 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, Bacillus licheniformis BFP011 has been cultivated for producing antimicrobial compounds, which were concentrated from the crude supernatant and purified using thin-layer chromatography. The antimicrobial activity was investigated in the presence of several organic solvents and detergents, and inhibiting effects to various Gram-negative and Gram-positive human pathogenic and food spoilage bacteria were observed. Three bands (F4, F5 and F6) showing antimicrobial activity against Salmonella typhi ATCC 5784 were subjected to reversed-phase high-performance liquid chromatography purification. Two peaks of fraction F5, F5-P3 and F5-P4, showed 100% inhibition against S. typhi ATCC 5784. The contained mixture of antimicrobial compounds consists of macrolactins and amicoumacins, which induced the collapse and breaking of S. typhi ATCC 5784 cell membranes in a time-dependent manner. Taken collectively, the results indicate that these compounds may represent promising candidates for the development of food preservative agents of natural origin, as well as novel antimicrobial drug candidates against multiresistant bacterial strains.
RESUMEN
En el presente estudio, se cultivó Bacillus licheniformis BFP011 para producir compuestos antimicrobianos; estos fueron concentrados del sobrenadante crudo y purificado empleando cromatografía en capa fina (CCF). En presencia de varios solventes y detergentes orgánicos se analizó la actividad antimicrobiana, observándose, además, los efectos inhibidores de varias bacterias gram-negativas y gram-positivas, patógenas para los seres humanos y causantes del deterioro de los alimentos. Mediante RP-HPLC se purificaron tres franjas (F4, F5 y F6) que mostraron actividad antimicrobiana contra la Salmonella typhi ATCC 5784, comprobándose que los dos picos de la fracción F5 (F5-P3 y F5-P4) provocan una inhibición del 100% contra la S. typhi ATCC 5784. La mezcla de compuestos antimicrobianos contiene macrolactinas y amicoumacinas; estas produjeron el colapso y el rompimiento de las membranas celulares de S. typhi ATCC 5784 a medida que fue transcurriendo el tiempo. Considerados en su conjunto, estos resultados indican que es posible que dichos compuestos sean sustancias prometedoras para la creación de agentes conservantes de alimentos de origen natural, y para el desarrollo de medicamentos antimicrobianas novedosas contra cepas bacterianas multirresistentes.
1. Introduction
Throughout half a century, numerous industrially important species belonging to the genus of Bacillus have been discovered (Nascimento & Martins, Citation2006; Sun et al., Citation2015; Suwanmanon & Hsieh, Citation2014) and found to be particularly valuable for genetic and biochemical studies. Several hundred wild-type forms of Bacillus sp. have been studied in terms of the potential to produce numerous antibiotics, predominantly peptides including surfactins, fengycins and iturins (Lee et al., Citation2012; Oh et al., Citation2011; Schneider et al., Citation2007; Stein, Citation2005). Antibiotics derived from Bacillus sp. were shown to inhibit the growth of Gram-positive and Gram-negative human pathogenic bacteria and phytopathogens (Lee et al., Citation2012; Wu et al., Citation2005). Apart from peptides, polyketides are the other dominant family of secondary metabolites having antimicrobial, immunosuppressive, antitumor or other physiologically relevant bioactivities. Although polyketides are widespread secondary metabolites from bacteria, only a few have been isolated and characterized from Bacillus (Schneider et al., Citation2007). However, in a recent example employing polyketides produced by B. subtilis as non-peptide-based antibiotics, including macrolactin A, 7-O-macrolactin A and 7-O-succinyl macrolactin A, inhibitory effects on the growth of Staphylococcus aureus rather than killing properties were reported (Romero-Tabarez et al., Citation2006).
Bacillus licheniformis is a Gram-positive, spore-forming soil bacterium that is used for the production of enzymes, antibiotics, biochemicals and consumer products on an industrial scale (Li, Yang, & Mu, Citation2010; Rey et al., Citation2004; Xie, Guan, & He, Citation2012). Previously, sequence homology-based studies revealed the identity of proteins involved in the production of antibiotics, leading to the detection of two mesarcidin-like peptide lichecidins in the cell-free culture supernatant of B. licheniformis (ATCC 14580 and DSM 13, respectively). Both peptides exhibited antimicrobial activity against Listeria monocytogenes, methicillin-resistant S. aureus and vancomycin-resistant Enterococcus strains (Baruzzi, Quintieri, Morea, & Caputo, Citation2011; Dischinger, Josten, Szekat, Sahl, & Bierbaum, Citation2009).
In another recent study, antimicrobial compounds derived from B. licheniformis BFP011 isolated from papaya (Thailand) were shown to inhibit the growth of several important phytopathogens, as well as human pathogenic and food spoilage bacteria. After biochemical testing and preliminary characterization of the peptide properties, several other small peptides or molecules were proposed to contribute to the overall observed antimicrobial activity (Arbsuwan, Sirithorn, Daduang, Dhiravisit, & Thammasirirak, Citation2014). In the present study, partial purification and characterization of these compounds by liquid chromatography-electrospray ionization-tandem mass spectrometry as well as determination of their antimicrobial activity against Salmonella typhi ATCC 5784 were investigated in more detail. In addition, the mode of action of the antimicrobial compounds against S. typhi ATCC 5784 was elucidated using scanning electron microscopy (SEM).
2. Materials and methods
2.1. Microorganisms
B. licheniformis BFP011 was cultivated for the production of antimicrobial compounds (Arbsuwan et al., Citation2014). Gram-positive bacteria (B. pumilus TISTR 905, B. amyloliquences TISTR 1045, B. subtilis TISTR 008, B. subtilis TISTR 6633, B. licheniformis TISTR 1010, B. cereus ATCC 11778, B. megaterium [clinical isolate], S. aureus 1466, S. aureus ATCC 25923, S. epidermidis ATCC 12228) and Gram-negative bacteria (Escherichia coli ATCC 25922, E. coli O157:H7, Klebsiella pneumoniae ATCC 17736, S. typhi ATCC 5784, S. typhi DMST 22842 and Vibrio cholerae [clinical isolate]) were used as indicator strains for antimicrobial activity determination. All bacteria were propagated in fresh nutrient broth (NB) and nutrient agar (NA) (HiMedia, Mumbai, India) before use.
