile increased, the strain viability decreased. However, each group of microorganisms (based on the product from which they were isolated) behaved differently, and L. casei Shirota showed the highest tolerance in the three concentrations that were tested. Those findings – along with the observations made in this study, in which 25% of the strains did not survive in the presence of bile salts () – show that tolerance to bile is strain dependent. Each of these particular characteristics of each microorganism could make them more suitable for use as probiotics in the development of functional foods and supplements.
Figure 2. Bile tolerance of FIS (2–9) and RS strains (11–12). Results are expressed in per cent survival per 8-h exposure to a concentration of 0.3% w/v bile salt (oxgall, cholic acid, or taurocholic acid) (n = 2).
Figura 2. Tolerancia a bilis de cepas FIS (2–9) y RS (11–12). Resultados son expresados en porcentaje de supervivencia por 8 horas de exposición a 0,3% p/v de sales biliares (oxgall, ácido cólico y ácido taurocólico) (n = 2).
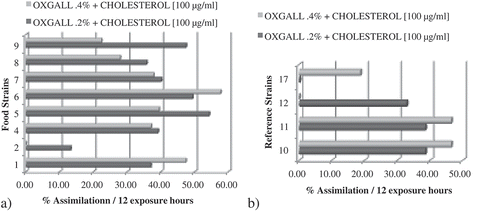
Cholesterol assimilation assay
Some probiotic strains exhibit the ability to absorb cholesterol from the culture medium by various mechanisms, which suggests that when these microorganisms are consumed, they could perform a similar function in the intestine, thus helping to control plasma cholesterol. The microorganisms were subjected to two concentrations of oxgall (0.2% or 0.4% w/v) and 100 μg/mL of cholesterol.
In FIS (1 to 9), the percentage of cholesterol assimilation was negatively affected by increasing the concentration of bile salt in 6/9 strains. In L. lactis lactis and L. fermentum (FIS 1, 6), the percentage of assimilation increased when a higher concentration of bile salt was used. In L. rhamnosus (FIS 2), the percentage of cholesterol assimilation decreased to nearly zero when the concentration of bile salt was increased ().
In RS (10–17), the cholesterol assimilation percentage was lower when compared to the percentage of assimilation by food isolates. In two of these strains (L. fermentum and B. lactis DSM10140), the cholesterol assimilation percentage was directly proportional to the concentration of bile salt. For example, in B. lactis DSM10140 (RS 17), in which slight cholesterol assimilation (0.08%) was detected at 0.2% oxgall concentration but when the concentration of oxgall was increased to 0.4%, the percentage of cholesterol assimilation increased to 18.69%. In contrast, L. paracasei paracasei LB30 (RS 13) show that when the concentration of idle salt is increased, the percentage of cholesterol assimilation was close to zero.
The Levene’s test was performed to assess the quality of the variances, showing that there was no homogeneity between strains and treatments and no significant difference (p < 0.05) was found between the percentages of cholesterol assimilation. When the strains were subjected to a concentration of 0.2% w/v of oxgall, the percentages of cholesterol assimilation ranged from 0.08% to 54.26%. The strains that showed the highest percentage of cholesterol assimilation were L. acidophilus (54.26%), L. fermentum (49.34%), and B. lactis (47.39%), all of them FIS. The strains that showed the lowest percentage of cholesterol assimilation were L. rhamnosus (13.21%) and L. pentosus (4.31%), which were FIS, as well as the RS B. lactis DSM10140 (0.08%). When the concentration of oxgall was increased to 0.4% w/v, cholesterol assimilation varied from 0% to 57.65%. The strains that assimilated the most cholesterol were the FIS L. fermentum (57.65%), L. pentosus (52.54%), and L. lactis lactis (47.36%). The strains that showed the minimum cholesterol assimilation were B. lactis DSM10140 (18.69%), L. paracasei paracasei LB30, all of them RS; and L. rhamnosus (FIS), which showed no assimilation. According to findings by Liong and Shah (Citation2005) and by Tahri, Crociani, Ballongue, and Schneider (Citation1995), strains from Bifidobacterium sp. in the growth phase were able to reduce the amount of cholesterol in the media via cholesterol assimilation when oxgall was used as the bile salt. This is consistent with results, in which we found that strain 8 (FIS) is able to assimilate 35.73% or 27.89% of cholesterol when the concentration of oxgall was 0.2% or 0.4%, respectively.
