 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A set of four maltodextrins (MXs) with different degree of polymerization were employed as carrying agents for the spray drying of liquid orange juice (OJ) without collapse in the microstructure; this was visually observed as fine powders non-agglomerated were obtained with a characteristic color. The powders were subjected to water adsorption and characterized. The effect of the MX added was the increase in the adsorption of water and the subsequent depression in the glass transition temperature (Tg). The microstructure did not crystalize at any water activity, but the phase changed from an amorphous solid to a solid covered by saturated liquid. Additionally, a simple mathematical model based on the molar fractions and the Tg of the individual components was proposed for predicting the overall Tg of the ternary system water–OJ–MX. The results presented herein may contribute to the processing of sugar-rich systems and in the stability of food products.
RESUMEN
Se emplearon cuatro maltodextrinas (MX) con diferente grado de polimerización (GP) como agente acarreador para el secado por aspersión sin colapso de la microestructura de jugo de naranja líquido (OJ); esto fue observado visualmente como un polvo de color blanco y apariencia fina sin aglomeración. Los polvos fueron sometidos a un proceso de adsorción de agua y caracterizados. El efecto de la adición de la MX fue el incremento en la adsorción de agua y la subsecuente depresión en el valor de la temperatura de transición vítrea (Tg). Para todas las actividades de agua evaluadas la microestructura no cristalizó, pero la fase cambió de un sólido amorfo a un sólido cubierto por líquido saturado. Adicionalmente se propuso para la predicción del valor de Tg del sistema ternario agua-OJ-MX, el uso de un modelo matemático simple basado en las fracciones molares y los valores de Tg de los componentes individuales. Los resultados obtenidos pueden contribuir para el procesamiento de sistemas ricos en azúcares y en la estabilidad de productos alimenticios.
1. Introduction
Maltodextrin (MX) is a polysaccharide derivate from the acid or enzymatic hydrolysis of starch with a nutrimental contribution of only four calories per gram. The chemical structure of MX is composed of a mixture of carbohydrates with different degree of polymerization (DP) and branching, i.e. amylose (linear) and amylopectin (branched). MX is considered as a D-glucose polymer joined by glycosidic bonds α-(1–4) and α-(1–6). The physicochemical and functional properties of MX and hence the potential applications depend on the DP, the molecular weight distribution (MWD) and the initial state of the powder (Hadnađev et al., Citation2014; Udomrati & Gohtani, Citation2015). Additionally, to the high cost-benefit, the MXs are largely used in industry because they improve the body and texture of the product without imparting a floury taste, help in the control of both, sweetness and hygroscopicity, and reduce the crystallization (Hadnađev et al., Citation2014). Due to the high solubility in water, the MX is one of the additives most used in the encapsulating industry, mainly in spray drying processes, which comprises the transformation of a food product from the liquid state into a dry powder of solid particles (Donhowe, Flores, Kerr, Wicker, & Kong, Citation2014; Santiago et al., Citation2015). In this process, the liquid food product is sprayed inside a conical chamber containing a hot-air flow, producing in this way the abrupt evaporation of water from the product. The main part of the evaporation process is carried out at the wet-bulb temperature. Thus, among the drying processes for powdered foods such as fluidized bed, lyophilization and flash evaporation, the spray-drying process is preferred because it offers low-cost equipment investment, easy operation and control, high drying rates and low residence times (Porras et al., Citation2014; Silva, Navarro, & Morim, Citation2008). These features allow that the heat-sensible food products can be dehydrated at relatively high temperatures while retaining most of the properties, such as flavor, color, odor and nutrients (Castro, Barragán, & Yáñez, Citation2015; Paini et al., Citation2015). However, the drying of sugar-rich food products such as fruit juices is not a trivial task because of the high water content, low molecular weight species and the low glass transition temperatures of the individual components of juice (Jakubczyk, Ostrowska, & Gondek, Citation2010; Karaaslan & Dalgıç, Citation2014). The glass transition temperature (Tg) is a transition dependent on time and comprises the change in any direction from the vitreous state into the rubbery state of a substance subjected to changes in temperature or humidity (Saavedra-Leos et al., Citation2012; Van Sleeuwen, Zhang, & Normand, Citation2012).
Some food products containing water, when they are put in contact with a hot-air flow at a temperature higher than its Tg, tend to stick to the walls of the dryer, generating an agglomerated product instead of a dry powder. This results in low yields, operation problems and low-quality products (Saavedra-Leos et al., Citation2014). In this context, MXs may offer a potential solution based on their physical properties such as high molecular weight, low viscosity and wide Tg range (100–188°C). These properties have been reported that depend on the dextrose equivalent (DE) of MXs, which is an indirect measure of the free glucose functional groups (aldehydes) responsible of granting the reducing power of polysaccharides. However, Saavedra-Leos et al. (Citation2015) recently reported that other physical parameters such as the DP and Tg were more suitable for describing the behavior of MXs (Saavedra et al., Citation2015). Furthermore, based on these parameters, it was possible to indicate the potential technological application of MXs. For example, it was found that the physical state of MXs with a DP of 2–16, Tg in the range of 90–100°C and degradation temperature onset (Td onset) higher than 190°C remained unaffected even at higher conditions of humidity.
Based on the previous, the aim of the present work was to study the influence of MXs when used as a carrier agent in the preparation of a sugar-rich food product such as orange juice (OJ). For this purpose, it was employed a set of four MXs with different DP.