2.2. Culture conditions and production of antimicrobial compounds
Bacterial cell culturing and production of antimicrobial compounds was performed as described elsewhere (Arbsuwan et al., Citation2014). Briefly, B. licheniformis BFP011 was grown on NA for 48 h at 30°C. A single bacterial colony was inoculated in 20 mL fresh NB. The culture medium was shaken at 120 rpm for 12 h at 30°C. Then, the culture was transferred to 200 mL of the fresh medium (OD600 = 0.1). The cultures were incubated under the same conditions for 48 h before the supernatant was obtained by centrifugation at 12,000 × g for 15 min at 4°C. The cell-free culture medium was filtered through a 0.2-µm pore size membrane (Sartorius Stedim Biotech, Göttingen, Germany) and concentrated at 50°C using a rotary vacuum evaporator to yield a 100-fold decrease in total volume. The concentrated crude BFP011 supernatant was stored at −20°C until use.
2.3. Purification of antimicrobial compounds
2.3.1. Thin-layer chromatography (TLC)
The concentrated crude BFP011 supernatant was dissolved in methanol and spotted onto TLC silica gel plates F254 (20 × 20 cm, Merck, Darmstad, Germany). The sample was eluted using n-butanol:ethanol:acetic acid:water (v/v, 6:12:1:6) as the mobile phase system. Afterwards, the TLC plate was dried at 80°C and the spots observed under ultraviolet (UV) at 254 and 365 nm. Each separated spot was analyzed in terms of its Rf value. The substance was then extracted by eluting overnight with methanol at 4°C. Afterwards, the samples were separated by centrifugation at 4°C for 10 min and concentrated using a speed vacuum concentrator. The sample was dissolved in deionized water for further experiments (Arbsuwan et al., Citation2014).
2.3.2. Reversed-phase high-performance liquid chromatography (RP-HPLC)
Compounds obtained from the TLC assay were purified by RP-HPLC according to the method of Sharma, Singh, and Kakkar (Citation2010) with small modifications. Briefly, the samples were filtered through a 0.2-µm membrane (Vertical, Thailand) before applying to a C18 reversed-phase column (ODS C18, 5 µm particle size, 250 × 4.6 mm ID, Japan). The antimicrobial compounds were separated using a gradient solvent system of 0.1% trifluoroacetic acid in deionized water (A) and 60% acetonitrile in 0.1% trifluoroacetic acid (B) as follows: 0–50 min (50% B), 50–80 min (100% B), 80–85 min (0% B) with a flow rate of 1.0 mL/min. The progress of the elution was monitored at 220 nm and the eluted peaks were collected and dried using a speed vacuum concentrator. Each peak fraction was dissolved in deionized water for determination of antimicrobial activity via a liquid growth inhibition assay.
2.4. Protein concentration determination
Protein concentrations were determined by the method of Bradford using bovine serum albumin as the standard (Bradford, Citation1976).
2.5. Biochemical properties determination
Depending on the individual experiment, 50% v/v of organic solvent (acetone, n-butanol, chloroform, methanol or toluene), 10% v/v of detergent (SDS, triton X-100 or tween 20) or 10 mM ethylenediaminetetraacetic acid (EDTA) were used in the assay. The concentrated crude BFP011 supernatant treated with the respective organic solvents, detergents or EDTA was incubated at 37°C for 1 h. The remaining antimicrobial activity against S. typhi ATCC 5784 was determined using a disc diffusion assay. The pure organic solvents, detergents or EDTA and concentrated crude BFP011 supernatant dissolved in NB were used as negative and positive controls, respectively.
2.6. Antimicrobial activity determination
2.6.1. Disc diffusion assay
A disc diffusion assay was used to preliminarily determine the ability of the antimicrobial substances to inhibit the growth of various Gram-positive bacteria (B. amyloliquences TISTR 1045, B. cereus ATCC 11778, B. licheniformis TISTR 1010, B. megaterium [clinical isolate], B. pumilus TISTR 905, B. subtilis TISTR 008, B. subtilis TISTR 6633, S. aureus ATCC 25923, S. aureus 1466 and S. epidermidis ATCC 12228) and Gram-negative bacteria (E. coli ATCC 25922, E. coli O157:H7, K. pneumoniae ATCC 17736, S. typhi ATCC 5784, S. typhi DMST 22842 and V. cholerae [clinical isolate]). Briefly, the indicator strain suspensions (OD600 ~ 0.01) were spread on NA plates. Thirty microliters of concentrated crude BFP001 supernatant diluted in NB by two-fold serial dilution were spotted on paper discs (6.0 mm), corresponding to 0.15–39 µg protein per disc, which were placed on plates inoculated with pathogenic bacteria. Plates were incubated overnight at 30°C (V. cholerae) or 37°C (other indicator strains). The inhibition zones were measured around the discs and all susceptibility determination experiments against individual indicator strains were performed in triplicate. In addition, NB and NB containing 10 µg streptomycin (Oxoid, Hampshire, UK) were used as a negative and positive control, respectively.