Gilliland et al. (Citation1985) showed that cholesterol assimilation only occurred when cultures were grown in the presence of bile salts under anaerobic conditions, that this assimilation was directly proportional to the concentration of oxgall (from 0.1% to 0.4%), and that cholesterol assimilation stabilized when the concentration of oxgall reached 0.5%. These observations were seen in the L. acidophilus NCFM strain (human origin). This research shown similar results in strains 1, 3, 10 (FIS), and 17 (RS), in which increasing the bile concentration from 0.2% to 0.4% led to an increase in cholesterol assimilation of between 10.42% and 48.21%. However, the same behaviour was not observed on the other eight strains that were analysed.
Singhal, Joshi, and Chaudhary (Citation2011) found that probiotic strains, specifically Lactobacillus sp., showed the highest cholesterol assimilation when the initial cholesterol concentration in the media was 100 μg/mL (the same concentration used in this study), which suggests that the cholesterol assimilation percentages observed may be the maximum values for the strains analysed.
Using in-vitro studies, Belviso, Giordano, Dolci, and Zeppa (Citation2009) examined the ability of eight Lactobacillus plantarum and five L. paracasei strains, isolated from Italian cheese, to reduce cholesterol. Two strains of L. plantarum and three strains of L. paracasei showed the highest cholesterol assimilation, 19.4% and 6.8%, respectively. These results and those observed here show that even when strains are closely related, their ability to assimilate cholesterol is strain dependent. Similar observations were made by Dilmi-Bouras (Citation2006), noticed considerable variation in cholesterol assimilation among the different species that were studied as well as between strains from the same species. In recent studies, the results observed are similar. Shehata, El Sohaimy, El-Sahn, and Youssef (Citation2016) observed until 43.7% of cholesterol removal ability of Lactococcus lactic subsp. lactis and Tokatl et al. (Citation2015) obtained that L. plantarum and L. brevis strains studied possessed desirable in-vitro properties, as cholesterol assimilation, with percentages between 48.56% and 1.57% and 16.62% and 1.61%, respectively, all being strain dependent. Also (Archer & Halami, Citation2015), L. fermentum strains remained cholesterol since 49–76% ratio. In those studies, researchers mentioned that strains studied could be potentially used in functional food and health products specially where cholesterol reduction is the target, but in vivo studies are required because the mechanism(s) involved in the removal of cholesterol by those probiotic isolates are not completely elucidated.
Conclusion
The ability to tolerate the acid and bile and maintain viability are particular characteristics of each strain and are essential requirements that must be taken into account at the time of choosing a microorganisms for use as probiotic, already that these characteristics would ensure the survival of these strains to the colon which is finally where they perform most functions with documented health benefits. All strains were able to absorb cholesterol; however, the percentage of assimilation is influenced by the type of strain and by the concentration of bile salts, which are exposed.
The characteristics and properties of the probiotics are strain dependent, so it is necessary to notice them up to the level of species or strain, as well as to study its interaction with other organisms and intestinal epithelial cells, and the different arrays that can be managed, to thus ensure the viability and desired effect when using them for the development of functional foods and supplements.
Acknowledgements
The authors thanked Universidad Autónoma de Nuevo León, México for technical and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Saleh, A.A., Metwalli, A.A.M., & Abu-Tarboush, H.M. (2006). Bile salts and acid tolerance and cholesterol removal from media by some lactic acid bacteria and Bifidobacteria. Journal of Saudi Society for Food and Nutrition, 1(1), 1–17.
- Archer, A.C., & Halami, P.M. (2015). Probiotic attributes of Lactobacillus fermentum isolated from human faeces and dairy products. Applied Microbiology and Biotechnology, 99(19), 8113–8123. doi:10.1007/s00253-015-6679-x
- Belviso, S., Giordano, M., Dolci, P., & Zeppa, G. (2009). In vitro cholesterol-lowering activity of Lactobacillus plantarum and Lactobacillus paracasei strains isolated from the Italian Castelmagno PDO cheese. Dairy Science and Technology, 89(2), 169–176. doi:10.1051/dst/2009004
- Berrada, N., Lemeland, J.F., Laroche, G., Thouvenot, P., & Piaia, M. (1991). Bifidobacterium from fermented milks: Survival during gastric transit. Journal of Dairy Science, 74(2), 409–413. doi:10.3168/jds.S0022-0302(91)78183-6
- Chou, L.S., & Weimer, B. (1999). Isolation and characterization of acid-and bile-tolerant isolates from strains of Lactobacillus acidophilus. Journal of Dairy Science, 82(1), 23–31. doi:10.3168/jds.S0022-0302(99)75204-5
- Dilmi-Bouras, A. (2006). Assimilation (in vitro) of cholesterol by yogurt bacteria. Annals of Agricultural and Environmental Medicine, 13(1), 49.