The result of the chemical characterization was employed to set the conditions to obtain a dried food product without microstructural collapse. The powders were subjected to different water adsorption conditions, and thermal analysis was performed. Additionally, a ternary mathematical model was developed to estimate the Tg of the systems. The calculated Tg values were compared with those determined experimentally.
2. Experimental procedure
2.1. Sample preparation
OJ was prepared in the laboratory according to the following composition (w/w): 8.5% of total sugars (53% sucrose, 25.5% fructose and 21.5% glucose), 5.4% of other components (proteins, vitamins and minerals) and 86.1% of water. Four MX powders (MXs) extracted from starch and with different DE were employed as the carrying agent. The MX powders were identified as commercial MX (Mc) and according to the DE: DE 10 (M10), DE 20 (M20) and DE 40 (M40). MXs were acquired commercially in Mexico: Mc powder from INAMALT and M10, M20 and M40 from INGREDION. OJ–MX solutions were prepared at a composition of 70–30 w/w. The dehydration of the OJ–MX solutions was carried out in a Mini Spray Dryer B290 (Buchi, Switzerland). Sample was supplied into the dryer at a temperature of 40°C, with a volumetric flow of 7 mL/min and a constant pressure of 1.5 bar. Air flow was set at 28 m3/h, and inlet and outlet temperatures were recorded at 200°C and 70°C, respectively. For obtaining a fully dried product, OJ–MX powders were placed for 7 days into a desiccator containing phosphorous oxide (V) (P2O5).
2.2. Sugar profile determination
The determination of total sugars (glucose, fructose and sucrose) was carried out by high-performance liquid chromatography (HPLC) in an Acquity Arc System (Waters Corp., USA), following the method proposed by Zuleta and Sambucetti (Citation2001). The HPLC system consisted in a vacuum degasser, a quaternary pump, a column heating chamber and a refractive index detector. The injected solution was separated into a microparticulate cation-exchange packed column Sugar Pak™ I column WAT085188 (Water Corp., USA) with dimensions of 6.5 mm of internal diameter and 300 mm in length. The mobile phase was deionized water (HPLC grade) with a rate flow of 1 mL/min. The injection volume of sample was 20 µL. The calibration curve was done employing a sugar standard Carbohydrate kit CAR-10 (Sigma-Aldrich, USA), containing 10 carbohydrates/sugars such as arabinose, fructose, galactose, glucose, α-lactose, maltose, mannose, ribose, sucrose and xylose. The data was analyzed employing the software Empower 2 included with the HPLC apparatus. Each experiment was performed in triplicate.
2.3. Physical characterization and stability
2.3.1. Stabilization of the powders
For stabilization of the OJ–MX powders, six reagents were used to generate an atmosphere with different water activity (aw): NaOH (0.070), MgCl2 (0.328), K2CO3 (0.434), Mg(NO3)2 (0.528), SrCl (0.718) and NaCl (0.75). Approximately 2 g of OJ–MX powder was placed into hermetic containers and incubated for 30 days at 30°C, until reaching the equilibrium at the given aw. After the incubation time was elapsed, water content was measured according to the Association of Official Analytical Chemists (AOAC) method, which requires drying the sample in an oven at 110°C for 2 h. As well, aw was determined employing an Aqualab Series 3 Water Activity Meter (Decagon Devices, Inc., USA). Each experiment was performed in triplicate.
2.3.2. Structural characterization by X-ray diffraction (XRD)
To determine the structure of the OJ–MX powders by XRD, scans were performed in the 2θ range of 10–100°, with step size of 0.016° and 20 s per step in an X’Pert Empyrean diffractometer (Panalytical, The Netherlands) with Cu-Kα radiation (λ=1.5406 Å) operated at 45 kV, 40 mA and equipped with a X’Celerator detector in a Bragg-Brentano geometry.
2.3.3. Thermal characterization by modulated differential scanning calorimetry (MDSC)
Thermograms required to determine the glass transition temperature (Tg) of the OJ–MX samples were acquired in the temperature range of −90°C to 250°C, with a modulation period of 40 s and a temperature amplitude of 1.5°C. Thermal characterization was performed with a modulated DSC Q200 (TA Instruments, USA) equipped with an RCS90 cooling system. Samples ranging in 5–10 mg were encapsulated in Tzero® aluminum pans. Each experiment was repeated three times.
2.4. Glass transition temperature prediction
To evaluate the effect of sugar composition (fructose, glucose and sucrose) on the Tg value of the OJ, the Scheffe’s cubic model was applied to the experimental data. This model is presented in Equation (1):
where TgJ is the calculated glass transition temperature of the OJ in Celsius degrees, XG, XF and XS are the calculated percentages of glucose, fructose and sucrose, respectively, and the αi are the regression coefficients of the model.