2.6.2. Microtiter plate-based antibacterial assay
The antimicrobial activity against S. typhi ATCC 5784 of all fractions obtained from RP-HPLC purification was tested by liquid growth inhibition assays in 96-well microtiter plates. Briefly, a single colony of bacteria was inoculated into NB and cultured at 37°C for 6 h. One hundred microliters of mid-logarithmic phase bacteria culture (OD600 = 0.001) were transferred into 96-well microtiter plates, followed by addition of 15 µL samples of the respective RP-HPLC peaks (triplicate for each peak). Bacterial growth was assayed by measurement of the optical density at 550 nm after 18–20 h incubation at 37°C. Deionized water and 10 µg/µL streptomycin were used as a negative and positive control, respectively. Finally, the percentage of inhibition was calculated using the formula:
2.7. Characterization of antimicrobial compounds using Nanoflow-liquid chromatography-quadropole-time-of-flight-mass spectrometry (Nanoflow-LC-Q-TOF-MS) technique
The LC-MS analysis was performed on a nano-LC (Easy-nLC II, Bruker Daltonics, Billerica, MA, USA) directly connected to a Hybrid Quadrupole-Time-of-Flight (MicrOTOF-Q II, Bruker Daltonics) mass spectrometer equipped with a Captive Spray Ionization (Bruker Daltonics). Easy-Columns were used as analytical (10 cm, ID 75 μm, 3 μm particle size, C18) and trapping columns (2 cm, ID 100 μm, 5 μm particle size, C18), respectively. Samples were injected as 2–3 µL (~500–750 ng) aliquots. The separation on the analytical column was achieved at a flow rate of 500 nL/min. The sample was eluted using a linear gradient from 5% of solution A (0.1% formic acid) to 55% of solution B (0.1% formic acid in acetonitrile) up to 25 min and 80% of solution B up to 30 min. The instrument was operated in positive ion mode using a range of 50–3000 m/z. The capillary voltage of the ion source, flow rate of dry gas and dry temperature were set as 1500 V, 3.0 L/min and 160°C, respectively.
2.8. Scanning electron microscopy
SEM was performed according to the method of Thirabunyanon and Thongwittaya (Citation2012) with modifications. S. typhi ATCC 5784 was grown for 6 h at 37°C in NB until reaching the logarithmic phase. The cell pellets were collected by centrifugation at 3000 × g for 5 min and washed three times with phosphate-buffered saline, pH 7.0. Afterwards, the pellets were resuspended to 106 CFU/mL final concentrations. One hundred microliters of bacteria suspension were incubated with 30 µL of samples (F5-P3 or F5-P4) from RP-HPLC separation at 37°C for 1 and 2 h. One hundred and thirty microliters of samples were loaded onto a 0.2-µm polycarbonate membrane filter (Whatman, Dassel, Germany) and the cells were carefully fixed on the membrane with equal volumes of 2.5% w/v of glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 1 h. The fixed cells were dehydrated by rinsing (for 15 min) repeatedly with ethanol solutions, which were elevated stepwise to 30%, 50%, 70%, 90% and 100% ethanol. The dehydrated material in absolute ethanol was dried overnight. Dried material was coated using a sputter coater (SC7620; Polaron, Hertfordshire, England) with gold palladium and examined by SEM (SNE-4500M, SEC, Kyunggido-do, South Korea) operating at 30 kV. Deionized water was used as a negative control.
2.9. Statistical analysis
Statistical values of all experiment results were calculated using analysis of variance, followed by least significant different. These data are presented as mean ± SD. A value of p < 0.05 was accepted as being significant (*p < 0.05).
3. Results and discussion
3.1. Purification of antimicrobial compounds
3.1.1. Partial purification of crude BFP011 supernatant by TLC technique
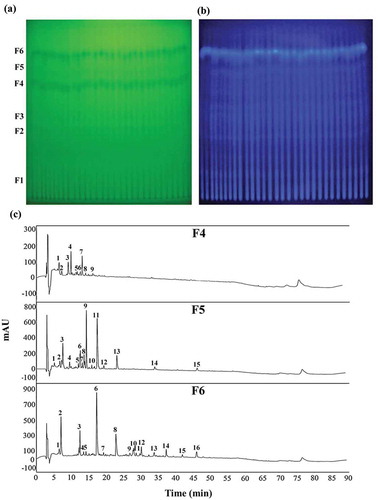
A previous study conducted in our lab demonstrated that the antimicrobial substances of B. licheniformis BFP011 produced in stationary phase cultures might be secondary metabolites (Arbsuwan et al., Citation2014). Analysis of extracts partially purified using TLC technique revealed six spots, which easily quenched UV at 254 nm ()). This phenomenon is well-known to be caused by compounds comprising conjugated double bonds, such as macrolactins, bacillaene, difficidin and dihydrobacillaene (Butcher et al., Citation2007; Schneider et al., Citation2007). In addition, one spot appearing under UV at 365 nm ()) could be stained with 0.3% ninhydrin (UNIVAR, Ingleburn, NSW, Australia, 0.3 g ninhydrin in 100 mL n-butanol and 3 mL acetic acid) (data not shown). This result indicated the presence of free amino acids in these antimicrobial substances. The Rf values of compounds visible under UV at 254 nm were calculated to be 0.12 ± 0.00, 0.24 ± 0.00, 0.29 ± 0.00, 0.35 ± 0.00, 0.48 ± 0.00 and 0.55 ± 0.01 for fractions F1, F2, F3, F4, F5 and F6, respectively. From our previous study, it was known that the antimicrobial substances of B. licheniformis BFP011 comprise peptides and unsaturated fatty acids and contain free amino groups (Arbsuwan et al., Citation2014). The antimicrobial activity of all fractions was determined by a disc diffusion assay, showing that three factions (F4, F5 and F6) exhibited higher antimicrobial activity against S. typhi ATCC 5784 than other fractions (data not shown). Therefore, the most active fractions (F4, F5 and F6) were selected for further purification and characterization by RP-HPLC.
Figure 1. TLC-based analysis of antimicrobial compounds from concentrated crude Bacillus licheniformis BFP011 cell culture supernatant. TLC separation was conducted using a solvent mixture of n-butanol:ethanol:acetic acid:water (v/v, 6:12:1:6) and compounds were detected under UV at 254 (a) and 365 nm (b). Fractions F1–F6 were subjected to Rf calculation and a chromatographic separation of active antimicrobial compounds (F4, F5 and F6) was constructed using C18 RP-HPLC (c). Individual peaks were detected by UV absorption at 220 nm.