- Giannella, R.A., Broitman, S.A., & Zamcheck, N. (1972). Gastric acid barrier to ingested microorganisms in man: Studies in vivo and in vitro. Gut, 13(4), 251–256. doi:10.1136/gut.13.4.251
- Gilliland, S.E., Nelson, C.R., & Maxwell, C. (1985). Assimilation of cholesterol by Lactobacillus Acidophilus.. Applied and Environmental Microbiology, 49(2), 377–381.
- González Martínez, B.E. (2005). Queso fresco como vehículo para microorganismos probióticos y su efecto sobre el crecimiento de Salmonella enteritidis var. Typhimurium, Compendio de Tesis Doctoral, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León. México: Universidad Autónoma de Nuevo León.
- Kumar, M., Nagpal, R., Kumar, R., Hemalatha, R., Verma, V., Kumar, A., ... Yadav, H. (2012). Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Journal of Diabetes Research, 2012, 1–14. https://doi.org/10.1155/2012/902917
- Ledesma Velazco, M.S. (2011). Las Enfermedades Cardiovasculares en México. ANCAM: www.ancam.org.mx/html/dos/docs/plat-z/DR%2520MARIANO%2520LEDESMAECV%2520EN%2520MEXICO%2520ZACATECAS%2520JUNIO%25202011.ppt
- Liong, M.T., & Shah, N.P. (2005). Acid and bile tolerance and cholesterol removal ability of Lactobacilli strains. Journal of Dairy Science, 88(1), 55–66. doi:10.3168/jds.S0022-0302(05)72662-X
- Mann, G.V., & Spoerry, A. (1974). Studies of a surfactant and cholesteremia in the Maasai. The American Journal of Clinical Nutrition, 27(5), 464–469.
- Manson, J.E., Tosteson, H., Ridker, P.M., Satterfield, S., Hebert, P., O’Connor, G.T., … Hennekens, C.H. (1992). The primary prevention of myocardial infarction. New England Journal of Medicine, 326(21), 1406–1416. doi:10.1056/NEJM199205213262107
- Ooi, L.-G., & Liong, M.-T. (2010). Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. International Journal Of Molecular Sciences, 11(6), 2499–2522. doi:10.3390/ijms11062499
- Pereira, D.I., & Gibson, G.R. (2002). Cholesterol assimilation by lactic acid bacteria and Bifidobacteria isolated from the human gut. Applied and Environmental Microbiology, 68(9), 4689–4693. doi:10.1128/AEM.68.9.4689-4693.2002
- Ranadheera, R.D.C.S., Baines, S.K., & Adams, M.C. (2010). Importance of food in probiotic efficacy. Food Research International, 43(1), 1–7. doi:10.1016/j.foodres.2009.09.009
- Sahadeva, R.P.K., Leong, S.F., Chua, K.H., Tan, C.H., Chan, H.Y., Tong, E.V., & Chan, H.K. (2011). Survival of commercial probiotic strains to pH and bile. International Food Research Journal, 18(4), 1515–1522.
- Shehata, M.G., El Sohaimy, S.A., El-Sahn, M.A., & Youssef, M.M. (2016). Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Annals of Agricultural Sciences, 61(1), 65–75. doi:10.1016/j.aoas.2016.03.001
- Singhal, K., Joshi, H., & Chaudhary, B. (2011). Influence of initial concentrations of cholesterol on the uptake of cholesterol by the standard lactobacillus strains and lactobacillus isolates. IJPI’s Journal of Biotechnology and Biotherapeutics, 1(4), 2–6.
- Tahri, K., Crociani, J., Ballongue, J., & Schneider, F. (1995). Effects of three strains of Bifidobacteria on cholesterol. Letters in Applied Microbiology, 21(3), 149–151. doi:10.1111/j.1472-765X.1995.tb01028.x
- Tokatl, M., Gülgör, G., Bagder Elmacj, S., Arslankoz Egleyen, N., & Özçelik, F. (2015). In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. Biomed Research International, 2015, e315819. doi:10.1155/2015/315819
- Xie, N., Cui, Y., Yin, Y.N., Zhao, X., Yang, J.W., Wang, Z.G., … Lu, F.G. (2011). Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complementary and Alternative Medicine, 11(1), 53. doi:10.1186/1472-6882-11-53