The Tg of a binary matrix is commonly estimated based on the solid content according to Equation (2) of Gordon–Taylor:
where Tgb, Tgs and Tgw represent the glass transition temperature of the binary system, solids and water, respectively, Xs and Xw are the mass fraction of the solid and water, respectively and k is known as the Gordon–Taylor parameter that is proportional to the plasticizing effect of the solvent. Additionally, the Gordon–Taylor equation can be modified for ternary systems as Equation (3):
where the TgS, TgJ, Tgw and TgM are the overall glass transition temperature of the system, OJ, water and MX, respectively, XJ, Xw and XM are the corresponding mass fractions of the OJ, water and MX, and kw and kM are the adjusting parameters of model. However, because this model is based on the mass fractions of the components, the predicted Tg may show an inaccurate value. Then, with the aim of evaluating the effect of the molecular weight of MXs (Mi) on the overall Tg of the systems, in this work the extended Gordon–Taylor was modified, considering the molar fractions (Yi) instead of the mass fractions (Xi). The implemented model is presented in Equation (4):
where the YJ, Yw and YM are the corresponding molar fractions of the OJ, water and MX, and kw and kM are the adjusting parameters for water and MX, respectively.
3. Results and discussion
3.1. Carbohydrates profile
In general, fruit juices are composed of a mixture of low molecular weight sugars such as fructose, glucose and sucrose, representing about the 90% in weight of the total solids of the juice. The total sugars profile obtained by HPLC of the OJ showed a composition of sugars of 57%, 23% and 20% of sucrose, glucose and fructose, respectively. The results are in agreement with those reported by Arthey and Ashurst (Citation2001), and Jagtiani and Sakai (Citation1998) who reported the total composition of sugars in OJ as 53% of sucrose, 25.5% of fructose and 21.5% of glucose. In addition to this, Bhandari and Hartel (Citation2005), Gabas, Telis, Sobral, and Telis (Citation2007) and Roos (Citation2002) indicated that the total content of sugars, and the type and composition of the monosaccharides are factors that must be considered for the correct drying of the fruit juices. Because the spray drying is a rapid water removal process, the low molecular weight sugar-rich systems such as the fruit juices may develop an amorphous state because there is no enough time for crystallization. Therefore, the correct identification of the type and the quantification of the composition of the monosaccharides are of great importance for the accurate setting of the spray drying conditions.
3.2. Setting the spray drying conditions based on the Tg of the system
Carbohydrates such as sucrose, fructose and glucose typically present a Tg value in the range of 5–68°C (Jaya & Das, Citation2009). This wide Tg range may induce changes in the initial state of the powders subjected to drying processes such as spray drying (Shrestha, Ua-Arak, Adhikari, Howes, & Bhandari, Citation2007). During the contact with the hot-air flow in the drying process of sugar-rich food products, the microstructure of powders may relax, behaving as syrup and in consequence sticking on the walls of the dryer. Evidently, this produces operation problems and difficulties in predicting the quality of the product. In this sense, Tg value is a parameter largely employed in the prediction and optimization of spray drying processes (Roos, Citation2002). Thus, the total sugar content was employed to calculate the theoretical Tg value of the fruit juices, employing the Scheffe’s cubic model according to Equation (1). Based on this equation, the calculated theoretical TgJ value for the OJ prepared in this work was 41.48°C, which was very close to the value of 46.2°C, determined experimentally by Saavedra-Leos et al. (Citation2012). Although the use of an additive method for sugar-rich systems may be considered inaccurate, the result showed a good approximation to the experimental value, with a difference lower than 10%.
On the other hand, in order to avoid the sticking and structure collapsing observed during the spray drying of food products, some authors have reported the use of carrying agents. These agents must present a Tg value higher than that of the food product. For example, Saavedra-Leos et al. (Citation2014) reported the use of inulin as a carrying agent for the spray drying of OJ without microstructural collapse (Saavedra-Leos et al., Citation2014). They determined the Tg of inulin by MDSC and reported a value of 125°C. With the same purpose, Ruiz et al. (Citation2009) employed a mixture of lactose–MX for the spray drying of passion fruit juice without sticking problems (Ruiz et al., Citation2009). Additionally, Saavedra et al. (Citation2015) reported the Tg in the range of 52–100°C of a set of MXs with different DE and DP (Saavedra et al., Citation2015). Overall, for the drying of fruit juices it is preferred to employ a carrying agent with a Tg higher than 100°C and in a composition relative to the food product of 10–60% in weight (Mishra, Mishra, & Lata, Citation2014; Oberoi & Sogi, Citation2015; Shi, Fang, & Bhandari, Citation2013). It is worth to mention that in the case of multicomponent systems, the overall Tg of the system does not follow an additive behavior of the individual components, and additionally it depends on the amount of water adsorbed from the environment. Thus, the use of binary mathematical models has been employed in order to have a better prediction of the Tg of different dry fruit products (Choudhury, Sahu, & Sharma, Citation2011; Gordon & Taylor, Citation1952; Khalloufi, El-Maslouhi, & Ratti, Citation2000; Mrad, Boudhriou, Kechaou, Courtoisa, & Bonazzi, Citation2012; Symaladevi, Sablani, Tang, Powers, & Swanson, Citation2009). One of these models is