Figura 1. Análisis basado en la CCF de compuestos antimicrobianos procedentes del sobrenadante concentrado crudo del cultivo celular de Bacillus licheniformis BFP011. Mediante CCF se realizó la separación, utilizando para ello una mezcla de disolventes de n-butanol: etanol: ácido acético: Agua (v/v, 6: 12: 1: 6); los compuestos fueron detectados por medio de luz UV a 254 nm (a) y 365 nm (b). Las fracciones F1 a F6 fueron sometidas al cálculo Rf; y se construyó una separación cromatográfica de compuestos antimicrobianos activos (F4, F5 y F6) mediante C18 RP-HPLC (c). Los picos individuales fueron detectados empleando absorción UV a 220 nm.
3.1.2. Purification of antimicrobial compounds using RP-HPLC
The fractions F4, F5 and F6, which had been separated and extracted from TLC plates, were subjected to C18 RP-HPLC purification, resulting in 9, 15 and 16 separated peaks for fractions F4, F5 and F6, respectively ()). The chromatogram indicates mostly hydrophilic properties for fractions F4, F5 and F6.
3.2. Biochemical properties determination
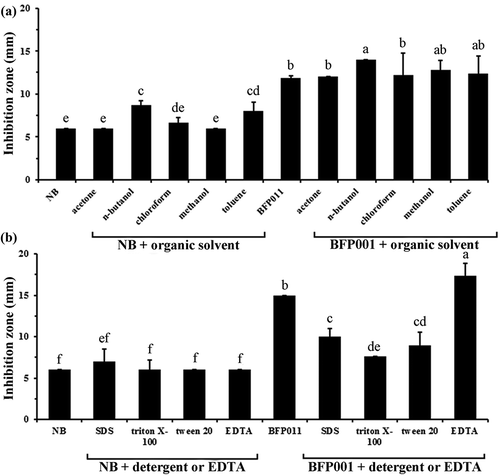
The effects of organic solvents, detergents and EDTA on the antimicrobial activity of concentrated crude BFP011 supernatant are shown in . Mixing the concentrated crude BFP011 supernatant with different organic solvents (acetone and chloroform) had no observable effect on the antibacterial activity against S. typhi ATCC 5784. In contrast, co-incubation of concentrated crude BFP011 supernatant with n-butanol, methanol and toluene slightly enhanced the observed antibacterial activity ()). However, the combination of concentrated crude BFP011 supernatant and different detergents (10% SDS, triton X-100, tween 20) resulted in diminished antibacterial activity, with the exception of 10 mM EDTA ()). A preceding study covering the stability of antimicrobial substances produced by B. licheniformis indicated that the antimicrobial activity was not affected by treatment with pronase and incubation at 37°C and 70°C (against B. megaterium), or by autoclaving at 100°C and 121°C (against S. typhi ATCC5784) (Arbsuwan et al., Citation2014). Previously, the bacteriocin-like inhibitory substance (BLIS) identified from B. licheniformis IITTRHR2 was found to possess remarkable tolerance for temperature and pH stress. However, this substance is sensitive to proteolytic enzymes (α-chymotrypsin, proteinase K and pronase E) and its activity is inhibited by the presence of α-amylase. In addition, it was shown to be resistant to various organic solvents, including chloroform, n-butanol and acetone, while on the other hand, BLIS was found labile in the presence of toluene, triton X-100 and TCA (Sharma et al., Citation2010). Another example of an antibiotic peptide produced from a thermotolerant B. licheniformis strain was addressed by Mendo, Faustino, Sarmento, Amado, and Moir (Citation2004). They reported a new antibiotic peptide, which is thermostable and highly resistant to several proteolytic enzymes, retaining about 85% and 20% of its activity after incubation for 6 h at 50°C and 100°C. Taken collectively, the results suggest that the antimicrobial properties of concentrated crude BFP011 supernatant are of nonprotein origin and small compounds or polyketides might instead be responsible for the observed activity.
Figure 2. Effect of organic solvents (a), detergents and EDTA (b) on the antimicrobial activity of concentrated crude BFP011 supernatant. The growth inhibition of Salmonella typhi ATCC 5784 was determined using a disc diffusion assay. Different letters indicate significant differences between individual groups (p < 0.05). Values represent means ± SD (N = 3).
Figura 2. Efecto de solventes orgánicos (a), detergentes y EDTA (b) en la actividad antimicrobiana del sobrenadante concentrado crudo BFP011. La inhibición del crecimiento de la Salmonella typhi ATCC 5784 se determinó mediante el uso de análisis de difusión por disco. Las letras diferentes indican diferencias significativas entre los grupos individuales (p < 0,05). Los valores representan medias ± DE (N = 3).
3.3. Antimicrobial activity determination
The antimicrobial activity and bacterial susceptibility of a two-fold serial dilution of concentrated crude BFP011 supernatant was determined using disc diffusion assays against various Gram-positive and Gram-negative pathogenic bacteria (). The results show that the antimicrobial compounds in crude BFP011 supernatant exhibit a broad spectrum of antimicrobial activity against all indicator strains. At concentrations higher than 19.5 µg, the growth of all Gram-positive and Gram-negative bacteria was effectively inhibited. Among the Gram-positive bacteria, which were generally found more susceptible to crude BFP011 supernatant than Gram-negative bacteria, B. megaterium (clinical isolated) and S. epidermidis ATCC 12228 displayed the highest susceptibility to crude BFP011 supernatant (≤0.15 µg). Within the tested Gram-negative bacteria, V. cholerae (clinical isolate) showed the greatest sensitivity towards the antimicrobial compounds in crude BFP011 supernatant (≤0.3 µg). In addition, the growth of three bacterial strains – specifically, E. coli O157:H7, S. typhi DMST 22842 and S. typhi ATCC 5784 – was found inhibited at a concentration of 9.75 µg of crude supernatant. This result is not surprising, since many antibacterial compounds act on the synthesis of peptidoglycan, a substance making up roughly 90% of the cell wall in Gram-positive bacteria, but only 10% in Gram-negatives. Therefore, Gram-positives are expected to be inherently more susceptible to such antibacterial compounds and antibiotics. Despite being a Gram-negative strain, S. typhi ATCC 5784 was chosen as the indicator strain for the following studies due its clear association with human diseases and its ability to growth at low temperature, which represents a severe risk for contamination in the food industry.