based on the mass fraction of water and solids contained in the mixture, which is intended to understand the plasticizing effect of water on the products subjected to a drying process. The Gordon–Taylor model assumes an additive volume law of the repeating monomer units, i.e. in the polymer chains of carbohydrate and protein systems. The k constant of the model indicates the extent of the interactions between the solid components and water. The increase in the k constant is an indication of the increment of plasticization of the system, which is evidenced by the depression in the Tg value with the addition of water. In the extended Gordon–Taylor model, there are two constants resulting from the fitting of the data, kW and kM. The former constant (kW) has the same physical mining as in the binary system, while the kM constant can be related to the hygroscopic nature of the carrying agent (Choudhury et al., Citation2011). Thus, in this work it was developed a simple mathematical model, which considers the molar fraction and the Tg values of the individual sugar components, MX and adsorbed water. The model was presented in Equation (4). The values of the parameters k and Tgs can be estimated by applying an iterative model such as the Newton–Raphson method, setting the value of Tgw as −135°C. The TgJ was calculated from Equation (1), while the values of the TgM were taken from those reported by Saavedra et al. (Citation2015). The values of the molar fractions were calculated by a simple mass balance, based on the dry amounts of each component employed in the preparation of the OJ–MXs mixture (the calculated values will be shown and discussed in Section 3.4). The calculated TgS values of the powders were employed as a reference to set the spray drying conditions of the OJ–MXs according to that described in Section 2. shows digital images of the resulting OJ–MX powders after the spray drying process. As observed, powders identified as OJ–Mc, OJ–M10 and OJ–M20 presented a white color appearance and a fine particulate morphology without microstructural collapse or sticking problems. However, the powder identified as OJ–M40 presented a different performance, showing an orange color appearance and the formation of large clusters of particles, which tend to stick together and on the walls of the dryer. It is worth to mention that the microstructure of the OJ–M40 collapsed with the exerted spray drying conditions, and in consequence, this system was no longer considered in the rest of this investigation. However, the intention of showing the corresponding digital image for the OJ–M40 in ) was to exhibit the collapsed structure and the change in appearance. The individual OJ obtained after the spray drying process was a syrup with a dark orange color appearance. This result was expected because the OJ is very hygroscopic due to the high sugar content, which hinders the complete removal of water. Clearly, the main effect on the addition of carrying agents is the increment on the overall Tg of the system, which is observed macroscopically as a structure free of collapse and appearance in white color. This behavior can be attributed to the differences in the chemical structure of the MXs employed as encapsulating agents in this work. Accordingly, to the work reported by Saavedra et al. (Citation2015) the MWD and specifically the DP of the MXs showed a linear relation with the DE, which was explained in terms of the differences in the molecular branching of the MXs. Thus, the branching increased in the following order: Mc, M10, M20 and M40. Evidently, the M40 was the MX with the more branched chemical structure and in consequence presented more available functional groups (aldehydes) for interacting with the water molecules, i.e. through van der Waals attractions and hydrogen bonding. These molecular interactions are strong enough to restrict the use of MXs such as M40 in applications where very low water adsorption is required. In contrast, the MWD and DP of the other three MXs (Mc, M10 and M20) resulted in relatively low chemical interactions between the functional groups of the MXs and the water molecules from the OJ, and then allowed the satisfactory spray drying of the OJ–MXs. These results are in agreement with those reported by Mishra, Mishra and Mahanta (Citation2014), Oberoi and Sogi (Citation2015) and Fang and Bhandari (Citation2010) who employed MXs with similar DE values to those employed in this work for Mc and M10, in the drying of watermelon juice, Indian gooseberry (Phyllanthus emblica) and bayberry (Myrica pensylvanica), respectively (Fang & Bhandari, Citation2010; Mishra et al., Citation2014; Oberoi & Sogi, Citation2015). In previous works, it has been reported that the main benefits of adding a carrying agent (MX) to a sugar-rich system (OJ) are the increase of the overall Tg and the subsequent preservation of the physicochemical properties (Araujo-Díaz, Leyva-Porras, Aguirre-Bañuelos, Alvarez-Salas, & Saavedra-Leos, Citation2017; Saavedra et al., Citation2015). Thus, for the effective removal of water, it is desirable that the Tg of the carrying agent presents a value above 100°C. The results presented here showed that the only MX with this feature was Mc, while the M10 and M20 showed values of 90°C and 80°C, respectively. However, after the processing of the OJ, the three systems presented a microstructure without collapse. Evidently, the rule does not hold for the systems OJ–M10 and OJ–M20. This is because the effective encapsulation of sugar-rich systems depends on factors such as the mass ratio of components (i.e. OJ and MX), the selected drying temperature, the chemical composition of the juice, and the MWD and DP of the carrying agent. The results of the spray drying showed that the use of these MXs as encapsulating agents was suitable for obtaining the OJ-MXs without processing problems.
3.3. Microstructural analysis
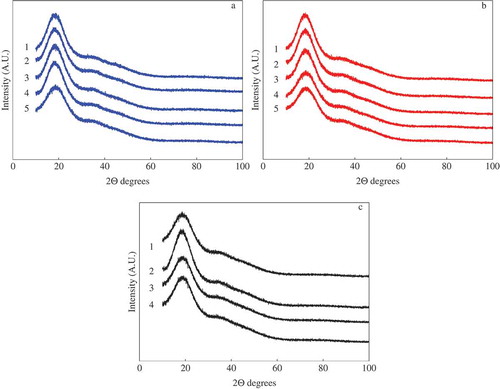
shows the diffraction patterns for the OJ–MXs at the different water activities and includes only the diffraction patterns of the OJ–MX samples that remained in the solid state after the adsorption of water at the given aw, i.e. only those samples where saturation of water was not presented. Overall, the OJ–Mc and OJ–M10 presented a similar behavior, where the microstructure remained in the amorphous state in the range of aw of 0.07–0.532. For a water activity greater than 0.532, the microstructure of the systems started to saturate of water, presenting liquid condensation and the subsequent liquid phase. In the case of the OJ–M20, the previous behavior was observed at lower values of aw. The microstructure of this system remained amorphous until a maximum aw value of 0.434 and above this activity liquid phase was observed. It is important to note that crystallization was not observed in any of the three systems at any value of aw. These results suggested that the use of MXs as carrying agents was suitable not only for obtaining dry powders of low molecular weight systems such as the OJ without the collapse of the microstructure, but additionally for avoiding the crystallization of the system subjected to water adsorption. Saavedra-Leos et al. (Citation2014) studied the effect of the addition of inulin as a carrying agent in the drying of OJ (Saavedra-Leos et al., Citation2014). They subjected the dried powders to water adsorption in the water activity range of 0.05–0.71 and observed the formation of crystals at water activities above 0.434. Furthermore, Saavedra et al. (Citation2015) showed the effect of the molecular weight on the physical properties of a set of MXs also subjected to water adsorption in the water activity range of 0.07–0.75 (Saavedra-Leos et al. Citation2015). They found by X-ray and optical microscopy that the microstructure of the MX remained unchanged, i.e. in the amorphous state in the whole range of aw for the MXs identified as Mc and M10, while the MX M20 presented the phase change at the water activity of 0.434. However, they mentioned that none of the MXs analyzed in the work presented evidence of crystallization during the adsorption of water but showed only phase change, first from solid to liquid condensation, and second the formation of the liquid phase. After comparing the results and observations reported in the works of Saavedra-Leos et al. (Citation2014, Citation2015) with those reported herein, it is possible to infer that the main difference in the behavior of the carrying agents such as MX and inulin lies in the chemical structure of each agent. Although both agents are considered as carbohydrate polymers, the chemical structure of the inulin is based on a polymer of fructose units terminated by glucose units, while the chemical structure of the MX is a polymer formed by linear and branched glucose units. This suggests that the fructose units in the inulin are more susceptible to crystallize when subjected to water adsorption than glucose units in the MX. Conversely, the glucose units in the MX did not crystallize with the adsorption of water, but presented only a phase change from amorphous dried powders into liquid saturation and further liquid condensation.
Figure 2. Diffractograms determined by XRD of the powders: (a) OJ–Mc, (b) OJ–M10 and (c) OJ–M20. The corresponding water activity (aw) is indicated by the number written next to each curve: (1) 0.07, (2) 0.328, (3) 0.434, (4) 0.528 and (5) 0.718.
Figura 2. Difractogramas obtenidos por XRD de los polvos: A) OJ-Mc, b) OJ-M10, y c) OJ-M20. La actividad de agua (aw) correspondiente se indica en cada curva con la siguiente numeración: 1) 0,07, 2) 0,328, 3) 0,434, 4) 0,528 y 5) 0,718.
3.4. Determination of the Tg by MDSC
The dried powders of fruit juices are characterized by a uniform vitreous microstructure, with high viscosity and reduced molecular mobility, which limits the diffusion phenomena (Santivarangkna, Aschenbrenner, Kulozik, & Foerst, Citation2011). One of the main parameters related to the physical stability during storage of dried powders of fruit juices is the glass transition temperature, where the degree of other phenomena such as agglomeration, caking and loss of volatiles depend on the Tg value of the system. If the system is stored at temperatures above its Tg or it is subjected to relatively high humidity conditions, the system may experience a sudden change in the microstructure, from the rigid amorphous state into the rubber state. In the rubber state is presented a decrease in the viscosity of the system, which increases the molecular mobility and in consequence it may promote changes such as the collapse of the microstructure, the formation of big agglomerates of particles and the sticking of the powder. Thus, the physical stability of fruit juices may be improved if the powder is stored at temperatures below the Tg. Therefore, in this lies the importance of accurately determining the Tg of dried food products such as OJ powder. One advantage of employing MDSC is that it may decompose the total heat signals into two components, the reversible and non-reversible contributions, leading to a more accurate determination of the Tg and the correct identification of other thermal events. While in the non-reversible heat flow curve the kinetic processes depending on the temperature are observed such as evaporation, crystallization, solid–solid transitions and thermal decomposition, in the reversible heat flow curve the time-dependent phenomena are included such as the glass transition temperature and melting. shows the total, reversible and non-reversible heat flow thermograms determined by MDSC of the OJ–MX systems at aw of 0.07. It is worth to mention that melting was not observed for any of the OJ–MX systems at any of the water activities. On the reversible heat flow curve the temperature range was included where the Tg onset was observed. Tg was determined from the stepped difference in the slope of the heat flow. summarizes the Tg values of the OJ–MX systems at the different water activities determined by MDSC. Overall, the tendency of the Tg was to decrease with the adsorption of water. This behavior is commonly observed in sugar-rich systems and may be attributed to the plasticizer effect caused by the enhanced adsorption of water, promoted by the hydrophilic groups. This causes an increase in the intermolecular free volume space, lowering the viscosity of the system and increasing the molecular mobility (Collares, Kieckbusch, & Finzer, Citation2002). The effect of the molecular weight of the MX was also observed in the results of . As reported by Saavedra et al. (Citation2015). the MXs employed in that study showed differences in the MWD and DP, i.e. the Mc and M10 showed DP values of 2–12 and 2–16, respectively, while the M20 showed a DP value of 2–21. The increase in the DP suggested differences in the branching of the MXs and in the availability of the functional groups available for establishing intra- and inter-molecular interactions with the water molecules. Since the MXs employed in the present work were similar to those reported by Saavedra-Leos et. al. (Citation2015), it was possible to associate the effect of the DP of the MXs with the Tg of the systems. Additionally, the complex chemical composition of the OJ depressed even more the Tg of the OJ–MX systems. This was caused by the increase of the functional groups added by the sugars (sucrose, glucose and fructose) contained in the OJ, generating a more complex system.