Table 1. Sensitivity of various pathogenic and food spoilage indicator strains toward crude antimicrobial supernatant from Bacillus licheniformis BFP011.
Tabla 1. Sensibilidad de varias cepas patógenas e indicadoras del deterioro de los alimentos ante el sobrenadante antimicrobiano crudo de Bacillus licheniformis BFP011.
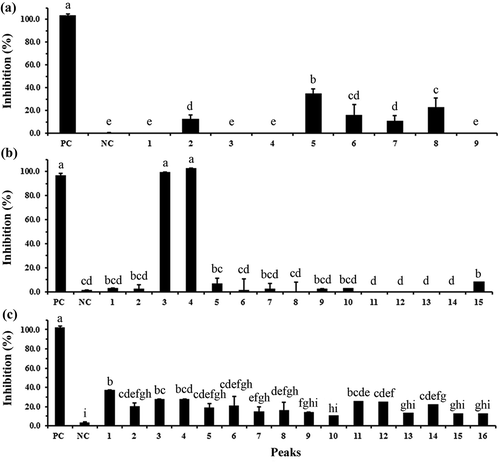
The individual constituents of crude BFP011 supernatant have been separated using a TLC-based approach. Among the resulting fractions, F4, F5 and F6 were found to possess the highest antimicrobial activity and have thus been further purified using RP-HPLC. The antimicrobial activity of the resulting peak fractions was then determined using a liquid growth inhibition assay in a 96-well plate format (). The results of this assay indicate fraction F4-P5 displays the highest bacterial growth inhibition (Equation (1)) of 29% ()) within TLC fraction F4. Among all fractions, the highest antimicrobial activity against pathogenic bacteria was found for fractions F5-P3 and F5-P4, each reaching 100% inhibition ()). Moreover, fractions F6-P1, F6-P3 and F6-P11 showed inhibition values against tested bacteria of 37%, 26% and 26%, respectively ()). Based on achieving the highest antimicrobial activity, fractions F5-P3 and F5-P4 were selected for elucidation of substance composition using Nanoflow-LC-Q-TOF-MS, as well as investigation of the mode of antibacterial action against S. typhi ATCC 5784 using SEM.
Figure 3. Antimicrobial activity of each separated peak from C18 RP-HPLC purification of fraction F4 (a), fraction F5 (b) and fraction F6 (c) against Salmonella typhi ATCC 5784 using a liquid growth inhibition assay in a 96-well plate. Different letters indicate significant differences between individual groups (p < 0.05). Values represent means ± SD (N = 3). Streptomycin (10 µg/µL) and deionized water were used as a positive (PC) and negative (NC) control, respectively.
Figura 3. Actividad antimicrobiana de cada pico por separado, procedente de la purificación mediante C18 RP-HPLC de la fracción F4 (a), de la fracción F5 (b) y de la fracción F6 (c) contra Salmonella typhi ATCC 5784, evaluando la inhibición del crecimiento líquido en una placa de 96 pocillos. Las distintas letras indican la presencia de diferencias significativas entre los grupos individuales (p < 0,05). Los valores representan las medias ± DE (N = 3). Se emplearon estreptomicina (10 µg/µL) y agua desionizada como control positivo (PC) y negativo (NC), respectivamente.
3.4. Nanoflow-LC-Q-TOF-MS analysis of purified antimicrobial compounds
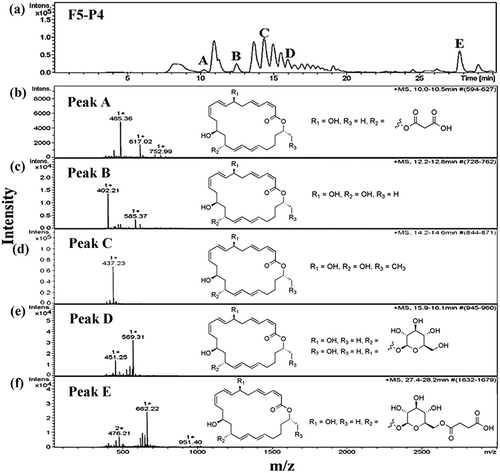
The molecular mass of the most antimicrobially active fractions F5-P3 and F5-P4 was determined using a Nanoflow-LC-Q-TOF-MS. The LC chromatogram of fraction F5-P3 revealed five individual peaks ()), which were then identified using electrospray ionization-tandem mass spectrometry. Mass spectrometric analysis of the fraction F5-P3 showed molecular mass clusters [M + H]+ at m/z ratios of 485.36 ()), 569.31 ()), 441.18 ()) and 409.16 ()) relative to peaks A, B, C and D, respectively. These compounds were identified as 7-O-malonyl macrolactin A (Romero-Tabarez et al., Citation2006), macrolactin B or C (Kang et al., Citation2012), N-acetylated amicoumacin B (Itoh, Omoto, Nishizawa, Kodama, & Inouye, Citation1982) and amicoumacin C (Pinchuk, Bressollier, Sorokulova, Verneuil, & Urdaci, Citation2002), respectively (). In addition, the five major peaks in the LC chromatogram for fraction F5-P4 ()) were associated with mass peaks [M + H]+ at m/z ratios of 485.36 ()), 402.21 ()), 437.23 ()), 569.31 ()) and 662.22 ()), which were identified as 7-O-malonyl macrolactin A, macrolactin A (Romero-Tabarez et al., Citation2006), macrolactin M (Nagao, Adachi, Sakai, Nishijima, & Sano, Citation2001), macrolactin B or C and, macrolactin D (Kang et al., Citation2012), respectively (). These results suggest that the pronounced antimicrobial activity against S. typhi ATCC 5784 is strongly dependent on the composition of macrolactins and amicoumacins in the mixture.