Figure 3. Thermograms determined by MDSC of the powders: (a) OJ-Mc, (b) OJ–M10 and (c) OJ–M20. Each plot contains three curves, identified in the following order as: total heat flow (on top), reversible heat flow (in the middle) and non-reversible heat flow (bottom curve).
Figura 3. Termogramas obtenidos por MDSC de los polvos: A) OJ-Mc, b) OJ-M10, y c) OJ-M20. Cada gráfica contiene tres curvas, que se identifican en el siguiente orden: flujo de calor total (curva superior), flujo de calor reversible (curva media) y flujo de calor no reversible (curva inferior).
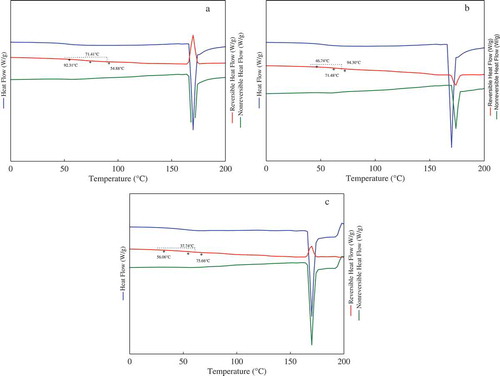
Table 1. Tg values of the OJ–MX systems at the different water activities determined by MDSC.
Table 1. Valores de Tg de los sistemas de OJ-MX´s a diferentes actividades de agua determinados por MDSC.
shows the calculated TgS values for each of the OJ–MX systems and the difference from those determined experimentally. The TgS values were calculated employing Equation (4), and the individual kw and kM are reported in . The largest difference was obtained for the OJ–Mc system at aw of 0.328 with a value of 15.7°C, while the lowest was obtained for the OJ–M20 at the same aw with a value of 0.006°C. All of the other calculated values were within this range, validating the proposed model as a simple approximation for determining the overall Tg of complex systems. The advantage of this model is that it is based on simple experimental measurements such as the molar fractions and the Tg of the individual components of the system. On the other hand, when the binary model (Equation (2)) was employed for calculating the overall Tg of the OJ–MX systems, the Tgb values were similar to those predicted by the ternary model (Equation (4)), but the difference between the calculated and experimental values was slightly larger. The disadvantage of the binary model is that it considers all the solid components as a single contribution, and therefore prevents to understand the interactions of the individual components of the system. shows the calculated values of kw and kM for each OJ–MXs system and the overall k values calculated considering the whole data set. The kw and kM values were in the range of 0.21–5.72, and the values tend to increase with the increase in the MWD of the MXs. The kw and kM values were within the same range as those reported for fruits (Mrad et al., Citation2012; Ruiz et al., Citation2016; Symaladevi et al., Citation2009). According to Arvanitoyannis and Biliaderis (Citation1999), the larger values of the Gordon–Taylor parameter, the greater is the plasticization effect. These results suggested that the OJ–MX systems presented a greater plasticizing effect with the increment of MWD of the corresponding MX. As discussed in the previous sections, the higher the MWD, the more the chemical interactions between the components of the OJ, MX and water. Therefore, it is possible to infer that the k values were related to the chemical interactions developed in the OJ–MX systems when subjected to different conditions of humidity. Additionally, the overall k values reported in may be employed as the starting values for solving the model presented in Equation (3), avoiding the use of random values in the iterative model.
Table 2. Comparison of the TgS values calculated with Gordon–Taylor equation and the Tg values determined by MDSC of the OJ–MX systems at the different water activities.
Table 2. Comparación de los valores de TgS calculados mediante la ecuación de Gordon-Taylor y los valores de Tg determinados mediante MDSC de los sistemas de OJ-MX´s a diferentes actividades de agua.
Table 3. The kw and kM values of Gordon–Taylor equation of the OJ–MX systems.
Table 3. Valores de los parámetros kw y kM de la ecuación de Gordon-Taylor de los sistemas de OJ-MX´s.
4. Conclusion
A set of four MXs with different MWD and DP were employed as carrying agents for the spray drying of liquid OJ. The OJ–MX without microstructural collapse was observed as white color appearance and a non-agglomerated fine powder. The Scheffe’s model showed a good prediction on the Tg of the OJ when compared to that determined experimentally (41.48°C and 46.2°C, respectively). The OJ–MX powders subjected to water adsorption in the water activity range (aw) of 0.07–0.75 resulted in an increase in the amount of adsorbed water, induced by the high hygroscopic MX added. The collapse in the microstructure was analyzed by XRD, showing that above an aw of 0.532 (OJ–Mc and OJ–M10) and 0.434 (OJ–M20), the phase changed from an amorphous solid into a solid covered with saturated liquid. However, at any aw the glucose units contained in the MX suppressed the crystallization of the microstructure. The Tg presented a depression produced by the affinity between the polar functional groups of the OJ and the MX with the water molecules. The effect of the MWD of the MXs on the OJ–MX powders was directly related to the adsorption of water and inversely with the Tg. Additionally, a simple mathematical model was proposed for predicting the overall Tg of the ternary system water–OJ–MX. The model showed good prediction results and was based on the molar fractions and the Tg of the individual components. Overall, the MX can be successfully employed as a carrying agent in the spray drying of OJ without the collapse of the microstructure and in consequence, avoiding processing problems such as sticking to the walls of the dryer, change in appearance and the formation of particle agglomerates.
Acknowledgment
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) in Mexico, through the grant No. 247906.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Araujo-Díaz, S.B., Leyva-Porras, C., Aguirre-Bañuelos, P., Alvarez-Salas, C., & Saavedra-Leos, Z. (2017). Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydrate Polymers, 167, 317–325. doi:10.1016/j.carbpol.2017.03.065
- Arthey, D., & Ashurst, P. (2001). Fruit processing nutrition, products and quality management (2nd ed., pp. 312). Aspen, Colorado: An Aspen Publication.
- Arvanitoyannis, I., & Biliaderis, C.G. (1999). Physical properties of polyol-plasticized edible blends made of methylcellulose and soluble starch. Carbohydrates Polymers, 38, 47–58. doi:10.1016/S0144-8617(98)00087-3
- Bhandari, B.R., & Hartel, R.W. (2005). Phase transitions during food powder production and powder stability. In C. Onwulata (Ed.), Encapsulated and powdered foods (pp. 61–292). Boca Raton: CRC Press-Taylor & Francis Group.
- Castro, M.R., Barragán, H.B.E., & Yáñez, F.J. (2015). Use of gelatin-maltodextrin composite as an encapsulation support for clarified juice from purple cactus pear (Opuntia stricta). LWT - Food Science Technology, 62, 242–248. doi:10.1016/j.lwt.2014.09.042
- Choudhury, D., Sahu, J.K., & Sharma, G.D. (2011). Moisture sorption isotherms, heat of sorption and properties of sorbed water of raw bamboo (Dendrocalamus longispathus) shoots. Industrial Crops and Products, 33, 211–216. doi:10.1016/j.indcrop.2010.10.014
- Collares, F.P., Kieckbusch, T.G., & Finzer, J.R.D. (2002). Review glass transition in food products. Brazilian Journal Food Technology, 5, 117–130.
- Donhowe, E.G., Flores, F.P., Kerr, W.L., Wicker, L., & Kong, F. (2014). Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. Food Science and Technology, 57, 4–48.
- Fang, Z., & Bhandari, B. (2010). Encapsulation of polyphenols – A review. Trends in Food Science and Technology, 21, 510–523. doi:10.1016/j.tifs.2010.08.003
- Gabas, A.L., Telis, V.R.N., Sobral, P.J.A., & Telis, R.J. (2007). Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. Journal of Food Engineering, 82, 246–252. doi:10.1016/j.jfoodeng.2007.02.029
- Gordon, M., & Taylor, J.S. (1952). Ideal copolymers and the second-order transitions of synthetic rubbers. i. Non-crystalline copolymers. Journal of Chemical Technology and Biotechnology, 2, 493–500.
- Hadnađev, M., Hadnađev, T.D., Dokic, L., Pajin, B., Torbica, A., Saric, L., & Ikonic, P. (2014). Physical and sensory aspects of maltodextrin gel addition used as fat 495-replacers in confectionery filling systems. Food Science and Technology, 59, 495–503.
- Jagtiani, J.C., Jr., & Sakai, W.S. (1998). Food science and technology: A series of monographics: Tropical fruit processing (pp. 184). San Diego, California, USA: Academic, Pres. Inc.