Table 2. Antimicrobial compounds from Bacillus licheniformis BFP011 detected by Nanoflow-liquid chromatography-quadropole-time-of-flight-mass spectrometry (Nanoflow-LC-Q-TOF-MS).
Tabla 2. Compuestos antimicrobianos de la Bacillus licheniformis BFP011 detectados por el sistema LC de nanoflujo-cuadripolo-espectrometría de masas de tiempo de vuelo (Nanoflow-LC-Q-TOF-MS).
Figure 4. Chromatogram of antimicrobial compounds of fraction F5-P3 analyzed by HPLC (a) and ESI-MS (b–e). Peak A was identified as 7-O-malonyl macrolactin A (b), Peak B was identified as macrolactin B or C (c), Peak C was identified as N-acetylated amicoumacin B (d) and Peak D was identified as amicoumacin C (e). All mass spectra were detected in positive ion mode (m/z represents mass-to-charge ratio).
Figura 4. Cromatograma de compuestos antimicrobianos de la fracción F5-P3 analizada por HPLC (a) y ESI-MS (b-e). El pico A fue identificado como 7-O-malonil macrolactina A (b), el pico B como macrolactina B o C (c), el pico C como N-acetilatada amicoumacina B (d) y el pico D como amicoumacina C (e). Todos los espectros de masa fueron detectados en el modo de ion positivo (m/z representa la relación masa/carga).
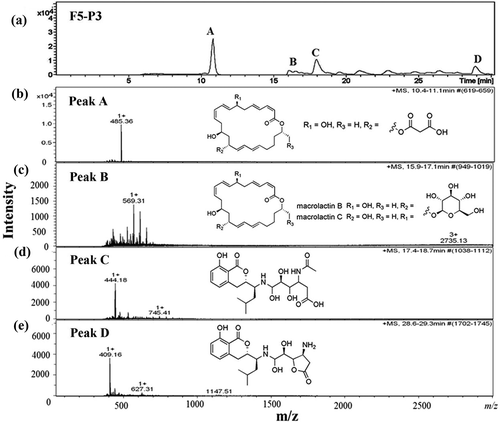
Figure 5. Chromatogram of antimicrobial compounds of fraction F5-P4 analyzed by HPLC (a) and ESI-MS (b–f). Peak A was identified as 7-O-malonyl macrolactin A (b), Peak B was identified as macrolactin A (c), Peak C was identified as macrolactin M (d), Peak D was identified as macrolactin B or C (e) and Peak E was identified as macrolactin D (f). All mass spectra were recorded in positive ion mode (m/z indicates mass-to-charge ratio).
Figura 5. Cromatograma de compuestos antimicrobianos de la fracción F5-P4 analizada por HPLC (a) y ESI-MS (b-f). El pico A fue identificado como 7-O-malonil macrolactina A (b), el pico B como macrolactina A (c); el pico C como macrolactina M (d); el pico D como macrolactina B o C (e); y el pico E como macrolactina D (f). Todos los espectros de masa fueron detectados en el modo de ion positivo (m/z representa la relación masa/carga).
3.5. Scanning electron microscopy
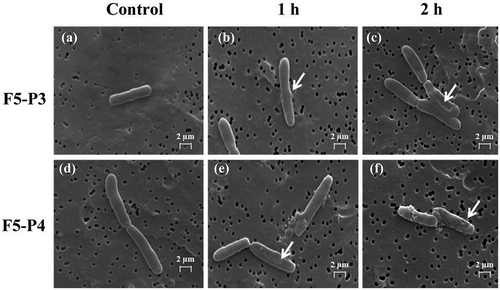
The morphology of S. typhi ATCC 5784 cell membranes was examined after treatment with purified antimicrobial compound fractions F5-P3 and F5-P4 using SEM (). Changes of the cell membrane conformation were observed after treatment with both antimicrobial compounds mixtures. Fraction F5-P3 was found to induce deformation of the cell membranes (,)), while treatment with fraction F5-P4 resulted in formation of blebs on the cell surface as well as changes of cell conformation after incubation for 1 and 2 h (,)). Conversely, untreated cells were characterized by regular, smooth surface membranes (,)). From these results, a high antimicrobial activity was concluded for both peaks (P3 and P4) of fraction F5. Although the detailed mechanism of action for fractions F5-P3 and F5-P4 remains speculative, it is assumed to involve the induction of a number of deleterious morphology changes to the affected bacterial cells, ultimately resulting in time-dependent breakage of the cell membrane. An investigation of the related mechanism of 7-O-malonyl macrolactin A on vancomycin-resistant Enterococci (VREs) and methicillin-resistant S. aureus was conducted by Romero-Tabarez et al. (Citation2006), revealing that the compound inhibited the growth of the tested strain via inducing disruption of cell division. Thus, macrolactin is assumed to restrict the growth of bacteria (bacteriostatic action) rather than to effect direct killing (bactericidal action) (Yuan et al., Citation2012). Amicoumacin has been classified as a group of six compounds sharing the same backbone, termed amicoumacin A, B, C, D, E and F (Itoh et al., Citation1982; Shimojima, Hayashi, Ooka, & Shibukawa, Citation1982; Shimojima, Hayashi, Ooka, Shibukawa, & Iitaka, Citation1982, Citation1984). Among these, amicoumacin A and B are considered the major representative compounds of the family (Shinkaruk et al., Citation2008). The mode of action of amicoumacin A has been associated with inhibition of protein synthesis by suppressing the progression of the ribosome along the mRNA (Polikanov et al., Citation2014). Taken collectively, these results suggest that the mixture of antimicrobial compounds contained in crude B. licheniformis BFP011 supernatant may prove useful to prevent infections with food-borne pathogens. Based on the suggested mode of action, this compound mixture may also provide an effective alternative treatment option specifically against multidrug-resistant Gram-positive bacterial pathogens, including methicillin-resistant S. aureus, VREs and a small-colony variant of Burkholderia cepacia (Kim et al., Citation2011; Romero-Tabarez et al., Citation2006). Notably, amicoumacin antibiotics have further been associated antifungal, anticancer and anti-inflammation activity (Park, Perez, Perry, & Crawford, Citation2016), which may broaden the scope of potential applications for substances produced by B. licheniformis BFP011 even beyond the use as antimicrobial agents.