- Jakubczyk, E., Ostrowska, L.E., & Gondek, E. (2010). Moisture sorption characteristics and glass transition temperature of apple puree powder. Journal of Food Science and Technology, 45, 2515–2523. doi:10.1111/j.1365-2621.2010.02425.x
- Jaya, S., & Das, H. (2009). Glass transition and sticky point temperatures and stability/mobility diagram of fruit powders. Food Bioprocess Technology, 2, 89–95. doi:10.1007/s11947-007-0047-5
- Karaaslan, I., & Dalgıç, A.C. (2014). Spray drying of liquorice (Glycyrrhiza glabra) extract. Journal of Food Science and Technology, 51, 3014–3025. doi:10.1007/s13197-012-0847-0
- Khalloufi, S., El-Maslouhi, Y., & Ratti, C. (2000). Mathematical model for prediction of glass transition temperature of fruit powders. Journal of Food Science, 65, 842–848. doi:10.1111/jfds.2000.65.issue-5
- Mishra, P., Mishra, S., & Lata, C. (2014). Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of Amla (Emblica officinalis) juice powder. Food and Bioproducts Processing, 92, 252–258. doi:10.1016/j.fbp.2013.08.003
- Mrad, N.D., Boudhriou, N., Kechaou, N., Courtoisa, F., & Bonazzi, C. (2012). Influence for air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food and Bioproducts Processing, 90, 433–441. doi:10.1016/j.fbp.2011.11.009
- Oberoi, D.P.S., & Sogi, D.S. (2015). Drying kinetics, moisture diffusivity and lycopene retention of watermelon pomace in different dryers. Journal Food Science and Technology, 52, 7377–7384. doi:10.1007/s13197-015-1863-7
- Paini, M., Aliakbarian, B., Casazza, A.A., Lagazzo, A., Botter, R., & Perego, P. (2015). Microencapsulation of phenolic compounds from olive pomace using spray drying: A study of operative parameters. LWT - Food Science and Technology, 62, 177–186. doi:10.1016/j.lwt.2015.01.022
- Porras, S.J., Palacios, G.E., Lartundo, R.L., Garibay, F.V., Yáñez, F.J., Hernández, S.H., … Alamilla, B.L. (2014). Microstructural properties and distribution of components in microparticles obtained by spray-drying. Journal of Food Engineering, 152, 105–112. doi:10.1016/j.jfoodeng.2014.11.014
- Roos, Y. (2002). Importance of glass transition and water activity to spray drying and stability of dairy powders. Le Lait, INRA EDP Science, 82, 475–484. doi:10.1051/lait:2002025
- Ruiz, C.M.A., Espinosa, M.C.L., Aviles, A.C., González, G.R., Moscosa, S.M., Grajales, L.A., & Abud, A.M. (2009). Spray-drying of passion fruit juice using lactose maltodextrin blends as the support material. Brazilian Archives of Biology and Technology. an International Journal, 52, 1011–1018. doi:10.1590/S1516-89132009000400026
- Ruiz-Cabrera, M.A., Rivera-Bautista, C., Grajales-Lagunes, A., González-García, R., & Schmidt, S.J. (2016). State diagrams for mixtures of low molecular weight carbohydrates. Journal of Food Engineering, 171, 185–193. doi:10.1016/j.jfoodeng.2015.10.038
- Saavedra, L.Z., Leyva, P.C., Araujo, D.S.B., Toxqui, T.A., & Borrás, E.A.J. (2015). Technological application of maltodextrins according to the degree of polymerization. Molecules, 20, 21067–21081. doi:10.3390/molecules201219746
- Saavedra-Leos, M.Z., Álvarez-Salas, C., Esneider-Alcalá, M.A., Toxqui-Terán, A., Pérez-García, S.A., & Ruiz-Cabrera, M.A. (2012). Towards an improved calorimetric methodology for glass transition temperature determination in amorphous sugars. Cyta-Journal of Food, 10, 258–267. doi:10.1080/19476337.2011.639960
- Saavedra-Leos, M.Z., Leyva-Porras, C., Martínez-Guerras, E., Pérez-García, S.A., Aguilar-Martínez, J.A., & Álvarez-Salas, C. (2014). Physical properties of inulin and inulin–orange juice: Physical characterization and technological application. Carbohydrate Polymers, 105, 10–19. doi:10.1016/j.carbpol.2013.12.079
- Santiago, A.R., Medina, T.L., Gallegos, I.J.A., Calderas, F., González, L.R.F., Rocha, G.N.E., … Bernard, B.M.J. (2015). Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. Food Science and Technology, 64, 571–577.
- Santivarangkna, C., Aschenbrenner, M., Kulozik, U., & Foerst, P. (2011). Role of glassy state on stabilities of freeze-dried probiotics. Journal of Food Science, 76, 152–156. doi:10.1111/j.1750-3841.2011.02347.x
- Shi, Q., Fang, Z., & Bhandari, B. (2013). Effect of addition of whey protein isolate on spray-drying behavior of honey with maltodextrin as a carrier material. Drying Technology: An International Journal, 31, 1681–1692. doi:10.1080/07373937.2013.783593
- Shrestha, A.K., Ua-Arak, T., Adhikari, B.P., Howes, T., & Bhandari, B.R. (2007). Glass transition behavior of spray dried orange juice powder measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT). International Journal of Food Properties, 10, 661–673. doi:10.1080/10942910601109218
- Silva, C.L., Navarro, D.N., & Morim, A. (2008). Spray drier atomization of milk. In M.M. Vieira & P. Ho (Eds.), Experiments in unit operations and processing of food, Part II (pp. 77–80). New York, U.S.A: Springer Science + Business Media, LLC.
- Symaladevi, R.M., Sablani, S.S., Tang, J., Powers, J., & Swanson, B.G. (2009). State diagram and water adsorption isotherm of raspberry (Rubus idaeus). Journal Food Engineering, 91, 460–467. doi:10.1016/j.jfoodeng.2008.09.025
- Udomrati, S., & Gohtani, S. (2015). Tapioca maltodextrin fatty acid ester as a potential stabilizer for Tween 80-stabilized oil-in-water emulsions. Food Hydrocolloides, 44, 23–31. doi:10.1016/j.foodhyd.2014.08.015
- Van Sleeuwen, R.M.T., Zhang, S., & Normand, V. (2012). Spatial glass transition temperature variations in polymer glass: Application to a maltodextrin – water system. Biomacromolecules, 13, 787–797. doi:10.1021/bm201708w
- Zuleta, A., & Sambucetti, M. (2001). Inulin determination for food labeling. Journal Agriculture and Food Chemistry, 49, 4570−4572. doi:10.1021/jf010505o