Figure 6. Cell morphology changes of Salmonella typhi ATCC 5784 observed using scanning electron microscopy. Tested cells were treated with fraction F5-P3 and F5-P4 for 1 and 2 h. Negative control (a) and (d), F5-P3 treated cells after 1 h (b), F5-P3 treated cells after 2 h (c), F5-P4 treated cells after 1 h (e) and F5-P4 treated cells after 2 h (f).
Figura 6. Cambios en la morfología celular de Salmonella typhi ATCC 5784 observados mediante microscopía electrónica de barrido. Las células investigadas fueron tratadas con las fracciones F5-P3 y F5-P4 durante 1 y 2 horas. Control negativo (a) y (d), células tratadas con F5-P3 después de 1 hora (b), células tratadas con F5-P3 después de 2 horas (c), células tratadas con F5-P4 después de 1 hora (e) y células tratadas con F5-P4 después de 2 horas (f).
4. Conclusion
The present work aimed to identify antimicrobial substances contained in the crude supernatant of cultivated B. licheniformis BFP011. Taken collectively, the obtained results suggest that the majority of the antimicrobial substances secreted by this bacterial strain are non-peptidic metabolites, which display a broad spectrum of antimicrobial activity. In addition, elucidation of the identity of these compounds revealed several isoforms of macrolactins and amicoumacins to be the main antimicrobial constituents of B. licheniformis BFP011 cell culture supernatant. It is concluded from the observed spectrum of antibacterial activity that these compounds may serve as useful templates for the development of food preservative agents of natural origin. Notably, the scope of potential applications would not be limited to suppressing the bacterial growth in food products, but also include novel antimicrobial therapeutics, which could help to promote human health by lowering the risks associated to infection with multiresistant bacteria.
Acknowledgments
The authors wish to thank in particular the Department of Biochemistry, Faculty of Sciences, Khon Kaen University, Khon Kaen, Thailand, and Department of Systems Biotechnology, Chung-Ang University, Anseong, Republic of Korea, for providing essential research facilities. This research was partially supported by the Center of Excellence on Agricultural Biotechnology, Science and Technology Postgraduate Education and Research Development Office, Office of Higher Education Commission, Ministry of Education (AG-BIO/PERDO-CHE) and Agricultural Biotechnology Research Center for Sustainable Economy, Khon Kaen University; Protein and Proteomics Research Center for Commercial and Industrial Purposes, Fermentation Research Center for Value Added Agricultural Products, Faculty of Science and Faculty of Technology, Khon Kaen University.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arbsuwan, N., Sirithorn, P., Daduang, S., Dhiravisit, A., & Thammasirirak, S. (2014). Purification and characterization of antimicrobial substances from Bacillus licheniformis BFP001. Applied Biochemistry and Microbiology, 50, 580–587. doi:10.1134/S0003683814110015
- Baruzzi, F., Quintieri, L., Morea, M., & Caputo, L. (2011). Antimicrobial compounds produced by Bacillus spp. and application in food. In A. Méndes-Vilas (Eds.), Science against microbial pathogens: Communicating current research and technological advances (Vol. 2, pp. 1102–1111). Badajos, Spain: Formatex research center.
- Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Butcher, R.A., Schroeder, F.C., Fischbach, M.A., Straight, P.D., Kolter, R., Walsh, C.T., & Clardy, J. (2007). The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proceedings of the National Academy of Sciences of the United States of America, 104, 1506–1509. doi:10.1073/pnas.0610503104
- Dischinger, J., Josten, M., Szekat, C., Sahl, H.G., & Bierbaum, G. (2009). Production of the novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13. PLoS One, 4, e6788. doi:10.1371/journal.pone.0006788
- Itoh, J., Omoto, S., Nishizawa, N., Kodama, Y., & Inouye, S. (1982). Chemical structures of amicoumacins produced by Bacillus pumilus. Agricultural and Biological Chemistry, 46, 2659–2665. doi:10.1271/bbb1961.46.2659
- Kang, J.S., Kim, C.G., Kim, D.H., Kim, K.M., Kim, D.H., Lee, J.Y., … Hong, Y.G. (2012). U.S. Patent No. 20100087516A1. Washington, DC: U.S. Patent and Trademark Office.
- Kim, D.H., Kim, H.K., Kim, K.M., Kim, C.K., Jeong, M.H., Ko, C.Y., … Kang, J.S. (2011). Antibacterial activities of macrolactin a and 7-O-succinyl macrolactin a from Bacillus polyfermenticus KJS-2 against vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Archives of Pharmacal Research, 34, 147–152. doi:10.1007/s12272-011-0117-0
- Lee, G.W., Ko, J.A., Oh, B.T., Choi, J.R., Lee, K.J., Chae, J.C., & Kamala-Kannan, S. (2012). Biological control of postharvest diseases of apples, peaches and nectarines by Bacillus subtilis S16 isolated from halophytes rhizophere. Biocontrol Science and Technology, 22, 351–361. doi:10.1080/09583157.2012.658553
- Li, Y., Yang, S., & Mu, B. (2010). The surfactin and lichenysin isoforms produced by Bacillus licheniformis HSN 221. Analytical Letters, 43, 929–940. doi:10.1080/00032710903491047
- Mendo, S., Faustino, N.A., Sarmento, A.C., Amado, F., & Moir, A.J.G. (2004). Purification and characterization of a new peptide antibiotic produced by a thermotolerant Bacillus licheniformis strain. Biotechnology Letters, 26, 115–119. doi:10.1023/B:BILE.0000012888.72489.3f
- Nagao, T., Adachi, K., Sakai, M., Nishijima, M., & Sano, H. (2001). Novel macrolactins as antibiotic lactones from a marine bacterium. The Journal of Antibiotics, 54, 333–339. doi:10.7164/antibiotics.54.333
- Nascimento, W.C.A., & Martins, M.L.L. (2006). Studies on the stability of protease from Bacillus sp. and its compatibility with commercial detergent. Brazilian Journal of Microbiology, 37, 307–311. doi:10.1590/S1517-83822006000300020
- Oh, B.T., Hur, H., Lee, K.J., Shanthi, K., Soh, B.Y., Lee, W.J., … Kamala-Kannan, S. (2011). Suppression of phytophthora blight on pepper (Capsicum annuum L.) by bacilli isolated from brackish environment. Biocontrol Science and Technology, 21, 1297–1311. doi:10.1080/09583157.2011.618264
- Park, H.B., Perez, C.E., Perry, E.K., & Crawford, J.M. (2016). Activating and attenuating the amicoumacin antibiotics. Molecules, 21, 824–840. doi:10.3390/molecules21070824
- Pinchuk, I.V., Bressollier, P., Sorokulova, I.B., Verneuil, B., & Urdaci, M.C. (2002). Amicoumacin antibiotic production and genetic diversity of Bacillus subtilis strains isolated from different habitats. Research in Microbiology, 153, 269–276. doi:10.1016/S0923-2508(02)01320-7
- Polikanov, Y.S., Osterman, I.A., Szal, T., Tashlitsky, V.N., Serebryakova, M.V., Kusochek, P., … Sergiev, P.V. (2014). Amicoumacin a inhibits translation by stabilizing mRNA interaction with the ribosome. Molecular Cell, 56, 531–540. doi:10.1016/j.molcel.2014.09.020
- Rey, M.W., Ramaiya, P., Nelson, B.A., Brody-Karpin, S.D., Zaretsky, E.J., Tang, M., … Berka, R.M. (2004). Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biology, 5, R77. doi:10.1186/gb-2004-5-10-r77
- Romero-Tabarez, M., Jansen, R., Sylla, M., Lünsdorf, H., Häussler, S., Santosa, D.A., … Molinari, G. (2006). 7-O-malonyl macrolactin A, a new macrolactin antibiotic from Bacillus subtilis active against methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, and a small variant of Burkholderia cepacia. Antimicrobial Agents and Chemotherapy, 50, 1701–1709. doi:10.1128/AAC.50.5.1701-1709.2006
- Schneider, K., Chen, X.H., Vater, J., Franke, P., Nicholson, G., Borriss, R., & Süssmuth, R.D. (2007). Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. Journal of Natural Products, 70, 1417–1423. doi:10.1021/np070070k
- Sharma, S., Singh, R.L., & Kakkar, P. (2010). Bacillus licheniformis IITRHR2: A novel source of antimicrobial proteinaceous food substance. Journal of Microbiology and Antimicrobials. 2, 127–133. Retrieved from http://www.academicjournals.org/journal/JMA/article-abstract/1A08FC610026
- Shimojima, Y., Hayashi, H., Ooka, T., & Shibukawa, M. (1982). Production, isolation and pharmacological studies of AI-77s. Agricultural and Biological Chemistry, 46, 1823–1829. doi:10.1080/00021369.1982.10865335
- Shimojima, Y., Hayashi, H., Ooka, T., Shibukawa, M., & Iitaka, Y. (1982). (Studies on AI-77s, microbial products with pharmacological activity) structures and the chemical nature of AI-77s. Tetrahedron Letters, 23, 5435–5438. doi:10.1016/0040-4039(82)80150-0
- Shimojima, Y., Hayashi, H., Ooka, T., Shibukawa, M., & Iitaka, Y. (1984). Studies on AI-77s, microbial products with gastroprotective activity. Structure and the Chemical Nature of AI-77s. Tetrahedron, 40, 2519–2527. doi:10.1016/S0040-4020(01)83504-3
- Shinkaruk, S., Bennetau, B., Babin, P., Schmitter, J.M., Lamothe, V., Bennetau-Pelissero, C., & Urdaci, M.C. (2008). Original preparation of conjugates for antibody production against amicoumacin-related anti-microbial against. Bioorganic & Medicinal Chemistry, 16, 9383–9391. doi:10.1016/j.bmc.2008.08.017
- Stein, T. (2005). Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Molecular Microbiology, 56, 845–857. doi:10.1111/j.1365-2958.2005.04587.x
- Sun, H., Yao, X., Wang, X., Wu, Y., Liu, Y., Tang, J., & Feng, J. (2015). Chemical composition and in vitro antioxidant property of peptides produced from cottonseed meal by solid-state fermentation. CyTa – Journal of Food, 13, 264–272. doi:10.1080/19476337.2014.948072
- Suwanmanon, K., & Hsieh, P.C. (2014). Isolating Bacillus subtilis and optimizing its fermentative medium for GABA and nattokinase production. CyTa – Journal of Food, 12, 282–290. doi:10.1080/19476337.2013.848472
- Thirabunyanon, M., & Thongwittaya, N. (2012). Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella Enteritidis infection. Research in Veterinary Science, 93, 74–81. doi:10.1016/j.rvsc.2011.08.008
- Wu, S., Jia, S., Sun, D., Chen, M., Chen, X., Zong, J., & Huan, L. (2005). Purification and characterization of two novel antimicrobial peptides subpeptin JM4-A and subpeptin JM4-B produced by Bacillus subtilis JM4. Current Microbiology, 51, 292–296. doi:10.1007/s00284-005-0004-3
- Xie, B.H., Guan, Z., & He, Y.H. (2012). Biocatalytic knoevenagel reaction using alkaline protease from Bacillus licheniformis. Biocatalysis and Biotransformation, 30, 238–244. doi:10.3109/10242422.2012.662961
- Yuan, J., Li, B., Zhang, N., Waseem, R., Shen, Q., & Huang, Q. (2012). Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 suppressing soilborne plant pathogens. Journal of Agricultural and Food Chemistry, 60, 2976–2981. doi:10.1021/jf204868z
